Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Cyclerion Therapeutics, Inc. | tm202191d1_ex99-1.htm |
| 8-K - FORM 8-K - Cyclerion Therapeutics, Inc. | tm202191d1_8k.htm |
Exhibit 99.2
 J.P. Morgan Healthcare Conference January 13, 2020 Peter Hecht, CEO
J.P. Morgan Healthcare Conference January 13, 2020 Peter Hecht, CEO
 Safe Harbor Statement This presentation contains forward-looking statements. Any statements contained in this presentation that are not historical facts may be deemed to be forward looking statements. Words such as “anticipate,” “believe,” “potential,” “expect,” “may,” “will,” “should,” “could,” “plan,” “estimate,” “target,” “project,” “contemplate,” “intend,” “future,” “will,” “predict,” “continue,” and the negative of these terms and similar expressions are intended to identify these forward-looking statements. These forward-looking statements are based on Cyclerion’s current expectations, projections and trends, are only predictions and involve known and unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements. Investors are cautioned not to place undue reliance on these forward-looking statements, which include but are not limited to statements about possible or assumed future results of operations; preclinical, clinical and non-clinical studies, the interpretation of data therefrom and the ability to replicate findings from such studies; business strategies, research and development plans, collaborations, partnerships, out-licensing (including without limitation with respect to praliciguat), regulatory activities and any timing thereof; competitive position, potential growth or commercial opportunities; the clinical potential, application, commercialization or potential markets of or for any proposed products; the anticipated timing of release of data from any clinical trials; and the size and design of those clinical trials. Applicable risks and uncertainties include those listed under the heading “Risk Factors” and elsewhere in our Registration Statement on Form S-1 filed with the Securities and Exchange Commission (SEC) on April 18, 2019, and in our subsequent SEC filings, including our Quarterly Report on Form 10-Q filed with the SEC on August 12, 2019. These forward-looking statements speak only as of the date of this presentation, and we undertake no obligation and do not intend to update these forward-looking statements. 2
Safe Harbor Statement This presentation contains forward-looking statements. Any statements contained in this presentation that are not historical facts may be deemed to be forward looking statements. Words such as “anticipate,” “believe,” “potential,” “expect,” “may,” “will,” “should,” “could,” “plan,” “estimate,” “target,” “project,” “contemplate,” “intend,” “future,” “will,” “predict,” “continue,” and the negative of these terms and similar expressions are intended to identify these forward-looking statements. These forward-looking statements are based on Cyclerion’s current expectations, projections and trends, are only predictions and involve known and unknown risks and uncertainties that could cause actual results to differ materially from those expressed or implied in such statements. Investors are cautioned not to place undue reliance on these forward-looking statements, which include but are not limited to statements about possible or assumed future results of operations; preclinical, clinical and non-clinical studies, the interpretation of data therefrom and the ability to replicate findings from such studies; business strategies, research and development plans, collaborations, partnerships, out-licensing (including without limitation with respect to praliciguat), regulatory activities and any timing thereof; competitive position, potential growth or commercial opportunities; the clinical potential, application, commercialization or potential markets of or for any proposed products; the anticipated timing of release of data from any clinical trials; and the size and design of those clinical trials. Applicable risks and uncertainties include those listed under the heading “Risk Factors” and elsewhere in our Registration Statement on Form S-1 filed with the Securities and Exchange Commission (SEC) on April 18, 2019, and in our subsequent SEC filings, including our Quarterly Report on Form 10-Q filed with the SEC on August 12, 2019. These forward-looking statements speak only as of the date of this presentation, and we undertake no obligation and do not intend to update these forward-looking statements. 2
 Priorities for 2020 1 2 3 DN praliciguat partnering: out-license discussions based on promising phase 2 SCD olinciguat phase 2 completion: potential for raising standard four therapeutic domains of care across CNS IW-6463 advancement: advancement from successful phase 1 in healthy volunteers to ongoing study in elderly subjects 3 sGC
Priorities for 2020 1 2 3 DN praliciguat partnering: out-license discussions based on promising phase 2 SCD olinciguat phase 2 completion: potential for raising standard four therapeutic domains of care across CNS IW-6463 advancement: advancement from successful phase 1 in healthy volunteers to ongoing study in elderly subjects 3 sGC
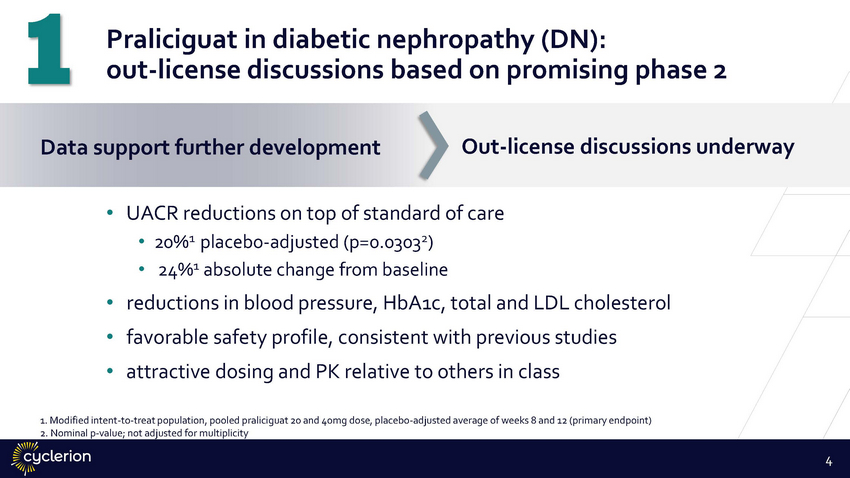 1 Data Praliciguat in diabetic nephropathy (DN): out-license discussions based on promising phase 2 Out-license discussions underway support further development • UACR reductions on top of standard of care •20%1 placebo-adjusted (p=0.03032) • 24%1 absolute change from baseline • • • reductions in blood pressure, HbA1c, total and LDL cholesterol favorable safety profile, consistent with previous studies attractive dosing and PK relative to others in class 1. Modified intent-to-treat population, pooled praliciguat 20 and 40mg dose, placebo-adjusted average of weeks 8 and 12 (primary endpoint) 2. Nominal p-value; not adjusted for multiplicity 4
1 Data Praliciguat in diabetic nephropathy (DN): out-license discussions based on promising phase 2 Out-license discussions underway support further development • UACR reductions on top of standard of care •20%1 placebo-adjusted (p=0.03032) • 24%1 absolute change from baseline • • • reductions in blood pressure, HbA1c, total and LDL cholesterol favorable safety profile, consistent with previous studies attractive dosing and PK relative to others in class 1. Modified intent-to-treat population, pooled praliciguat 20 and 40mg dose, placebo-adjusted average of weeks 8 and 12 (primary endpoint) 2. Nominal p-value; not adjusted for multiplicity 4
 Phase 2 showed promising improvement in UACR 20 % UACR change from baseline LS mean (90% CI) change UACR in (mg/g) (mITT)1 0 -20 -40 (90 % CIs by dose & timepoint) 0 1 4 8 12 weeks of treatment 1. mITT: modified intent-to-treat population n=133 excludes data from site 00A where data inconsistencies were observed in both the treatment and control groups 5
Phase 2 showed promising improvement in UACR 20 % UACR change from baseline LS mean (90% CI) change UACR in (mg/g) (mITT)1 0 -20 -40 (90 % CIs by dose & timepoint) 0 1 4 8 12 weeks of treatment 1. mITT: modified intent-to-treat population n=133 excludes data from site 00A where data inconsistencies were observed in both the treatment and control groups 5
 2 Olinciguat in sickle cell disease (SCD): potential for raising standard of care across four therapeutic domains Dynamic mechanism - alone or complementing approved treatments Topline phase 2 results mid-2020 • • upstream + downstream pharmacological intervention impact potential in four clinical domains: anemia, daily symptoms, VOC, and organ protection--not fully addressed by approved therapies oral, QD drug for use in a broad range of SCD patients STRONG SCD trial designed to assess safety and pharmacodynamic effects across full dose range • • 6
2 Olinciguat in sickle cell disease (SCD): potential for raising standard of care across four therapeutic domains Dynamic mechanism - alone or complementing approved treatments Topline phase 2 results mid-2020 • • upstream + downstream pharmacological intervention impact potential in four clinical domains: anemia, daily symptoms, VOC, and organ protection--not fully addressed by approved therapies oral, QD drug for use in a broad range of SCD patients STRONG SCD trial designed to assess safety and pharmacodynamic effects across full dose range • • 6
 Olinciguat: upstream and downstream intervention in SCD Upstream • increased HbF leads to reduced proportion of sickled RBCs1 Increased hemolysis leads to reduced nitric oxide state sGC restores deficient nitric oxide signaling Downstream • • improved blood flow decreased vascular inflammation & cell adhesion improved endothelial integrity • 1. Conran, N., & Torres, L. (2019). cGMP modulation therapeutics for sickle cell disease. Experimental Biology and Medicine, 244(2), 132–146. 7
Olinciguat: upstream and downstream intervention in SCD Upstream • increased HbF leads to reduced proportion of sickled RBCs1 Increased hemolysis leads to reduced nitric oxide state sGC restores deficient nitric oxide signaling Downstream • • improved blood flow decreased vascular inflammation & cell adhesion improved endothelial integrity • 1. Conran, N., & Torres, L. (2019). cGMP modulation therapeutics for sickle cell disease. Experimental Biology and Medicine, 244(2), 132–146. 7
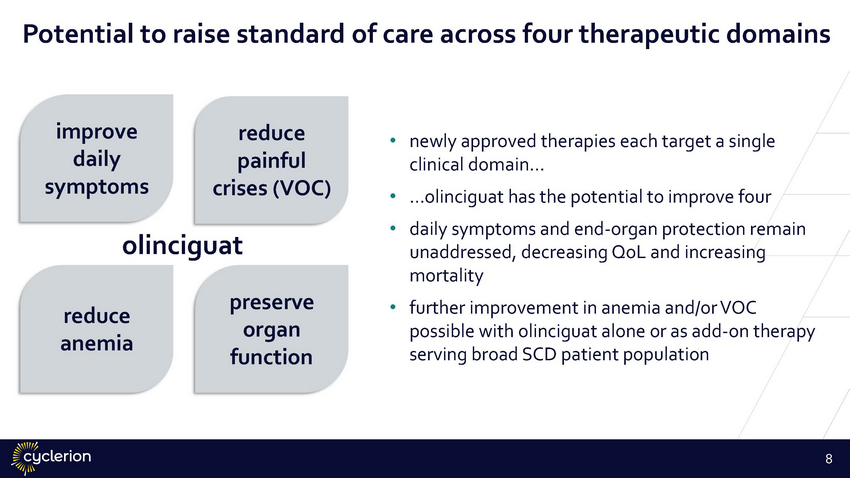 Potential to raise standard of care across four therapeutic domains improve daily symptoms reduce painful crises (VOC) • newly approved therapies each target a single clinical domain… …olinciguat has the potential to improve four daily symptoms and end-organ protection remain unaddressed, decreasing QoL and increasing mortality further improvement in anemia and/or VOC possible with olinciguat alone or as add-on therapy serving broad SCD patient population • • olinciguat preserve organ function • reduce anemia 8
Potential to raise standard of care across four therapeutic domains improve daily symptoms reduce painful crises (VOC) • newly approved therapies each target a single clinical domain… …olinciguat has the potential to improve four daily symptoms and end-organ protection remain unaddressed, decreasing QoL and increasing mortality further improvement in anemia and/or VOC possible with olinciguat alone or as add-on therapy serving broad SCD patient population • • olinciguat preserve organ function • reduce anemia 8
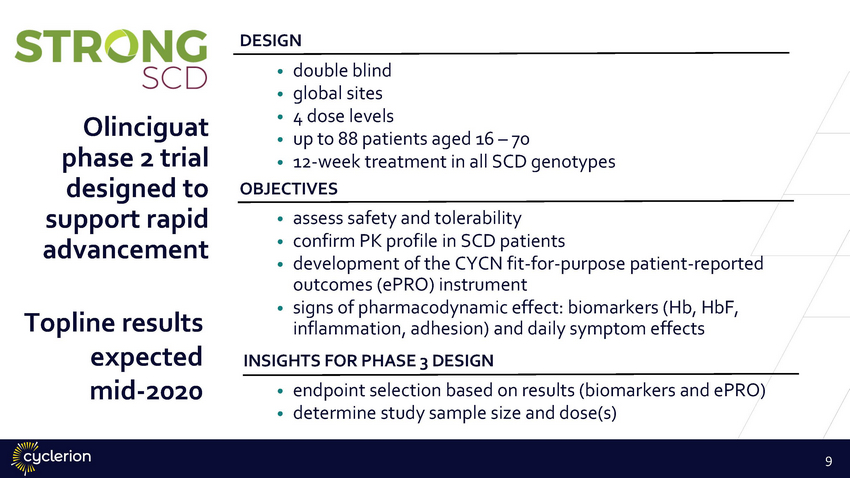 DESIGN •double blind •global sites •4 dose levels •up to 88 patients aged 16 – 70 •12-week treatment in all SCD genotypes OBJECTIVES Olinciguat phase 2 trial designed to support rapid advancement assess safety and tolerability confirm PK profile in SCD patients development of the CYCN fit-for-purpose patient-reported outcomes (ePRO) instrument signs of pharmacodynamic effect: biomarkers (Hb, HbF, inflammation, adhesion) and daily symptom effects • • • • Topline results expected mid-2020 INSIGHTS FOR PHASE 3 DESIGN •endpoint selection based on results (biomarkers and ePRO) •determine study sample size and dose(s) 9
DESIGN •double blind •global sites •4 dose levels •up to 88 patients aged 16 – 70 •12-week treatment in all SCD genotypes OBJECTIVES Olinciguat phase 2 trial designed to support rapid advancement assess safety and tolerability confirm PK profile in SCD patients development of the CYCN fit-for-purpose patient-reported outcomes (ePRO) instrument signs of pharmacodynamic effect: biomarkers (Hb, HbF, inflammation, adhesion) and daily symptom effects • • • • Topline results expected mid-2020 INSIGHTS FOR PHASE 3 DESIGN •endpoint selection based on results (biomarkers and ePRO) •determine study sample size and dose(s) 9
 3 IW-6463 in CNS: advancing development for treatment of serious neurodegenerative diseases Ph 1 showed safety, target Additional clinical studies in 2020 to accelerate and de-risk program engagement, CNS exposure Preclinical evidence: stimulation of nitric oxide-cGMP pathway improves determinants of brain health Enhanced NO-cGMP in the CNS leads to: • • • • enhanced neuronal function increased cerebral vascular function decreased microglial activity improved mitochondrial output Impaired brain function associated with low nitric oxide-cGMP Potential for improved brain health 1. Cyclerion's pre-clinical work www.cyclerion.com 10
3 IW-6463 in CNS: advancing development for treatment of serious neurodegenerative diseases Ph 1 showed safety, target Additional clinical studies in 2020 to accelerate and de-risk program engagement, CNS exposure Preclinical evidence: stimulation of nitric oxide-cGMP pathway improves determinants of brain health Enhanced NO-cGMP in the CNS leads to: • • • • enhanced neuronal function increased cerebral vascular function decreased microglial activity improved mitochondrial output Impaired brain function associated with low nitric oxide-cGMP Potential for improved brain health 1. Cyclerion's pre-clinical work www.cyclerion.com 10
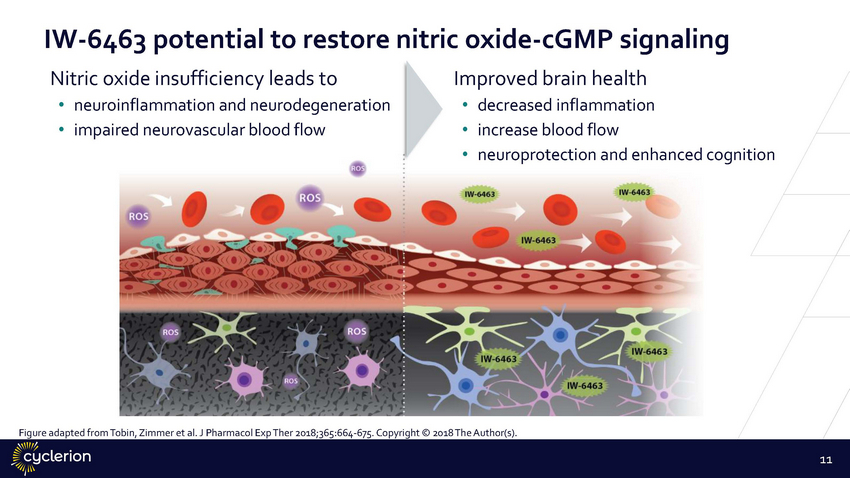 IW-6463 potential to restore Nitric oxide insufficiency leads to nitric oxide-cGMP signaling Improved brain health • • • • • neuroinflammation and neurodegeneration impaired neurovascular blood flow decreased inflammation increase blood flow neuroprotection and enhanced cognition Figure adapted from Tobin, Zimmer et al. J Pharmacol Exp Ther 2018;365:664-675. Copyright © 2018 The Author(s). 11
IW-6463 potential to restore Nitric oxide insufficiency leads to nitric oxide-cGMP signaling Improved brain health • • • • • neuroinflammation and neurodegeneration impaired neurovascular blood flow decreased inflammation increase blood flow neuroprotection and enhanced cognition Figure adapted from Tobin, Zimmer et al. J Pharmacol Exp Ther 2018;365:664-675. Copyright © 2018 The Author(s). 11
 Positive phase 1 IW-6463 results support further development Newly released—completed December 2019 Phase 1 study design Insights • CNS drug presence at levels expected to be pharmacologically active QD dosing, linear and predictable PK may be taken with or without food evidence of target engagement (blood pressure) well-tolerated at all dose levels all AEs mild in severity, no SAEs • • • • • 3 stage: SAD, MAD and food interaction 110 healthy volunteers age range 18-63 assessed safety, PK (blood & CSF) wide dose / exposure range tested • • • • • 12
Positive phase 1 IW-6463 results support further development Newly released—completed December 2019 Phase 1 study design Insights • CNS drug presence at levels expected to be pharmacologically active QD dosing, linear and predictable PK may be taken with or without food evidence of target engagement (blood pressure) well-tolerated at all dose levels all AEs mild in severity, no SAEs • • • • • 3 stage: SAD, MAD and food interaction 110 healthy volunteers age range 18-63 assessed safety, PK (blood & CSF) wide dose / exposure range tested • • • • • 12
 Clinical direction: accelerate and de-risk into high value 2019 CNS indications 2020 2021 translational pharmacology study in elderly subjects safety, tolerability, PK, biomarkers: CBF, cGMP completed December initiated January 2020 additional clinical studies in neurodegenerative disorders 2019 ongoing discovery work: •understand fundamental mechanisms •apply the power of sGC and NO signaling 13 IW-6463 ph1 SAD/MAD/FI
Clinical direction: accelerate and de-risk into high value 2019 CNS indications 2020 2021 translational pharmacology study in elderly subjects safety, tolerability, PK, biomarkers: CBF, cGMP completed December initiated January 2020 additional clinical studies in neurodegenerative disorders 2019 ongoing discovery work: •understand fundamental mechanisms •apply the power of sGC and NO signaling 13 IW-6463 ph1 SAD/MAD/FI
 Cyclerion 2020 • • catalysts across programs partnering clinical trials ~$102M cash1 and reduced our priorities into Q2 2021 burn support Team, talent and intensity to deliver 1. Preliminary, unaudited unrestricted cash, cash equivalents and restricted cash balance as of Dec 31, 2019 14
Cyclerion 2020 • • catalysts across programs partnering clinical trials ~$102M cash1 and reduced our priorities into Q2 2021 burn support Team, talent and intensity to deliver 1. Preliminary, unaudited unrestricted cash, cash equivalents and restricted cash balance as of Dec 31, 2019 14
 Priorities for 2020 1 2 3 DN praliciguat partnering: out-license discussions based on promising phase 2 SCD olinciguat phase 2 completion: potential for raising standard four therapeutic domains of care across CNS IW-6463 advancement: advancement from successful phase 1 in healthy volunteers to ongoing study in elderly subjects 15 sGC
Priorities for 2020 1 2 3 DN praliciguat partnering: out-license discussions based on promising phase 2 SCD olinciguat phase 2 completion: potential for raising standard four therapeutic domains of care across CNS IW-6463 advancement: advancement from successful phase 1 in healthy volunteers to ongoing study in elderly subjects 15 sGC
 J.P. Morgan Healthcare Conference January 13, 2020 Peter Hecht, CEO
J.P. Morgan Healthcare Conference January 13, 2020 Peter Hecht, CEO
