Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Tricida, Inc. | jpmorganconference.htm |

Treating Metabolic Acidosis and Slowing the Progression of Chronic Kidney Disease January 2020

Forward Looking Statements Any statements contained in this presentation or made during the accompanying oral presentation that are not statements of historical facts are forward-looking statements as defined under the Federal securities laws. Examples of such statements include our plans, beliefs, intentions, expectations and projections regarding, among other things: (i) our receipt of FDA approval for veverimer on a timely basis, or at all; (ii) the potential date of approval of our New Drug Application (“NDA”) for veverimer; (iii) advisory committee meeting matters; (iv) the safety, efficacy and other expected patient benefits of veverimer; (v) the market opportunity, competition and rate of adoption for veverimer; (vi) our future clinical trial and product development milestones; (vii) our disease awareness and commercialization efforts and strategies; (viii) our receipt of adequate reimbursement for veverimer, and (ix) our financial projections and cost estimates. Any such forward-looking statements are based on our current expectations and assumptions, but are subject to a number of risks and uncertainties that could cause our actual future results to differ materially from our current expectations or those implied by the forward-looking statements. In addition, this presentation contains industry and market data prepared by third parties or by us. We have not independently verified this third-party data, and our data is based on our estimates and assumptions, which are subject to uncertainty and risk. As a result, you should not place undue reliance on this industry and market data. The risks and uncertainties that could adversely affect our forward-looking statements and the industry and market data include, but are not limited to: (i) the prospects of veverimer, including our ability to obtain FDA approval; (ii) the potential that the FDA could identify issues regarding our NDA at any time during its review of the application; (iii) risks related to the enrollment, completion and results of our confirmatory postmarketing trial; (iv) risk related to the market acceptance or commercial success of veverimer, including receipt of adequate reimbursement; (v) risks related to competition, potential market size and the size of the patient population for veverimer; (vi) risks related to the safety, efficacy and clinical benefit of veverimer; (vii) risks related to our manufacturing, sales, marketing and distribution strategies and activities; and (viii) risks related to our capital requirements and ability to raise sufficient funds for our operations. These and other factors that may affect our future results and operations are identified and described in more detail in our filings with the Securities and Exchange Commission (the “SEC”), including the Company’s most recent Annual Report filed on Form 10-K and our subsequently filed Quarterly Report(s) on Form 10-Q. You should not place undue reliance on these forward-looking statements, which speak only as of the date of this presentation. Except as required by applicable law, the Company does not intend to update any of the forward-looking statements to conform these statements to actual results, later events or circumstances or to reflect the occurrence of unanticipated events. This presentation is intended for investor purposes only. 2

Our Goals for Veverimer* A Drug Candidate for the Treatment of Metabolic Acidosis in Patients with CKD First and ONLY FDA- Disease Improve How the Approved Treatment Modifying Patient Feels and Functions Significant unmet medical Slow CKD progression Enhance physical need to treat metabolic through the treatment of functioning and physical acidosis metabolic acidosis functioning-related quality of life * Veverimer is not yet approved 3
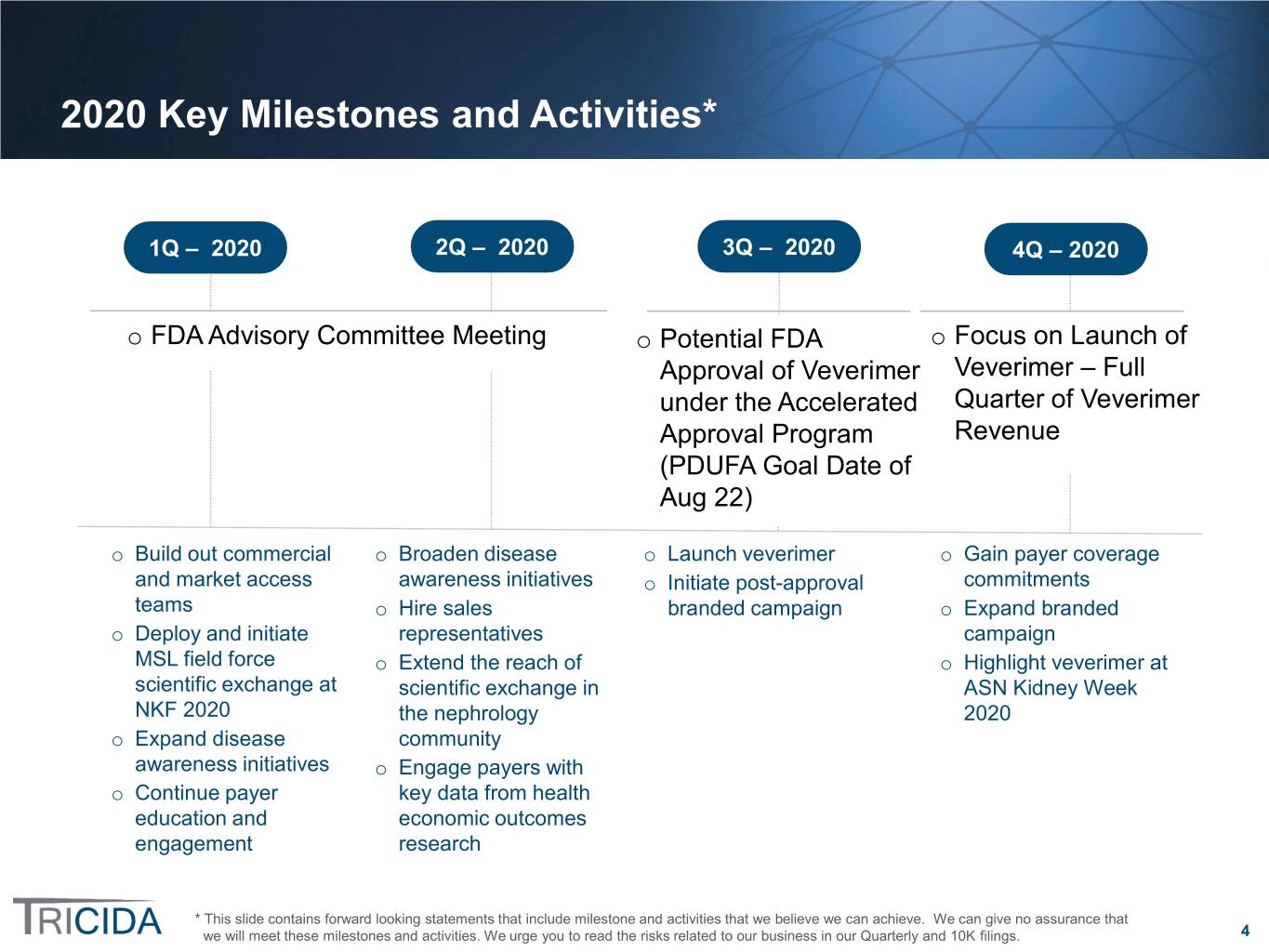
2020 Key Milestones and Activities* 1Q – 2020 2Q – 2020 3Q – 2020 4Q – 2020 o FDA Advisory Committee Meeting o Potential FDA o Focus on Launch of Approval of Veverimer Veverimer – Full under the Accelerated Quarter of Veverimer Approval Program Revenue (PDUFA Goal Date of Aug 22) o Build out commercial o Broaden disease o Launch veverimer o Gain payer coverage and market access awareness initiatives commitments o Initiate post-approval teams o Hire sales branded campaign o Expand branded o Deploy and initiate representatives campaign MSL field force o Extend the reach of o Highlight veverimer at scientific exchange at scientific exchange in ASN Kidney Week NKF 2020 the nephrology 2020 o Expand disease community awareness initiatives o Engage payers with o Continue payer key data from health education and economic outcomes engagement research * This slide contains forward looking statements that include milestone and activities that we believe we can achieve. We can give no assurance that we will meet these milestones and activities. We urge you to read the risks related to our business in our Quarterly and 10K filings. 4

Eight Things to Know about Metabolic Acidosis
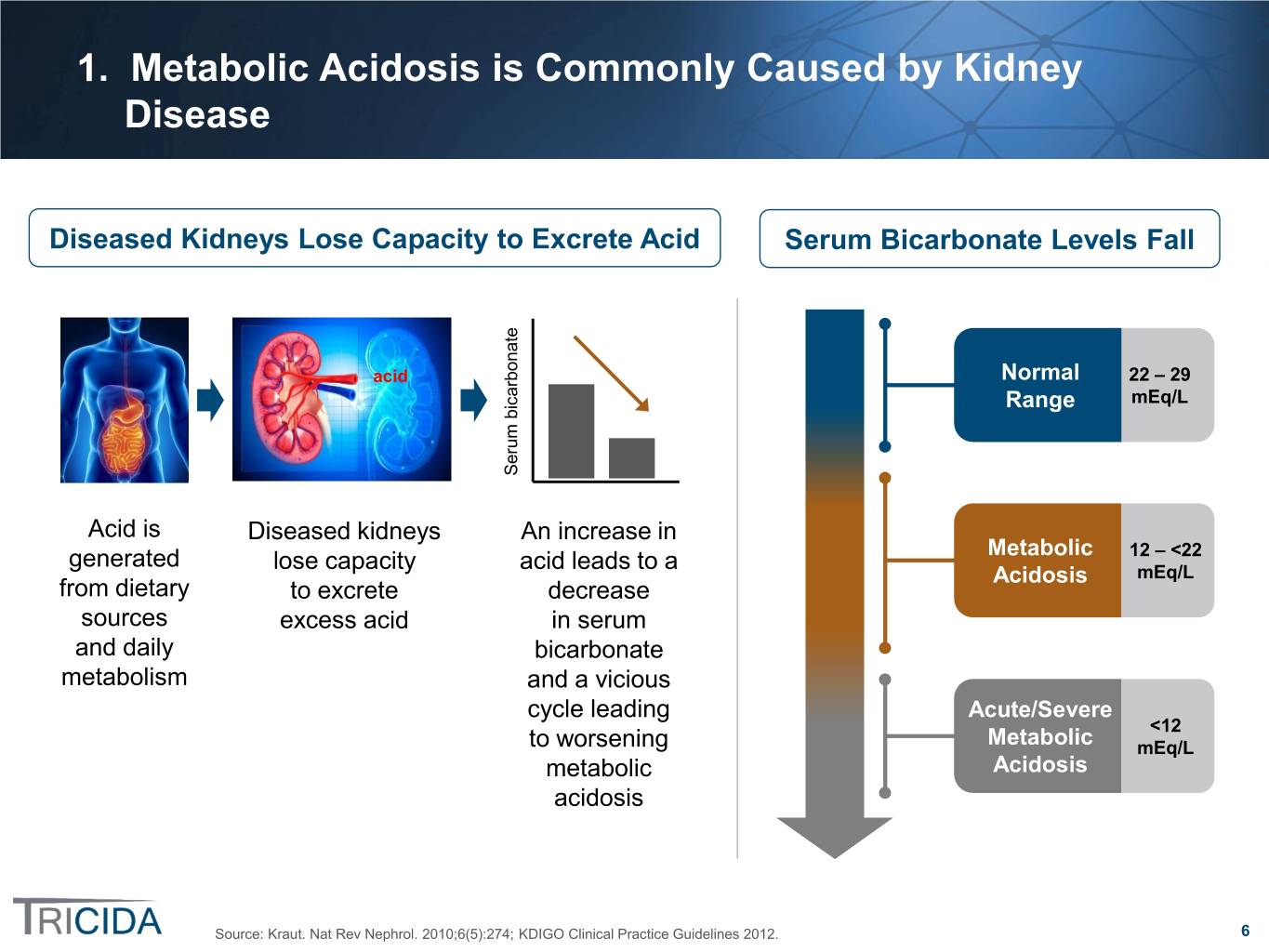
1. Metabolic Acidosis is Commonly Caused by Kidney Disease Diseased Kidneys Lose Capacity to Excrete Acid Serum Bicarbonate Levels Fall acid Normal 22 – 29 Range mEq/L Serum bicarbonate Acid is Diseased kidneys An increase in generated lose capacity acid leads to a Metabolic 12 – <22 Acidosis mEq/L from dietary to excrete decrease sources excess acid in serum and daily bicarbonate metabolism and a vicious cycle leading Acute/Severe <12 to worsening Metabolic mEq/L metabolic Acidosis acidosis Source: Kraut. Nat Rev Nephrol. 2010;6(5):274; KDIGO Clinical Practice Guidelines 2012. 6

2. Metabolic Acidosis is Highly Prevalent in CKD ~ 3 Million Patients with Metabolic Acidosis and CKD in the United States ~600,000 ~1.1 Million > % ~1.4 Million 30 18% 9% Stage 3a Stage 3b Stage 4&5 *Stage 5 pre-dialysis patients. Data on file. NHANES 1999-2004 reports prevalence of CKD Stages 3 and 4 for the US adult population ages 20 and older. CKD Stage 3 and 4 prevalence was calculated using NHANES prevalence and 2016 US Census data. Stage 3a (70%) and 3b (30%) were approximated using NCCD-CDC Surveillance System. MA prevalence by Stage 3a, 3b, and 4 reported in Inker LA et al., J Am Soc Nephrol 22:2322-31, 2011. 7

3. Metabolic Acidosis Can Impact Kidney, Bone and Muscle Health Metabolic Acidosis is Associated with an Increased Risk of CKD Progression and Adverse Effects On Bone and Muscle Reduced Renal Acid Increased Risk of CKD Secretion Leads to Acid Progression and Mortality Buildup Increased Risk of Acid Buffering Leads to Fractures, Renal Loss of Bone Density Osteodystrophy Acid Buffering Leads to Muscle Wasting and Increased Protein Reduced Physical Catabolism Functioning 8 Kraut JA et al., Adv Chronic Kidney Dis. 24:289-97, 2017.

4. Metabolic Acidosis is Linked to Worsening Kidney Disease Increased Acid Excretion Augmented Ammoniagenesis and Enhanced Proton Secretion Persistent Acid Activation of Retention Metabolic Endothelin 1 (ET-1), Sustained Expression of Aldosterone and Vicious Acidosis ET-1, Angiotensin II Aldosterone and Cycle Angiotensin II Inflammation, Fibrosis, Tubular Atrophy and Proteinuria Further Diminishing Kidney Function Laghmani K et al., J Clin Invest 107:1563-9, 2001. Wesson DE, J Am Soc Nephrol 12:1826-35, 2001. Wesson DE et al., Kidney Int 78:1128-35, 2010. Wesson DE et al., Kidney Int 82:1184-94, 2012. Wesson DE et al., Nephrol Dial Transplant 30:762-70, 2015. Phisitkul S et al., Kidney Int 77:617-23, 2010. Ruiz-Ortega M et al., J Hypertens Suppl 12:S51-58, 1994. Seccia TM et al., J Hypertens 26:2022-9, 2008. Wolf G et al., Nephron Physiol 93:P3-13, 2003. Greene EL et al., J Clin Invest 98:1063-8, 1996. Remuzzi G et al., Kidney Blood Press Res 19:182-3, 1996. Halperin ML et al., Am J Kidney Dis 14:267-71, 1989. Nath KA et al., J Clin Invest 76:667-75, 1985. Nath KA et al., Am J Kidney Dis 17:654-7, 1991. Chen W et al., BMC Nephrol 15:55, 2014. 9

5. Multiple Academic Studies Show that Increasing Serum Bicarbonate Translates to Clinical Benefit Publications Supporting Renal Benefits Publications Supporting Bone and Muscle Benefits 10

6. CKD Practice Guidelines Recommend Keeping Patients in a Normal Serum Bicarbonate Range “We suggest that in people with CKD and “In CKD Stages 3, 4, and 5, the serum level of serum bicarbonate concentrations total CO2 should be measured. The frequency <22 mmol/L treatment with oral bicarbonate of these measurements should be based on the supplementation be given to maintain serum stage of CKD (OPINION). bicarbonate within the normal range, unless contraindicated. In these patients, serum levels of total CO2 should be maintained at >22 mEq/L Serum bicarbonate concentrations less than (22 mmol/L). (EVIDENCE) If necessary, 22 mmol/L are associated with risk of CKD supplemental alkali salts should be given to progression and increased risk of achieve this goal. (OPINION).” death.” KDIGO: Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Inter, Suppl 3:1-150, 2013. Eknoyan G et al., Am J Kidney Dis. 42:1-201, 2003. 11

7. Oral Sodium Bicarbonate Has Not Been Established to be Safe and Efficacious (Not FDA-Approved) – Part 1 BiCARB Multi-Center, Double-Blind, Placebo Controlled, Randomized Trial • Population: 300 subjects enrolled at 27 • Efficacy Summary study sites in the UK – There is no evidence of a treatment • Double-blind, placebo-controlled 24-month benefit of oral sodium bicarbonate at study doses of 1.5 – 3 g/day vs placebo on physical function, quality of life or • Average Age: ~74 years CKD progression • Baseline Serum Bicarbonate: ~20-21 mEq/L • Safety Summary • Baseline eGFR: ~18-20 mL/min/1.73m2 – Death • 9.9% sodium bicarbonate vs 7.4% • Restrictive eligibility criteria, and corresponding restrictive population, resulted placebo in 4 years to enroll 300 subjects, of which – Early withdrawals only about half completed the study • 47% sodium bicarbonate vs 46% placebo Oral sodium bicarbonate did not improve a wide range of health measures in recent UK study BICARB Study protocol: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-015-0843-6 Link to BICARB Study results: https://www.clinicaltrialsregister.eu/ctr-search/trial/2011-005271-16/results 12

7. Oral Sodium Bicarbonate Has Not Been Established to be Safe and Efficacious (Not FDA-Approved) – Part 2 Sodium Attenuates the Beneficial Effects of ARBs and ACEi Sodium-Based Alkali Therapy Causes Deleterious Systemic Effects • In the absence of severe dietary NaCl restriction (<10 Low mEq/day), the volume effects Med (BP, sodium retention) of High Post-hoc analysis of RENAAL Post-Hoc Analysis of REIN Trials NaHCO3 are the same as and IDNT trials those from NaCl Risk of kidney events in patients Higher salt intake was associated with an receiving ARBs increases in direct increased risk of progression to ESRD in • Worsening edema/increased proportion to higher sodium intake patients taking an ACEi loop diuretics required* Post-hoc analysis of RENAAL and IDNT Studies: • Worsening “The renal and cardiovascular protective effects of ARB hypertension/increased therapy compared with non-RAASi–based therapy attenuated antihypertensive drugs in subjects with larger consumption of sodium so that in required* subjects with the highest sodium intake the treatment effects • Gastrointestinal intolerability on hard renal and cardiovascular outcomes were completely annihilated.” ARB: Angiotension II receptor blocker. ACE: Angiotension converting enzyme inhibitor. RAASi: Renal angiotension aldosterone system inhibitor. Abramowitz M et al., 2013. Bushinsky DA.,2019. Raphael KL et al., 2020. Lambers Heerspink HJ et al., 2012. Vegter S et al., 2012. *Meta-analysis of studies of CKD patients treated with sodium-based oral alkali for metabolic acidosis - Navaneethan SD et al., 2019. 13

8. Metabolic Acidosis is Undertreated Oral Alkali Treatment Rates Range from 3% to 15% 100% 90% 80% 70% The majority of patients 60% with metabolic acidosis are NOT treated 50% 40% 30% Receiving Receiving Oral Alkali Therapy 20% 15% % of Patients with Metabolic Acidosis %of Patients with Metabolic <10% 10% <6% <3% 0% CRIC Manitoba Tricida 301/301E Symphony *Based on a cohort of ~86,000 patients. Dobre M et al., Am J Kidney Dis. 62: 670-8, 2013. Tangi N. Manitoba (data on file). Wesson DE et al., The Lancet. March 8, 2019. Tangri N, American Society of Nephrology, Nov 7-10, 2019 (abstract 3231478). This study was sponsored by Tricida, Inc. 14

Three Key Takeaways Metabolic acidosis is common, harmful 1 and needs to be treated Metabolic acidosis is both a complication and 2 an underlying cause of kidney disease progression There is an urgent need for an FDA-approved 3 therapy to treat metabolic acidosis 15

Potential First-in-Class Polymer Designed to Have High Binding Capacity for the Removal of Excess Acid Veverimer Mechanism of Action Oral Ingestion Acid Binding in GI Tract Cl- Cl- + + + + NH2 NH2 NH3 NH3 NH3 NH3 H+ Cl- Excretion in Veverimer Veverimer Veverimer feces + + + + NH2 NH2 NH3 NH3 NH3 NH3 Cl- Cl- Non-absorbed Binds protons (H+) Excreted, polymer and then selectively resulting in binds chloride (Cl–) removal of HCl* in the GI tract GI: Gastrointestinal. HCl: Hydrochloric acid. *Veverimer’s maximum theoretical HCl binding capacity is approximately 10 mEq/gram. 16

High Selectivity for Chloride Ions Derived from Built-In Size Exclusion Mechanism GI Anions Ranked by Size The high degree of cross-linking in veverimer provides a Bile Acids size exclusion mechanism Fatty Acids that leads to high Veverimer selectivity for binding chloride (the Citrate smallest anion) over larger anions Phosphate Chloride 17

Veverimer Disease Modifying Data Published in The Lancet Veverimer-Treated Subjects Experienced a Mean Increase from TRCA-301 / TRCA-301E Study Results Baseline in Serum Bicarbonate of 4.5 mEq/L (p<0.0001) Prespecified Time-to-Event Analyses (52 Weeks) 65% Reduction in the Annualized Event Rate for Veverimer-Treated Subjects 22 21.7 21.6 22.0 21.6 21.7 4% DD50 21 20.8 incidence rate 20.2 20 4.5 12% DD50 incidence rate 0 mEq/L 0 19 19.0 18.6 18.9 18.6 18.4 18.5 18 18.1 17.3 17.3 17 BL Week 1 Week 2 Week 4 Week 6 Week 8 Week Week 10 12 Placebo Veverimer KDQOL Physical Function Domain Repeated Chair Stand Test Improved physical function Improvedphysical 11.4 function Improvedphysical -1.4 0 -4.3 -0.7 0 12 Time since randomisation (weeks) 40 52 BL: Baseline. W: Week. Error bars: Standard error of the mean. DD50: Death, dialysis or ≥50% reduction in eGFR. Wesson DE et al., Lancet 2019; 393:1417-27. Wesson DE et al., Lancet 2019; 394: 396-406. 18
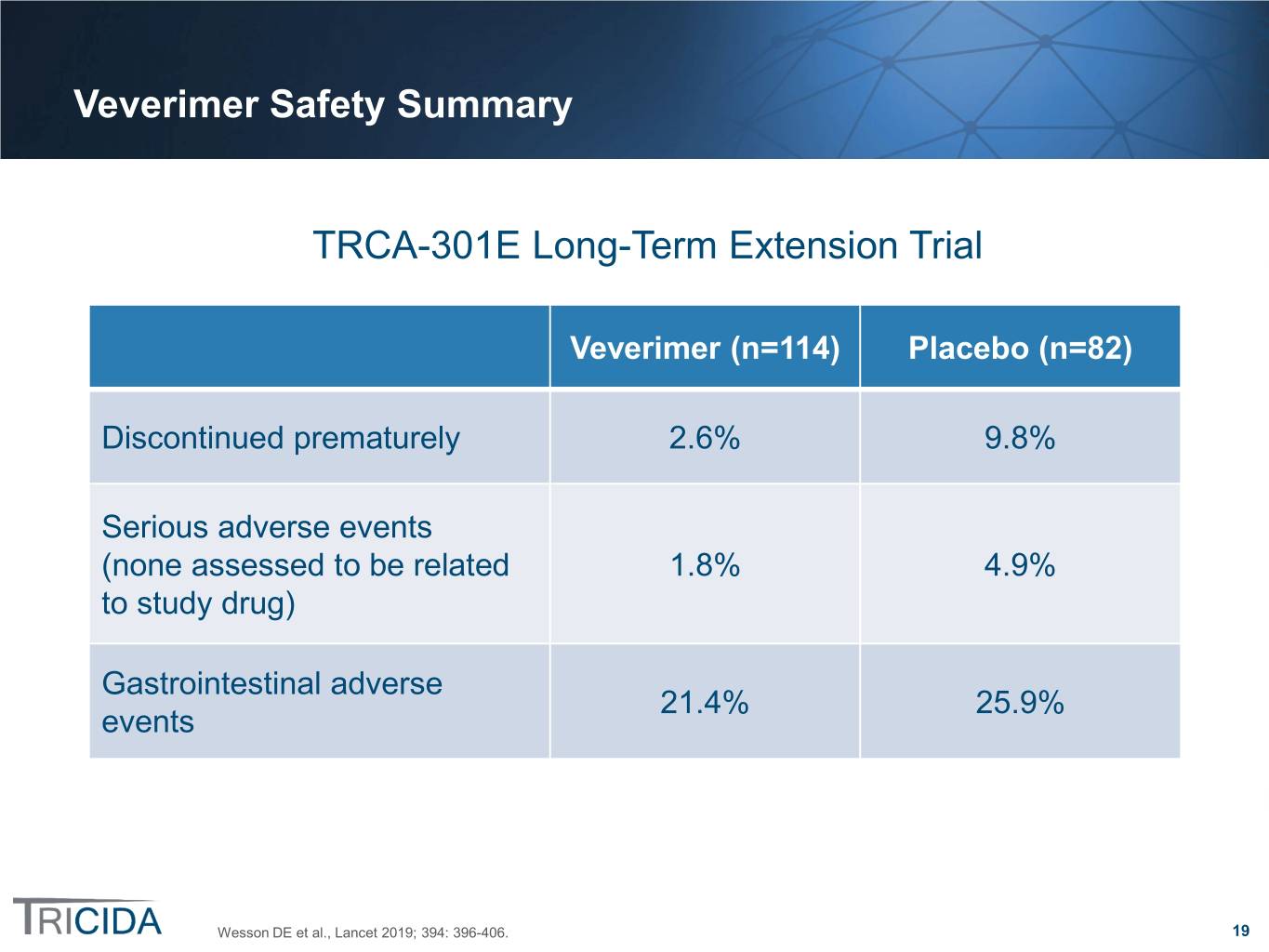
Veverimer Safety Summary TRCA-301E Long-Term Extension Trial Veverimer (n=114) Placebo (n=82) Discontinued prematurely 2.6% 9.8% Serious adverse events (none assessed to be related 1.8% 4.9% to study drug) Gastrointestinal adverse 21.4% 25.9% events Wesson DE et al., Lancet 2019; 394: 396-406. 19

Summary of TRCA-301 and TRCA-301E Clinical Trials TRCA-301 Results TRCA-301E Results 20

Veverimer is Being Reviewed through the Accelerated Approval Program – PDUFA Goal Date of August 22 Accelerated Approval Veverimer NDA Accepted for Review through Program Requirements the Accelerated Approval Program Increasing blood bicarbonate may Treats a Serious, Life- slow progression to ESRD for Threatening Condition patients with CKD and metabolic acidosis There are no FDA-approved Meaningful Advantage therapies for the treatment of Over Standard of Care metabolic acidosis Surrogate Endpoint Increasing blood bicarbonate has Reasonably Likely to been linked to the slowing of Predict Clinical Benefit CKD progression 21 Source: FDA Guidance for Industry—Expedited Programs for Serious Conditions—Drugs and Biologics.

Our Goal is to Launch Veverimer in the United States for Patients with CKD and Metabolic Acidosis Treated by Nephrologists 22

Significant US Market Opportunity 3,000,000 Patients with Increased awareness drives higher CKD and metabolic acidosis diagnosis and treatment metabolic acidosis 1,100,000 beyond nephrologists Expansion Patients Under provides additional opportunity Physician Care Focus on patients seen by ~600,000 nephrologists provides initial Patients Under opportunity for veverimer Nephrologist Care Data on file. NHANES 1999-2004 reports prevalence of CKD Stages 3 and 4 for the US adult population ages 20 and older. CKD Stage 3 and 4 prevalence was calculated using NHANES prevalence and 2016 US Census data. Stage 3a (70%) and 3b (30%) were approximated using NCCD-CDC Surveillance System. MA prevalence by Stage 3a, 3b, and 4 reported in Inker LA et al., J Am Soc Nephrol 22:2322-31, 2011. Symphony Health Solutions Claims Data. 23

A High Number of Patients with Metabolic Acidosis Are Treated By a Relatively Small Number of Nephrologists Highly Concentrated Market 100% 80% of Potential Target Patient Population 80% 60% Cumulative % Potential 40% 20% 0% ~5,000 Nephrologists Cumulative % of nephrologists with highest number of pre-dialysis patients with Stages 3-5 CKD Symphony Health Solutions IDV® 24

We Will Target ~5,000 Community Nephrologists at Launch Optimize Reach with 2 Regional Managers, 10 Division Heads and ~85 Sales Representatives # Nephrologist Symphony Health Solutions IDV® 25

Most Community Nephrologists Understand the Importance of Treating Metabolic Acidosis to Slow Kidney Disease Progression Q: Importance of Treating Metabolic Acidosis in Slowing Kidney Disease Progression? 22% 84% 29% 94% 91% 47% Extremely Important 23% Very Important 41% Moderately Important 29% 29% Slightly Important Not Important 19% 24% 15% 6% 7% 5% 1% 3% Stage 3 Stage 4 Stage 5 Tricida Primary Market Research, IQVIA Quant Q3’19 Nephrologist n=100 26

Expanding our Disease Awareness Campaign Website and Neph+ App Digital Ads and Congresses ASN Kidney Week Meeting www.metabolicacidosisinsights.com >1,000 Engagements with HCPs Now ranked 1st on Google search 27

Increasing Awareness through Peer-to-Peer Engagement • Metabolic Acidosis Working Group Meeting – 30 key opinion leaders in nephrology – Key takeaway: new evidence supports guideline updates • Publications / presentations on clinical data and complications of metabolic acidosis TRCA-301 Results TRCA-301E Results – A cause of CKD progression March 2019 June 2019 Clinical Evidence – Associated with increased fractures/falls and failure 2019 to thrive – Associated with increased cardiovascular outcomes – Underdiagnosed and undertreated Sodium is Sodium Six Presentations 2018 2019 • Initiation of the ULTIMA-CKD Patient Registry – Engagement with high-volume community-based ULTIMA-CKD Metabolic Acidosis Patient Registry nephrology practices to understand natural history of metabolic acidosis To Be Managed by a Field Force of ~20 MSLs 28

Positive Reaction to Veverimer Profile and Anticipated Adoption Supports Market Opportunity to Address a High Unmet Need Nephrologists Were Asked about Their Nephrologists Were Asked to Estimate Likelihood of Prescribing Veverimer* Veverimer Share at One Year* 79% Would Definitely or Probably Prescribe 52% Veverimer Share For Their Other 18% Patients, it will be Might “Watch and Wait” Prescribe Definitely/Probably Might Would Not Definitely Would Not Source: IQVIA Quant Q3’19 Nephrologist n=100 *Based on a target product profile including information from Wesson DE et al., Lancet. 2019; 393:1417- 1427. Wesson DE et al., Lancet. 2019; 394:396-406. Quotation from Tricida Primary Market Research Qual Survey 2019, n=15. If approved, the veverimer prescribing information may differ. *We believe this estimate is more representative of peak share opportunity 29

Engaging with Payers to Facilitate Optimal Coverage $$$ $$$ $$$$ $$$ Managed Market Employing Multiple Payer Working Team Actively Avenues for Handling Group Meeting Meeting with Payers Prior Authorizations • Met with 4 payers • Building field-based payer • Mirror nephrologists’ representing over 150 team of 12 current practice million lives representatives ˗ Preferred pharmacy (retail and • Key takeaways: • Met with 34 payers specialty, as preferred) ˗ First-in-class, must cover representing ~ 220 million ˗ Electronic Prior Authorization ˗ Prior authorization likely lives System (confirm serum bicarbonate ˗ Goal is to meet with 100 ˗ Patient Assistance Hub and sodium sensitivities) payers representing ~95% of ˗ Nephrologist staff education by ˗ Positive reactions to health covered lives prior to approval 10 field reimbursement economics analysis at least once managers 30

Ongoing Payer Discussions Include Health Impact of Metabolic Acidosis Supportive Data from Analysis of Major Payer Database Shows Significant Health Impact of Metabolic Acidosis Over a Two-Year Period 3X 3.6X 1.5X Higher Higher Higher Likelihood Likelihood of Likelihood to of Death Starting Dialysis Progress 1 or More CKD Stages 31% of CKD patients with 18% of CKD patients with 38% of CKD patients with metabolic acidosis at baseline metabolic acidosis at baseline metabolic acidosis at baseline died versus 10% of CKD started dialysis versus 5% of progressed 1 or more stages of patients with normal serum CKD patients with normal serum CKD versus 25% of CKD bicarbonate at baseline bicarbonate at baseline patients with normal serum bicarbonate at baseline Optum® EMR, De-identified electronic Health Record dataset 2007–2017. Presents at AMCP 2019 and ASN Kidney Week 2019. Data on file. 31

Ongoing Payer Discussions Include Compelling HEOR Analysis Compelling Health Economic Outcomes Research (HEOR) Message for Payers Impact of increase in serum Impact of metabolic acidosis bicarbonate as a continuous comparing all-cause cost in an variable in combined acidotic and acidotic vs. non-acidotic population non-acidotic population ~$40K of added costs/year Each 1 mEq/L increase in serum CKD stage 3 to 5 non-dialysis patients bicarbonate was associated with metabolic acidosis costs ~$40K more with a 7% decrease in all cause than CKD patients with normal serum monthly healthcare costs (p<0.0001) bicarbonate Optum® EMR, De-identified electronic Health Record dataset 2007–2017. Presented at AMCP 2019 and ASN Kidney Week 2019. 32

Initial Focus will be on Commercial, Medicare with Subsidy, Medicaid and VA/DOD Patients, Representing ~62% of Target Population Anticipated Payer Mix in Target Population 1% VA/ DOD Uninsured5% Cash Medicaid Over half of the initial 10% Medicaid targeted patients with CKD 33% and metabolic acidosis will Medicare with no subsidy have either low copay (Medicaid, Medicare with 33% Commercial subsidy and VA/DOD) or 18% may have assistance with Medicare with subsidy their copay (commercial) Symphony Health Integrated Dataverse, 2018 USRDS Annual Report, Kaiser Family Foundation 33

Veverimer Has the Opportunity to Achieve Over $2 Billion Peak Revenue in the US Alone 3,000,000 Potential Peak US Revenue Metrics Patients with CKD and metabolic acidosis Est. WAC price: ~$2,000 - $3,000/month Est. net price (~30% GtN): ~$1,400 - $2,100/month 1,100,000 Patients Under Est. compliance: Physician Care 65% (~8 months/year) ~600,000 Patients Under Nephrologist Care Potential Peak US Revenue ~$14,000 revenue PPPY at midpoint ~25% Penetration* X ~150,000 patients Over $2 billion / year PPPY: Per Patient Per Year. *Incorporates intent to treat and managed care coverage assumptions 34

Veverimer Comprehensive Worldwide Patent Estate United States Europe Rest of World • Six issued Orange • Two issued • Issued patents in Australia, Book eligible patents patents Japan and Mexico • Several pending • Several pending • Corresponding pending patent applications patent applications patent applications in other commercially significant countries Patent protection expected to provide exclusivity for veverimer through at least 2034* in Australia, European Union, Japan, Mexico, and the United States. Corresponding applications are pending in China, India and certain other markets *Exclusivity does not include extensions related to Hatch-Waxman. 35

Strong Financial Position ~ $355 million year-end cash, cash equivalents and investments of as of December 31, 2019* $60 million currently drawn under a $200 million debt facility with Hercules (including $20 million drawn in December 2019) – Additional draw down availability of: • $15 million prior to December 15, 2020 • $75 million subject to FDA approval of veverimer and prior to December 15, 2020 • $50 million subject to approval by Hercules ~ 49.8 million total shares outstanding as of December 31, 2019 * Unaudited. Anticipate reporting full 4Q 2019 financial results in late February 36

THANK YOU
