Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PIERIS PHARMACEUTICALS, INC. | form8-k.htm |

INVESTOR PRESENTATION JANUARY 2020

Forward Looking Statements This presentation contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward- looking statements. Such forward-looking statements include, among other things, references to novel technologies and methods and our business and product development plans, including the advancement of our proprietary and co-development programs into and through the clinic. Actual results could differ from those projected in any forward-looking statements due to numerous factors. Such factors include, among others, our ability to raise the additional funding we will need to continue to pursue our business and product development plans; the inherent uncertainties associated with developing new products or technologies and operating as a development stage company; our ability to develop, complete clinical trials for, obtain approvals for and commercialize any of our product candidates, including our ability to recruit and enroll patients in our studies; our ability to address the requests of the FDA; competition in the industry in which we operate and market conditions. These forward-looking statements are made as of the date of this press release, and we assume no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. Investors should consult all of the information set forth herein and should also refer to the risk factor disclosure set forth in the reports and other documents we file with the SEC available at www.sec.gov, including without limitation the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2018 and the Company's Quarterly Reports on Form 10-Q. 2

What are Anticalin® proteins? A Novel Therapeutic Class with Underpinned by a Powerful Favorable Drug-Like Properties Target Drug Discovery Platform • Derived from lipocalins (human • Highly diverse libraries (>1011) of extracellular binding proteins) potential drug candidates… • TLC and NGAL lipocalins used as • Automated high-throughput drug “templates” for drug development screening technology (phage • Engineerable binding pocket for display)… robust target engagement • Extensive protein engineering • Monomeric, monovalent, small size know-how… (~18 kDa vs 150kDa mAbs) • …resulting in high hit rates, • Can be formulated for inhalable quick-to-development candidates delivery • Can be formatted into novel bi/multispecific constructs Anticalin • Broad IP position Protein 3

Company Snapshot Pipeline Highlights Anchor Partnerships 2020 Catalysts • Respiratory: • PRS-060: Inhaled IL4-Rα antagonist • Validation through three anchor PRS-060 phase 2a trial initiation for moderate-to-severe asthma partnerships Data and rationale for advancement (partnered with AstraZeneca) • $120+M in upfront payments and into IND-enabling studies for wholly- • Next-generation respiratory: milestones since January 2017 owned inhaled program Includes 4 discovery-stage inhaled • Each partnership includes options for • IO: therapeutics programs (2 proprietary, 2 co-development & US-focused PRS-343 complete monotherapy phase 1 escalation data partnered with AstraZeneca) commercialization rights PRS-343 complete combination with • PRS-343: 4-1BB/HER2 bispecific for • Value-driving opt-in for PRS-060 after atezolizumab phase 1 escalation data solid tumors phase 2a completion PRS-343 phase 1 expansion initiation • PRS-344: 4-1BB/PD-L1 bispecific PRS-344 IND 1H2020 (partnered with Servier) 4

Partnerships • PRS-060 + 4 additional novel inhaled • PRS-344: PD-L1/4-1BB antibody-Anticalin • 3-program partnership based on tumor- Anticalin protein programs bispecific localized costimulatory bispecific fusion proteins • Retained co-development and co- • 5-program deal (all bispecific fusion commercialization (US) options on PRS- proteins) • Pieris retains opt-in rights for 50/50 global 060 and up to 2 additional programs profit split and U.S. commercialization • Pieris retains option for full U.S. rights for 3 rights on one of the programs • $57.5M upfront & 2017 milestone out of 5 programs • $30M upfront payment, ~$1.2B milestone • ~$2.1B in milestone potential, plus double- • ~$31M upfront payment, ~$1.8B milestone potential digit royalties potential Two preclinical milestones achieved • Up to double-digit royalties on non-co- • AZ funds all PRS-060 development costs developed products through post-phase 2a co-development for PRS-344 opt-in decision • Up to low double-digit royalties on non-co- • Access to complementary formulation and developed products device know-how for inhaled delivery Strong Partners • Significant Cash Flow • Retained Commercial Rights 5
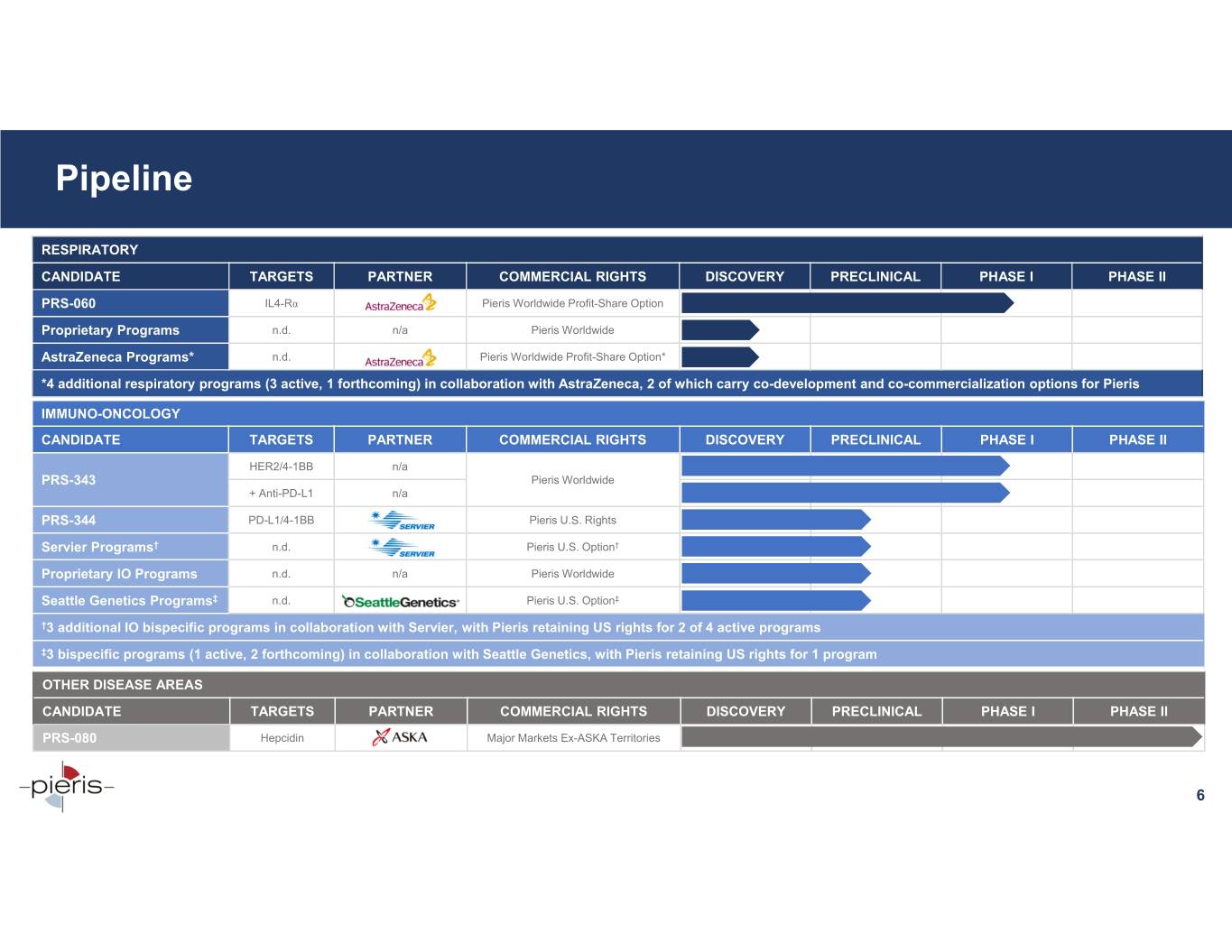
Pipeline RESPIRATORY CANDIDATE TARGETS PARTNER COMMERCIAL RIGHTS DISCOVERY PRECLINICAL PHASE I PHASE II PRS-060 IL4-Rα Pieris Worldwide Profit-Share Option Proprietary Programs n.d. n/a Pieris Worldwide AstraZeneca Programs* n.d. Pieris Worldwide Profit-Share Option* *4 additional respiratory programs (3 active, 1 forthcoming) in collaboration with AstraZeneca, 2 of which carry co-development and co-commercialization options for Pieris IMMUNO-ONCOLOGY CANDIDATE TARGETS PARTNER COMMERCIAL RIGHTS DISCOVERY PRECLINICAL PHASE I PHASE II HER2/4-1BB n/a PRS-343 Pieris Worldwide + Anti-PD-L1 n/a PRS-344 PD-L1/4-1BB Pieris U.S. Rights Servier Programs† n.d. Pieris U.S. Option† Proprietary IO Programs n.d. n/a Pieris Worldwide Seattle Genetics Programs‡ n.d. Pieris U.S. Option‡ †3 additional IO bispecific programs in collaboration with Servier, with Pieris retaining US rights for 2 of 4 active programs ‡3 bispecific programs (1 active, 2 forthcoming) in collaboration with Seattle Genetics, with Pieris retaining US rights for 1 program OTHER DISEASE AREAS CANDIDATE TARGETS PARTNER COMMERCIAL RIGHTS DISCOVERY PRECLINICAL PHASE I PHASE II PRS-080 Hepcidin Major Markets Ex-ASKA Territories 6
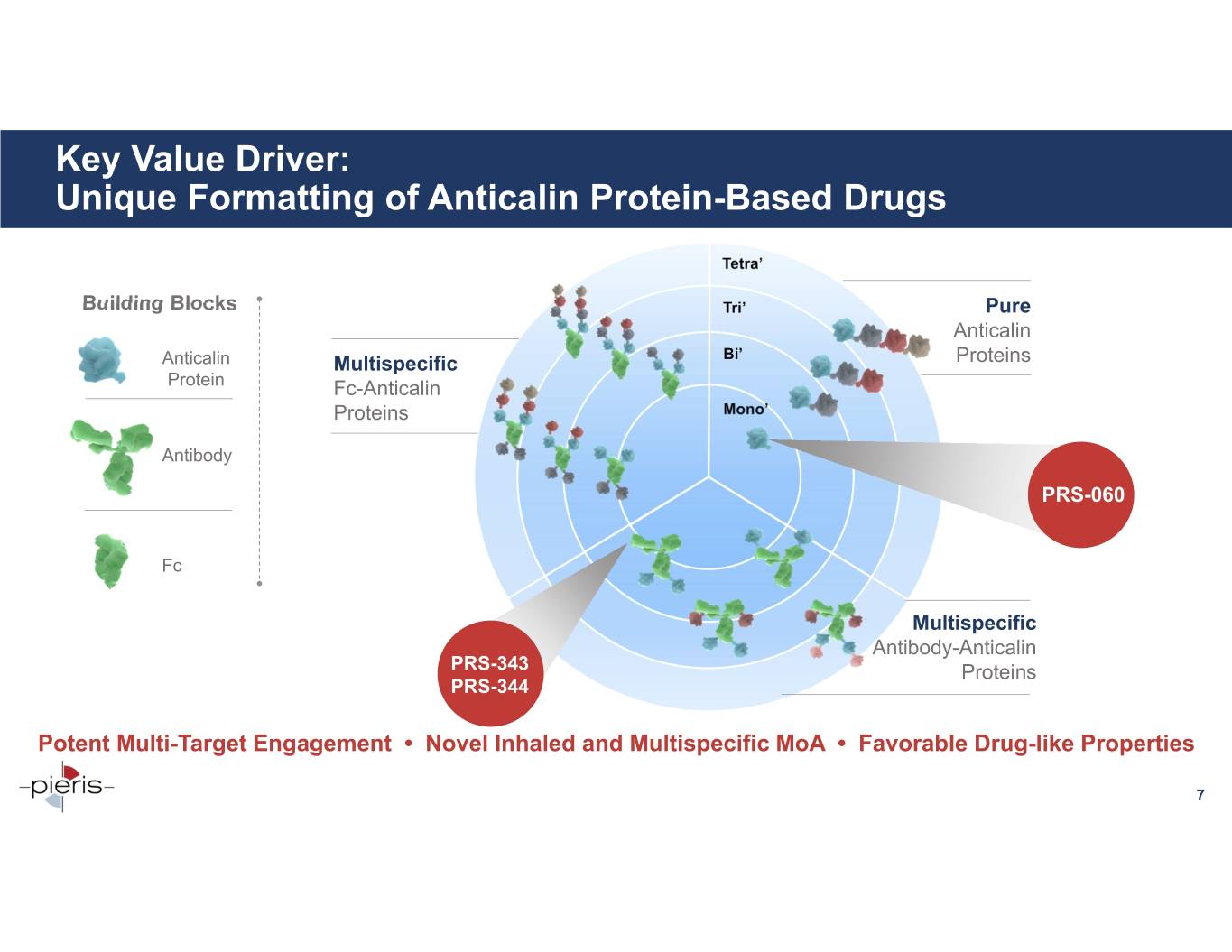
Key Value Driver: Unique Formatting of Anticalin Protein-Based Drugs Building Blocks Pure Anticalin Anticalin Multispecific Proteins Protein Fc-Anticalin Proteins Antibody PRS-060 Fc Multispecific Antibody-Anticalin PRS-343 Proteins PRS-344 Potent Multi-Target Engagement • Novel Inhaled and Multispecific MoA • Favorable Drug-like Properties 7

Anticalin Technology Advantages: Differentiated Respiratory Platform Tear lipocalin (TLC) is abundant in human lung and permeates lung epithelium Very low predicted immunogenicity Stable, monovalent molecules with high melting temperatures and insensitivity to mechanical stress Inhalation pharmacokinetics suitable for once or twice daily administration and compatible with flexible treatment regimes 8

PRS-060: IL-4Rα Antagonist Candidate PRS-060 Inhibiting IL4-Rα (disrupts IL-4 & IL-13 Function/MoA signaling) Indications Moderate-to-severe asthma Development Phase 1 multiple-ascending dose trial ongoing PRS-060 Co-development and U.S. co-commercialization Commercial Rights rights, including gross margin share 9

PRS-060’s Potency is Similar to that of Dupilumab PRS-060 reduces levels of pSTAT6, eotaxin-3, TARC and MDC in a comparable manner to dupilumab IC [nM] IC [nM] IC [nM] IC [nM] Drug 50 50 50 50 pSTAT6 Eotaxin-3 TARC MDC PRS-060 1.3 2.1 1.3 2.0 Dupilumab 0.8 1.5 0.8 1.1 PRS‐060 Katerina Pardali et al. AZD1402/PRS-060, an inhaled Anticalin® IL4-Rα antagonist, potently inhibits IL-4 induced functional effects in human whole blood, which can PRS‐060 be employed translationally in clinical studies. Poster presented at: European Respiratory Society International Congress 2018; 2018 Sep 19; Munich, Germany. 10

FeNO is a Validated Biomarker in Allergic Asthma Interventions Elevated fractional exhaled nitric oxide (FeNO) is a marker Biologics that have demonstrated a meaningful of allergic asthma reduction in FeNO (dupilumab, tezepelumab) have subsequently produced clinically- significant improvements in lung function and superior exacerbation improvements versus Nitric Oxide (NO) drugs that had no on effect FeNO Dupilumab was recently approved by the EMA for severe asthma in patients with either high eosinophils (EOs) or high FeNO We are exploring FeNO reduction versus placebo in a multi-dose ascending phase 1 study of PRS-060 Positive FeNO data from this study would support continued development to assess the Normal epithelial cells During airway inflammation, activated potential to improve lung function (FEV1) in release minimal NO epithelial cells increase production of NO uncontrolled asthmatics 11

PRS-060 Phase I Multiple Ascending Dose Trial Ascertain PK/PD with a reliable biomarker to confirm local target engagement and Strategic Objectives inform Phase II dosage regimen Dosing patients with mild asthma with elevated FeNO levels (≥35 ppb), to receive Trial Design Highlights inhaled PRS-060 or pbo b.i.d.* over a 10-day period *q.d. on Day 10 Initiated in July 2018 Evaluating safety, tolerability, PK, PD and will also evaluate FeNO reduction vs. placebo Measuring safety, tolerability and FeNO changes days 1-10,17, and 40 Pieris is sponsoring the trial, AstraZeneca is reimbursing Pieris for all associated costs 12

Phase 1b Interim Results: Favorable Safety Profile • All doses of AZD1402/PRS-060 tested in the study were well tolerated • No treatment-related serious AEs were observed Placebo AZD1402/PRS-060c Overall System organ class (N = 12) (N = 30) (N = 42) AE Preferred Termsb n (%) m n (%) m n (%) m Gastrointestinal disorders 4 (33.3) 4 13 (43.4) 14 17 (40.5) 18 Dry mouth 1 (8.3) 1 2 (6.7) 2 3 (7.1) 3 Nausea 1 (8.3) 1 3 (10.0) 3 4 (9.5) 4 Infections and infestations 1 (8.3) 1 7 (23.3) 8 8 (19.0) 9 Upper respiratory tract infection 1 (8.3) 1 3 (10.0) 4 4 (9.5) 5 Nervous system disorders 5 (41.7) 9 13 (43.4) 18 18 (42.9) 27 Headache 3 (25.0) 6 5 (16.7) 7 8 (19.0) 13 Presyncope 0 4 (13.3) 6 4 (9.5) 6 Respiratory, thoracic and mediastinal disorders 6 (50.0) 6 14 (46.7) 15 20 (47.6) 21 Cough 1 (8.3) 1 4 (13.3) 4 5 (11.9) 5 Rhinorrhoea 2 (16.7) 2 1 (3.3) 1 3 (7.1) 3 Wheezing 2 (16.7) 2 4 (13.3) 5 6 (14.3) 7 13

Phase 1b Interim Results: Robust FeNO Reduction PRS-060 Relative FeNO Reduction (Emax Analysis) PRS-060 Relative FeNO Reduction (ANCOVA Analysis) Mean change from baseline in FeNO levels at 0.5h (A) and 2h (B) post-dose on Day 10 in participants with mild asthma Reduction vs. PRS-060, mg n placebo, % (95% p-value (delivered) CI) 2 6 24.0 (1.8–41) 0.04 6 6 24.3 (2.7–41) 0.03 20 12 36.4 (22–48) <0.0001 60 6 30.5 (10–46) 0.005 Placebo 12 14
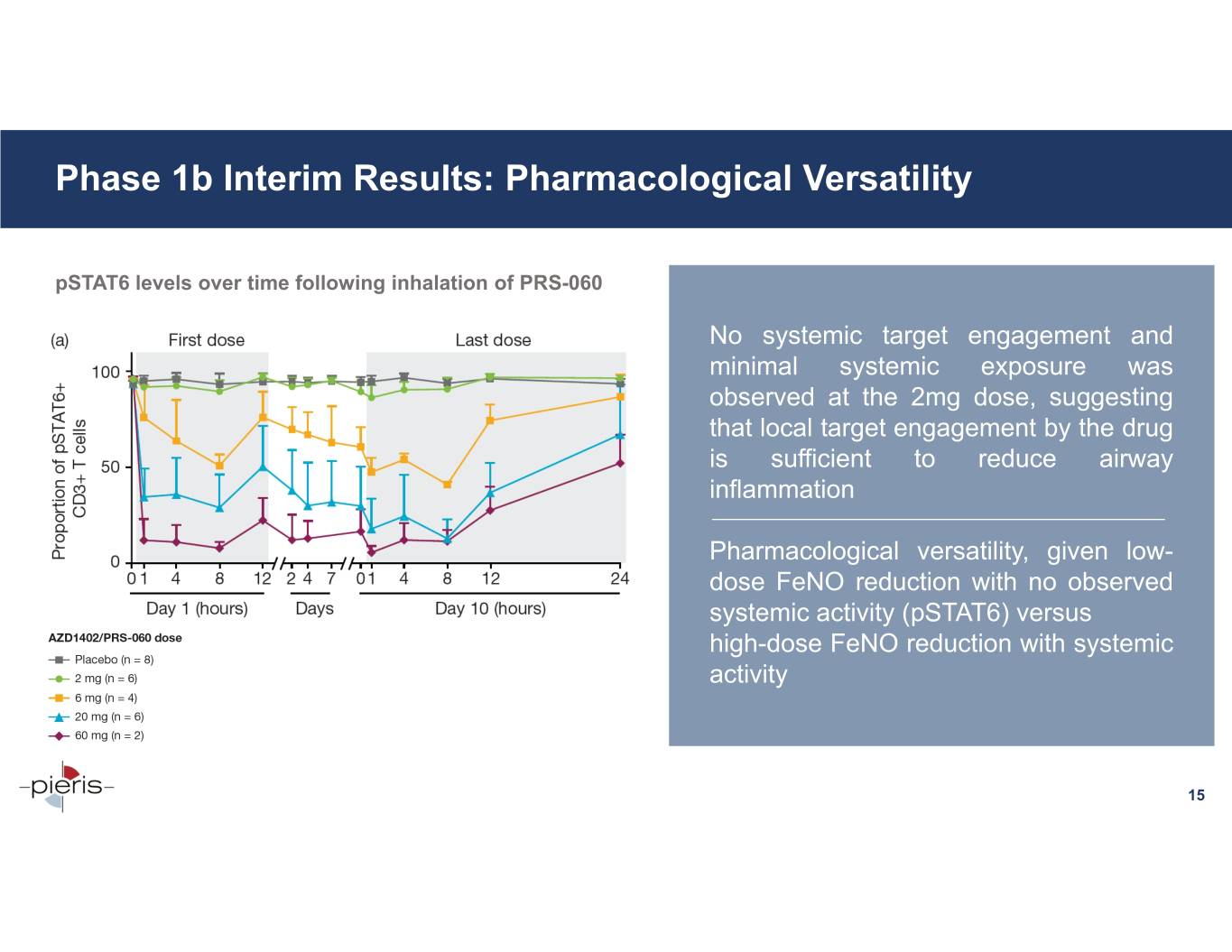
Phase 1b Interim Results: Pharmacological Versatility pSTAT6 levels over time following inhalation of PRS-060 No systemic target engagement and minimal systemic exposure was observed at the 2mg dose, suggesting that local target engagement by the drug is sufficient to reduce airway inflammation Pharmacological versatility, given low- dose FeNO reduction with no observed systemic activity (pSTAT6) versus high-dose FeNO reduction with systemic activity 15

Moderate-to-Severe Asthma Market Opportunity U.S. EU asthma patients over 12 asthma patients over 12 19.0M years of age in the U.S. 19.0M47.8M years of age in the EU with moderate-to- 21.5M with moderate-to- 7.8M7.8M severe asthma (41%) severe asthma (45%) 3.1M3.1M uncontrolled (40%) 8.6M uncontrolled (40%) 1.9M high EOs (60%) 1.2M low EOs (40%) 5.2M high EOs (60%) 3.4M low EOs (40%) All numbers reflect 2016 estimates. Source: Artisan Healthcare Consulting analysis, including the following: CDC, Eurostat, Rabe (2004), Cazzoletti (2007), Colice (2012), Hekking (2015). 16
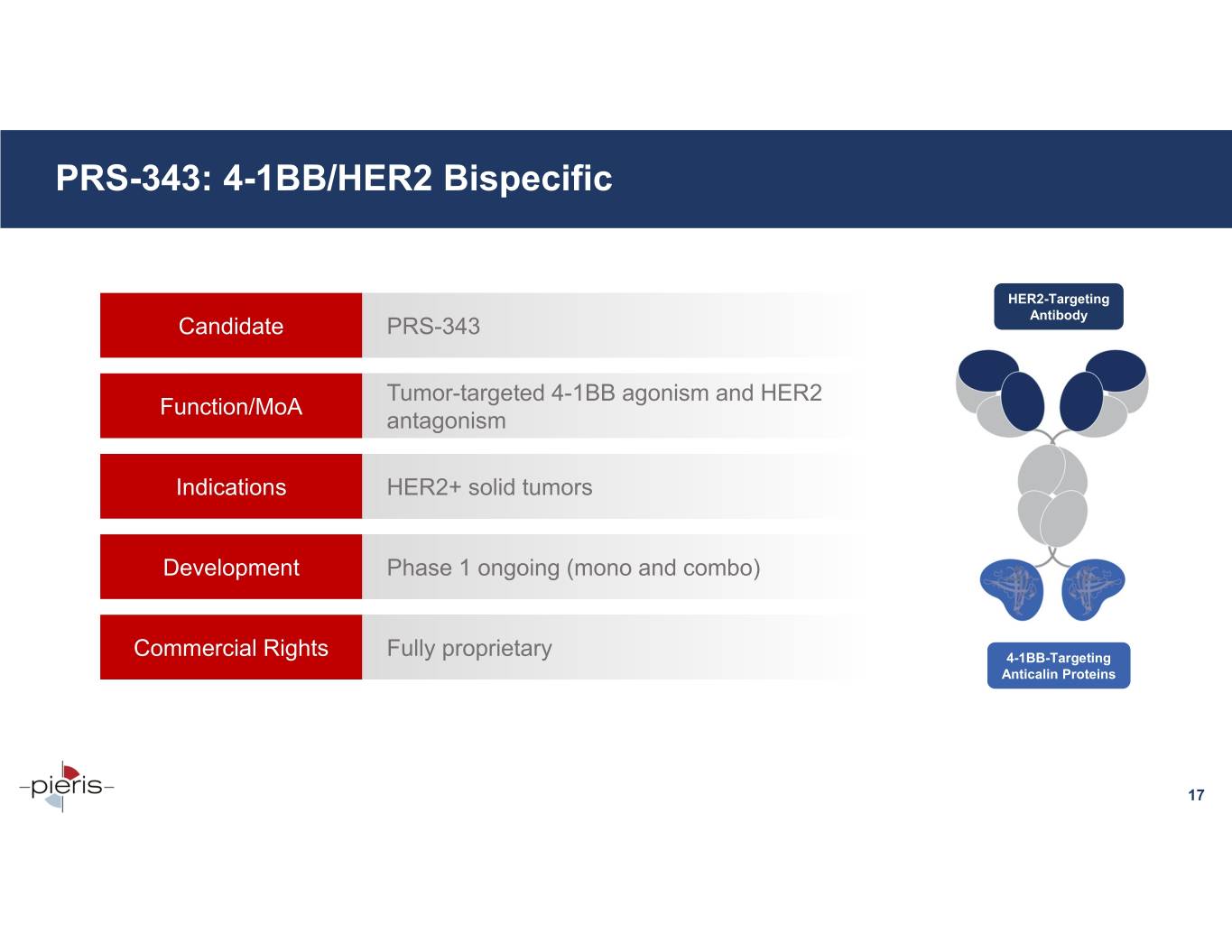
PRS-343: 4-1BB/HER2 Bispecific HER2-Targeting Candidate PRS-343 Antibody Tumor-targeted 4-1BB agonism and HER2 Function/MoA antagonism Indications HER2+ solid tumors Development Phase 1 ongoing (mono and combo) Commercial Rights Fully proprietary 4-1BB-Targeting Anticalin Proteins 17

PRS-343: Modes of Action HER2‐ targeting HER2‐targeting moiety Antibody of the drug localizes to the tumor HER2+ microenvironment and facilitates Tumor Cell 4‐1BB cross‐linking PRS‐343 4‐1BB cross‐linking ameliorates T‐cell exhaustion and is critical 4‐1BB‐ Tumor‐specific targeting T Cell for T‐cell expansion Anticalin Proteins 18
PRS-343 Phase 1 Escalation and Expansion Trials Enrolling patients with HER2+ solid tumors Bladder Dose-escalation trial ongoing; expansion initiation pending positive escalation data Gastric Presented PK, safety, tolerability, biomarker data and clinical response data at SITC 2019 Presented initial data from study of PRS- Other(s) 343 in combination with atezolizumab at R&D Day on November 19, 2019 ESCALATION
MD Anderson Cancer Center 19
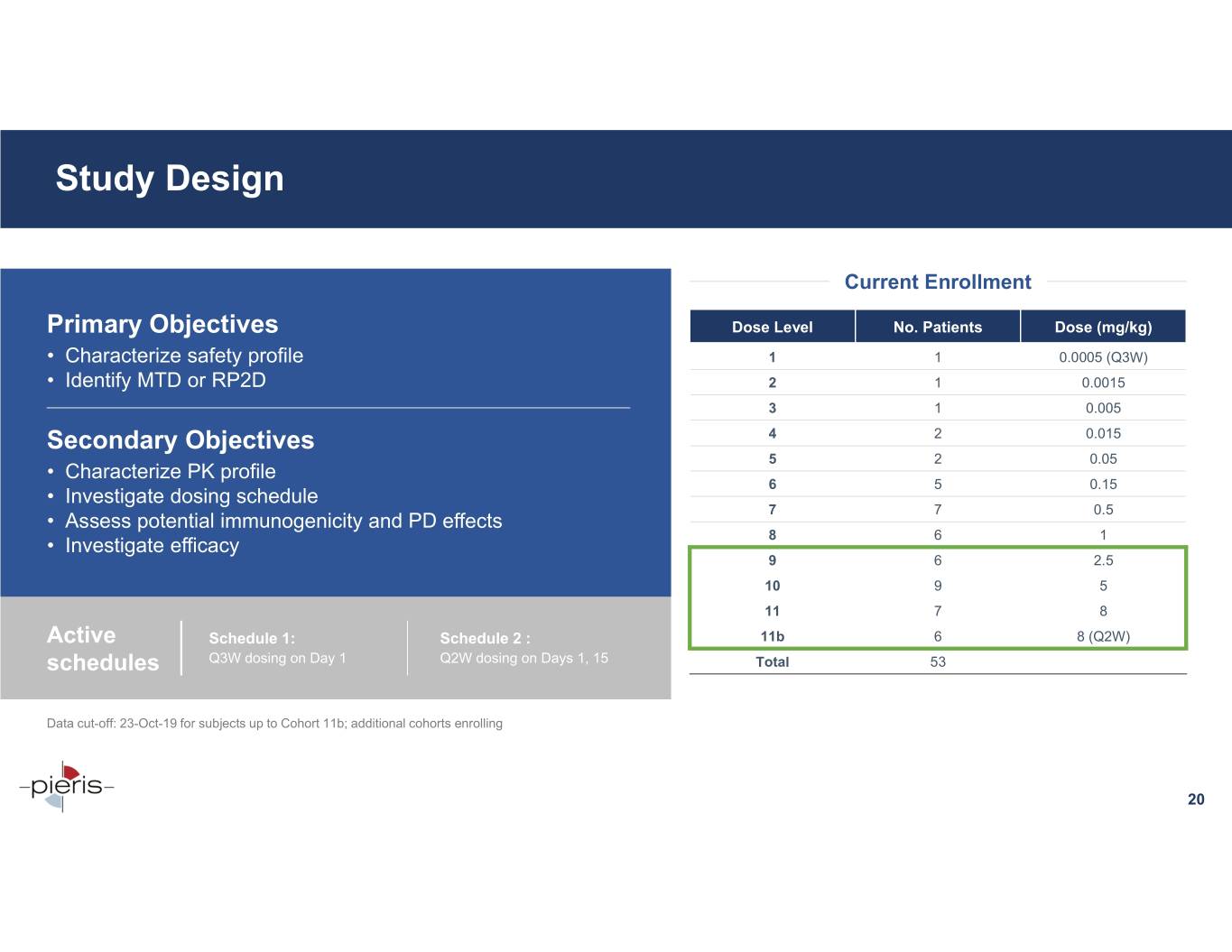
Study Design Current Enrollment Primary Objectives Dose Level No. Patients Dose (mg/kg) • Characterize safety profile 1 1 0.0005 (Q3W) • Identify MTD or RP2D 2 1 0.0015 3 1 0.005 Secondary Objectives 4 2 0.015 5 20.05 • Characterize PK profile 6 50.15 • Investigate dosing schedule 7 70.5 • Assess potential immunogenicity and PD effects 8 61 • Investigate efficacy 9 62.5 10 95 11 78 Active Schedule 1: Schedule 2 : 11b 68 (Q2W) schedules Q3W dosing on Day 1 Q2W dosing on Days 1, 15 Total 53 Data cut-off: 23-Oct-19 for subjects up to Cohort 11b; additional cohorts enrolling 20
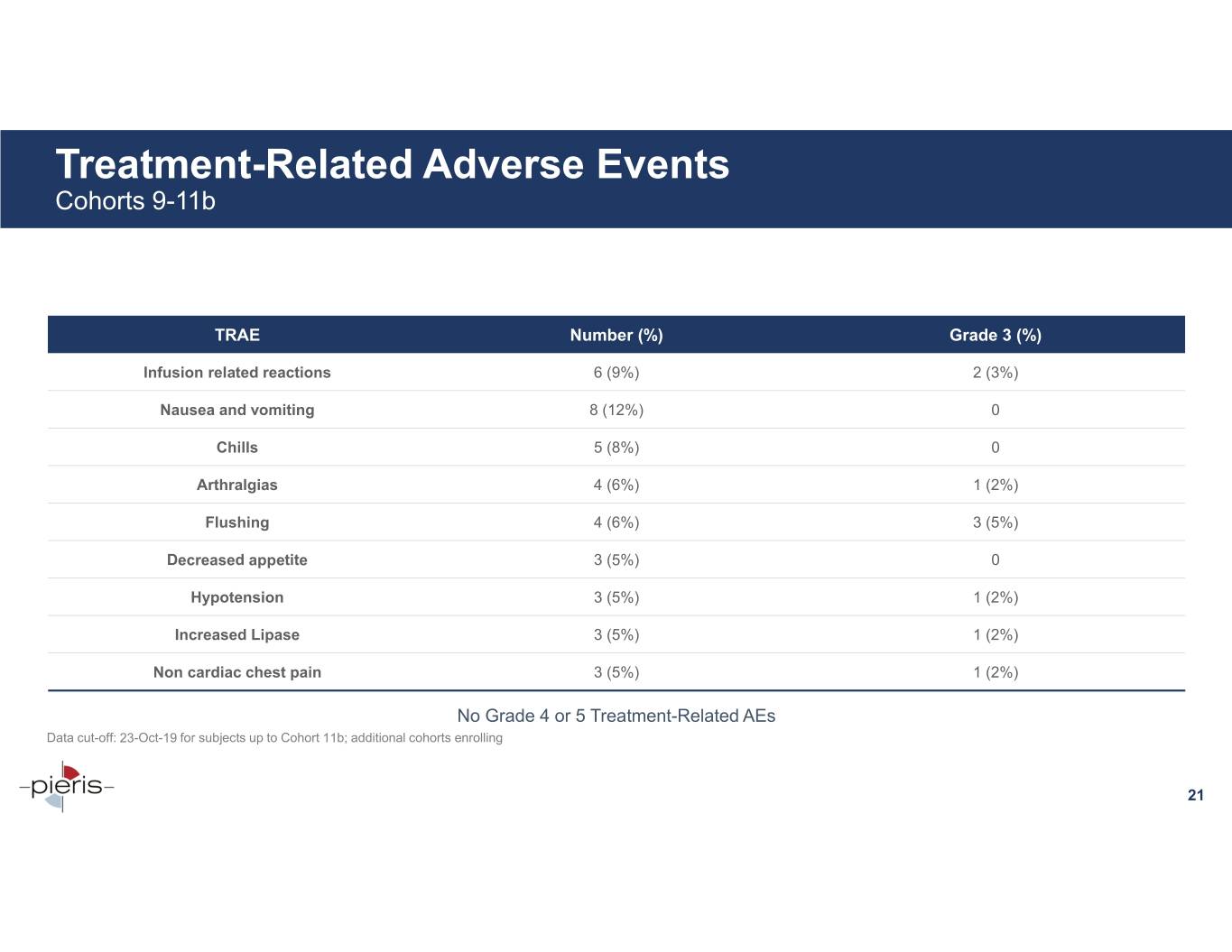
Treatment-Related Adverse Events Cohorts 9-11b TRAE Number (%) Grade 3 (%) Infusion related reactions 6 (9%) 2 (3%) Nausea and vomiting 8 (12%) 0 Chills 5 (8%) 0 Arthralgias 4 (6%) 1 (2%) Flushing 4 (6%) 3 (5%) Decreased appetite 3 (5%) 0 Hypotension 3 (5%) 1 (2%) Increased Lipase 3 (5%) 1 (2%) Non cardiac chest pain 3 (5%) 1 (2%) No Grade 4 or 5 Treatment-Related AEs Data cut-off: 23-Oct-19 for subjects up to Cohort 11b; additional cohorts enrolling 21

Increased CD8+ T Cell Numbers in Tumor Biopsies Post-Treatment Biopsy Biopsy Pre-dose PRS-343 PRS-343 Post-dose (Cycle 1 Day 1) (Cycle 2 Day 1) (Cycle 2 Days 2-8) Tumor Stroma Pronounced increase in CD8+ T cell 17 17 numbers is observed post-treatment in 16 16 patients receiving doses ≥ 2.5 mg/kg 6 6 5 5 tumor area tumor area 2 2 4 4 Patients benefiting from treatment (SD 3 3 > 120 days (blue) and PR (green) had 2 2 Fold induction CD8+ induction Fold Fold induction CD8+ induction Fold + T cells/mm T cells/mm 1 1 more pronounced increase in CD8 T 0 0 cell number in tumor vs. stroma Cohort 1-8 Cohort 9-11b Cohort 1-8 Cohort 9-11b Data cut-off: 23-Oct-19 for subjects up to Cohort 11b; additional cohorts enrolling 22

Summary of Responses at Active Dose Range of PRS-343 Based on clinical data, serum concentration of > 20 µg/ml defines active dose range (beginning at Cohort 9) Cohort 11b 11 10 9 Total Best Response 8 mg/kg, Q2W 8 mg/kg, Q3W 5 mg/kg, Q3W 2.5 mg/kg, Q3W Response Evaluable Patients 544518 PR 2- - - 2 SD 32128 PD -2338 ORR 40% 0% 0% 0% 11% DCR 100% 50% 25% 40% 55% Data cut-off: 23-Oct-19 for subjects up to Cohort 11b; additional cohorts enrolling 23

Best Response in Target Lesions Cohorts 9-11b 60% 40% Cohort 9 – 2.5 mg/kg Cohort 10 – 5 mg/kg Disease Progression 20% Cohort 11 – 8 mg/kg Cohort 11b – 8 mg/kg (Q2W) 0% -20% Best change from baseline (%) Best change from Partial Response -40% -60% 9 9 9 11101011b9 11101011b9 101011b11b Cohort Data cut-off: 23-Oct-19 for subjects up to Cohort 11b; additional cohorts enrolling 24
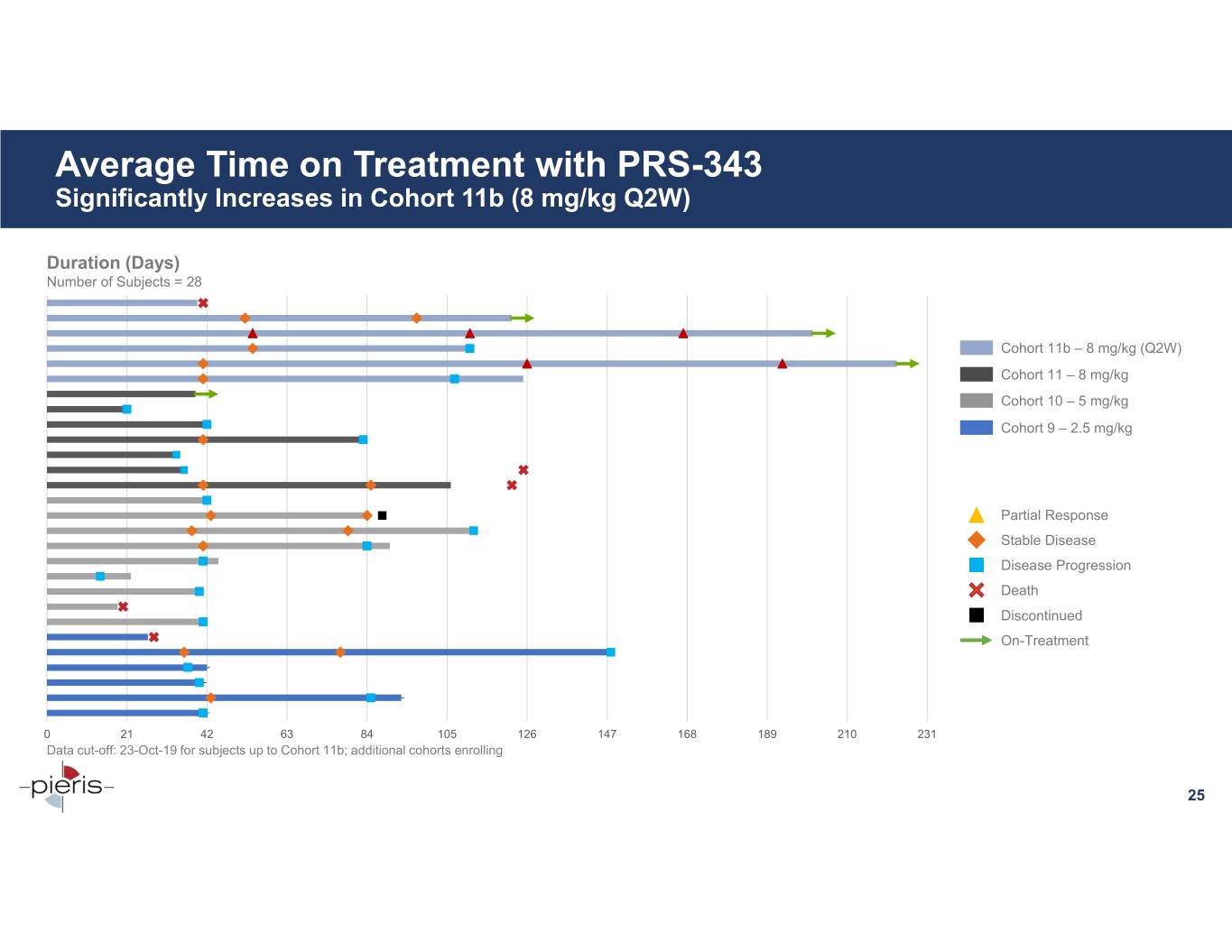
Average Time on Treatment with PRS-343 Significantly Increases in Cohort 11b (8 mg/kg Q2W) Duration (Days) Number of Subjects = 28 Cohort 11b – 8 mg/kg (Q2W) Cohort 11 – 8 mg/kg Cohort 10 – 5 mg/kg Cohort 9 – 2.5 mg/kg Partial Response Stable Disease Disease Progression Death Discontinued On-Treatment 0 21 42 63 84 105 126 147 168 189 210 231 Data cut-off: 23-Oct-19 for subjects up to Cohort 11b; additional cohorts enrolling 25

Case Study #1: Gastric Cancer Patient with Confirmed Partial Response Patient Profile, Treatment History and Treatment Outcome Patient Profile Oncology Treatment History Duration Best Response • Cohort 11b | 8 mg/kg every two weeks • 80-year old woman; initial diagnosis in June 2017 • Stage IV gastric adenocarcinoma Trastuzumab, Pembrolizumab + July 2017 – June 2018 Stable Disease • Metastases to liver, lymph node and adrenal glands Capecitabine/oxaliplatin • HER2 IHC 3+; PD-L1 positive (CPS=3) • NGS: ERBB2 amplification, TP53 mutation, alteration Nivolumab with IDO1 inhibitor Aug 2018 – Jan 2019 Stable Disease of CDK12 and SF3B1 (investigational drug) Lesion Size (mm) Lesions Lesion Site Baseline C2 Post-treatment C3 Post-treatment C4 Post-treatment C6 Post-treatment Target 1 Liver 14 12 10 9 9 Target 2 Liver 20 16 10 8 8 Target 3 Pancreas 19 16 14 14 14 % Change from Baseline -17% -36% -42% -42% Non-target 1 Lung Present Present Present Present Present Non-target 2 Stomach Present Present Present Present Absent Non-target 3 Stomach Present Present Present Present Absent PR, partial response; HER2, human epidermal growth factor receptor 2; PD-L1, Programmed Death Ligand 1; CPS, combined positive score; NGS, next-generation sequencing Data cut-off: 23-Oct-19 26

CD8+ T Cell Numbers Increase Post-Treatment in Responding Gastric Cancer Patient Tumor Stroma CD8 fold change: 5.7 CD8 pre [n/mm2]: 38 CD8 fold change: 4 CD8 pre [n/mm2]: 66 ) ) 2 2 Pre-Treatment (CD8: Teal | Ki67: Red) CD8 + T cells (n/mm CD8 + T cells (n/mm Pre Post Pre Post Post-Treatment (CD8: Teal | Ki67: Red) CD8+ T cell numbers increase post-treatment. This is more pronounced in tumor tissue and consistent with the predicted MoA of PRS-343. Data cut-off: 23-Oct-19 27

Case Study #2: Fallopian Tube Cancer Patient with Partial Response Patient Profile, Treatment History and Treatment Outcome • 59 year old female, initial diagnosis on September 19, 2017 • Fallopian tube carcinoma Cohort 11b • ERBB2 2+; MSI stable; TMB 4 Muts/Mb; PDL-1 status not known 8 mg/kg PRS-343 (Q2W) • CD8 fold change in tumor: Not known as multiple post- treatment core biopsies did not contain cancer cells Oncology Treatment History Duration Best Response Taxol/Carboplatin October 2017 - November 2017 Stable Disease Taxotere/Carboplatin December 2017 - May 2018 Stable Disease Doxil October 2018 – February 2019 Progressive Disease Lesion Size (mm) Lesions Lesion Site Baseline C2 Post-treatment C4 Post-treatment C6 Post-treatment Target 1 Liver – Dome of left lobe 18 10 12 8 % Change from Baseline -44% -33% -55% Data cut-off: 23-Oct-19 28

Case Study #3: Urothelial Cell Carcinoma Patient with Stable Disease Patient Profile, Treatment History and Treatment Outcome • 92 year old male, initial diagnosis in August 2015 Cohort 9 • Urothelial cell carcinoma Stage 3 2.5 mg/kg PRS-343 (Q3W) • HER2 FISH positive; MSS; TMB high16 mut/Mbp • CD8 fold change in tumor: 5.1 Oncology Treatment History Duration Best Response Cisplatin + gemcitabine September 2015 – September 2015 Toxicity Carboplatin + gemcitabine October 2015 – December 2015 Progressive Disease Atezolizumab December 2016 – June 2017 Stable Disease MEDI-0562 + durvalumab August 2017 – May 2018 Stable Disease Lesion Size (mm) Lesions Lesion Site Baseline C2 Post-treatment C4 Post-treatment C6 Post-treatment Target 1 Left para-aortic lymph node 27 24 24 25 Target 2 Right para-aortic lymph node 17 18 18 18 Target 3 Paraesophageal lymph node 18 19 19 20 % Change from Baseline -1.6% -1.6% 1.6% Data cut-off: 23-Oct-19 29

PRS-343-Atezolizumab Combination Trial Current Enrollment Number of Patients PRS-343 Dose Dose Level Atezolizumab (mg) Primary Objectives Enrolled (mg/kg) • Characterize safety profile of PRS-343 in combination with atezolizumab • Identify MTD or RP2D for PRS-343 in combination 1 3 0.05 1200 with a fixed dose of atezolizumab 2 1 0.15 1200 Secondary Objectives 3 2 0.5 1200 • Characterize PK profile 4 3 1.0 1200 • Investigate dosing schedule • Assess potential immunogenicity and PD effects • Investigate efficacy 5 8 2.5 1200 6 9 5.0 1200 Dosing schedule Q3W dosing on Day 1 7 9 8.0 1200 Data presented at R&D Day in New York on November 19, 2019. 30
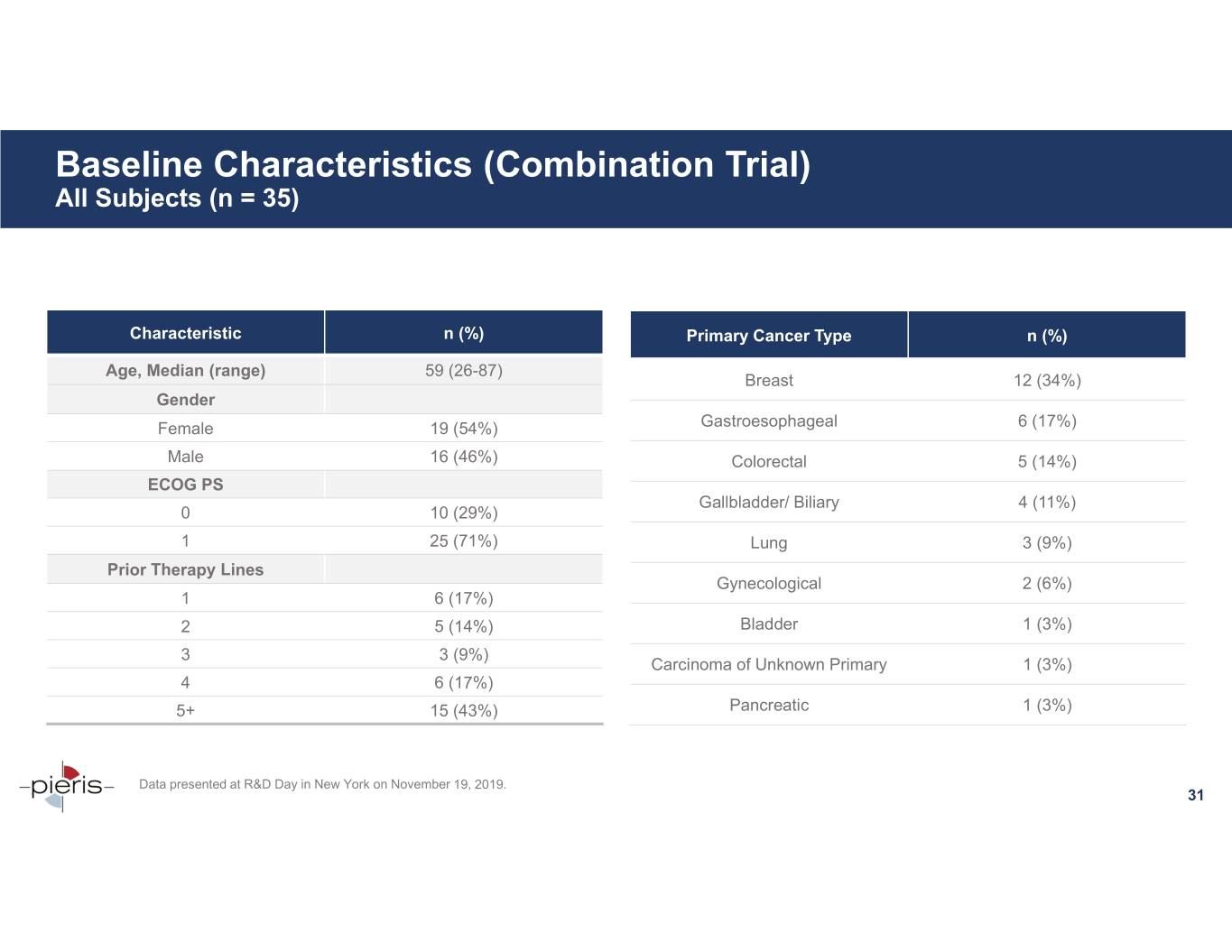
Baseline Characteristics (Combination Trial) All Subjects (n = 35) Characteristic n (%) Primary Cancer Type n (%) Age, Median (range) 59 (26-87) Breast 12 (34%) Gender Female 19 (54%) Gastroesophageal 6 (17%) Male 16 (46%) Colorectal 5 (14%) ECOG PS Gallbladder/ Biliary 4 (11%) 0 10 (29%) 1 25 (71%) Lung 3 (9%) Prior Therapy Lines Gynecological 2 (6%) 1 6 (17%) 2 5 (14%) Bladder 1 (3%) 33 (9%) Carcinoma of Unknown Primary 1 (3%) 4 6 (17%) 5+ 15 (43%) Pancreatic 1 (3%) Data presented at R&D Day in New York on November 19, 2019. 31

Treatment-Related Adverse Events (Combination Trial) Cohorts 4-7 TRAE n = 85 % Grade 3 Infusion related reaction 17 (20%) Nausea and Vomiting 7 (9%) Diarrhea 6 (7%) 1 (1%) Fatigue 6 (7%) Rash 4 (5%) Anemia 3 (4%) 1 (1%) Chills 2 (2%) Decreased appetite 2 (2%) Dizziness 2 (2%) Dysgeusia 2 (2%) Malaise 2 (2%) Myalgia 2 (2%) Neutrophil count decreased 2 (2%) 1 (1%) Platelet count decreased 2 (2%) 1 (1%) Pyrexia 2 (2%) White blood cell count decreased 2 (2%) 1 (1%) No Grade 4 or 5 PRS-343 Treatment-Related AEs Data presented at R&D Day in New York on November 19, 2019. 32

Best Response in Target Lesions (Combination Trial) Combination Study Cohorts 4-7 (n = 21) 100% 80% 60% 40% Cohort 4 – 1 mg/kg Disease Progression (20%) 20% Cohort 5 – 2.5 mg/kg Cohort 6 - 5 mg/kg 0% Cohort 7 - 8 mg/kg -20% -40% Partial Response (-30%) Best from Change (%) Baseline -60% -80% 554656674755576767746 Cohort Data presented at R&D Day in New York on November 19, 2019. 33

Case Study #1: Breast Cancer Patient with Partial Response Patient Profile and Treatment History • 64 year old female, initial diagnosis October 16, 2000 Cohort 4 • Stage 4 breast carcinoma 1 mg/kg PRS-343 (Q3W) + • ER/PR-; HER2 3+ (IHC biopsy collected in Jan 2010), FISH+ atezolizumab 1200 mg • PD-L1, MSI and TMB status not known • CD8 fold change in tumor: 8.5 Oncology Treatment History Duration Best Response AC X4, tamoxifen (X1 yr), arimidex (X5 yrs) 2000-2006 Stable Disease Carboplatin/Taxotere then Taxotere/Herceptin then Xeloda/ Lapatinib then May 2006 – September 2009 Complete Response Herceptin/ Navelbine Vinorelbine and Herceptin February 2010 – May 2011 Unknown Trastuzumab/Lapatinib Ditosylate (Tykerb) May 2011 – May 2012 Progressive Disease Trastuzumab/Gemzar May 2012 – Feb 2013 Unknown ADT (TDM1, Kadcyla) May 2013 – Jun 2015 Stable Disease Trastuzumab/Pertuzumab (Perjeta) Sep 2017 – Dec 2017 Stable Disease ADT (TDM1, Kadcyla) Dec 2017 – Jul 2018 Stable Disease Trastuzumab/Capecitabine (Xeloda) Aug 2018 – Jan 2019 Stable Disease Data presented at R&D Day in New York on November 19, 2019. 34

Case Study #1: Breast Cancer Patient with Partial Response Treatment Outcome Lesion Size (mm) Lesions Lesion Site Baseline C2 Post-treatment C4 Post-treatment C6 Post-treatment C8 Post-treatment C12 Post-treatment Target 1 Sub-cranial lymph node 15 8 5 8 8 6 Target 2 Right neck lymph node 15 9 7 7 6 5 % Change from Baseline -43% -60% -50% -53% -63% Data presented at R&D Day in New York on November 19, 2019. 35

Case Study #2: Breast Cancer Patient with Stable Disease Patient Profile, Treatment History and Treatment Outcome • 53 year old male, initial diagnosis July 28, 2011 • Stage 4 invasive ductal breast carcinoma (metastatic to Cohort 6 mediastinal lymph nodes, bones and lung) 5 mg/kg PRS-343 (Q3W) + • ER+/PR-, HER2- (IHC), FISH+ (biopsy collected in March atezolizumab 1200 mg 2019) • PD-L1, MSI and TMB status not known • CD8 fold change in tumor: 8 Oncology Treatment History Duration Best Response Trastuzumab + Carboplatin + Docetaxel + Tamoxifen September 2011 – July 2013 not known Trastuzumab + Perjeta + Navelbine August 2013 – January 2016 not known TDM-1 + Fulvestrant November 2017 – March 2018 not known Lapatinib + Capecitabine March 2018 – March 2019 not known Anastrozole + Ibrance April 2019 – May 2019 not known Lesion Size (mm) Lesions Lesion Site Baseline C2 Post-treatment C4 Post-treatment C6 Post-treatment Target 1 Lymph node 16 18 15 13 % Change from Baseline +13% -6% -19% Data presented at R&D Day in New York on November 19, 2019. 36

Case Study #3: NSCLC Patient with Partial Response Patient Profile, Treatment History and Treatment Outcome Cohort 6 • 65 year old male, initial diagnosis Feb 6, 2018 • Stage 4 NSCLC squamous 5 mg/kg PRS-343 (Q3W) + • Foundation One HER2 amplification atezolizumab 1200 mg • CD8 fold change in tumor: Results to be presented Oncology Treatment History Duration Best Response Carboplatin/paclitaxel + RT March 2018 – April 2018 Partial Response Stable Disease (treatment ended upon disease Atezolizumab August 2018 – May 2019 progression) Lesion Size (mm) Lesions Lesion Site Baseline C2 Post-treatment C4 Post-treatment Target 1 Lung 42 26 20 Target 2 Lung 16 0 0 % Change from Baseline -55% -66% Non-target 1 Lung Present Absent Absent Non-target 2 Lung Present Present Absent Data presented at R&D Day in New York on November 19, 2019. 37

PRS-344: 4-1BB/PD-L1 Bispecific PD-L1-Targeting Candidate PRS-344 Antibody Function/MoA Localized 4-1BB agonism with PD-L1 antagonism Indications N.D. Development 1H20 IND expected (in co-dev with Servier) Opt-in for co-development with full U.S. Commercial Rights commercial rights; royalty on ex-U.S. sales 4-1BB-Targeting Anticalin Proteins 38

Financial Overview (As of 9/30/19) $86.2* M $0.0 49.4** M Cash & Cash Debt CSO Equivalents $120+ M non-dilutive capital since January 2017 * excludes $32M of gross proceeds from Nov ‘19 private placement ** following Nov ‘19 private placement, CSO + “toothless” preferred = ~70.0 M shares 39
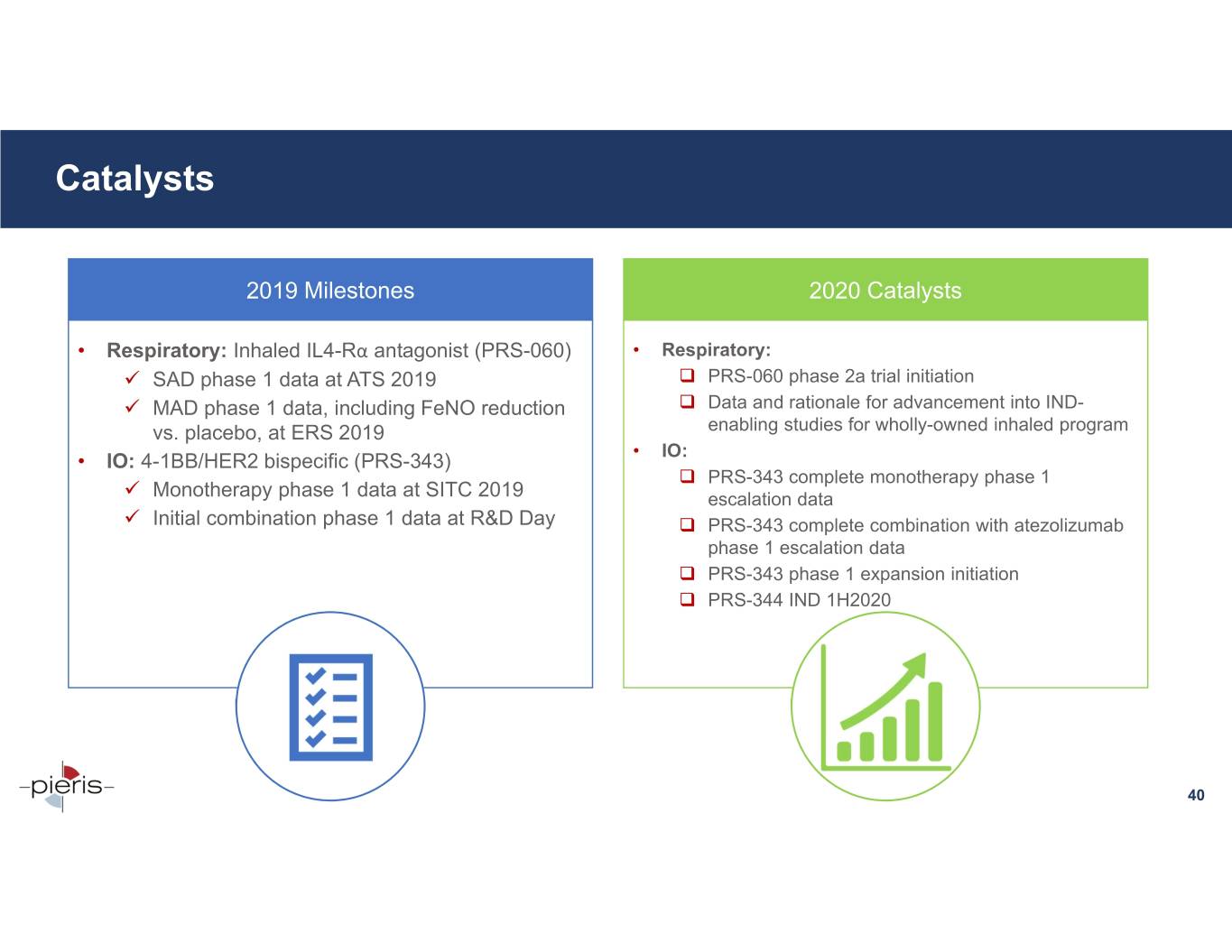
Catalysts 2019 Milestones 2020 Catalysts • Respiratory: Inhaled IL4-Rα antagonist (PRS-060) • Respiratory: SAD phase 1 data at ATS 2019 PRS-060 phase 2a trial initiation MAD phase 1 data, including FeNO reduction Data and rationale for advancement into IND- vs. placebo, at ERS 2019 enabling studies for wholly-owned inhaled program • IO: • IO: 4-1BB/HER2 bispecific (PRS-343) PRS-343 complete monotherapy phase 1 Monotherapy phase 1 data at SITC 2019 escalation data Initial combination phase 1 data at R&D Day PRS-343 complete combination with atezolizumab phase 1 escalation data PRS-343 phase 1 expansion initiation PRS-344 IND 1H2020 40

Pieris Pharmaceuticals 255 State Street Lise-Meitner-str. 30 Boston, MA 02109 85254 Freising USA Germany IR: kelman@pieris.com BD: niemeier@pieris.com NASDAQ: PIRS www.pieris.com
