Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - ViewRay, Inc. | vray-ex991_247.htm |
| 8-K - 8-K - ViewRay, Inc. | vray-8k_20200113.htm |
January 2020 Exhibit 99.2

FORWARD-LOOKING STATEMENTS This presentation has been prepared solely for use at this meeting and is intended for investors and analysts only. The material is given in conjunction with an oral presentation and should not be taken out of context. Unless the context requires otherwise, references to “ViewRay,” “the company,” “we,” “us” and “our,” refer to ViewRay, Inc. Except for historical information, ViewRay’s written and accompanying oral presentation may contain forward-looking statements, including statements about the overall industry, including but not limited to: our current expectations of the market; growth drivers; future trends; demand for radiation oncology products and features; and innovation and growth opportunities. Forward-looking statements also include, but are not limited to, statements about ViewRay’s: future orders; backlog or earnings growth; future financial results; and market acceptance of ViewRay’s existing products, future products, or technology. Words such as “could,” “anticipates,” “expects,” “outlook,” “intends,” “plans,” “believes,” “seeks,” “vision,” “estimates,” “may,” “will,” “future,” “horizon,” “aiming,” “driving,” “target” (or variations of them,) and similar statements, are forward-looking statements. These types of statements express management’s beliefs based on the information available to us as of the date of this presentation, are subject to change, and are not guarantees of future performance. Forward-looking statements involve risks, uncertainties, and assumptions that are difficult to predict and could cause ViewRay’s results to differ materially from those presented. These risks, uncertainties, and assumptions include, but are not limited to, changes in: the regulatory environment; global economics; trade compliance requirements, duties or tariffs; third-party reimbursement levels; currency exchange rates; taxation, healthcare law, and product clearance requirements, as well as those related to: adverse publicity about ViewRay and our products; our reliance on sole or limited source suppliers; our ability to commercialize our products successfully; the impact of competitive products and pricing, and all other risks listed from time to time in the company’s filings with the Securities and Exchange Commission, which are incorporated into this Forward-Looking Statements disclosure by this reference. We do not assume any obligation to update or revise the forward-looking statements in ViewRay’s written or oral presentation, whether based on future events, new or additional information or otherwise. ViewRay’s written and oral presentation does not constitute an offer to sell, or the solicitation of an offer to buy, securities. MEDICAL ADVICE DISCLAIMER ViewRay is a medical device manufacturer and cannot and does not recommend specific treatment approaches. Individual results may vary. ADDITIONAL INFORMATION We have filed with the Securities and Exchange Commission (SEC) a registration statement on Form S-3 (including a prospectus) and will file with the SEC a prospectus supplement to the prospectus for the offering to which this presentation relates. Before you invest, you should read the prospectus supplement and accompanying prospectus in that registration statement and the documents incorporated by reference or filed as exhibits to the registrations statement for more complete information about ViewRay and this offering. You may obtain these documents and other documents for free by visiting EDGAR on the SEC website. Alternatively, copies of the preliminary prospectus supplement and accompanying prospectus, when available, may be obtained from Piper Jaffray & Co. by telephone at (800) 747-3924 or by e-mail at prospectus@pjc.com. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of these securities in any jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of such state or jurisdiction. FORWARD-LOOKING STATEMENTS & DISCLAIMER

Clearly Differentiated Technology Moving to personalized care RADIATION ONCOLOGY SURGERY MEDICAL ONCOLOGY
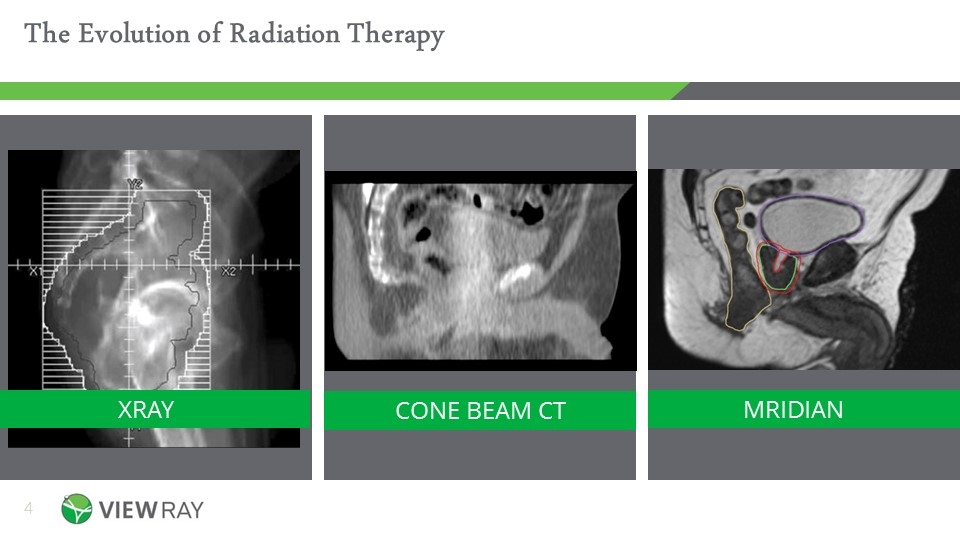
MRIDIAN XRAY CONE BEAM CT The Evolution of Radiation Therapy
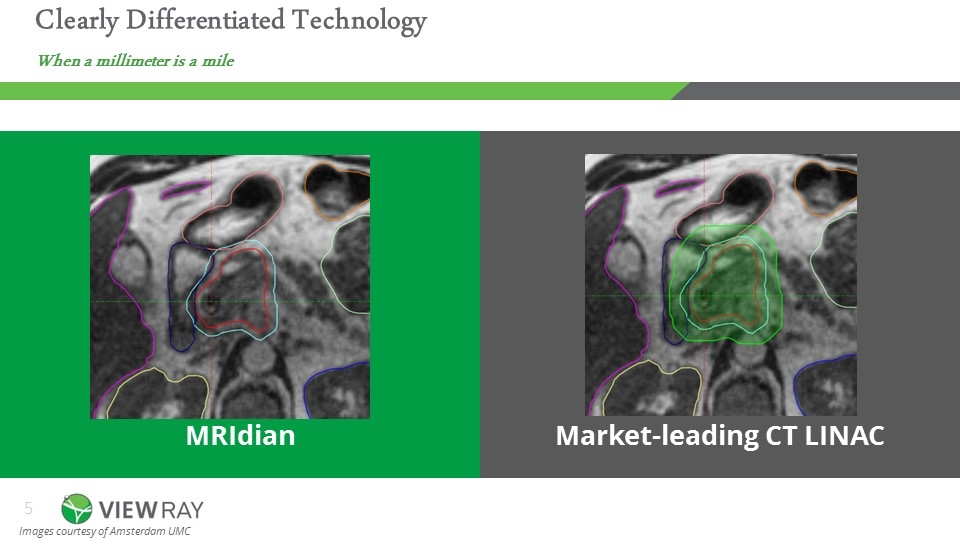
MRIdian Market-leading CT LINAC Images courtesy of Amsterdam UMC Clearly Differentiated Technology When a millimeter is a mile

Proven next-generation radiation therapy Differentiated: safely delivers high-dose, adaptive, beam-gated SBRT Significant reduction in toxicities across a variety of tumor sites1,2,3,4,5,6 Surpassing 6,500* patients 100s of peer-reviewed journal articles and abstracts proving clinical utility MRIdian MRI-Guided Radiotherapy * As of November 2019

MRIdian is the only system that: Offers personalized, precision radiation therapy Sees and tracks tumors in real-time Provides automatic beam gating for precise and accurate dosing Enables complete, on-table re-optimization of treatment plans Defining & Delivering A NEW STANDARD OF CARE
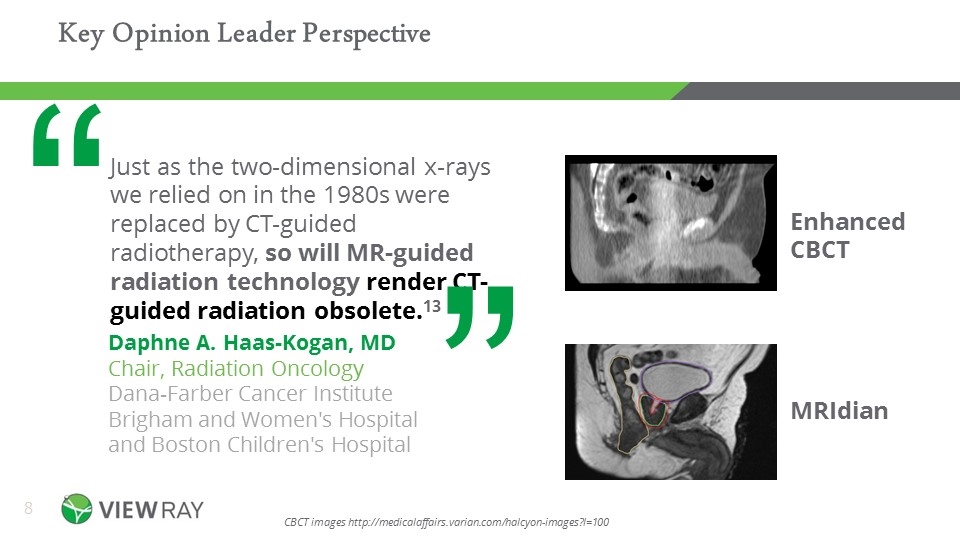
Key Opinion Leader Perspective Daphne A. Haas-Kogan, MD Chair, Radiation Oncology Dana-Farber Cancer Institute Brigham and Women's Hospital and Boston Children's Hospital Just as the two-dimensional x-rays we relied on in the 1980s were replaced by CT-guided radiotherapy, so will MR-guided radiation technology render CT-guided radiation obsolete.13 “ MRIdian Therapeutically Relevant Imaging MRIdian Enhanced CBCT CBCT images http://medicalaffairs.varian.com/halcyon-images?l=100 “
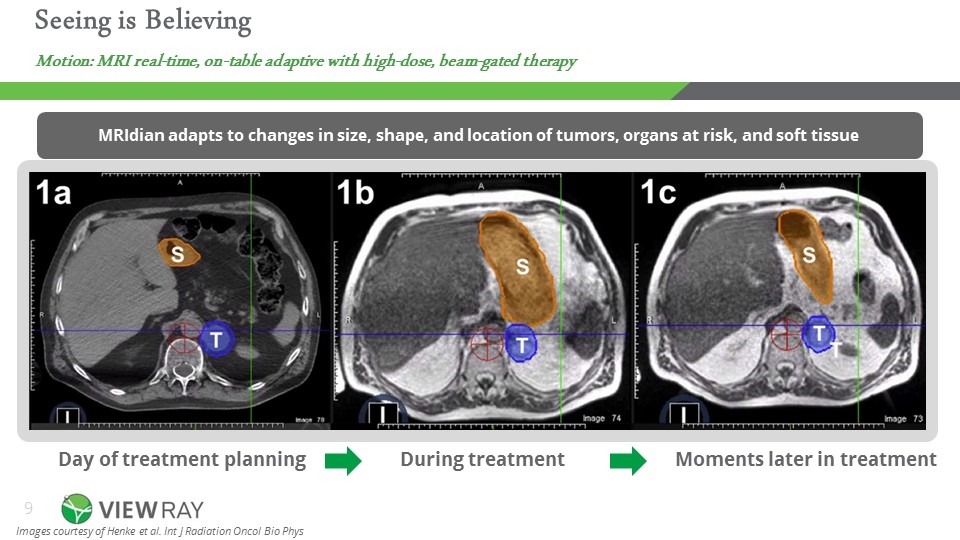
Moments later in treatment Day of treatment planning During treatment MRIdian adapts to changes in size, shape, and location of tumors, organs at risk, and soft tissue Images courtesy of Henke et al. Int J Radiation Oncol Bio Phys Motion: MRI real-time, on-table adaptive with high-dose, beam-gated therapy Seeing is Believing

Making the Invisible Visible M. Salim Siddiqui, MD Director, Stereotactic Radiation Program and MR Simulation Program Henry Ford Cancer Center This would have been unimaginable before MRIdian. “ Image and quote courtesy of Siddiqui, S. (2018). MR Image-Guided RT Case Review: Making the Invisible, Visible. Miami Symposium. Miami, Florida. Retrieved from https://vimeo.com/293473054 “

Beyond a Treatment We are advancing cancer care. In the MRIdian vault, this is where we cure cancer. Down the hall they treat it. “ Clinician Amsterdam UMC Upon completing 36Gy in a single fraction to treat lung cancer “
How We Win Commercial Organization Customer Service Operational Excellence Therapy Adoption via Innovation, Clinical Data, & Training
Bold Commercial Strategy Providing access and serving more patients Built commercial team, building distribution network Initial focus on United States, EMEA, and Japan This is ~70% of market opportunity Markets & Markets, 2015 Radiotherapy Market Global Forecasts 2020

Relentless Focus on Operational Excellence Vault readiness Installation Manufacturing efficiencies, supply chain management Driving efficiency across the organization

Customer Service Radical customer delight World-class service and support Clinical workflow Innovation, clinical data, and training
Extending our leadership position Investing in innovation Machine vision, biologic imaging Simplified workflow Faster treatment times “Groundbreaking” long-term pipeline Game-changing Technological Innovation Therapy Adoption

Increase in Overall Survival with simultaneous vanishing of Grade 3+ Toxicity in unresectable pancreatic cancer1,2,3,4 Ability to treat prostate with very high doses in few sessions with very low toxicity5, even in high risk populations12 Ability to treat partial breast with very high doses in few sessions with excellent cosmesis6,7,8,9,10 50% less treatment volumes in whole breast11 Many more examples in lung, liver, oligomets in the abdomen, adrenal tumors, etc. Over 6,500 Patients Treated Globally - Early, Compelling Data Therapy Adoption
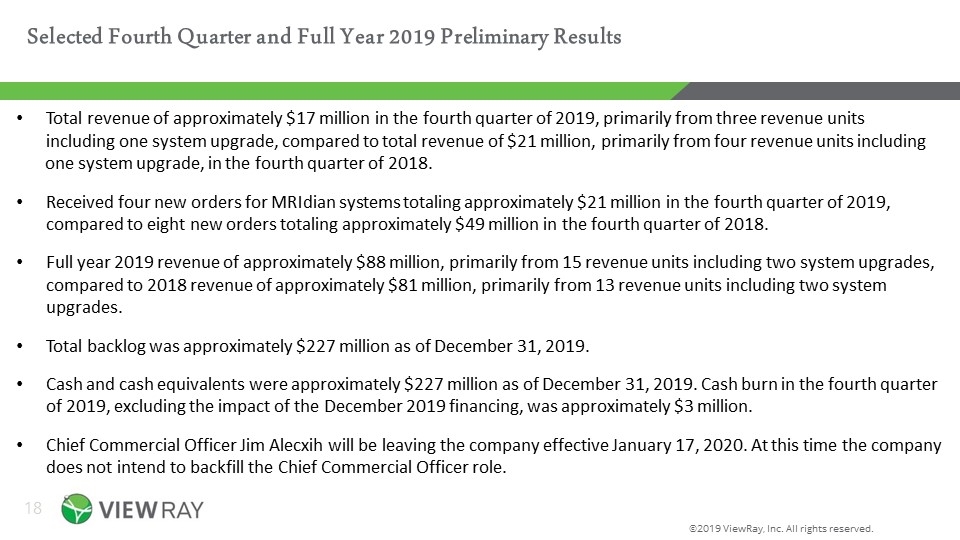
Selected Fourth Quarter and Full Year 2019 Preliminary Results ©2019 ViewRay, Inc. All rights reserved. Total revenue of approximately $17 million in the fourth quarter of 2019, primarily from three revenue units including one system upgrade, compared to total revenue of $21 million, primarily from four revenue units including one system upgrade, in the fourth quarter of 2018. Received four new orders for MRIdian systems totaling approximately $21 million in the fourth quarter of 2019, compared to eight new orders totaling approximately $49 million in the fourth quarter of 2018. Full year 2019 revenue of approximately $88 million, primarily from 15 revenue units including two system upgrades, compared to 2018 revenue of approximately $81 million, primarily from 13 revenue units including two system upgrades. Total backlog was approximately $227 million as of December 31, 2019. Cash and cash equivalents were approximately $227 million as of December 31, 2019. Cash burn in the fourth quarter of 2019, excluding the impact of the December 2019 financing, was approximately $3 million. Chief Commercial Officer Jim Alecxih will be leaving the company effective January 17, 2020. At this time the company does not intend to backfill the Chief Commercial Officer role.
Investment Highlights Large Market Opportunity Paradigm Shift in MR-technology to Standard of Care Experienced Management Team Growing Clinical Data Compendium In-line with Market Trends: Non-invasive, Precise, Personalized Medicine Investing in Innovation to Extend Lead

BOLDLY INNOVATIVE. CLINICALLY DRIVEN. COMMITTED TO CONQUERING CANCER.

1 Dr. Henke, ASTRO 2018, https://vimeo.com/298626732#t=458 2 Dr. Bassetti, Miami Symposium 2018, https://vimeo.com/293472071#t=1224 3 Dr. Parikh ASTRO 2017 Abstract, ASTRO 2017 multi-institutional, https://www.redjournal.org/article/S0360-3016(17)32096-5/fulltext 4 Washington University, Abstract from ASTRO 2018, https://www.redjournal.org/article/S0360-3016(18)31888-1/fulltext 5 Dr. Ben Slotman, Amsterdam Symposium March 2018, https://vimeo.com/264778067#t=145 6 Dr. Zoberi, Washington University School of Medicine, ASTRO 2018, https://vimeo.com/300878157#t=980 7 ASTRO Abstract 2018 – single fraction, https://www.redjournal.org/article/S0360-3016(18)31564-5/fulltext 8 ASTRO Abstract 2017 – single fraction, https://www.redjournal.org/article/S0360-3016(17)31788-1/fulltext 9 ASTRO 2016 Abstract – APBI 10 FX BID, https://www.redjournal.org/article/S0360-3016(16)31037-9/fulltext 10 Red Journal Manuscript, https://www.redjournal.org/article/S0360-3016(16)33065-6/abstract 11 Dr. Zoberi, Washington University School of Medicine, ASTRO 2018, https://vimeo.com/300878157#t=860 12 AUMC Prostate Data https://www.redjournal.org/article/S0360-3016(19)33640-5/fulltext 13 Dargan, R. (2017, November). Opportunities abound for radiation oncology in the era of personalized medicine, RSNA: Daily Bulletin, https://rsna2017.rsna.org/dailybulletin/index.cfm?pg=17thu01 Appendix
