Attached files
| file | filename |
|---|---|
| 8-K - 8-K - EXACT SCIENCES CORP | exas-20200113x8xk.htm |
| EX-99.1 - EXHIBIT 99.1 - EXACT SCIENCES CORP | exas-20200113xex991.htm |

Exhibit 99.2 Building the Leading Cancer Diagnostics Company Kevin Conroy, Chairman and CEO January 15, 2020 Exact Sciences

Safe harbor statement This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended, that are intended to be covered by the “safe harbor” created by those sections. Forward-looking statements, which are based on certain assumptions and describe our future plans, strategies and expectations, can generally be identified by the use of forward-looking terms such as “believe,” “expect,” “may,” “will,” “should,” “would,” “could,” “seek,” “intend,” “plan,” “goal,” “project,” “estimate,” “anticipate” or other comparable terms. All statements other than statements of historical facts included in this presentation regarding our strategies, prospects, financial condition, operations, costs, plans and objectives are forward-looking statements. Examples of forward-looking statements include, among others, statements we make regarding expected future operating results, anticipated results of our sales, marketing and patient adherence efforts, expectations concerning payer reimbursement, the anticipated results of our product development efforts and the anticipated benefits of our acquisition of Genomic Health, including estimated synergies and other financial impacts. Forward-looking statements are neither historical facts nor assurances of future performance. Instead, they are based only on our current beliefs, expectations and assumptions regarding the future of our business, future plans and strategies, projections, anticipated events and trends, the economy and other future conditions. Because forward-looking statements relate to the future, they are subject to inherent uncertainties, risks and changes in circumstances that are difficult to predict and many of which are outside of our control. Our actual results and financial condition may differ materially from those indicated in the forward-looking statements. Therefore, you should not rely on any of these forward-looking statements. Important factors that could cause our actual results and financial condition to differ materially from those indicated in the forward-looking statements include, among others, the following: our ability to successfully and profitably market our products and services; the acceptance of our products and services by patients and healthcare providers; our ability to meet demand for our products and services; the willingness of health insurance companies and other payers to cover our products and services and adequately reimburse us for such products and services; the amount and nature of competition from other cancer screening and diagnostic products and services; the effects of the adoption, modification or repeal of any law, rule, order, interpretation or policy relating to the healthcare system, including without limitation as a result of any judicial, executive or legislative action; the effects of changes in pricing, coverage and reimbursement for our products and services, including without limitation as a result of the Protecting Access to Medicare Act of 2014; recommendations, guidelines and quality metrics issued by various organizations such as the U.S. Preventive Services Task Force, the American Cancer Society, and the National Committee for Quality Assurance regarding cancer screening or our products and services; our ability to successfully develop new products and services and assess potential market opportunities; our ability to effectively utilize strategic partnerships, such as through our Promotion Agreement with Pfizer, Inc., and acquisitions; our success establishing and maintaining collaborative, licensing and supplier arrangements; our ability to maintain regulatory approvals and comply with applicable regulations; expectations regarding our international expansion and opportunities; the potential effects of foreign currency exchange rate fluctuations and our efforts to hedge such effects; the possibility that the anticipated benefits from our acquisition of Genomic Health cannot be realized in full or at all or may take longer to realize than expected; the possibility that costs or difficulties related to the integration of Genomic Health’s operations will be greater than expected; the outcome of any litigation, government investigations, enforcement actions or other legal proceedings; and the other risks and uncertainties described in the Risk Factors and in Management's Discussion and Analysis of Financial Condition and Results of Operations sections of our most recently filed Annual Report on Form 10-K and our subsequently filed Quarterly Reports on Form 10-Q. We undertake no obligation to publicly update any forward- looking statement, whether written or oral, that may be made from time to time, whether as a result of new information, future developments or otherwise. Exact Sciences 2

We strive to change lives through earlier detection and smarter answers for cancer patients. Exact Sciences 3

2019 Screening results Preliminary, unaudited financials Q4 2019 2019 Revenue $229-230M $809.5-810.5M Cologuard® tests 477K 1.68M 78% 2019 Y/Y revenue New Cologuard growth providers 12K 50K Exact Sciences 4

2019 Precision Oncology results Preliminary, unaudited financials Partial Proforma Proforma Q4 2019* Q4 2019** 2019** Revenue $65-66M $118-119M $455-456M 16% Oncotype DX® 2019 Y/Y revenue tests 23K 41K 156K growth *Revenue reported on a GAAP basis from the close of the Genomic Health combination through the end of 2019 (11/8/19-12/31/19) **Revenue that would have been recognized for the full fourth quarter and year if Genomic Health were a standalone entity Exact Sciences 5
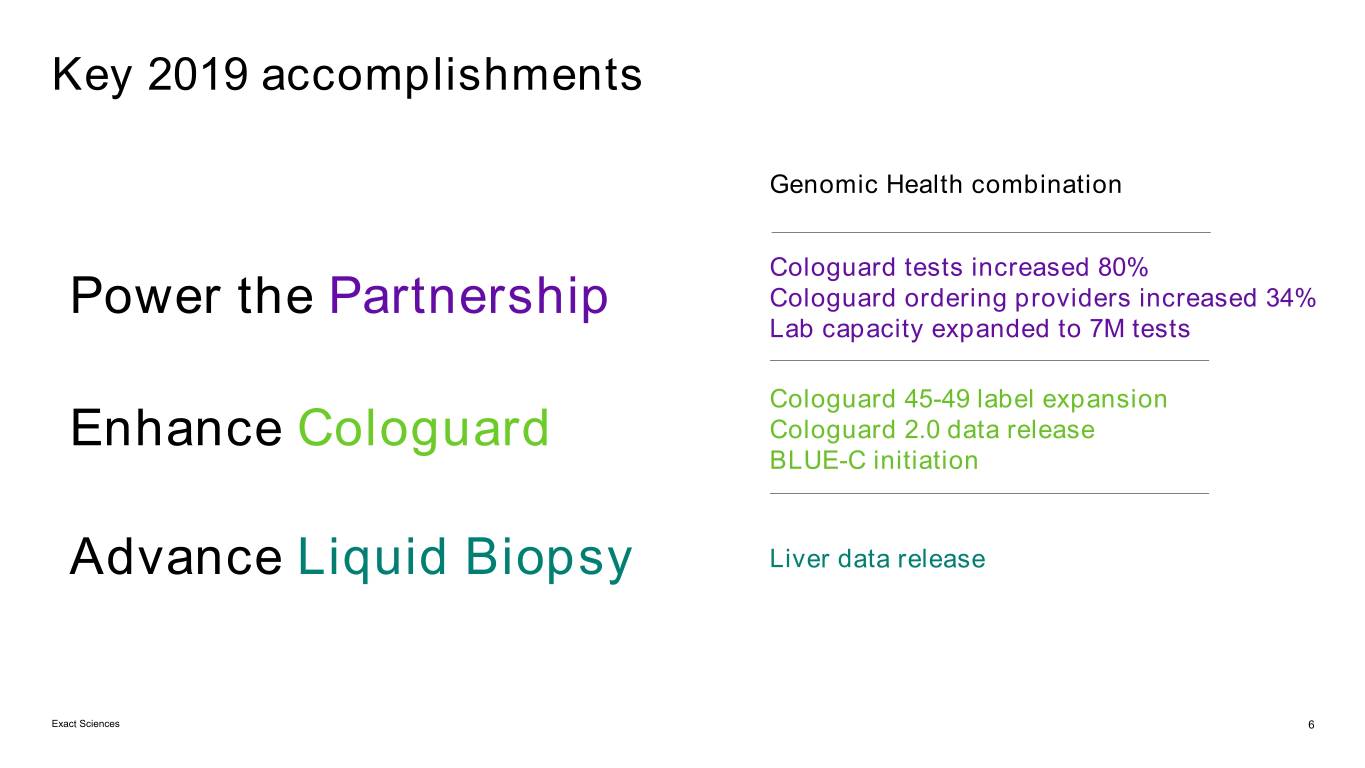
Key 2019 accomplishments Genomic Health combination Cologuard tests increased 80% Power the Partnership Cologuard ordering providers increased 34% Lab capacity expanded to 7M tests Cologuard 45-49 label expansion Enhance Cologuard Cologuard 2.0 data release BLUE-C initiation Advance Liquid Biopsy Liver data release Exact Sciences 6
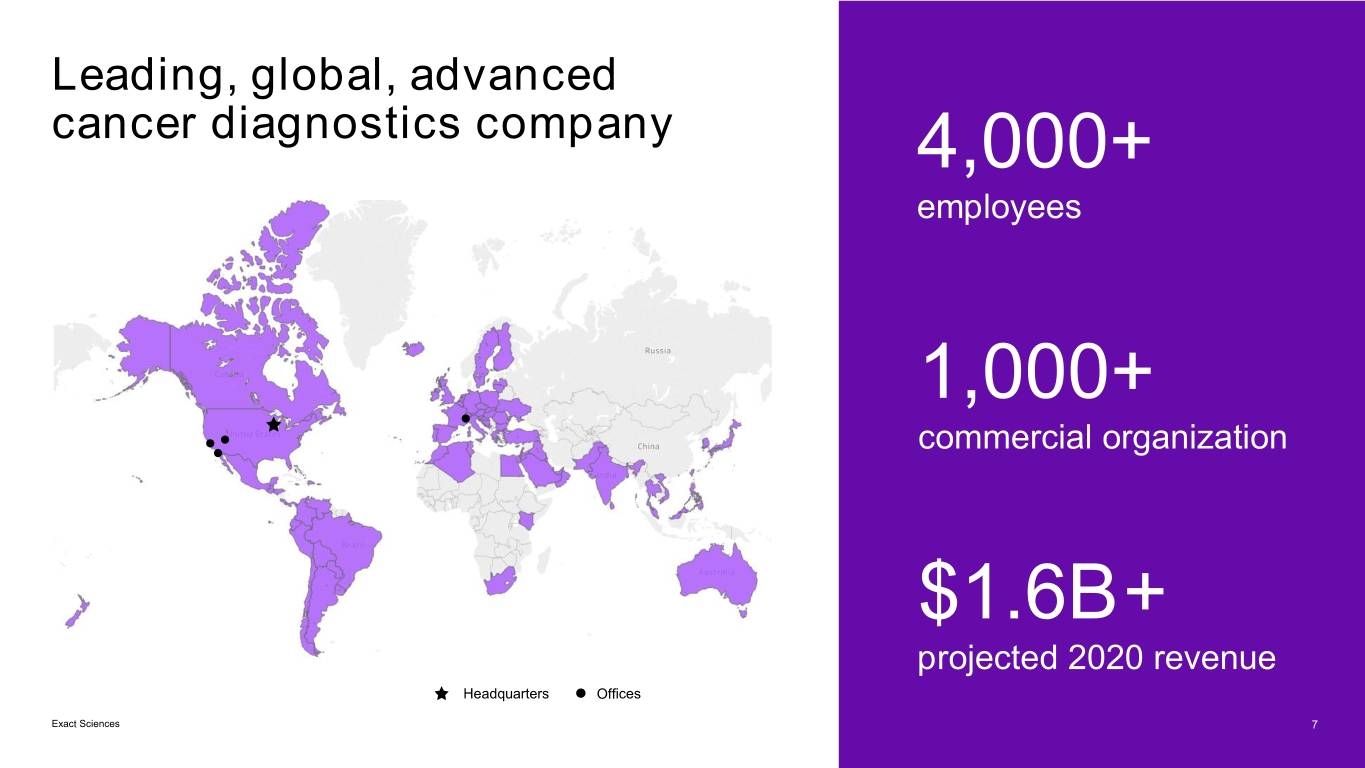
Leading, global, advanced cancer diagnostics company 4,000+ employees 1,000+ commercial organization $1.6B+ projected 2020 revenue Headquarters Offices Exact Sciences 7

Positioned for long-term success PRECISION SCREENING ONCOLOGY INTERNATIONAL PIPELINE Field teams Field teams Field team/presence R&D team 500 primary care 100 oncology 40 oncology 250 people Liver 60 gastroenterology urology countries 50 90 Pancreatic health system Esophageal 35 Bladder Exact Sciences 8

2020 Corporate Priorities Deliver More Answers Enhance Customer Experience Power New Growth Exact Sciences 9

Screening Exact Sciences 10
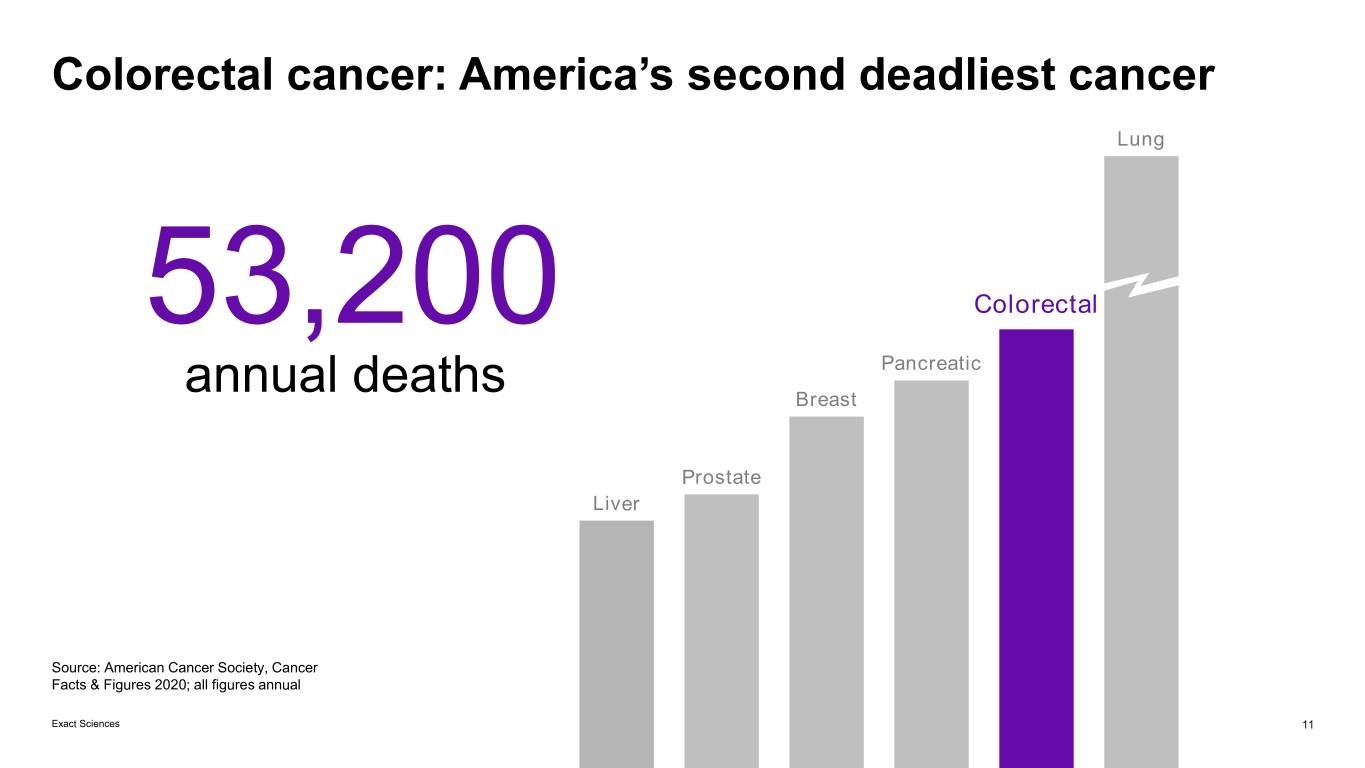
Colorectal cancer: America’s second deadliest cancer Lung 53,200 Colorectal Pancreatic annual deaths Breast Prostate Liver Source: American Cancer Society, Cancer Facts & Figures 2020; all figures annual Exact Sciences 11

Detecting colorectal cancer earlier is critical Diagnosed in Stages I or II Diagnosed in Stage IV 9/10 survive 5 years 1/10 survive 5 years Source: SEER 18 2009-2015, colorectal cancer statistics Exact Sciences 12

Cologuard: addressing the colorectal cancer challenge 94% Early-stage cancer sensitivity* Easy to use No preparation No time off work Non-invasive No sedation 24/7 customer support For adults 45 years or older and at average risk Developed with *For stage I and II cancers; 92% sensitivity overall, 87% specificity Source: Imperiale TF et al., N Engl J Med (2014) Exact Sciences 13

Demand fueling strong Cologuard growth $229-230M* 3.5M+ people screened since launch $35M 4Q16 4Q19 *Preliminary, unaudited Screening business unit Q4 2019 revenue Exact Sciences 14

Capturing a large U.S. screening opportunity 5.4% adoption 106M 40% Americans goal $18B total addressable U.S. colorectal cancer market screening population Source: Exact Sciences estimates, assuming 106 million average-risk, asymptomatic people ages 45-85, revenue per test of $500-$525, and 3-year interval for Cologuard; market share = (477,000 completed tests in 4Q19 x 4 to annualize x 3 to account for interval) / 106M Exact Sciences 15

Cologuard growth opportunities Near-term Sales team productivity Mid-term 19M people ages 45-49 Long-term Cologuard 2.0 enhancements Exact Sciences 16

Precision Oncology Exact Sciences 17

Breast cancer: America’s fourth deadliest cancer Lung 42,690 Colorectal Pancreatic annual deaths Breast Prostate Liver Source: American Cancer Society, Cancer Facts & Figures 2020; all figures annual Exact Sciences 18

Guiding therapy for early-stage breast cancer Prognostic and predictive Early stage: HER2 (-) ER (+) Node (-) Node (+) 1-2 Substantial No Chemotherapy Benefit 2 Chemotherapy Benefit (0-25) (26-100) Recurrence Score® 0 (RS) Result 26 100 1. Sparano et al. N Engl J Med. 2018 2. Geyer et al. NPJ Breast Cancer. 2018 Exact Sciences 19

Oncotype DX: standard of care in breast cancer 1M+ 96K+ 150+ patients tested patients worldwide with published publications prospective outcomes ASCO® NCCN® ESMO ST. GALLEN NICE Exact Sciences 20
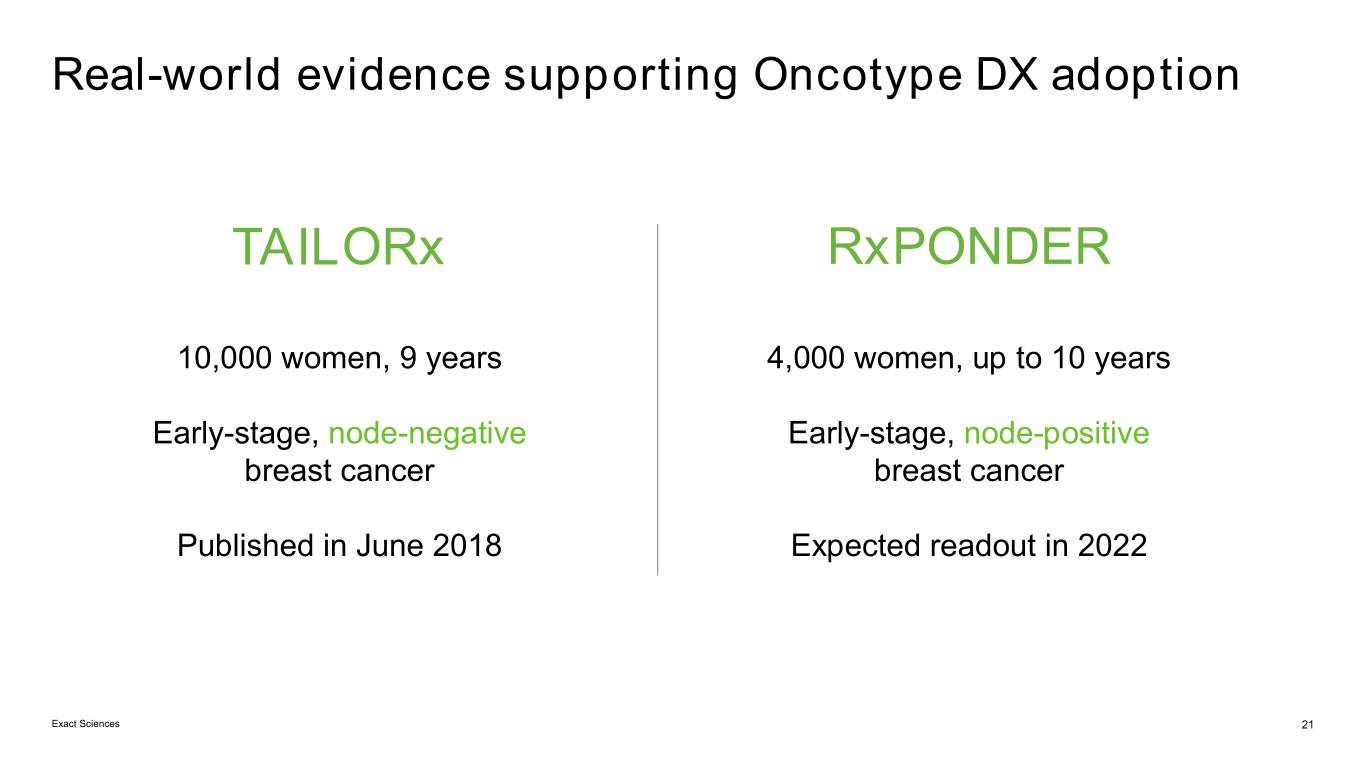
Real-world evidence supporting Oncotype DX adoption TAILORx RxPONDER 10,000 women, 9 years 4,000 women, up to 10 years Early-stage, node-negative Early-stage, node-positive breast cancer breast cancer Published in June 2018 Expected readout in 2022 Exact Sciences 21

Capturing a large global breast cancer opportunity U.S. opportunity International opportunity* 68% adoption 14% adoption 140K $500M 200K $400M people opportunity people opportunity Source: Exact Sciences estimates *Opportunity in key strategic markets Exact Sciences 22

Helping inform prostate cancer treatment recommendations Predicts clinical risk and tumor aggressiveness surveillance treatment For low- and 33K annual Helps inform Changes 15% adoption intermediate- prostate cancer active surveillance treatment in $440M U.S. risk patients deaths in U.S.1 vs. aggressive recommendation opportunity3 treatment for 1/4 men2 1. American Cancer Society, Cancer Facts & Figures 2020; all figures annual 2. Badani et al. Urol Pract. 2015 3. Exact Sciences estimates Exact Sciences 23

Pipeline Exact Sciences 24

Exact Sciences 25

Deep R&D capabilities Multiple classes of biomarkers Proprietary DNA chemistry State-of-the-art labs Flexible, automated platform Exact Sciences 26 26
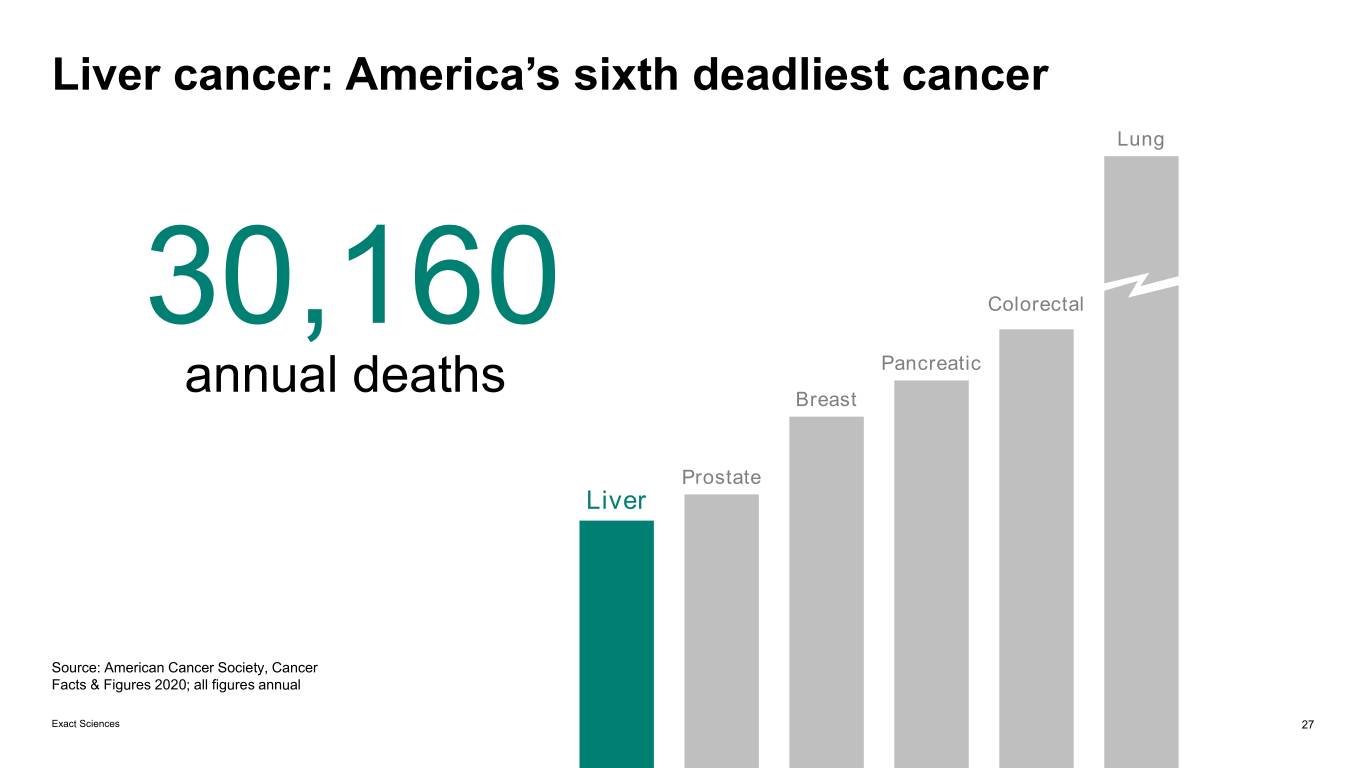
Liver cancer: America’s sixth deadliest cancer Lung 30,160 Colorectal Pancreatic annual deaths Breast Prostate Liver Source: American Cancer Society, Cancer Facts & Figures 2020; all figures annual Exact Sciences 27

Regular liver cancer testing leads to better outcomes Under regular testing Not under regular testing 6/10 survive 3 years 3/10 survive 3 years Source: Kuo, YH et al., Eur J Cancer (2010) Exact Sciences 28

Next steps to capturing a large U.S. liver cancer opportunity 3M Additional data in Americans 2020 $1.5B Test available in total U.S. 2H20 addressable market Note: Expect additional data in 2020 and to make the liver cancer test available in 2H20 Source: Exact Sciences estimates; total addressable market assumes revenue per test of $500 and 3M Americans tested annually Exact Sciences 29
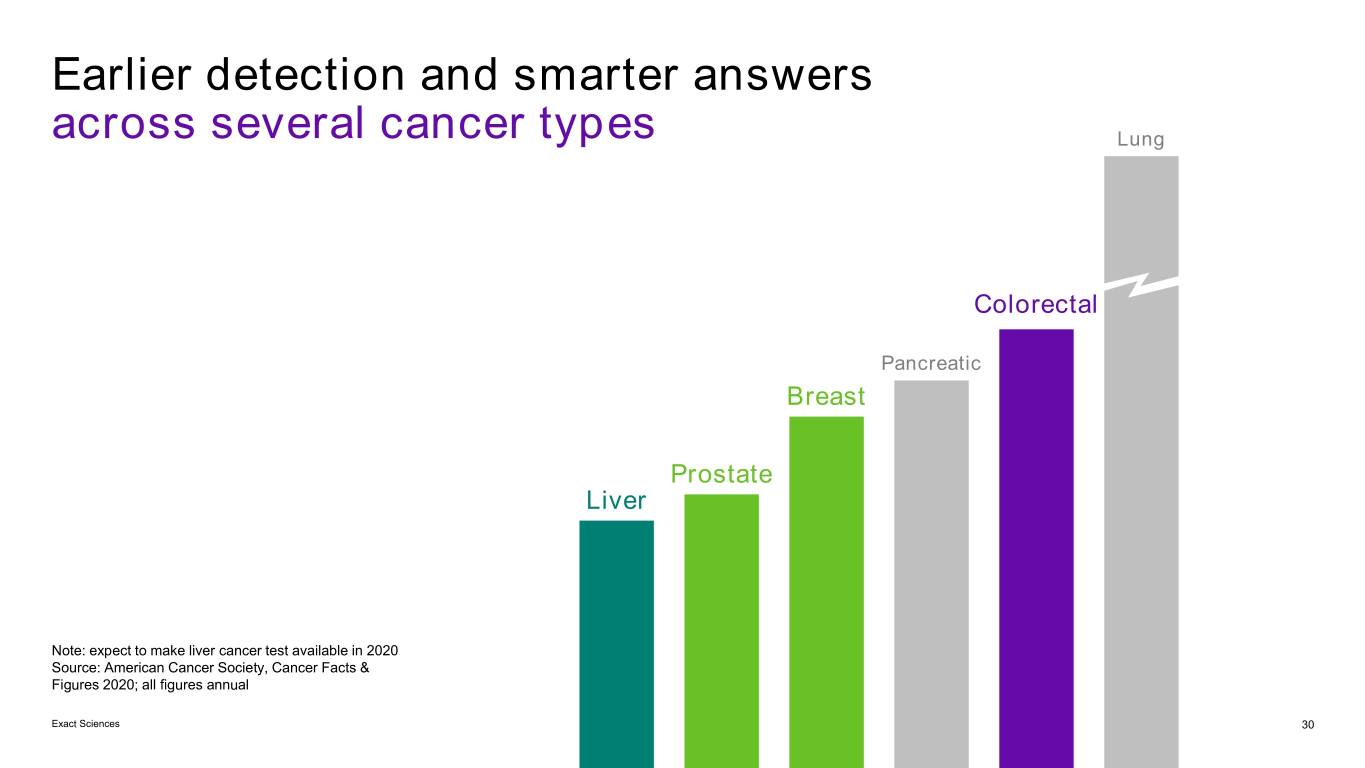
Earlier detection and smarter answers across several cancer types Lung Colorectal Pancreatic Breast Prostate Liver Note: expect to make liver cancer test available in 2020 Source: American Cancer Society, Cancer Facts & Figures 2020; all figures annual Exact Sciences 30

The leading global cancer diagnostics company $1.6B+ projected 2020 revenue 4.7M+ people tested to-date $1.2B+ and growing projected 2020 gross profit Note: 4.7M+ total people tested with Cologuard and Oncotype DX products combined Exact Sciences 31

Exact Sciences
