Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Neoleukin Therapeutics, Inc. | d867852d8k.htm |
Exhibit 99.1

De novo proteins for immunotherapy January 2020

Forward Looking Statements Certain of the statements made in these slides and the accompanying oral presentation are forward looking, including those relating to Neoleukin’s business, strategy, future operations, advancement of its product candidates and product pipeline, clinical development of its product candidates, including expectations regarding timing of regulatory submissions and initiation of clinical trials, regulatory requirements for initiation of clinical trials and registration of product candidates, the sufficiency of its cash resources and other statements containing the words “anticipate,” “believe,” “expect,” “may,” “plan,” “project,” “potential,” “will,” “would,” “could,” “continue,” and similar expressions. These statements are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, risks and uncertainties related to: whether results of early clinical trials or preclinical studies will be indicative of the results of future trials, the adequacy of any clinical models, uncertainties associated with regulatory review of clinical trials; our ability to identify or acquire additional clinical candidates, our ability to obtain and maintain regulatory approval for any product candidates and the potential safety, efficacy or clinical utility of or any product candidates, and other factors discussed in the “Risk Factors” section of the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2019 as filed with the Securities and Exchange Commission. Actual results or developments may differ materially from those projected or implied in these forward-looking statements. More information about the risks and uncertainties faced by the Company is contained in its Quarterly Report on Form 10-Q for the quarter ended September 30, 2019, and subsequent reports, filed with the Securities and Exchange Commission. The Company disclaims any intention or obligation to update or revise any forward-looking statements, whether as a result of new information, future events or otherwise. © 2020 Neoleukin Therapeutics. All Rights Reserved. Do not distribute or reproduce 2

Leader in De Novo Protein Design for Immunotherapy Neoleukin Therapeutics (NASDAQ: NLTX) founded in 2018; merged with Aquinox Pharmaceuticals to become public company in August 2019 Closed follow-on offering in December 2019 Platform technology utilizes computational methods for de novo protein design of NeoleukinTM cytokine mimetics Lead program: NL-201, a highly potent, non-alpha, agonist of IL-2 and IL-15 receptors for cancer immunotherapy Headquarters in Seattle; 39 employees © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 3

Leadership Team Jonathan Drachman, M.D. Kamran Alam Daniel-Adriano Silva, Ph.D. Chief Executive Officer Chief Financial Officer (Interim) VP, Head of Research Previous: CMO, EVP R&D, Previous: CFO Aquinox Previous: Translational Investigator, Seattle Genetics IPD, UW Carl Walkey, Ph.D. Umut Ulge, M.D., Ph.D. Leslie Aberman, J.D. Samantha Willing VP, Corporate Development VP, Translational Medicine VP, IP & Legal Affairs VP, People Previous: Postdoctoral Fellow, Previous: Postdoctoral Fellow, Previous: IDRI, Previous: Seattle Genetics, IPD, UW UW Seattle Genetics Microsoft © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 4

Board of Directors Jonathan G. Drachman, M.D. Todd Simpson Cantey Boyd Director Lead Independent Director Director CEO and President, CFO Managing Director, Neoleukin Therapeutics Seattle Genetics Baker Brothers Advisors Sarah Noonberg, M.D. Lewis T. Williams, M.D., Ph.D. Sean Nolan Director Director Director Previous: CMO, Nohla Therapeutics CEO Walking Fish Therapeutics Previous: CEO, AveXis Previous: CEO, FivePrime Therapeutics 5 © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce
Functional De Novo Proteins: Better Immunotherapies by Design Computational algorithms invented by Daniel-Adriano Silva at UW IPD NL-201: IL-2/IL-15 cytokine mimetic (Neoleukin™) for cancer immunotherapy Discovery published in Nature January 2019 Company formed to advance technology platform and lead asset © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 6
Neoleukin Therapeutics: Pioneers in De Novo Protein Design Technology originated at University of Washington’s Institute for Protein Design, led by David Baker, PhD Scientific founders are world leaders in de novo protein design Exclusive license obtained for commercialization of NL-201 and other de novo protein assets Investigating additional NeoleukinTM cytokine mimetics for cancer, inflammation, and autoimmunity © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 7
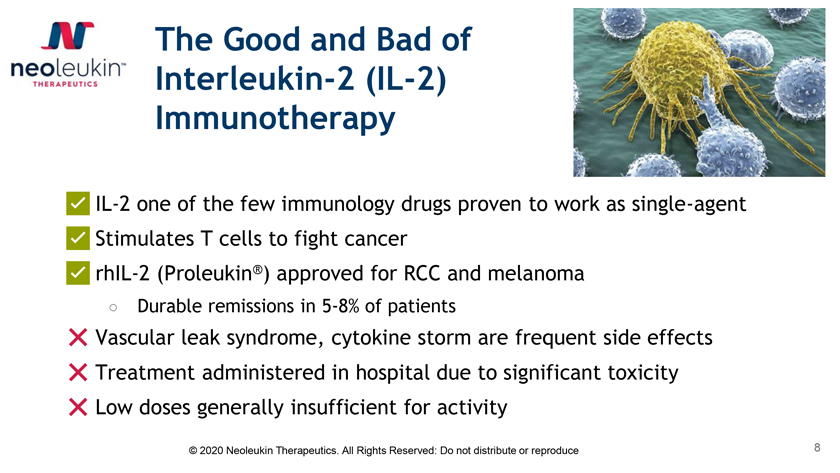
The Good and Bad of Interleukin-2 (IL-2) Immunotherapy ✅IL-2 one of the few immunology drugs proven to work as single-agent ✅Stimulates T cells to fight cancer ✅rhIL-2 (Proleukin®) approved for RCC and melanoma â—‹ Durable remissions in 5-8% of patients âŒVascular leak syndrome, cytokine storm are frequent side effects âŒTreatment administered in hospital due to significant toxicity âŒLow doses generally insufficient for activity © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 8
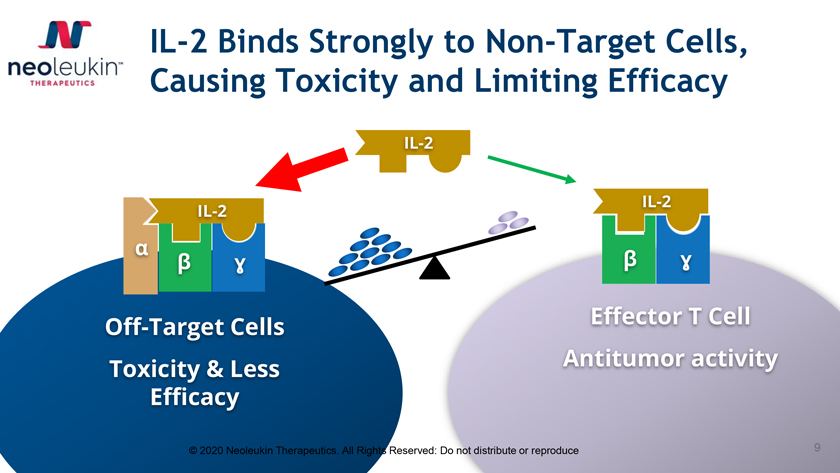
IL-2 Binds Strongly to Non-Target Cells, Causing Toxicity and Limiting Efficacy IL-2 IL-2 IL-2 á â â É£ É£ Off-Target Cells Effector T Cell Toxicity & Less Antitumor activity Efficacy © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 9
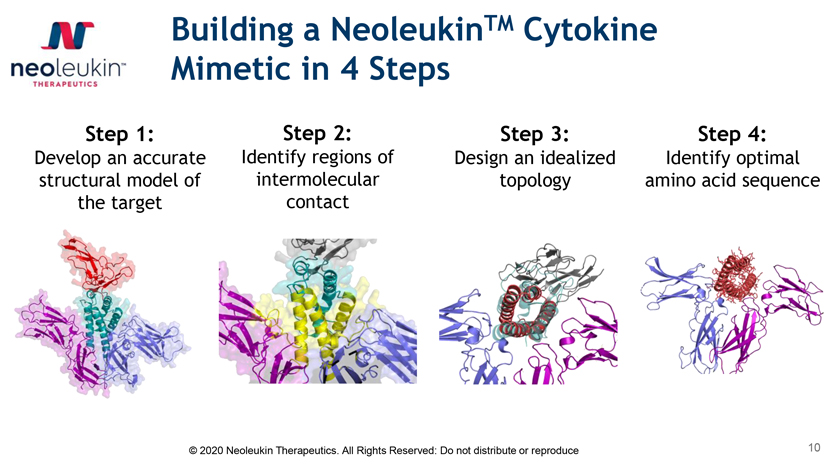
Building a NeoleukinTM Cytokine Mimetic in 4 Steps Step 1: Step 2: Step 3: Step 4: Develop an accurate Identify regions of Design an idealized Identify optimal structural model of intermolecular topology amino acid sequence the target contact © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 10
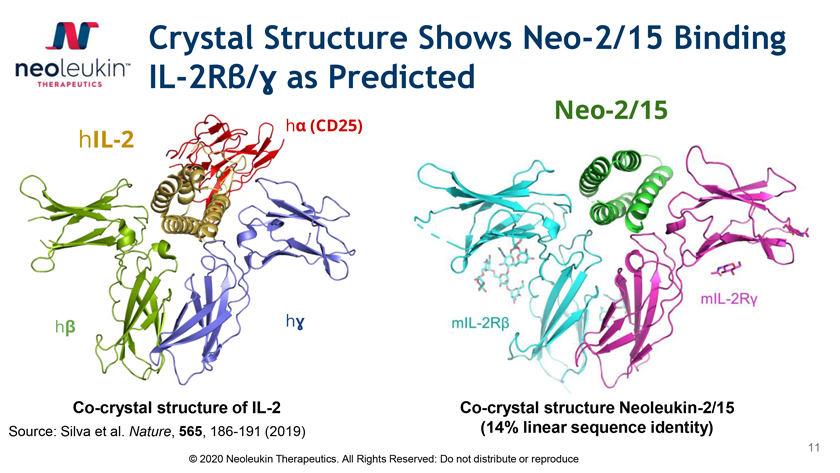
Crystal Structure Shows Neo-2/15 Binding IL-2Râ/É£ as Predicted Neo-2/15 há (CD25) hIL-2 hâ hÉ£ Co-crystal structure of IL-2 Co-crystal structure Neoleukin-2/15 Source: Silva et al. Nature, 565, 186-191 (2019) (14% linear sequence identity) 11 © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce
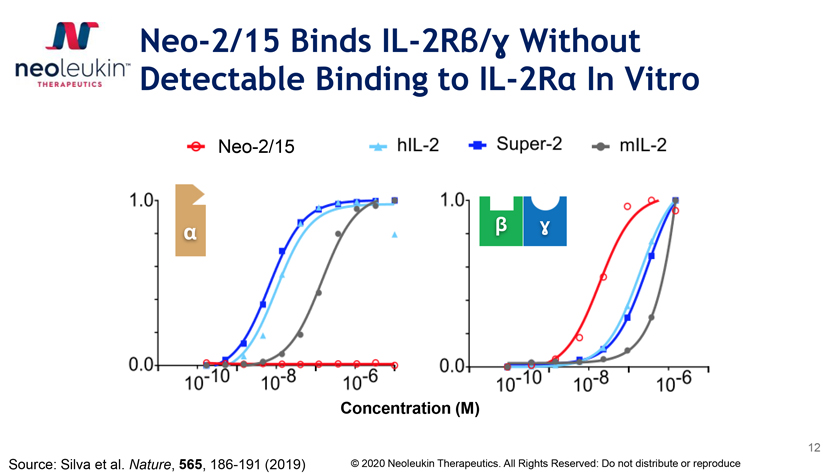
Neo-2/15 Binds IL-2Rβ/É£ Without Detectable Binding to IL-2Rα In Vitro Neo-2/15 á â É£ Concentration (M) 12 Source: Silva et al. Nature, 565, 186-191 (2019) © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce
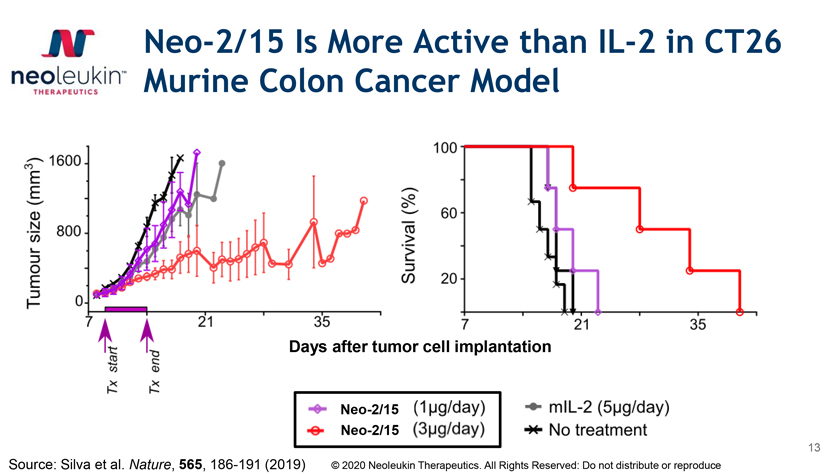
Neo-2/15 Is More Active than IL-2 in CT26 Murine Colon Cancer Model mor cell implantation Neo-2/15 Neo-2/15 13 Source: Silva et al. Nature, 565, 186-191 (2019) © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce
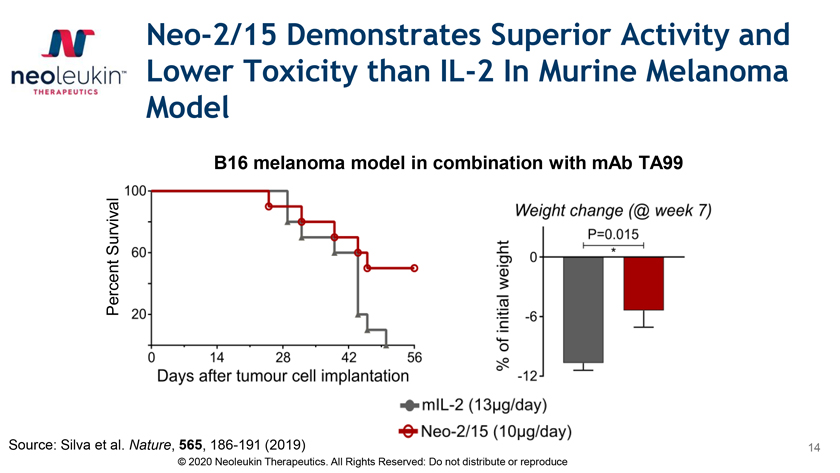
Neo-2/15 Demonstrates Superior Activity and Lower Toxicity than IL-2 In Murine Melanoma Model B16 melanoma model in combination with mAb TA99 Survival Percent Source: Silva et al. Nature, 565, 186-191 (2019) 14 © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce
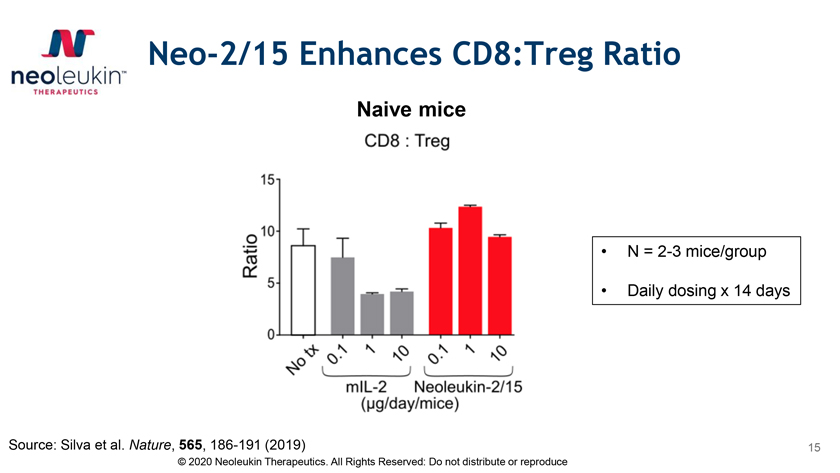
Neo-2/15 Enhances CD8:Treg Ratio Naive mice • N = 2-3 mice/group • Daily dosing x 14 days Source: Silva et al. Nature, 565, 186-191 (2019) 15 © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce
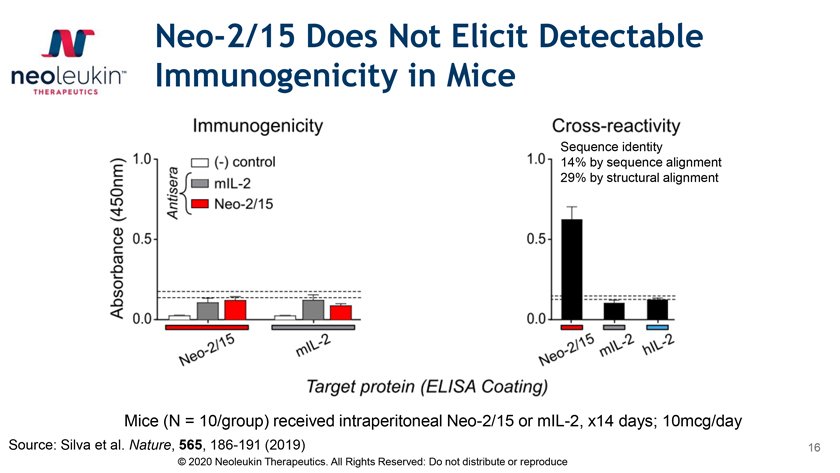
Neo-2/15 Does Not Elicit Detectable Immunogenicity in Mice Sequence identity 14% by sequence alignment 29% by structural alignment Mice (N = 10/group) received intraperitoneal Neo-2/15 or mIL-2, x14 days; 10mcg/day Source: Silva et al. Nature, 565, 186-191 (2019) 16 © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce
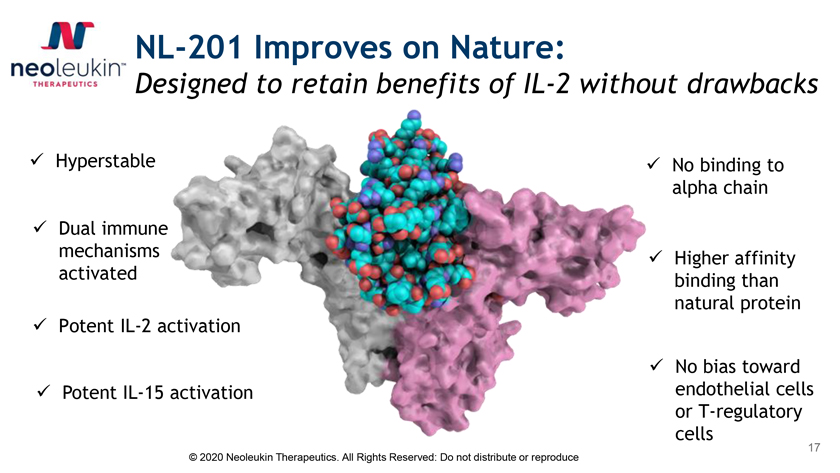
NL-201 Improves on Nature: Designed to retain benefits of IL-2 without drawbacks ✓ Hyperstable ✓ No binding to alpha chain ✓ Dual immune mechanisms ✓ Higher affinity activated binding than natural protein ✓ Potent IL-2 activation ✓ No bias toward ✓ Potent IL-15 activation endothelial cells or T-regulatory cells © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 17

IL-2/IL-15 Competitive Landscape Other significant IL-2/IL-15 immunotherapies in development are modified versions of native IL-2 or IL-15 IL-2 modification often results in a less potent, less stable molecule, with some residual binding to the alpha subunit We believe NL-201 is the only IL-2 agonist specifically designed with no alpha-binding region NL-201 potently agonizes both IL-2 and IL-15 receptors, potentially stimulating complementary cancer-fighting pathways © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 18
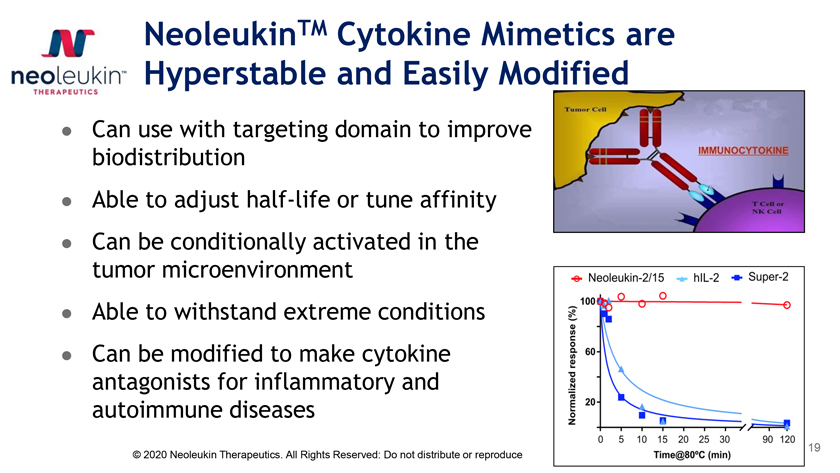
NeoleukinTM Cytokine Mimetics are Hyperstable and Easily Modified Can use with targeting domain to improve biodistribution Able to adjust half-life or tune afinity Can be conditionally activated in the tumor microenvironment Able to withstand extreme conditions Can be modified to make cytokine antagonists for inflammatory and autoimmune diseases 19 © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce
NL-201: On Track for IND by End of 2020 CMC: process developed and GLP tox batch successfully produced Toxicology: multi-dose, non-GLP and GLP tox studies conducted in rats and non-human primates â—‹ GLP in-life dosing complete; no unexpected toxicity observed Bioassays: GLP and clinical bioassays in development and on track to support IND submission and clinical studies Pharmacology: multiple models under evaluation 20 © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce

Financial Highlights $66.3 million cash & cash equivalents as of Sept. 30, 2019 Follow-on offering in December 2019 raised ~$81M (net) ~38 million common shares outstanding and ~11 million pre-funded warrants after follow-on Cash and cash equivalents expected to fund operations through 2022 © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 21
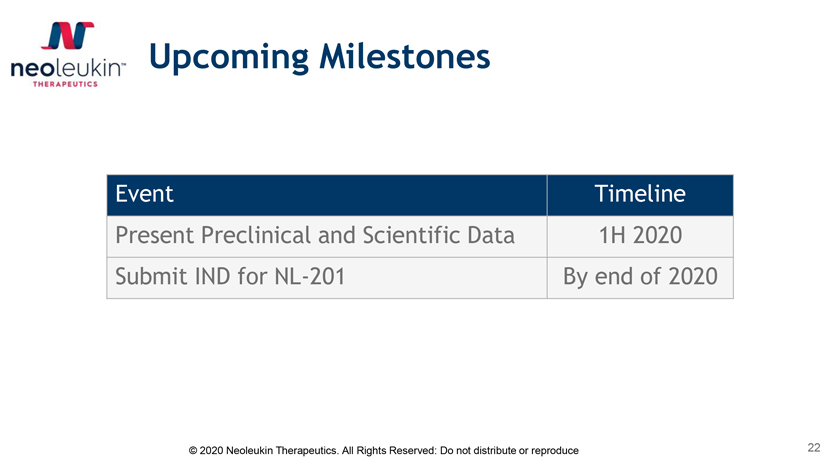
Upcoming Milestones Event Timeline Present Preclinical and Scientific Data 1H 2020 Submit IND for NL-201 By end of 2020 © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce 22

life. 23 © 2020 Neoleukin Therapeutics. All Rights Reserved: Do not distribute or reproduce
