Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Rubius Therapeutics, Inc. | tm201897d1_ex99-1.htm |
| 8-K - FORM 8-K - Rubius Therapeutics, Inc. | tm201897-1_8k.htm |
Exhibit 99.2

Pablo J. Cagnoni , M.D., Chief Executive Officer JANUARY 2020 REALIZING THE POWER OF RED ™ : A NEW ERA IN CELLULAR MEDICINE

Forward - Looking Statements This presentation may contain forward - looking statements . Forward - looking statements are neither historical facts nor assurances of future performance . Instead, they are based on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, our development plans, our clinical results and other future conditions . All statements other than statements of historical facts contained in this presentation, including statements regarding our future financial or business performance, conditions, plans, prospects, trends or strategies and other financial and business matters ; our current and prospective product candidates, planned clinical trials and preclinical activities, including timing related to such trials and expected results, research and development costs, current and prospective collaborations ; the estimated size of the market for our product candidates, the timing and success of our development and commercialization of our anticipated product candidates ; and the availability of alternative therapies for our target market . New risks and uncertainties may emerge from time to time, and it is not possible to predict all risks and uncertainties . Except as required by applicable law, we do not plan to publicly update or revise any forward - looking statements contained herein, whether as a result of any new information, future events, changed circumstances or otherwise . Although we believe the expectations reflected in such forward - looking statements are reasonable, we can give no assurance that such expectations will prove to be correct . Accordingly, readers are cautioned not to place undue reliance on these forward - looking statements . No representations or warranties (expressed or implied) are made about the accuracy of any such forward - looking statements . Certain information contained in this presentation relates to or is based on studies, publications, surveys and other data obtained from third - party sources and our own internal estimates and research . While we believe these third - party sources to be reliable as of the date of this presentation, we have not independently verified, and make no representation as to the adequacy, fairness, accuracy or completeness of, any information obtained from third - party sources . In addition, all of the market data included in this presentation involves a number of assumptions and limitations, and there can be no guarantee as to the accuracy or reliability of such assumptions . Finally, while we believe our own internal research is reliable, such research has not been verified by any independent source . Rubius recommends that investors independently evaluate specific investments and strategies . For further information regarding these risks, uncertainties and other factors, you should read the “Risk Factors” section of our Quarterly Report on Form 10 - Q filed for the period ended September 30 , 2019 , and subsequent filings with the Securities and Exchange Commission . This presentation may contain tradenames, trademarks or servicemarks of other companies . Rubius does not intend the use or display of other parties’ tradenames, trademarks or servicemarks to imply a relationship with, or endorsement or sponsorship of, these other parties . 2

2019 Achievements: Setting the Stage for Success 3 STRENGTHENED ORGANIZATION & DELIVERED MILESTONES Accelerated Rubius manufacturing site buildout and GMP operations Manufactured clinical - grade RTX - 134 at contract manufacturing organization Filed IND and received clearance for first - ever RCT – RTX - 134 Filed first oncology IND for RTX - 240 Completed GMP engineering runs for RTX - 240 at Rubius manufacturing site Grew patent estate to 10 issued U.S. patents Presented preclinical oncology data at AACR, AACR - NCI - EORTC & SITC Strengthened leadership team with Chief Medical Officer, Chief Scientific Officer, Head of BD, Chief Legal Officer and Chief Quality Officer
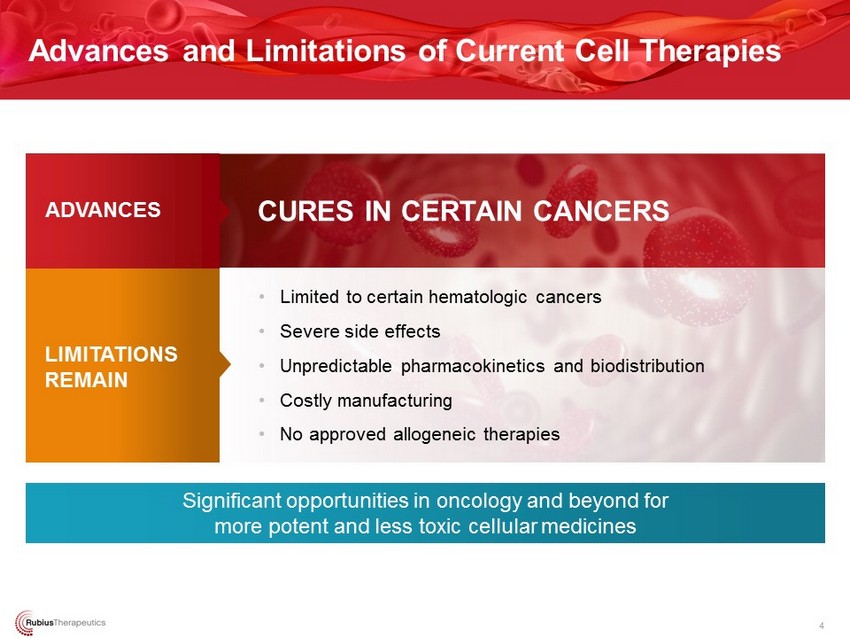
Advances and Limitations of Current Cell Therapies 4 Significant opportunities in oncology and beyond for more potent and less toxic cellular medicines • Limited to certain hematologic cancers • Severe side effects • Unpredictable pharmacokinetics and biodistribution • Costly manufacturing • No approved allogeneic therapies CURES IN CERTAIN CANCERS ADVANCES LIMITATIONS REMAIN

Harnessing the Unique Properties of Red Blood Cells 5 • Have a circulation time of ~120 days • Lack a nucleus • Have a predictable biodistribution in the vasculature • > 100 - year history of administering blood to patients WHY RED BLOOD CELLS? O NEGATIVE RBCs CAN BE GIVEN TO ~ 95% OF PEOPLE
Red Cell Therapeutics™ 6 POTENTIALLY TRANSFORMATIVE CELLULAR THERAPIES OFF - THE - SHELF BROAD THERAPEUTIC APPLICATIONS PREDICTABLE BIODISTRIBUTION DEFINED LIFE IN CIRCULATION AND CONVENIENT DOSING ADVANTAGEOUS TOLERABILITY SCALABLE MANUFACTURING RARE DISEASE CANCER AUTOIMMUNE

The Promise of Red Cell Therapeutics™: Highly Potent, Allogeneic and Off - the - Shelf 7 Red Cell Therapeutic (RCT) dose expected to be <1% of normal red cell volume GENETIC E NGINEERING SINGLE HEALTHY O - DONOR EXPANSION & DIFFERENTIATION RED CELL THERAPEUTIC ENUCLEATION & MATURATION RED PLATFORM ® 100 - 1000’s OF DOSES EARLY PROGENITOR CELLS

RED PLATFORM ® Enables Multiple Modalities 8 RARE ENZYME DEFICIENCIES ENZYME REPLACEMENT CELLULAR SHIELDING ENZYMES WITHIN THE RCT CANCER IMMUNE STIMULATION POTENT CELL - CELL INTERACTION AUTOIMMUNE DISEASES IMMUNE MODULATION ANTIGEN - SPECIFIC IMMUNOMODULATORS T CELL NK CELL ANTIGEN - SPECIFIC IMMUNOSTIMULANTS T CELL NON - SPECIFIC IMMUNOMODULATORS NON - SPECIFIC IMMUNOSTIMULANTS TOLERANCE INDUCTION

Building a Broad and Diverse Pipeline 9 PRODUCT CATEGORY PATIENT POPULATION PROGRAM PRECLINICAL IND ENABLING PHASE 1 RARE DISEASES 50,000+ RTX - 134 FPI* & Initial Clinical Data Expected 100,000+ RTX - Uricase 2,000 - 4,000 RTX - CBS 20,000+ RTX - OxOx CANCER 100,000+ RTX - 240 IND on File with FDA 10,000+ RTX - 240 10,000+ RTX - 321 aAPC (HPV+) IND Filing Expected by Year - End 100,000+ RTX - 224 AUTOIMMUNE DISEASES Antigen - Specific RTX - T1D Non - Specific RTX - PV R/R aPD1 Solid Tumors Refractory Gout Type 1 Diabetes Pemphigus Vulgaris R/R aPD1 Solid Tumors R/R AML Post HSCT R/R HPV+ Solid Tumors Phenylketonuria Homocystinuria Hyperoxaluria *FPI: First Patient In

10 MANUFACTURING

Manufacturing Update 11 • Expected to provide supply for RTX - 240 and RTX - 321 trials • CMO producing RTX - 134 for current Phase 1b clinical trial • Rubius manufacturing site operational • Delivered nine months ahead of schedule • Potential to expand manufacturing capabilities based on future needs

12 PHENYLKETONURIA
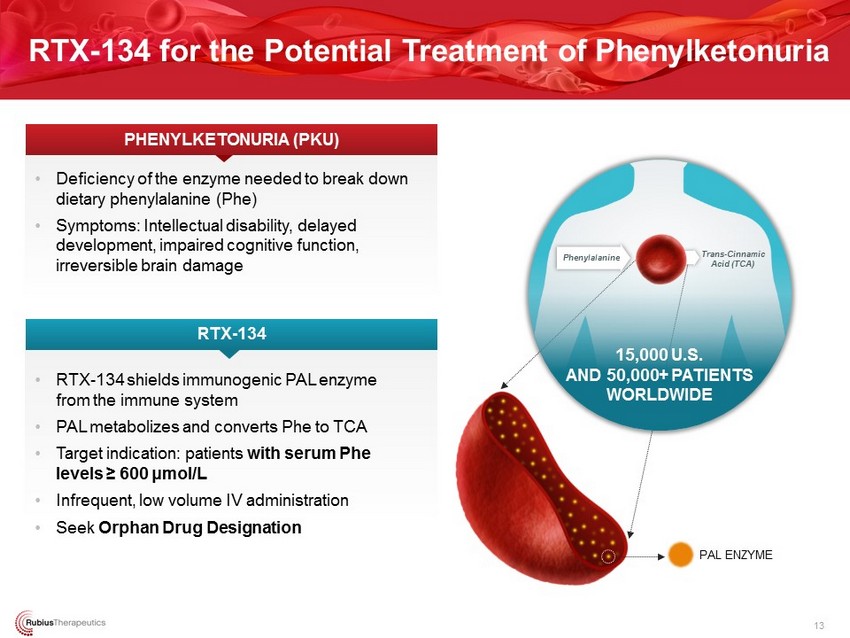
• RTX - 134 shields immunogenic PAL enzyme from the immune system • PAL metabolizes and converts Phe to TCA • Target indication: patients with serum Phe levels ≥ 600 μ mol/L • Infrequent, low volume IV administration • Seek Orphan Drug Designation • Deficiency of the enzyme needed to break down dietary phenylalanine (Phe) • Symptoms: Intellectual disability, delayed development, impaired cognitive function, irreversible brain damage RTX - 134 PHENYLKETONURIA (PKU) PAL ENZYME 15,000 U.S. AND 50,000+ PATIENTS WORLDWIDE Phenylalanine Trans - Cinnamic Acid (TCA) RTX - 134 for the Potential Treatment of Phenylketonuria 13

Trans - Cinnamic Acid (TCA) Phenylalanine ( Phe ) RTX - 134: Simple Three - Step Mechanism of Action 14 Phe Naturally Enters RTX - 134 as it Circulates 1 TCA Exits RTX - 134 and is Processed by the Body 3 PAL Enzyme Breaks Down Phe into TCA inside RTX - 134 2

SINGLE ASCENDING DOSE OF RTX - 134 N=UP TO 12 PATIENTS RTX - 134 Phase 1b Clinical Trial Design 15 Cohort 1 Cohort 2 Cohort 3 Cohort 4 n=2 n=2 n=2 n=2 Optional Cohort 5 Optional Cohort 6 Study design provides flexibility to enroll additional patients to certain cohorts or add additional cohorts PRIMARY OBJECTIVES: • Evaluate the safety and tolerability of RTX - 134 • Correlate dose with percent reduction in serum Phe levels relative to baseline • Determine a preliminary dose to achieve serum Phe levels < 360 µmol/L and < 600 µmol/L SECONDARY OBJECTIVES: • To evaluate pharmacodynamics of RTX - 134, including measurement of TCA Additional optional cohorts at lower, intermediate or higher dose levels may be explored

Initial Clinical Results 16 INITIAL CLINICAL DATA EXPECTED TO INCLUDE: PHASE 1B SINGLE DOSE ADMINISTRATION CLINICAL STUDY • Preliminary safety • Longevity of cells in circulation • Production of TCA as biomarker of MOA

17 CANCER

Challenges of Agonist Monoclonal Antibodies and Recombinant Cytokines 18 1 Barthowiak , et. al., Clinical Cancer Research ; 24(5) March 1, 2018 AGONIST ANTIBODIES AND RECOMBINANT CYTOKINES • Artificially engineered • Limited combinability due to toxicity • Systemic administration floods the liver and may lead to hepatotoxicity 1 • Narrow therapeutic window

Rubius Therapeutics’ Differentiated Oncology Approaches BROAD IMMUNE SYSTEM STIMULATION 19 • Stimulate adaptive and innate immunity through immune cell agonists – Presentation of synergistic co - stimulatory ligands and cytokines – Vascular compartmentalization may reduce toxicities – Potential broad therapeutic window ANTIGEN - SPECIFIC IMMUNE STIMULATION • Drive unique, in vivo antigen - specific immune responses – Co - stimulatory ligand induces significant quantity of CD8+T cells – Cytokine potently stimulates desired quality of killer T cells Natural ligand presentation drives potent cell - cell interactions in preclinical models

A Potentially Transformative Approach to Oncology: IND Under Review with FDA & FPI Expected in 2H’20 20 BROAD IMMUNE SYSTEM STIMULATION POTENTIAL BENEFITS: • Improved anti - tumor activity • Overcome resistance to immunotherapy • Reduced toxicity given vascular compartmentalization 4 - 1BBL NK CELL 4 - 1BBL IL - 15TP T CELL STIMULATE ADAPTIVE AND INNATE IMMUNE CELL AGONISTS RTX - 240 | (4 - 1BBL + IL - 15TP)

U t o m i l + X - l i n k i n g A b R T X - 2 1 2 R T X - C T R L 0 25 50 75 100 125 N F k B p a t h w a y a c t i v a t i o n ( f o l d i n c r e a s e o v e r n o t r e a t m e n t ) Realizing the Promise of the 4 - 1BB Pathway: RTX - 240 Stimulated Potent Activation of Immune System 21 NF k B ACTIVATION BY RTX - 240 IN T CELLS RTX - 240 is ~10 - Fold Superior to 4 - 1BB Agonist Antibody in Preclinical Models 100 nM 10 nM 1 nM 0.1 nM 0.01 nM 310 K 155 K 78 K 39 K 19 K 400 K 200 K 100 K 50 K 25 K 4 - 1BB agonist Ab RTX - 240 RCT CTRL RTX/RCT cell # or Ab concentration Dugast , et. al., American Association for Cancer Research ; Poster #3272, 2019

mRCT - 240 Inhibits Tumor Growth as Monotherapy or in Combination with Anti - PD - 1 mAb 22 m R C T - C T R L I V P D 1 m A b I V m R C T - 2 4 0 + P D 1 m A b I V 0 50 100 150 200 250 M e t a s t a s e s p e r L u n g p = 0.0025 mRCT - 240 + anti - PD - 1 mAb reduced tumor burden vs. anti - PD - 1 mAb alone mRCT - 240 reduced tumor burden and was equivalent to 4 - 1BB mAb m R C T - C T R L I V m R C T - I L 1 5 T P I V m R C T - 4 - 1 B B L I V m R C T - 2 4 0 I V 4 - 1 B B m A b I V 0 50 100 150 200 M e t a s t a s e s p e r L u n g p=0.0096 p<0.0001 p<0.0001 p=0.02 p<0.0001 ns B16F10 LUNG METASTASES MODEL B16F10 LUNG METASTASES MODEL m = murine

Lack of Liver Toxicity in Preclinical Models Suggests Better Therapeutic Window than 4 - 1BB mAbs 23 m R C T - C T R L m R C T - 2 1 2 1 E 9 P D 1 m A b 1 5 0 u g m R C T - 2 1 2 1 E 9 + P D 1 m A b 1 5 0 u g 4 - 1 B B m A b 2 0 0 u g 4 - 1 B B m A b m A b 5 0 u g 0 1 10 7 2 10 7 3 10 7 4 10 7 # F 4 / 8 0 Liver Macrophages m R C T - C T R L m R C T - 2 1 2 1 E 9 P D 1 m A b 1 5 0 u g m R C T - 2 1 2 1 E 9 + P D 1 m A b 1 5 0 u g 4 - 1 B B m A b 2 0 0 u g 4 - 1 B B m A b 5 0 u g 0 5 10 4 1 10 5 1 10 6 2 10 6 3 10 6 4 10 6 5 10 6 # C D 8 + / E o m e s + / K L R G 1 + Liver CD8+/ Eomes+ /KLRG1+ m R C T - C T R L m R C T - 2 1 2 1 E 9 P D 1 m A b 1 5 0 u g m R C T - 2 1 2 1 E 9 + P D 1 m A b 1 5 0 u g 4 - 1 B B m A b 2 0 0 u g 4 - 1 B B m A b 5 0 u g 0 5 10 6 1 10 7 1.5 10 7 # C D 8 + T C e l l s Liver Infiltrating T Cells m R C T - C T R L m R C T - 2 1 2 1 E 9 P D 1 m A b 1 5 0 u g m R C T - 2 1 2 1 E 9 + P D 1 m A b 1 5 0 u g 4 - 1 B B m A b 2 0 0 u g 4 - 1 B B m A b 5 0 u g 0 50 100 150 200 250 A L T / S G P T ( U / L ) Serum ALT Normal Range Dugast , et. al., American Association for Cancer Research ; Poster #3272, 2019
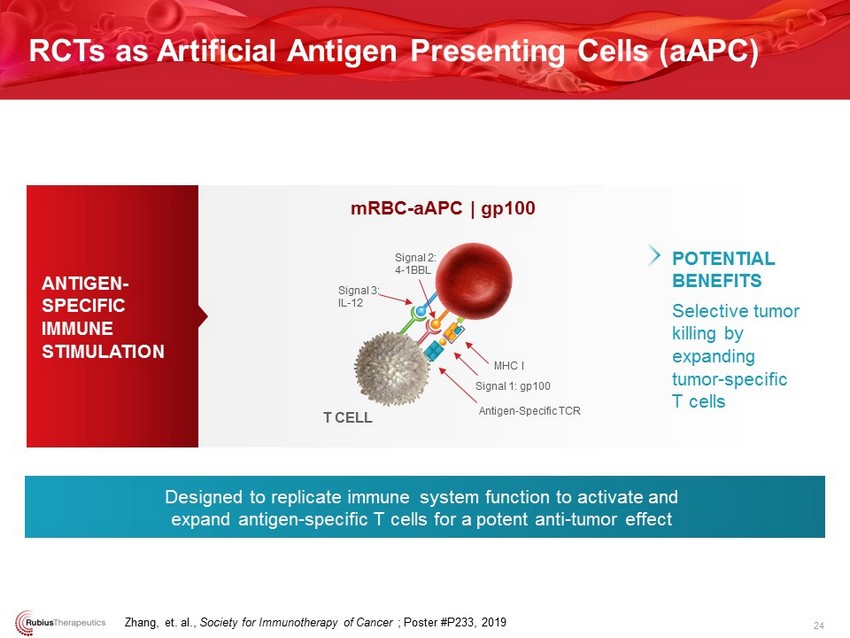
ANTIGEN - SPECIFIC IMMUNE STIMULATION RCTs as Artificial Antigen Presenting Cells ( aAPC ) 24 Selective tumor killing by expanding tumor - specific T cells POTENTIAL BENEFITS Designed to replicate immune system function to activate and expand antigen - specific T cells for a potent anti - tumor effect mRBC - aAPC | gp100 T CELL Signal 2: 4 - 1BBL Antigen - Specific TCR MHC I Signal 1: gp100 Signal 3: IL - 12 Zhang, et. al., Society for Immunotherapy of Cancer ; Poster #P233, 2019

mRBC - aAPC (gp100) Promotes Antigen - Specific T - Cell Expansion 25 CIRCULATION SPLEEN m R B C - C T R L 1 1 0 9 2 . 5 1 0 8 6 1 0 7 0 1 10 5 2 10 5 3 10 5 4 10 5 Pmel Number C D 9 0 . 1 + C D 8 + n u m b e r ** mRBC-gp100-4-1BBL-IL-12 Pmel Number Pmel Number Antigen - specific T cell expansion occurs in circulation and secondary lymphoid organs Zhang, et. al., Society for Immunotherapy of Cancer ; Poster #P233, 2019 1 × 1 0 9 m R B C - C T R L 1 × 1 0 9 m R B C - g p 1 0 0 - 4 - B B L - I L - 1 2 2 . 5 × 1 0 8 m R B C - g p 1 0 0 - 4 - B B L - I L - 1 2 6 × 1 0 7 m R B C - g p 1 0 0 - 4 - B B L - I L - 1 2 0 1×10 4 2×10 4 3×10 4 4×10 4 5×10 4 C D 9 0 . 1 + C D 8 + n u m b e r i n 5 0 m l b l o o d Day 4 Day 7 Day 11

mRBC - aAPC (gp100) Nearly Eliminates Lung Metastases in Melanoma Model 26 Lung Metastases Count mRBC - CTRL m R B C - C T R L 1 × 1 0 9 2 . 5 × 1 0 8 6 × 1 0 7 0 50 100 150 200 250 Lung Metastasis Count L u n g M e t a s t a s i s C o u n t ** ** ** mRBC-gp100-4-1BBL-IL-12 mRBC - aAPC (gp100) Zhang, et. al., Society for Immunotherapy of Cancer ; Poster #P233, 2019

Artificial Antigen Presenting Cells to Target HPV 16+ Cancers 27 ANTIGEN - SPECIFIC IMMUNE STIMULATION Replicating immune system function to activate and expand antigen - specific T cells for a potent anti - tumor effect RTX - 321 | HPV 16+ Tumors T CELL Signal 2: 4 - 1BBL Antigen - Specific TCR MHC I Signal 1: HPV Signal 3: IL - 12 POTENTIAL BENEFITS: SELECTIVE TUMOR KILLING BY EXPANDING ANTIGEN - SPECIFIC T CELLS

RTX - 321 (HPV+) Demonstrates In Vitro Activity 28 0 1×10 6 2×10 6 3×10 6 4×10 6 0 2 4 6 8 10 RTX Cell Number H P V T C R A c t i v a t i o n ( F o l d c h a n g e i n L u m i n e s e n c e ) RTX-null RTX-321 (HPV+) RTX-aAPC (CMV) • HPV - driven cancers are a global health burden and a foremost cause of infection - related cancers 1 • In the U.S., 51% of cervical cancers and 85% of head and neck cancers are HPV 16 - positive 2 HPV - DRIVEN CANCERS DOSE - DEPENDENT HPV TCR ACTIVATION 3 1. Serrano B, et al. Best Pract Res Clin Obstet Gynaecol . 2018;47:14 - 26. 2. Goodman, M., et al. J Natl Cancer Inst . 2015; Jun; 107(6): djv086. 3. Zhang, et al., American Association for Cancer Research , Poster #3260, 2019

29 INTELLECTUAL PROPERTY

Potential to Realize Broad Depth of Value of Red Cell Therapeutics 30 BROAD AND DEEP INTELLECTUAL PROPERTY COVERING: RAPIDLY EXPANDING PATENT PORTFOLIO Pioneering processes for engineering and culturing RCT product candidates Issued U.S. patents cover RTX - 134, RTX - 240, RTX - 224 and RTX - 321 Cover composition of matter, method of treating and method of making Patent Families 32 Applications Pending Worldwide >165 Issued U.S. Patents 10

31 2020 MILESTONES

Anticipated 2020 Milestones: A Year of Execution 32 AN INTEGRATED DEVELOPMENT COMPANY RTX - 321 IND filing by year - end STRONG FINANCIAL POSITION • $324.7 million in cash, cash equivalents and investments as of September 30, 2019 GMP material for RTX - 240 and RTX - 321 trials from Rubius site RTX - 134 FPI and initial clinical results RTX - 240 IND clearance • Cash runway into mid - 2021

REALIZING THE POWER OF RED ™ : A NEW ERA IN CELLULAR MEDICINE
