Attached files
| file | filename |
|---|---|
| 8-K - 8-K - IDEAYA Biosciences, Inc. | idya-8k_20200113.htm |
IDEAYA Biosciences Corporate Presentation JP Morgan Conference January 2020 Exhibit 99.1 “[COMPANY LOGO]”

Safe Harbor Statement Certain statements in this presentation and the accompanying oral commentary are forward-looking statements. These statements relate to future events or the future financial performance of IDEAYA Biosciences, Inc. (the “Company”) and involve known and unknown risks, uncertainties and other factors that may cause the actual results, levels of activity, performance or achievements of the Company or its industry to be materially different from those expressed or implied by any forward-looking statements. In some cases, forward-looking statements can be identified by terminology such as “may,” “will,” “could,” “would,” “should,” “expect,” “plan,” “anticipate,” “intend,” “believe,” “estimate,” “predict,” “potential” or other comparable terminology. All statements other than statements of historical fact could be deemed forward-looking, including any expectations regarding the Company’s target discovery platform or new target validation efforts as creating opportunities for research and development initiatives; any projections of financial information, market opportunities, cash runway or profitability; any statements about historical results that may suggest trends for the Company's business; any statements of the plans, strategies, and objectives of management for development programs or future operations; any statements about the timing of preclinical research, clinical development, regulatory filings, manufacturing or release of data; any statements of expectation or belief regarding future events, potential markets or market size, or technology developments; and any statements of assumptions underlying any of the items mentioned. The Company has based these forward-looking statements on its current expectations, assumptions, estimates and projections. While the Company believes these expectations, assumptions, estimates and projections are reasonable, such forward-looking statements are only predictions and involve known and unknown risks and uncertainties, many of which are beyond the Company's control. These and other important factors may cause actual results, performance or achievements to differ materially from those expressed or implied by these forward-looking statements. The forward-looking statements in this presentation are made only as of the date hereof. For a further description of the risks and uncertainties that could cause actual results to differ from those expressed in these forward-looking statements, as well as risks relating to the business of the Company in general, see the Company's periodic filings with the Securities and Exchange Commission (the "SEC"), including its Quarterly Report on Form 10-Q for the quarter ended September 30, 2019 and any current and periodic reports filed thereafter. Except as required by law, the Company assumes no obligation and does not intend to update these forward-looking statements or to conform these statements to actual results or to changes in the Company's expectations. This presentation concerns anticipated products that are under clinical investigation and which have not yet been approved for marketing by the U.S. Food and Drug Administration (FDA). It is currently limited by Federal law to investigational use, and no representation is made as to its safety or effectiveness for the purposes for which it is being investigated. “[COMPANY LOGO]” 2
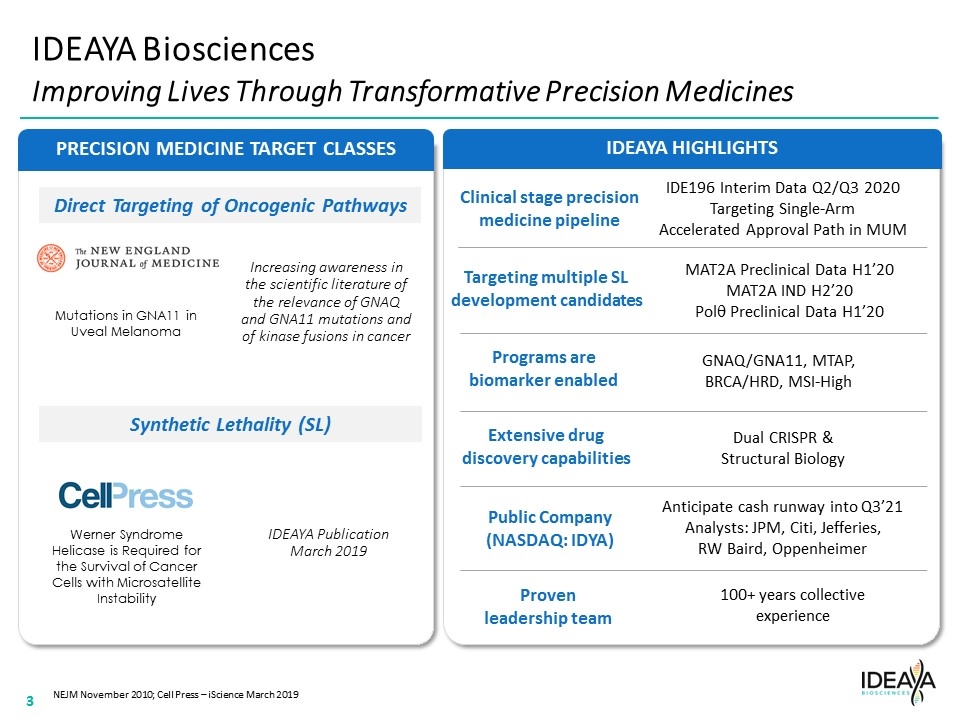
IDEAYA HIGHLIGHTS Synthetic Lethality (SL) Direct Targeting of Oncogenic Pathways PRECISION MEDICINE TARGET CLASSES IDEAYA Biosciences Improving Lives Through Transformative Precision Medicines Werner Syndrome Helicase is Required for the Survival of Cancer Cells with Microsatellite Instability Mutations in GNA11 in Uveal Melanoma NEJM November 2010; Cell Press – iScience March 2019 Clinical stage precision medicine pipeline Programs are biomarker enabled Extensive drug discovery capabilities Public Company (NASDAQ: IDYA) Proven leadership team Targeting multiple SL development candidates IDE196 Interim Data Q2/Q3 2020 Targeting Single-Arm Accelerated Approval Path in MUM MAT2A Preclinical Data H1’20 MAT2A IND H2’20 Polq Preclinical Data H1’20 GNAQ/GNA11, MTAP, BRCA/HRD, MSI-High Dual CRISPR & Structural Biology Anticipate cash runway into Q3’21 Analysts: JPM, Citi, Jefferies, RW Baird, Oppenheimer 100+ years collective experience Increasing awareness in the scientific literature of the relevance of GNAQ and GNA11 mutations and of kinase fusions in cancer IDEAYA Publication March 2019 “[COMPANY LOGO]” “[THE NEW ENGLAND JOURNAL OF MEDICINE LOGO]” 3
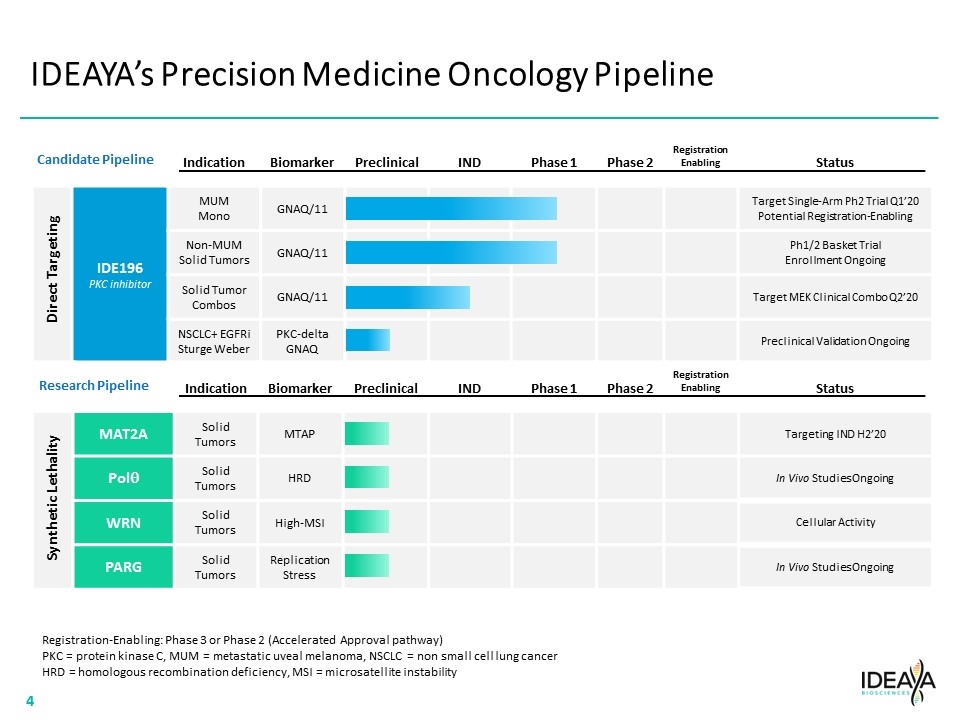
IDEAYA’s Precision Medicine Oncology Pipeline Indication Biomarker Preclinical IND Phase 1 Phase 2 Registration Enabling Status IDE196 PKC inhibitor MUM Mono GNAQ/11 Target Single-Arm Ph2 Trial Q1’20 Potential Registration-Enabling Non-MUM Solid Tumors GNAQ/11 Ph1/2 Basket Trial Enrollment Ongoing Solid Tumor Combos GNAQ/11 Target MEK Clinical Combo Q2’20 NSCLC+ EGFRi Sturge Weber PKC-delta GNAQ Preclinical Validation Ongoing Direct Targeting Indication Biomarker Preclinical IND Phase 1 Phase 2 Registration Enabling Status MAT2A Solid Tumors MTAP Targeting IND H2’20 Polq Solid Tumors HRD In Vivo Studies Ongoing WRN Solid Tumors High-MSI Cellular Activity PARG Solid Tumors Replication Stress In Vivo Studies Ongoing Synthetic Lethality Registration-Enabling: Phase 3 or Phase 2 (Accelerated Approval pathway) PKC = protein kinase C, MUM = metastatic uveal melanoma, NSCLC = non small cell lung cancer HRD = homologous recombination deficiency, MSI = microsatellite instability Candidate Pipeline Research Pipeline “[COMPANY LOGO]” 4
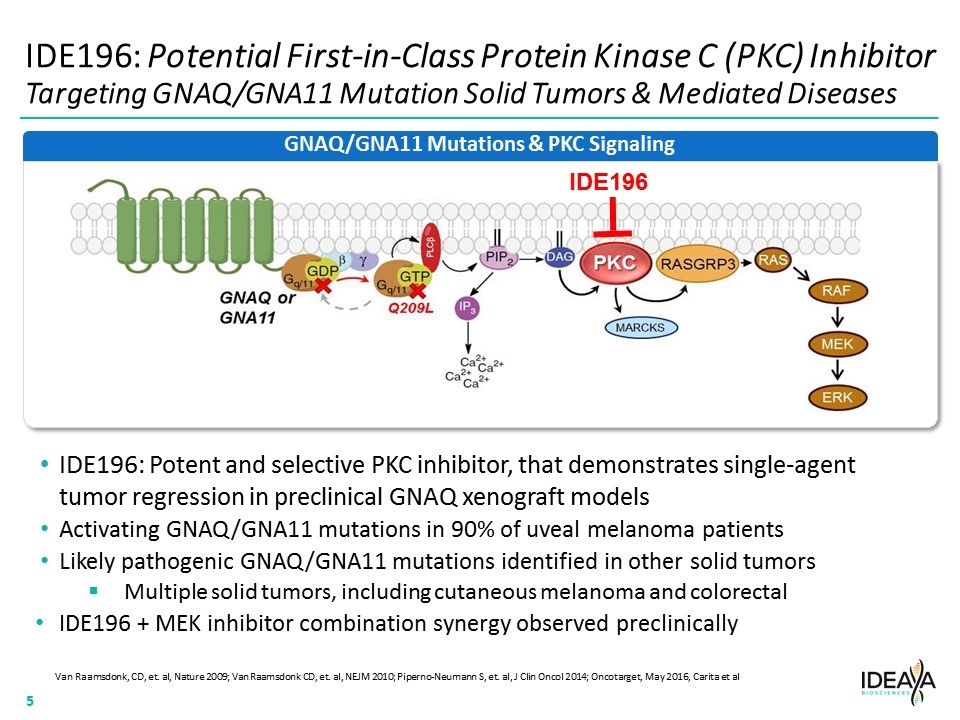
IDE196: Potential First-in-Class Protein Kinase C (PKC) Inhibitor Targeting GNAQ/GNA11 Mutation Solid Tumors & Mediated Diseases Van Raamsdonk, CD, et. al, Nature 2009; Van Raamsdonk CD, et. al, NEJM 2010; Piperno-Neumann S, et. al, J Clin Oncol 2014; Oncotarget, May 2016, Carita et al IDE196: Potent and selective PKC inhibitor, that demonstrates single-agent tumor regression in preclinical GNAQ xenograft models Activating GNAQ/GNA11 mutations in 90% of uveal melanoma patients Likely pathogenic GNAQ/GNA11 mutations identified in other solid tumors Multiple solid tumors, including cutaneous melanoma and colorectal IDE196 + MEK inhibitor combination synergy observed preclinically GNAQ/GNA11 Mutations & PKC Signaling IDE196 “[COMPANY LOGO]” “[Graphic]” 5
Metastatic Uveal Melanoma (MUM) Removal of the eye (”enucleation”) Surgery to save eye Radiation treatment - Iodine-125 plaque - Proton beam Other treatments - Laser, tumor resection Treatment Options for Primary Disease ~ 90% of uveal melanoma patients harbor activating GNAQ or GNA11 mutation ~ 50% of uveal melanoma patients get metastatic disease, primarily to the liver No FDA-approved therapies Median overall survival (OS) approximately 10 months Historical response rates observed in published studies generally between 0 - 10% across various experimental therapies Targeting Metastatic Uveal Melanoma (MUM) High Unmet Medical Need & Devastating Disease IDEAYA’s Vision Improving lives through transformative precision medicines Therapeutic Advances in Medical Oncology, Review, January 2018 “[COMPANY LOGO]” 6
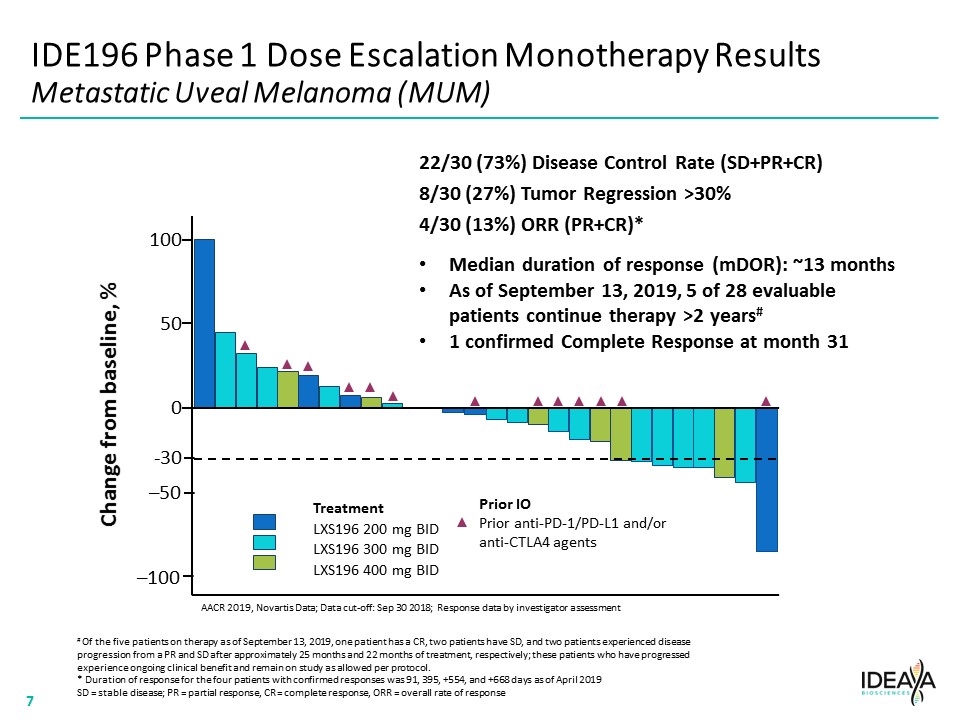
IDE196 Phase 1 Dose Escalation Monotherapy Results Metastatic Uveal Melanoma (MUM) AACR 2019, Novartis Data; Data cut-off: Sep 30 2018; Response data by investigator assessment Treatment LXS196 200 mg BID LXS196 300 mg BID LXS196 400 mg BID -30 22/30 (73%) Disease Control Rate (SD+PR+CR) 8/30 (27%) Tumor Regression >30% 4/30 (13%) ORR (PR+CR)* Change from baseline, % –100 –50 0 50 100 Prior IO Prior anti-PD-1/PD-L1 and/or anti-CTLA4 agents Median duration of response (mDOR): ~13 months As of September 13, 2019, 5 of 28 evaluable patients continue therapy >2 years# 1 confirmed Complete Response at month 31 # Of the five patients on therapy as of September 13, 2019, one patient has a CR, two patients have SD, and two patients experienced disease progression from a PR and SD after approximately 25 months and 22 months of treatment, respectively; these patients who have progressed experience ongoing clinical benefit and remain on study as allowed per protocol. * Duration of response for the four patients with confirmed responses was 91, 395, +554, and +668 days as of April 2019 SD = stable disease; PR = partial response, CR = complete response, ORR = overall rate of response “[COMPANY LOGO]” 7
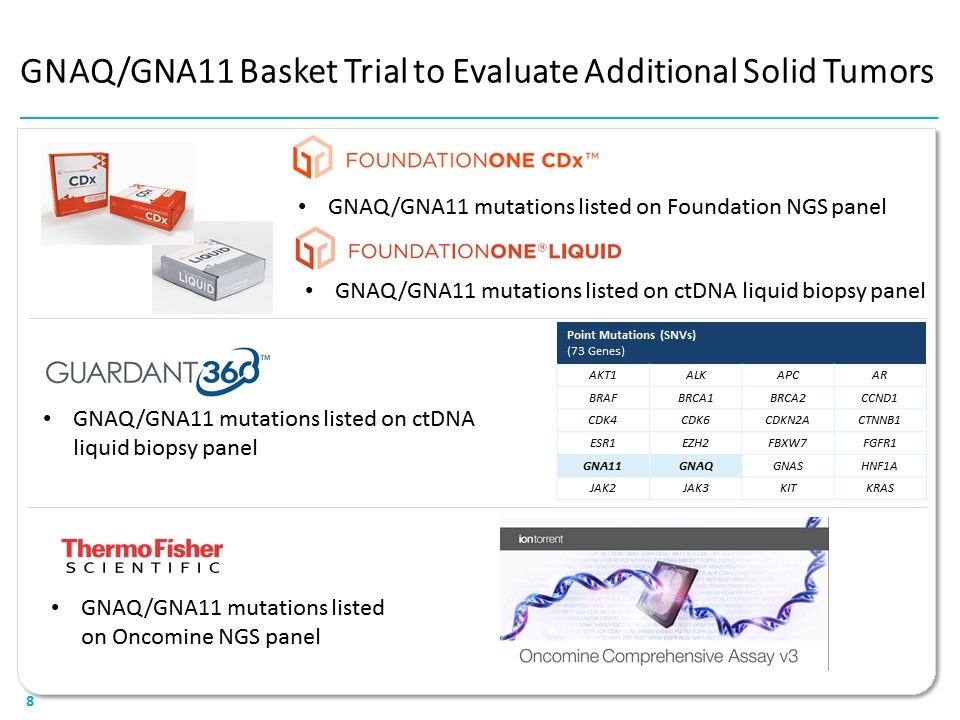
GNAQ/GNA11 Basket Trial to Evaluate Additional Solid Tumors GNAQ/GNA11 mutations listed on Foundation NGS panel GNAQ/GNA11 mutations listed on ctDNA liquid biopsy panel GNAQ/GNA11 mutations listed on ctDNA liquid biopsy panel Point Mutations (SNVs) (73 Genes) AKT1 ALK APC AR BRAF BRCA1 BRCA2 CCND1 CDK4 CDK6 CDKN2A CTNNB1 ESR1 EZH2 FBXW7 FGFR1 GNA11 GNAQ GNAS HNF1A JAK2 JAK3 KIT KRAS GNAQ/GNA11 mutations listed on Oncomine NGS panel “[CDX LOGO]” “[LIQUID LOGO]” “[GUARDANT360 LOGO] “[THERMO FISHER SCIENTIFIC LOGO] “[ion torrent LOGO] 8
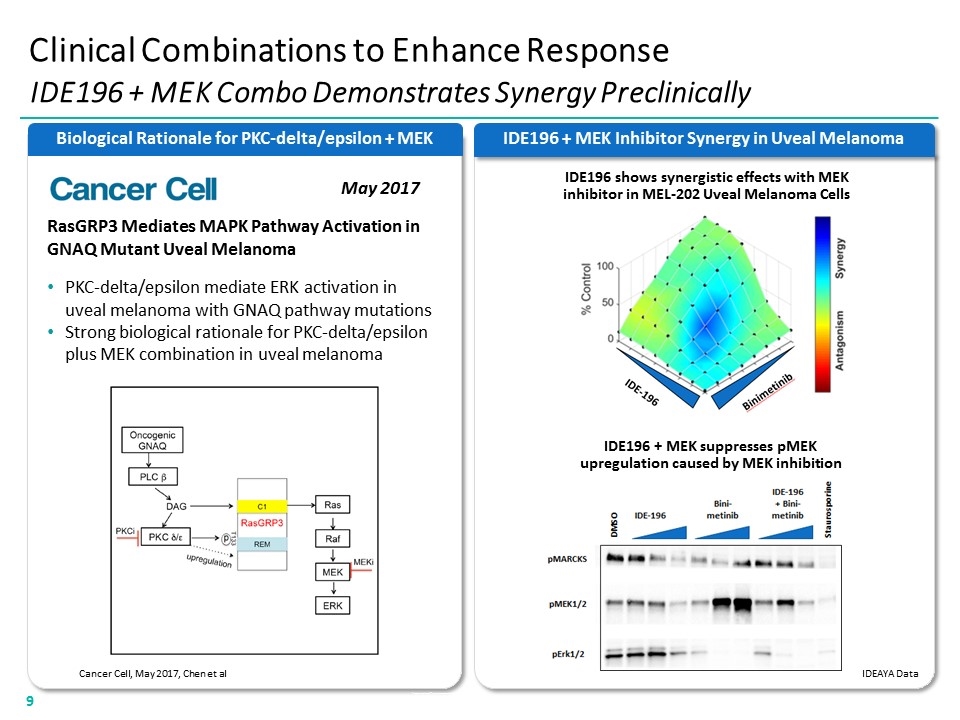
Biological Rationale for PKC-delta/epsilon + MEK Clinical Combinations to Enhance Response IDE196 + MEK Combo Demonstrates Synergy Preclinically RasGRP3 Mediates MAPK Pathway Activation in GNAQ Mutant Uveal Melanoma PKC-delta/epsilon mediate ERK activation in uveal melanoma with GNAQ pathway mutations Strong biological rationale for PKC-delta/epsilon plus MEK combination in uveal melanoma May 2017 Cancer Cell, May 2017, Chen et al IDE196 + MEK Inhibitor Synergy in Uveal Melanoma IDEAYA Data IDE196 shows synergistic effects with MEK inhibitor in MEL-202 Uveal Melanoma Cells IDE196 + MEK suppresses pMEK upregulation caused by MEK inhibition “[CANCER CELL CHART]” “[IDE196 SYNERGISTIC GRAPHICS]” “[IDE196 BAR CHART]” 9
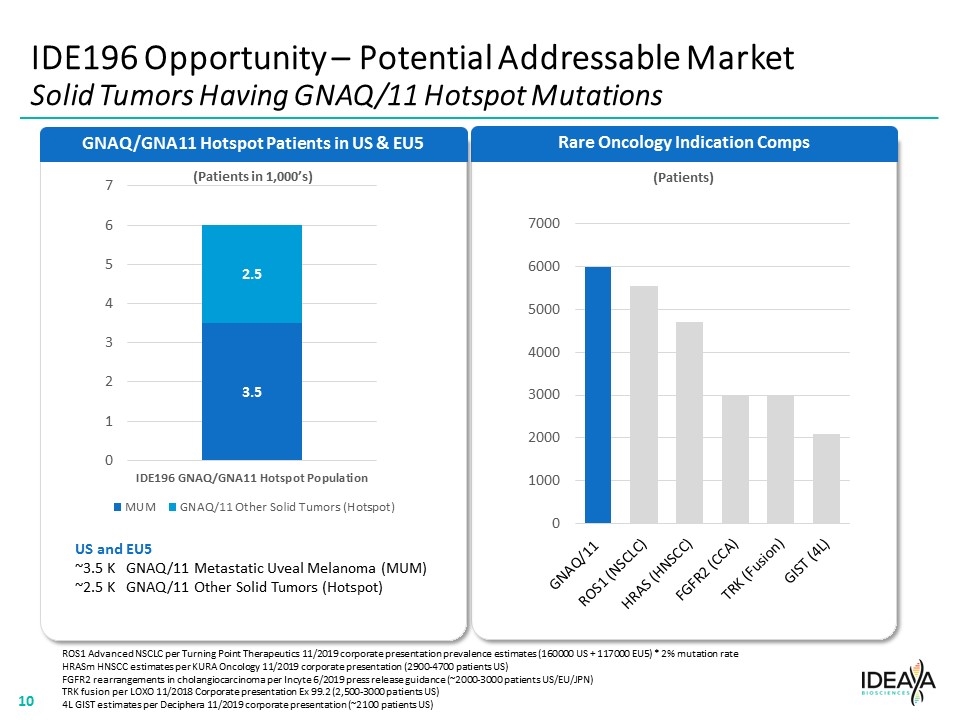
Rare Oncology Indication Comps IDE196 Opportunity – Potential Addressable Market Solid Tumors Having GNAQ/11 Hotspot Mutations GNAQ/GNA11 Hotspot Patients in US & EU5 (Patients in 1,000’s) US and EU5 ~3.5 K GNAQ/11 Metastatic Uveal Melanoma (MUM) ~2.5 K GNAQ/11 Other Solid Tumors (Hotspot) ROS1 Advanced NSCLC per Turning Point Therapeutics 11/2019 corporate presentation prevalence estimates (160000 US + 117000 EU5) * 2% mutation rate HRASm HNSCC estimates per KURA Oncology 11/2019 corporate presentation (2900-4700 patients US) FGFR2 rearrangements in cholangiocarcinoma per Incyte 6/2019 press release guidance (~2000-3000 patients US/EU/JPN) TRK fusion per LOXO 11/2018 Corporate presentation Ex 99.2 (2,500-3000 patients US) 4L GIST estimates per Deciphera 11/2019 corporate presentation (~2100 patients US) “[COMPANY LOGO]” “[GNAQ/GNA11 BAR CHART]” “[RARE ONCOLOGY BAR CHART]” 10
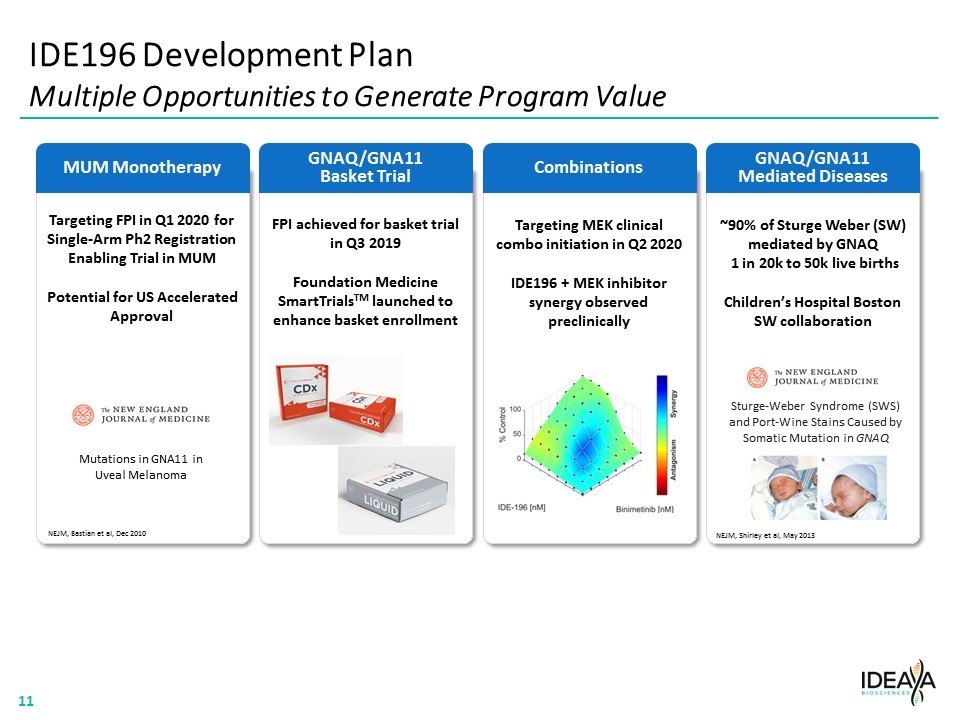
MUM Monotherapy IDE196 Development Plan Multiple Opportunities to Generate Program Value GNAQ/GNA11 Basket Trial Combinations GNAQ/GNA11 Mediated Diseases Targeting FPI in Q1 2020 for Single-Arm Ph2 Registration Enabling Trial in MUM Potential for US Accelerated Approval FPI achieved for basket trial in Q3 2019 Foundation Medicine SmartTrialsTM launched to enhance basket enrollment Targeting MEK clinical combo initiation in Q2 2020 IDE196 + MEK inhibitor synergy observed preclinically ~90% of Sturge Weber (SW) mediated by GNAQ 1 in 20k to 50k live births Children’s Hospital Boston SW collaboration NEJM, Shirley et al, May 2013 Mutations in GNA11 in Uveal Melanoma NEJM, Bastian et al, Dec 2010 Sturge-Weber Syndrome (SWS) and Port-Wine Stains Caused by Somatic Mutation in GNAQ “[COMPANY LOGO]” “[The New England Journal of Medicine Logo]” “[IDE196 GRAPH]” 11
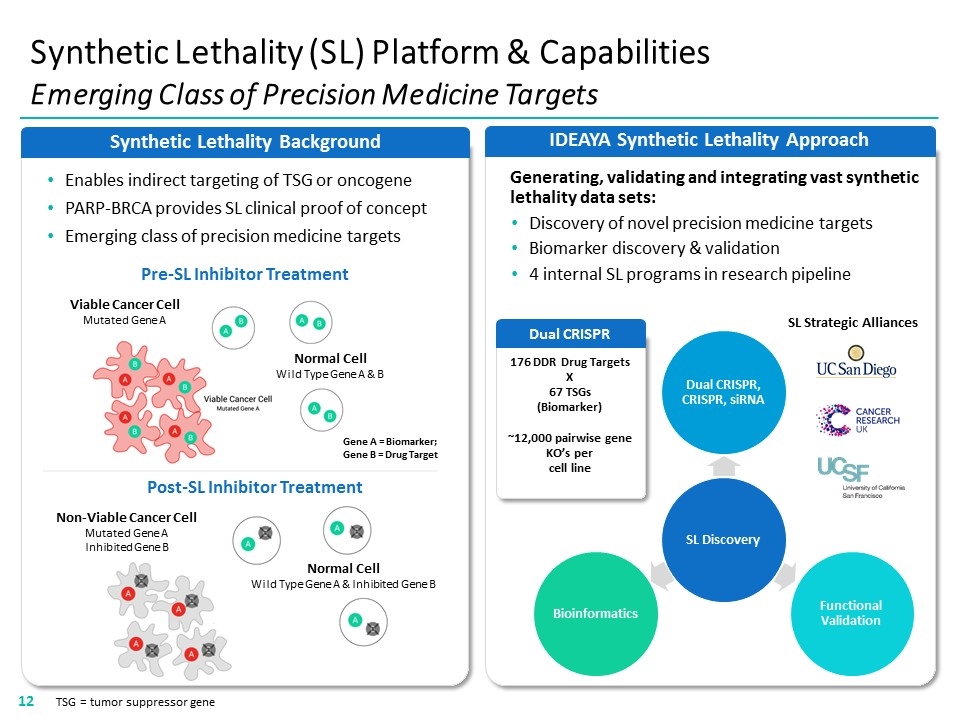
IDEAYA Synthetic Lethality Approach Synthetic Lethality Background Synthetic Lethality (SL) Platform & Capabilities Emerging Class of Precision Medicine Targets TSG = tumor suppressor gene Generating, validating and integrating vast synthetic lethality data sets: Discovery of novel precision medicine targets Biomarker discovery & validation 4 internal SL programs in research pipeline 176 DDR Drug Targets X 67 TSGs (Biomarker) ~12,000 pairwise gene KO’s per cell line Dual CRISPR SL Strategic Alliances Gene A = Biomarker; Gene B = Drug Target Pre-SL Inhibitor Treatment Post-SL Inhibitor Treatment Viable Cancer Cell Mutated Gene A Normal Cell Wild Type Gene A & B Enables indirect targeting of TSG or oncogene PARP-BRCA provides SL clinical proof of concept Emerging class of precision medicine targets Non-Viable Cancer Cell Mutated Gene A Inhibited Gene B Normal Cell Wild Type Gene A & Inhibited Gene B “[VIABLE CANCER CELL GRAPHIC]” “[UC SAN DIEGO LOGO]” “[UCSF LOGO]” “[CANCER RESEARCH UK LOGO]”12 SL Discovery Dual CRISPR, CRISPR, siRNA Functional Validation Bioinformatics
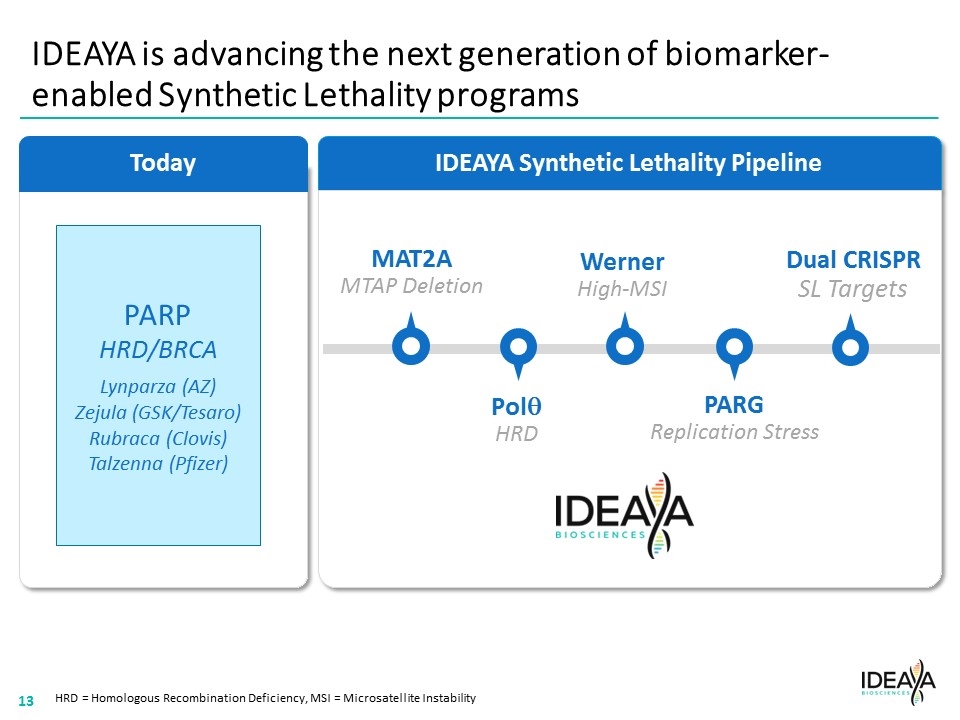
Today IDEAYA Synthetic Lethality Pipeline IDEAYA is advancing the next generation of biomarker-enabled Synthetic Lethality programs HRD = Homologous Recombination Deficiency, MSI = Microsatellite Instability MAT2A MTAP Deletion PARG Replication Stress Polq HRD Dual CRISPR SL Targets PARP HRD/BRCA Lynparza (AZ) Zejula (GSK/Tesaro) Rubraca (Clovis) Talzenna (Pfizer) Werner High-MSI “[COMPANY LOGO]” 13
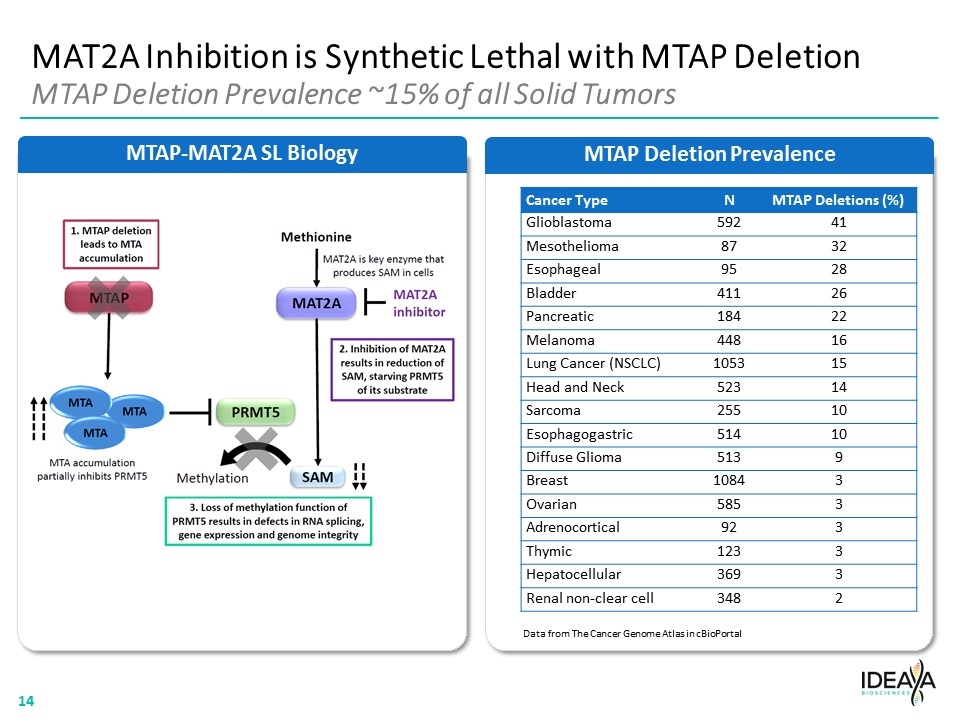
MAT2A Inhibition is Synthetic Lethal with MTAP Deletion MTAP Deletion Prevalence ~15% of all Solid Tumors MTAP Deletion Prevalence MTAP-MAT2A SL Biology Data from The Cancer Genome Atlas in cBioPortal Cancer Type N MTAP Deletions (%) Glioblastoma 592 41 Mesothelioma 87 32 Esophageal 95 28 Bladder 411 26 Pancreatic 184 22 Melanoma 448 16 Lung Cancer (NSCLC) 1053 15 Head and Neck 523 14 Sarcoma 255 10 Esophagogastric 514 10 Diffuse Glioma 513 9 Breast 1084 3 Ovarian 585 3 Adrenocortical 92 3 Thymic 123 3 Hepatocellular 369 3 Renal non-clear cell 348 2 “[MTAP FLOW CHART]” “[COMPANY LOGO]” 14
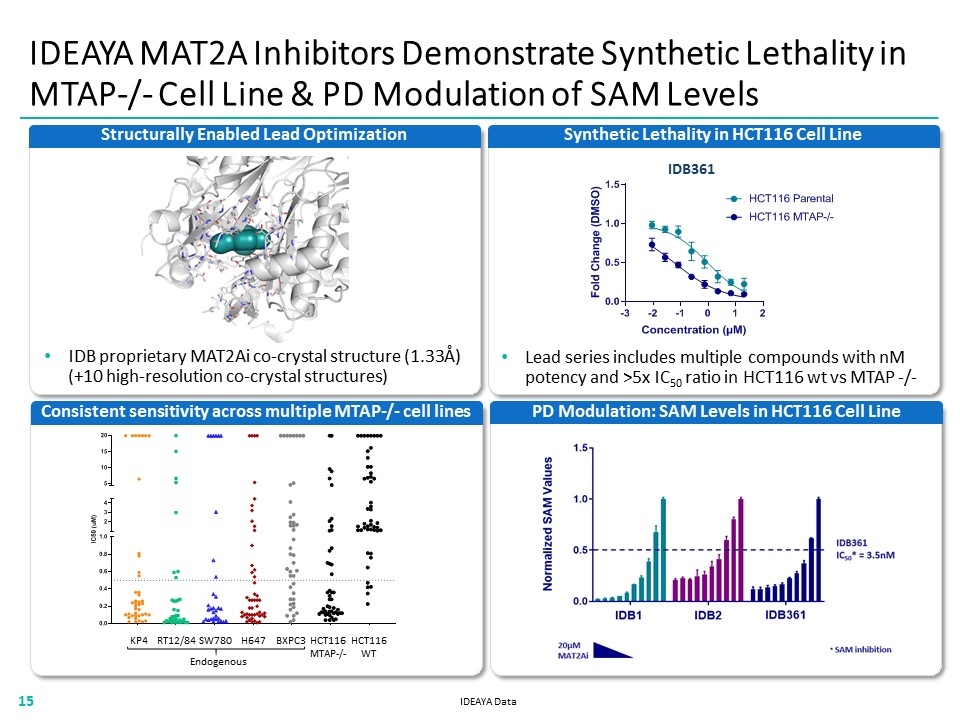
IDEAYA MAT2A Inhibitors Demonstrate Synthetic Lethality in MTAP-/- Cell Line & PD Modulation of SAM Levels PD Modulation: SAM Levels in HCT116 Cell Line Synthetic Lethality in HCT116 Cell Line IDB361 IDEAYA Data Structurally Enabled Lead Optimization IDB proprietary MAT2Ai co-crystal structure (1.33Å) (+10 high-resolution co-crystal structures) Consistent sensitivity across multiple MTAP-/- cell lines KP4 SW780 RT12/84 BXPC3 H647 HCT116 MTAP-/- HCT116 WT Lead series includes multiple compounds with nM potency and >5x IC50 ratio in HCT116 wt vs MTAP -/- Endogenous “[IDB361 LINE BAR CHART]” “[MTAP CHART]” “[HCT116 BAR CHART]”15
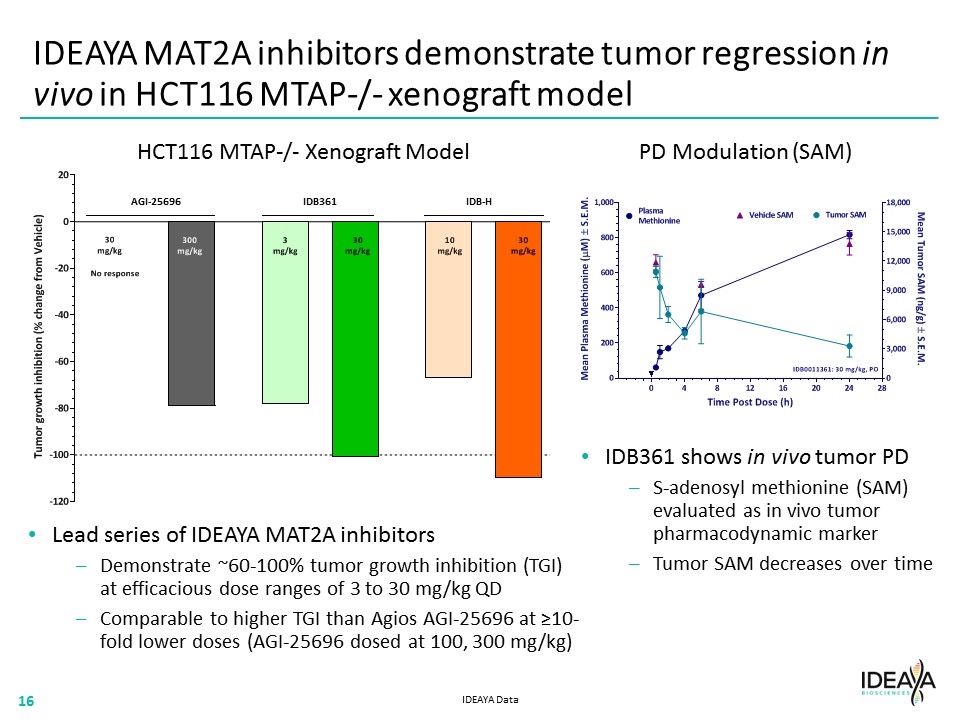
IDEAYA MAT2A inhibitors demonstrate tumor regression in vivo in HCT116 MTAP-/- xenograft model Lead series of IDEAYA MAT2A inhibitors Demonstrate ~60-100% tumor growth inhibition (TGI) at efficacious dose ranges of 3 to 30 mg/kg QD Comparable to higher TGI than Agios AGI-25696 at ≥10-fold lower doses (AGI-25696 dosed at 100, 300 mg/kg) IDB361 shows in vivo tumor PD S-adenosyl methionine (SAM) evaluated as in vivo tumor pharmacodynamic marker Tumor SAM decreases over time HCT116 MTAP-/- Xenograft Model PD Modulation (SAM) IDEAYA Data IDB361 IDB-H AGI-25696 “[HCT116 MTAP BAR CHART]” “[PD MODULATION CHART]” “[COMPANY LOGO]”16
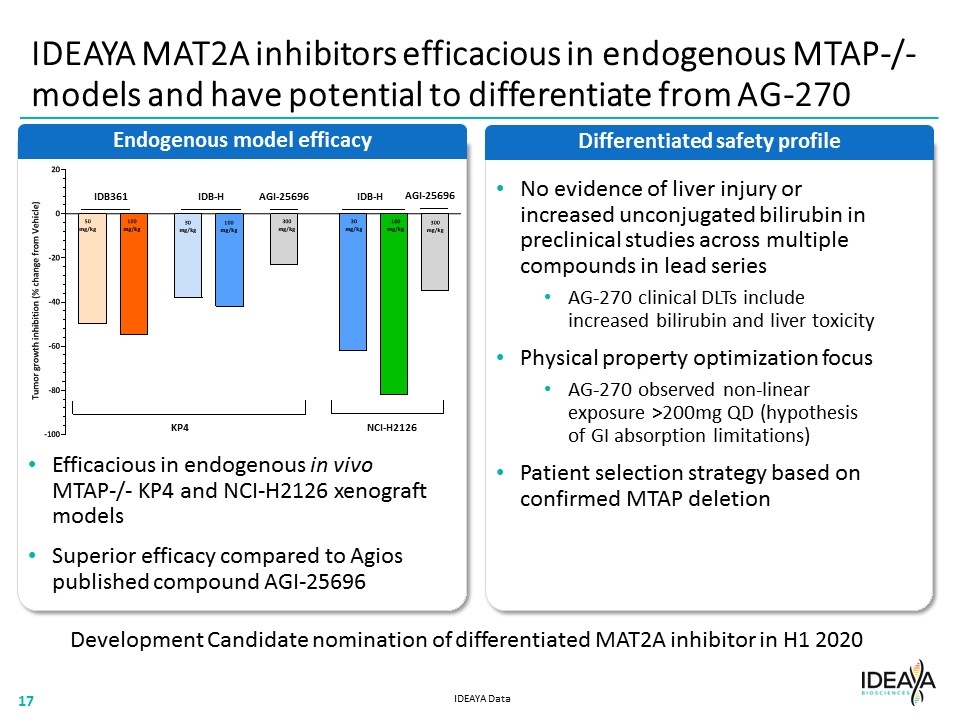
IDEAYA MAT2A inhibitors efficacious in endogenous MTAP-/- models and have potential to differentiate from AG-270 Differentiated safety profile Endogenous model efficacy Efficacious in endogenous in vivo MTAP-/- KP4 and NCI-H2126 xenograft models Superior efficacy compared to Agios published compound AGI-25696 No evidence of liver injury or increased unconjugated bilirubin in preclinical studies across multiple compounds in lead series AG-270 clinical DLTs include increased bilirubin and liver toxicity Physical property optimization focus AG-270 observed non-linear exposure >200mg QD (hypothesis of GI absorption limitations) Patient selection strategy based on confirmed MTAP deletion Development Candidate nomination of differentiated MAT2A inhibitor in H1 2020 IDEAYA Data IDB361 IDB-H IDB-H AGI-25696 AGI-25696 KP4 NCI-H2126 “[ENDOGENOUS MODEL BAR CHART]” “[COMPANY LOGO]”17
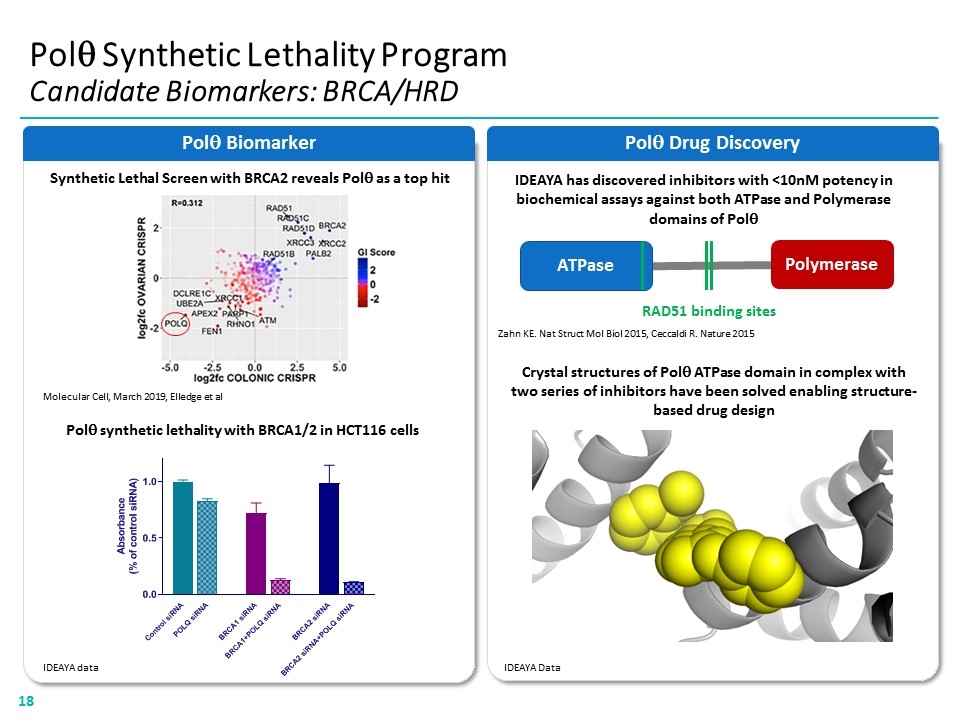
Polq Drug Discovery Polq Synthetic Lethality Program Candidate Biomarkers: BRCA/HRD Polq Biomarker Polq synthetic lethality with BRCA1/2 in HCT116 cells Synthetic Lethal Screen with BRCA2 reveals Polq as a top hit Molecular Cell, March 2019, Elledge et al ATPase Polymerase Zahn KE. Nat Struct Mol Biol 2015, Ceccaldi R. Nature 2015 RAD51 binding sites IDEAYA has discovered inhibitors with <10nM potency in biochemical assays against both ATPase and Polymerase domains of Polq IDEAYA Data IDEAYA data Crystal structures of Polq ATPase domain in complex with two series of inhibitors have been solved enabling structure- based drug design “[BIOMAKER GRAPH]” “[BAR CHART]”18
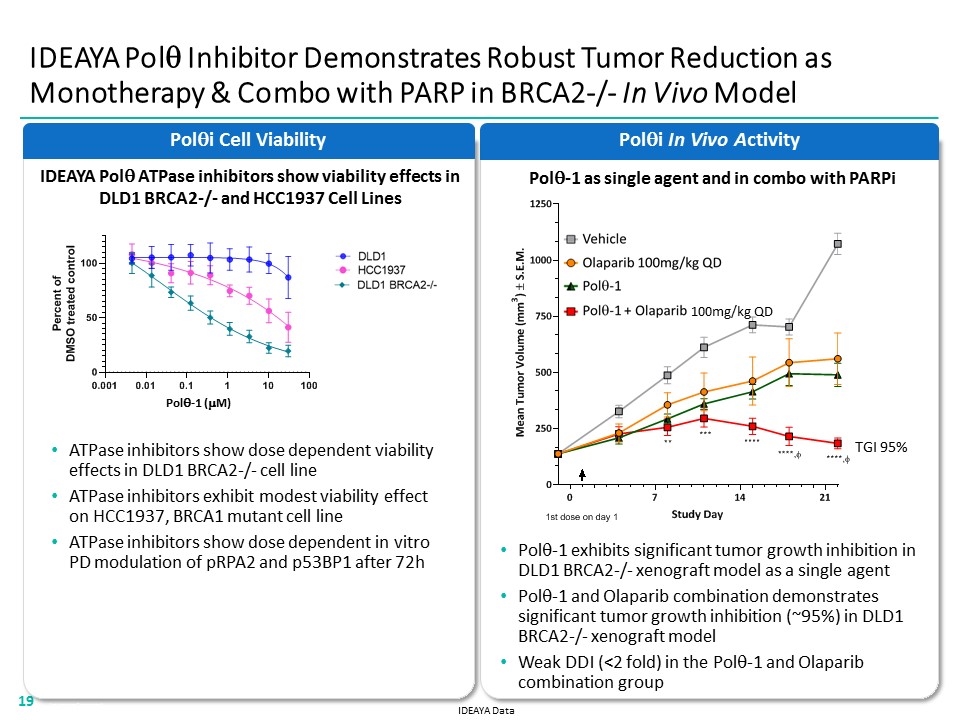
Polqi In Vivo Activity IDEAYA Polq Inhibitor Demonstrates Robust Tumor Reduction as Monotherapy & Combo with PARP in BRCA2-/- In Vivo Model Polqi Cell Viability ATPase inhibitors show dose dependent viability effects in DLD1 BRCA2-/- cell line ATPase inhibitors exhibit modest viability effect on HCC1937, BRCA1 mutant cell line ATPase inhibitors show dose dependent in vitro PD modulation of pRPA2 and p53BP1 after 72h IDEAYA Polq ATPase inhibitors show viability effects in DLD1 BRCA2-/- and HCC1937 Cell Lines Polq-1 exhibits significant tumor growth inhibition in DLD1 BRCA2-/- xenograft model as a single agent Polq-1 and Olaparib combination demonstrates significant tumor growth inhibition (~95%) in DLD1 BRCA2-/- xenograft model Weak DDI (<2 fold) in the Polq-1 and Olaparib combination group IDEAYA Data Polq-1 (mM) TGI 95% 100mg/kg QD Polq-1 as single agent and in combo with PARPi “[CELL VIABILITY LINE BAR CHART]” “[VIVO ACTIVITY LINE BAR CHART]” 19
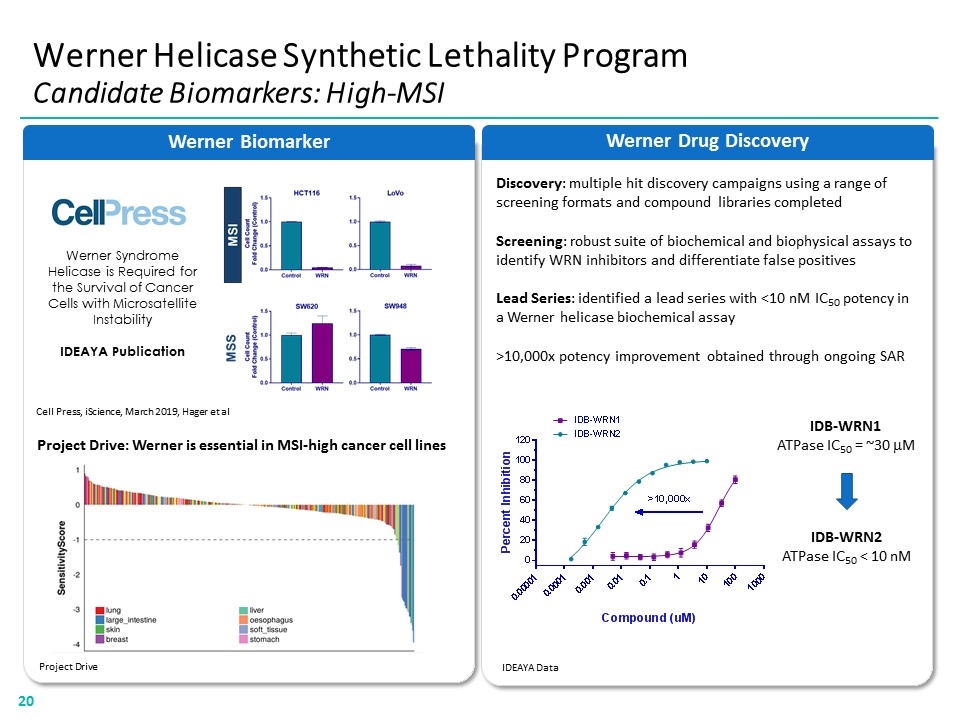
Discovery: multiple hit discovery campaigns using a range of screening formats and compound libraries completed Screening: robust suite of biochemical and biophysical assays to identify WRN inhibitors and differentiate false positives Lead Series: identified a lead series with <10 nM IC50 potency in a Werner helicase biochemical assay >10,000x potency improvement obtained through ongoing SAR Werner Drug Discovery Werner Biomarker Werner Helicase Synthetic Lethality Program Candidate Biomarkers: High-MSI Werner Syndrome Helicase is Required for the Survival of Cancer Cells with Microsatellite Instability IDEAYA Publication Cell Press, iScience, March 2019, Hager et al Project Drive: Werner is essential in MSI-high cancer cell lines Project Drive IDEAYA Data IDB-WRN1 ATPase IC50 = ~30 mM IDB-WRN2 ATPase IC50 < 10 nM “[CELL PRESS LOGO]” “[WERNER BIOMARKER BAR CHART]” “[CELL PRESS LINE BAR CHART] “[DRUG DISCOVERY LINE BAR CHART]20
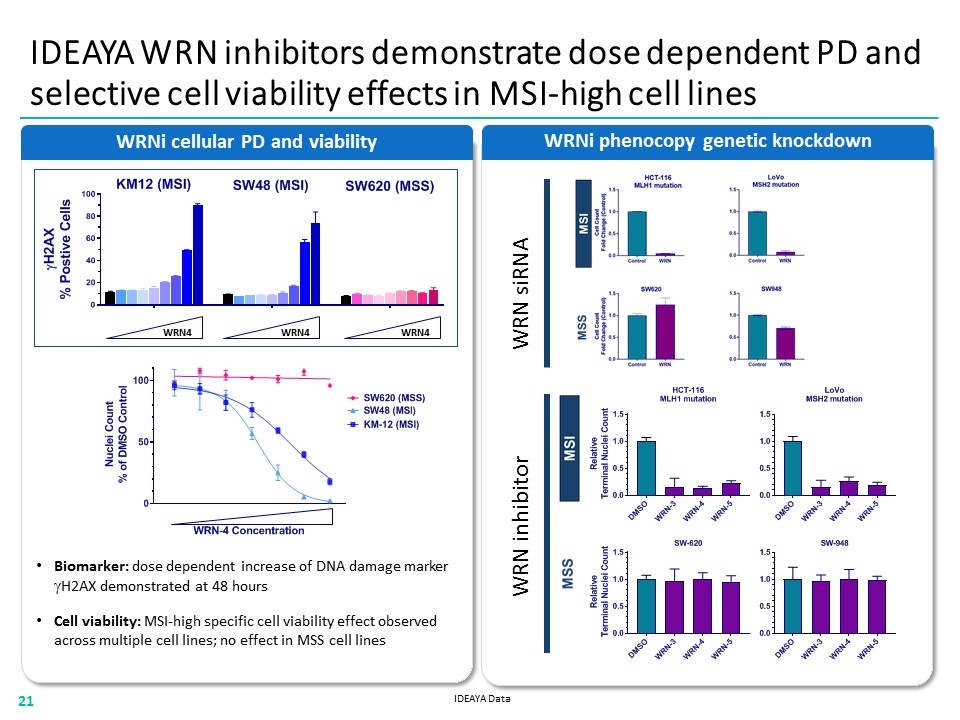
Biomarker: dose dependent increase of DNA damage marker gH2AX demonstrated at 48 hours Cell viability: MSI-high specific cell viability effect observed across multiple cell lines; no effect in MSS cell lines WRNi cellular PD and viability WRNi phenocopy genetic knockdown IDEAYA WRN inhibitors demonstrate dose dependent PD and selective cell viability effects in MSI-high cell lines WRN siRNA WRN4 WRN inhibitor WRN4 WRN4 IDEAYA Data “[WRNI CELLULAR PD AND VIABILITY BAR CHART AND LINE CHART]” “[WRNI PHENOCOPY GENETIC KNOCKDOWN BAR CHART]” 21
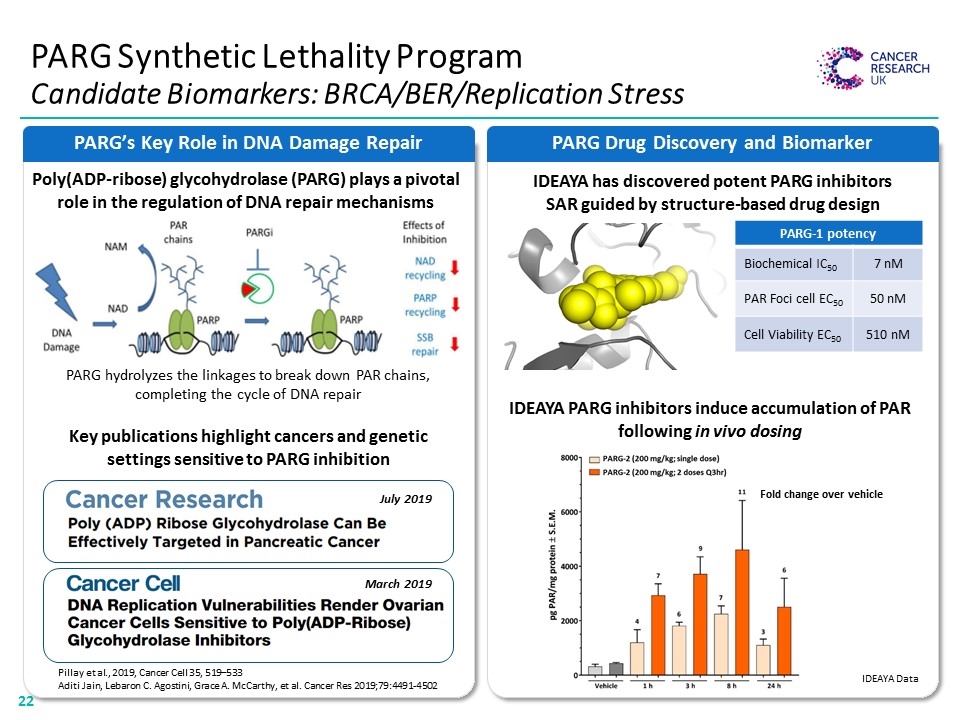
PARG Drug Discovery and Biomarker PARG Synthetic Lethality Program Candidate Biomarkers: BRCA/BER/Replication Stress PARG’s Key Role in DNA Damage Repair IDEAYA has discovered potent PARG inhibitors SAR guided by structure-based drug design Poly(ADP-ribose) glycohydrolase (PARG) plays a pivotal role in the regulation of DNA repair mechanisms IDEAYA PARG inhibitors induce accumulation of PAR following in vivo dosing PARG hydrolyzes the linkages to break down PAR chains, completing the cycle of DNA repair PARG-1 potency Biochemical IC50 7 nM PAR Foci cell EC50 50 nM Cell Viability EC50 510 nM Fold change over vehicle July 2019 March 2019 Key publications highlight cancers and genetic settings sensitive to PARG inhibition Pillay et al., 2019, Cancer Cell 35, 519–533 Aditi Jain, Lebaron C. Agostini, Grace A. McCarthy, et al. Cancer Res 2019;79:4491-4502 IDEAYA Data Cancer Research Cancer Cell “[PARG’S KEY ROLE IN DNA DAMAGE REPAIR GRAPHIC]” “[BAR CHART]”22
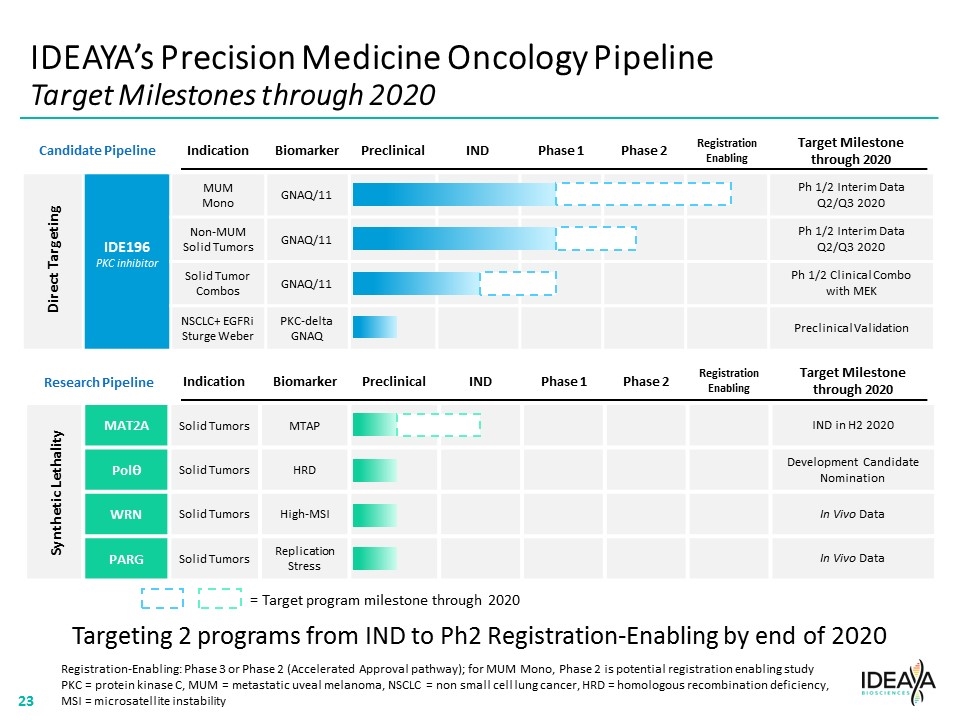
IDEAYA’s Precision Medicine Oncology Pipeline Target Milestones through 2020 Indication Biomarker Preclinical IND Phase 1 Phase 2 Registration Enabling Target Milestone through 2020 IDE196 PKC inhibitor MUM Mono GNAQ/11 Ph 1/2 Interim Data Q2/Q3 2020 Non-MUM Solid Tumors GNAQ/11 Ph 1/2 Interim Data Q2/Q3 2020 Solid Tumor Combos GNAQ/11 Ph 1/2 Clinical Combo with MEK NSCLC+ EGFRi Sturge Weber PKC-delta GNAQ Preclinical Validation Direct Targeting Indication Biomarker Preclinical IND Phase 1 Phase 2 Registration Enabling Target Milestone through 2020 MAT2A Solid Tumors MTAP IND in H2 2020 Polq Solid Tumors HRD Development Candidate Nomination WRN Solid Tumors High-MSI In Vivo Data PARG Solid Tumors Replication Stress In Vivo Data Synthetic Lethality Registration-Enabling: Phase 3 or Phase 2 (Accelerated Approval pathway); for MUM Mono, Phase 2 is potential registration enabling study PKC = protein kinase C, MUM = metastatic uveal melanoma, NSCLC = non small cell lung cancer, HRD = homologous recombination deficiency, MSI = microsatellite instability Targeting 2 programs from IND to Ph2 Registration-Enabling by end of 2020 = Target program milestone through 2020 Candidate Pipeline Research Pipeline “[COMPANY LOGO]” 23
