Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Biohaven Pharmaceutical Holding Co Ltd. | tm203198-1_8k.htm |
Exhibit 99.1

NYSE: BHVN © 2019 Biohaven Pharmaceuticals Inc. All rights reserved. NEURO INNOVATION Meeting Patient Needs. Growing Value. INVESTOR PRESENTATION | JANUARY 2020

Disclaimer This presentation contains forward - looking statements within the meaning of “safe harbor” provisions of The Private Securities L itigation Reform Act of 1995, including: statements about Biohaven Pharmaceutical Holding Company Ltd. (The ”Company”) and our plans to develop and commercialize our product candidates, our planned clinical trials for our rimegepant , vazegepant (BHV - 3500), troriluzole , BHV - 5000 and verdiperstat development programs, the timing of the availability of data from our clinical trials, the timing of our planned regulatory filings, the timing of and our ability to obtain and maintain regulatory approvals for our product candidates and the clinical potential utility of our product candidates, alone and as compared to other existing or potential treatment options. These st ate ments involve substantial known and unknown risks, uncertainties and other factors that may cause our actual results, levels of activity, p erf ormance or achievements to be materially different from the information expressed or implied by these forward - looking statements and from t he Company's current expectations. We may not actually achieve the plans, intentions or expectations disclosed in our forward - looki ng statements, and you should not place undue reliance on our forward - looking statements. The forward - looking statements in this presentation represent our views as of the date of this presentation. Subsequent events and developments may cause our views to change. However, while we may elect to update these forward - looking statements at some point in the future, we have no obligatio n to do so except to the extent required by applicable law. You should, therefore, not rely on these forward - looking statements as repre senting our views as of any date subsequent to the date of this presentation. For further information regarding these risks, uncertaintie s a nd other factors, you should read the “Risk Factors” section of the Company’s Annual Report on Form 10 - K for the year ended December 31, 2018, the Company’s Quarterly Report on Form 10 - Q filed with the Securities and Exchange Commission (the ”SEC”) on November 9, 2019 an d the Company’s other reports filed with the SEC. References to www.biohavenpharma.com in this presentation are not intended to , n or shall they be deemed to, incorporate information on such website into this presentation by reference. This presentation also contains market data and other statistical information that are based on independent industry publicat ion s, reports by market research firms or published independent sources. Some market data and statistical information are also based on the Company's good faith estimates, which are derived from management's knowledge of its industry and such independent sources re fer red to above. While the Company is not aware of any misstatements regarding the market and industry data presented herein, such d ata involve risks and uncertainties and are subject to change based on various factors. JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 2

At Biohaven, We Demand More for Patients. Meeting patient needs. Growing value. GOALS Launch rimegepant 1Q2020 Three marketed drugs by 2021 – 2022 Grow while maintaining a lean, efficient, high - performing culture ELLIE Rimegepant clinical trial participant for more than 2 years

JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 4 Rimegepant : formerly BHV - 3000; Vazegepant : formerly BHV - 3500; MPO: Myeloperoxidase; TDP - 43: TAR DNA - binding protein 43; UC1MT: monoclonal antibody targeting extracellula r metallothionein Late Stage Development Pipeline Pre - Clinical Amyotrophic Lateral Sclerosis TDP - 43 Neurological Indications UC1MT CGRP antagonism VAZEGEPANT Phase 2/3 Intranasal Acute Migraine COMPLETED - POSITIVE RIMEGEPANT Phase 3 Preventive Migraine 1Q20 Topline RIMEGEPANT Phase 3 Zydis ® ODT Acute Migraine COMPLETED - POSITIVE RIMEGEPANT Phase 3 Acute Migraine COMPLETED - POSITIVE RIMEGEPANT Phase 3 Long - Term Safety Study COMPLETED - POSITIVE GLUTAMATE modulation TRORILUZOLE Phase 3 Spinocerebellar Ataxia Initiated 1Q2019 TRORILUZOLE Phase 2/3 OCD 2Q2020 Topline TRORILUZOLE Phase 2/3 Alzheimer’s Disease (AD) Passed Interim Analysis TRORILUZOLE Phase 2/3 Generalized Anxiety Disorder 1Q20 Topline BHV - 5000 Phase 1 Neuropsychiatric Indications MPO inhibition VERDIPERSTAT Phase 3 Amyotrophic Lateral Sclerosis 2Q2020 Planned Start VERDIPERSTAT Phase 3 Multiple System Atrophy Initiated 3Q2019 Biohaven’s Neuroinnovation Portfolio

Advanced the Portfolio in 2019, Transitioning to Commercialization in 2020 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 5 Rimegepant • Filed NDA for acute migraine • Positive Phase 3 results • NEJM, Lancet • Completed enrollment of Phase 3 for prevention Vazegepant • Positive Phase 2/3 results with first and only intranasal CGRP - antagonist Troriluzole • Passed interim futility analysis in Alzheimer’s Disease • Enrolled Phase 3 studies for OCD and GAD • Initiated Phase 3 trial of troriluzole in SCA • Issued composition of matter patent until 2036 (likely 2039 with extensions) Verdiperstat • Initiated Phase 3 trial in MSA • Selected for ALS platform trial at Mass General Hospital/Healy Center for ALS Established launch - ready commercial organization to compete in blockbuster migraine therapeutic area CGRP GLUTAMATE MPO CORPORATE

CGRP - Antagonist Platform: Redefining the Treatment of Migraine

CGRP receptor CGRP Trigeminal nerve Central Glial cell Peripheral 1. Edvinsson L et al (2018). Nat Rev Neurol 14(6): 338 - 350. 2. Raddant AC et al (2011). Expert Rev Mol Med 13:e36. 3. Conway CM et al (2018). Headache 58(8):1295. 4. Data on file. Biohaven Pharmaceuticals. • CGRP is a peptide released by the trigeminal nerve during migraine attacks 1,2 • Blocking CGRP treats migraine without the vasoconstrictive effects of triptans 3 • CGRP mechanism of action is not associated with addiction potential or medication overuse headaches (MOH) 4 Oral CGRP Receptor Antagonists Developed to Address Unmet Needs JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 7
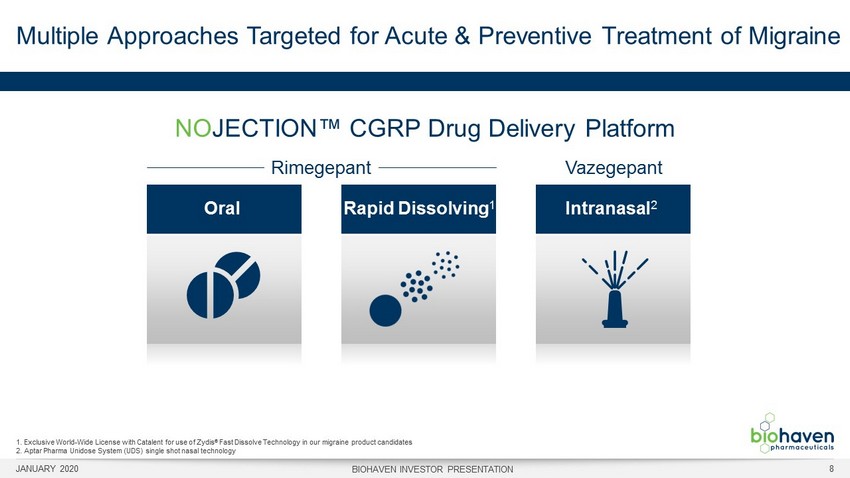
Multiple Approaches Targeted for Acute & Preventive Treatment of Migraine JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 8 NO JECTION™ CGRP Drug Delivery Platform 1. Exclusive World - Wide License with Catalent for use of Zydis ® Fast Dissolve Technology in our migraine product candidates 2. Aptar Pharma Unidose System (UDS) single shot nasal technology Oral Rapid Dissolving 1 Intranasal 2 Vazegepant Rimegepant

RIMEGEPANT ODT AND TABLET NDAS Rimegepant FDA Late - Cycle Communication Update (December 5, 2019) JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 9 Remaining Review Plans • Finalize reviews • Conduct labeling negotiations • Finalize post - marketing commitments No Substantive Review Issues Identified in Late - Cycle Communication Rimegepant NDA

OVER 3,700* SUBJECTS TREATED AND >113,000 RIMEGEPANT DOSES DELIVERED TO DATE Rimegepant: Three Positive Phase 3 Trials, 1 - Year Safety 1. Lipton, R. B., et al. (2019). " Rimegepant , an Oral Calcitonin Gene - Related Peptide Receptor Antagonist, for Migraine." N Engl J Med 381(2): 142 - 149. 2. Croop , R., et al. (2019). "Efficacy, safety, and tolerability of rimegepant orally disintegrating tablet for the acute treatment of migraine: a randomised , phase 3, double - blind, placebo - controlled trial." Lancet 394(10200): 737 - 745. *Patients may have been double counted if enrolled in both the long - term safety study 201, and a phase 3 trial. ODT, orally dis solving tablet. JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 10

Rimegepant 75 mg ODT: Preparing to Redefine Relief JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 11 In clinical trials, the safety and tolerability profile of rimegepant was similar to placebo FAST Dissolves in seconds and starts working in minutes LASTS Single dose demonstrated sustained efficacy across multiple measures for up to 48 hours Half life = 11 - 12 hours SIMPLE One 75 mg ODT, as needed up to once daily that can be taken anytime, anywhere

Rimegepant 75 mg ODT: Rapid Relief and Durable Effects with Single Dose JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 12 Croop R et al. Lancet. 2019;394(10200):737 - 745. Rapid Relief 2 - Hour Effects Durable Benefit (2h – 48h) FROM 2 – 24 HOURS POST DOSE • Sustained pain relief • Sustained freedom from MBS • Sustained ability to function normally • Sustained freedom from pain WITHIN 24 HOURS • No rescue medication FROM 2 – 24 HOURS POST DOSE • Sustained pain relief • Sustained freedom from MBS • Sustained ability to function normally • Sustained freedom from pain AT 2 HOURS POST DOSE • Freedom from pain • Freedom from MBS • Pain relief • Ability to function normally • Freedom from photophobia • Freedom from phonophobia AT 60 MIN POST DOSE • Pain relief • Ability to function normally AT 90 MIN POST DOSE • Ability to function normally • Pain relief • Freedom from MBS • Freedom from pain

Rimegepant 1 - Year Long - Term Safety to Capture Real World Patient Usage JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 13 SCREENING/ BASELINE Patients Received Free Access to Daily Supply 1 of Rimegepant for 1 year TREATMENT PERIOD up to 52 Weeks END of Treatment or Early D/C Visit 30 - day Observation Phase • Assess Long - Term Safety • Determine Patient Utilization • Assess Migraine Frequency and Disability Dispense a Start treatment Stop treatment 1. Patients were dispensed open label rimegepant 75 mg in bottles containing 30 tablets; A maximum of 1 tablet per calendar day was permitted

• PRN protocol allowed dosing up to once per calendar day Median Monthly Usage: < 8 pills/month over 1 - Year with PRN Dosing Rimegepant 75 mg Tablets Treated Subjects Average rimegepant exposure [tablets per 4 weeks] PRN Dosing 2 – 8 cohort {attack history/ mo } (n=1031) PRN Dosing 9 – 14 cohort {attack history/ mo } (n=481) Mean (SD) 5.6 (3.50) 8.5 (4.20) Median 4.9 7.8 Tablets per 4 weeks are based on 4 - week (28 - day) intervals in the long - term treatment period. Data on file. Study 201: Biohaven Pharmaceuticals; 2019. • To further test safety, patients in additional EOD+PRN group received scheduled every other day dosing (EOD) and could also dose PRN on nonscheduled dosing days for up to 3 months (n=286): median usage = 14.2 tablets per month (based on 28 - day month) JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 14

Rimegepant demonstrated improvements in mean change from baseline by ~50% for total MIDAS over the course of 1 - year Rimegepant Reduced Total Migraine Disability (MIDAS) by ~50% in 1 - Year Study 33.9 20.7 19.1 17.5 16.4 15 17 19 21 23 25 27 29 31 33 35 0 weeks 12 Weeks 24 Weeks 36 Weeks 52 weeks Mean MIDAS Total Score Migraine Disability Assessment Score P<0.0001 versus baseline for all time points Level of Disability Severe Moderate MIDAS, Migraine Disability Assessment. Validated, 5 - item instrument that queries migraine - related absenteeism and lost productiv ity at work, school, and home, as well as missed family, social, and leisure activities Reference: Biohaven presents data demonstrating reduction in migraine - related disability and improvement in patient reported outcomes after oral treatment with rimegepant at the international headache conference late breaking session. Biohaven Pharmaceuticals website. https://www.biohavenpharma.com/investors/news - event s/press - releases/09 - 09 - 2019. Published September 9, 2019. Accessed September 12, 2019. - 48.3% JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 15
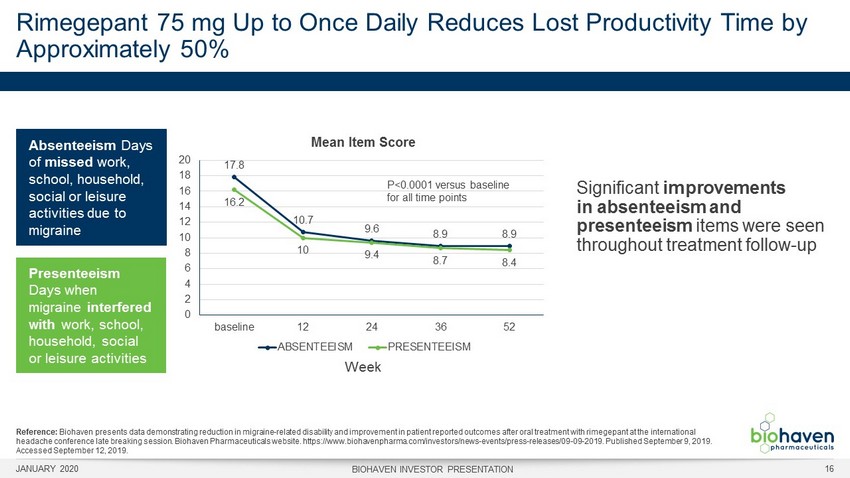
Significant improvements in absenteeism and presenteeism items were seen throughout treatment follow - up Rimegepant 75 mg Up to Once Daily Reduces Lost Productivity Time by Approximately 50% Mean Item Score Week Absenteeism Days of missed work, school, household, social or leisure activities due to migraine 17.8 10.7 9.6 8.9 8.9 16.2 10 9.4 8.7 8.4 0 2 4 6 8 10 12 14 16 18 20 baseline 12 24 36 52 ABSENTEEISM PRESENTEEISM Presenteeism Days when migraine interfered with work, school, household, social or leisure activities P<0.0001 versus baseline for all time points Reference: Biohaven presents data demonstrating reduction in migraine - related disability and improvement in patient reported outcomes after oral treatment with rimegepant at the international headache conference late breaking session. Biohaven Pharmaceuticals website. https://www.biohavenpharma.com/investors/news - event s/press - releases/09 - 09 - 2019. Published September 9, 2019. Accessed September 12, 2019. JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 16
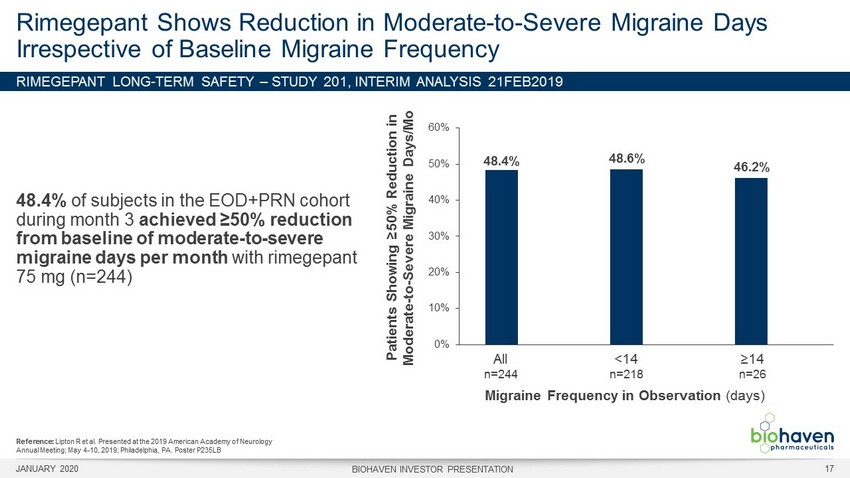
RIMEGEPANT LONG - TERM SAFETY – STUDY 201, INTERIM ANALYSIS 21FEB2 019 48.4% of subjects in the EOD+PRN cohort during month 3 achieved ≥50% reduction from baseline of moderate - to - severe migraine days per month with rimegepant 75 mg (n=244) Rimegepant Shows Reduction in Moderate - to - Severe Migraine Days Irrespective of Baseline Migraine Frequency 48.4% 48.6% 46.2% 0% 10% 20% 30% 40% 50% 60% Patients Showing ≥50% Reduction in Moderate - to - Severe Migraine Days/ M o Migraine Frequency in Observation (days) All n=244 <14 n=218 ≥14 n=26 Reference: Lipton R et al. Presented at the 2019 American Academy of Neurology Annual Meeting; May 4 - 10, 2019; Philadelphia, PA. Poster P235LB JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 17
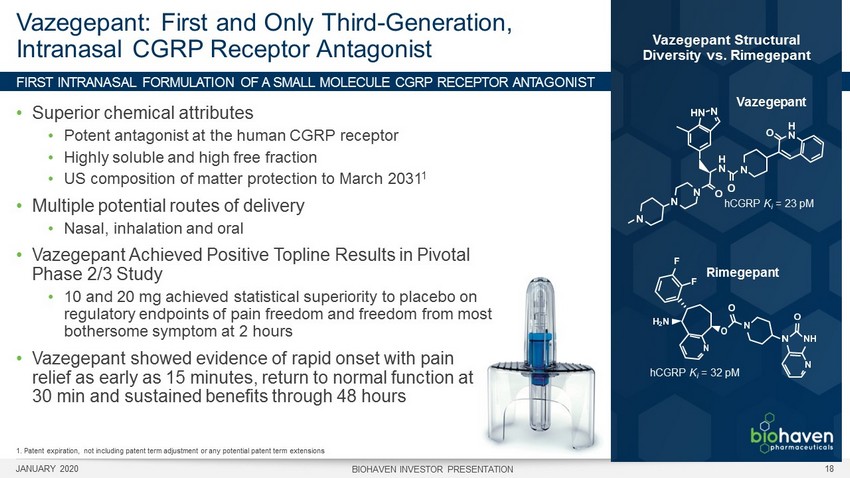
FIRST INTRANASAL FORMULATION OF A SMALL MOLECULE CGRP RECEPTOR A NTAGONIST Vazegepant : First and Only Third - Generation, Intranasal CGRP Receptor Antagonist • Superior chemical attributes • Potent antagonist at the human CGRP receptor • Highly soluble and high free fraction • US composition of matter protection to March 2031 1 • Multiple potential routes of delivery • Nasal, inhalation and oral • Vazegepant Achieved Positive Topline Results in Pivotal Phase 2/3 Study • 10 and 20 mg achieved statistical superiority to placebo on regulatory endpoints of pain freedom and freedom from most bothersome symptom at 2 hours • Vazegepant showed evidence of rapid onset with pain relief as early as 15 minutes, return to normal function at 30 min and sustained benefits through 48 hours hCGRP K i = 23 pM Vazegepant hCGRP K i = 32 pM Rimegepant 1. Patent expiration, not including patent term adjustment or any potential patent term extensions Vazegepant Structural Diversity vs. Rimegepant JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 18

VAZEGEPANT PHASE 2/3 — STUDY BHV3500 - 201, 10MG & 20MG INTRANASAL Vazegepant 10 mg and 20 mg Demonstrated Early Separation From Placebo at 15, 60 and 120 Minutes, Durable Through 24 – 48 Hours 1. Pain Relief is defined as patients who have either mild - pain or no - pain during the specified interval. 2. Sustained Pain Reli ef is defined as patients having mild - to - no - pain at 2 hours and continuing to the end of the specified interval. Estimates compu ted using the mITT population and CMH methods (mean ± Alpha’s Standard Error). Subjects using rescue medications at or before the assessment, and subjects not providing data, are cl assified as failures. 0 10 20 30 40 50 60 15 min 30 min 60 min 120 min 2-24 hr 2-48 hr Single Dose of Vazegepant , No Rescue Meds // 25 й 33 й 54 й 36 й 10 й 42 й % of Patients with Pain Relief Pain Relief to 2 Hours 1 & Sustained Pain Relief 2 - 24/48 Hours 2 Post - Single Dosing with Vazegepant Intranasal Time Vazegepant 20 mg (n=402) Placebo (n=401) Vazegepant 10 mg (n=391) 27 й 39 й 61 й 45 й 15 й 40 й 61 й 43 й 18 й 30 й 46 й 50 й JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 19

Commercial and Launch Readiness
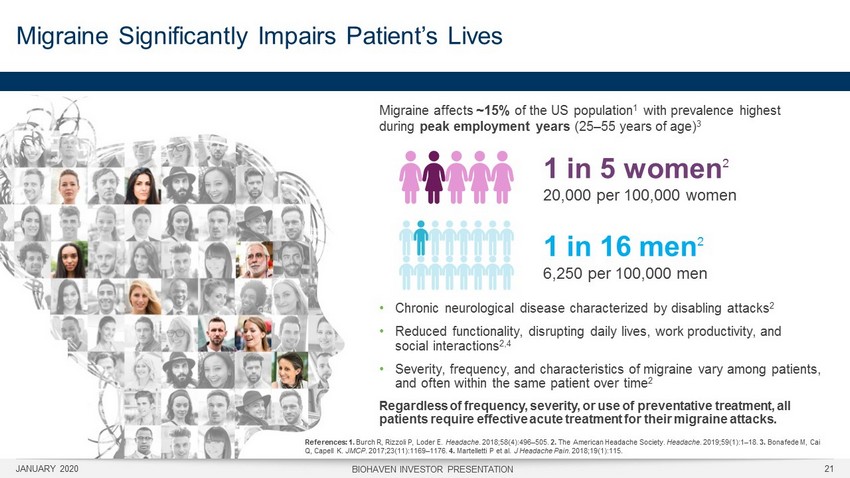
Migraine Significantly Impairs Patient’s Lives JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 21 References: 1. Burch R, Rizzoli P, Loder E. Headache . 2018;58(4):496 – 505. 2. The American Headache Society. Headache . 2019;59(1):1 – 18. 3. Bonafede M, Cai Q, Capell K. JMCP . 2017;23(11):1169 – 1176. 4. Martelletti P et al. J Headache Pain . 2018;19(1):115. • Chronic neurological disease characterized by disabling attacks 2 • Reduced functionality , disrupting daily lives, work productivity , and social interactions 2,4 • Severity, frequency, and characteristics of migraine vary among patients, and often within the same patient over time 2 Regardless of frequency, severity, or use of preventative treatment, all patients require effective acute treatment for their migraine attacks. Migraine affects ~15% of the US population 1 with prevalence highest during peak employment years (25 – 55 years of age) 3 1 in 16 men 2 6,250 per 100,000 men 1 in 5 women 2 20,000 per 100,000 women

Migraine Attacks: A Leading Cause of Economic Burden in US JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 22 References: 1. The American Headache Society. Headache . 2019;59(1):1 - 18. 2. Migraine Resource Center website. https://www.mdedge.com/neurology/ migraineresourcecenter /article/160613/headache - migraine/migraine - often - results. Accessed August 8, 2019. 3. Bonafede M, Cai Q, Cappell K. JMCP . 2017;23(11):1169 - 1176. 4. Bonafede M, Sapra S, Shah N et al. Headache . 2018;58(5):700 - 714. ~25% OF ER VISITS FOR MIGRAINE were followed by a second ER visit for headache in <6 month s 2 Direct health care costs associated with migraine (including interventions) are significant and affect patients, their families, and employer s 4 Annual total costs for migraine in the US are estimated at $27 BILLION 1 In a study of over 850,000 patients with migraine, patients with 2 or more migraine - related ER visits incurred $3,125 MORE total all - cause costs vs. those with less than 2 visits 3

Patients Report Low Satisfaction With Current Acute Treatments and High Abandonment Rates Across the Spectrum of Therapies JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 23 References: . 1. Migraine.com. https://migraine.com/infographic/migraine - is - more - than - a - headache - its - a - life - ache/. Published August 30, 2016. Acc essed September 9, 2019. 2. Data on file, Biohaven Pharmaceuticals 3 . Bonafede M, Cai Q, Cappell K. JMCP . 2017;23(11):1169 - 1176. • In a large survey, only 40% reported satisfaction with their current migraine treatment plan 2 • Migraine patients twice as likely to use opioids and had 1.8x the number of opioid scripts per patient 3 50% NSAIDs 45% PRESCRIPTION ANALGESICS 38% TRIPTANS 35% ERGOT DERIVATIVES ABANDONMENT RATES OF ACUTE MIGRAINE TREATMENTS 1

Oral CGRP Receptor Antagonists Address Treatment Needs Unmet by Triptans JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 24 References: Lipton RB et al. Headache. 2017;57:1026 - 40. Perry CM et al. Drugs. 1998;55:889 - 922. Cortelli P et al. Neurol Sci. 2011;32 Suppl 1:S95 - 8. Derry CJ et al. Cochrane Database Syst Rev. 2012;2(2):CD008615. Geraud G et al. Headache. 2003;43:376 - 88. Buse DC et al. Headache. 2017;57:31 - 44. Lipton RB et al. Headache. 2017;57:1507 - 21. GBD. Lancet. 2017;390:1211 – 59. Cameron C, Kelly S, Hsieh S - C et al. Headache . 2015;55(suppl 4):221 - 235. Nonresponders Cannot tolerate Recurrence Cardiovascular Contraindications Medication Overuse Headache (MOH) Inadequate Response in 34% 43% Have TEAEs Within 24 h 30% – 40% of Treated Patients ~2.6 Million US Triptans are among the most common causes of MOH
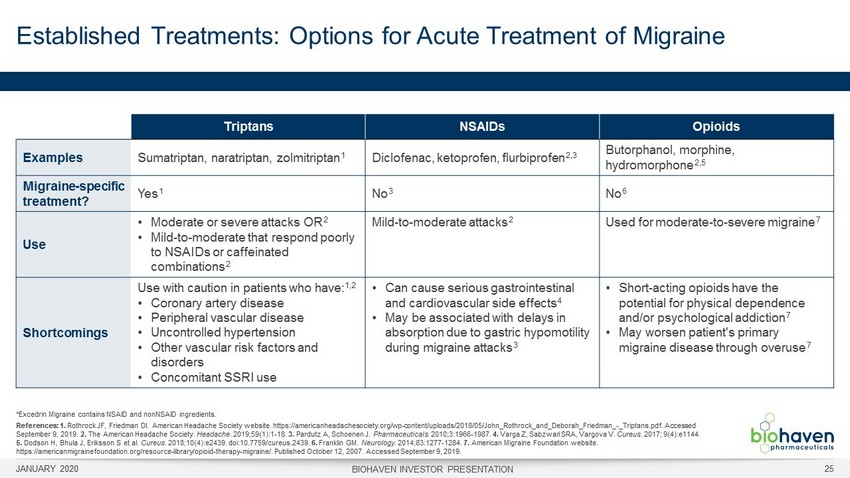
Triptans NSAIDs Opioids Examples Sumatriptan, naratriptan, zolmitripta n 1 Diclofenac, ketoprofen, flurbiprofe n 2,3 Butorphanol, morphine, hydromorphon e 2,5 Migraine - specific treatment? Ye s 1 N o 3 N o 6 Use • Moderate or severe attacks O R 2 • Mild - to - moderate that respond poorly to NSAIDs or caffeinated combination s 2 Mild - to - moderate attack s 2 Used for moderate - to - severe migrain e 7 Shortcomings Use with caution in patients who have : 1,2 • Coronary artery disease • Peripheral vascular disease • Uncontrolled hypertension • Other vascular risk factors and disorders • Concomitant SSRI use • Can cause serious gastrointestinal and cardiovascular side effect s 4 • May be associated with delays in absorption due to gastric hypomotility during migraine attack s 3 • Short - acting opioids have the potential for physical dependence and/or psychological addictio n 7 • May worsen patient's primary migraine disease through overus e 7 Established Treatments: Options for Acute Treatment of Migraine JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 25 *Excedrin Migraine contains NSAID and nonNSAID ingredients. References: 1. Rothrock JF, Friedman DI. American Headache Society website. https://americanheadachesociety.org/wp - content/uploads/2018/05/John _Rothrock_and_Deborah_Friedman_ - _Triptans.pdf. Accessed September 9, 2019. 2. The American Headache Society. Headache . 2019;59(1):1 - 18. 3. Pardutz A, Schoenen J. Pharmaceuticals . 2010;3:1966 - 1987. 4. Varga Z, Sabzwari SRA, Vargova V. Cureus . 2017; 9(4):e1144. 5. Dodson H, Bhula J, Eriksson S et al. Cureus . 2018;10(4):e2439. doi:10.7759/cureus.2439. 6. Franklin GM. Neurology . 2014;83:1277 - 1284. 7. American Migraine Foundation website. https://americanmigrainefoundation.org/resource - library/opioid - therapy - migraine/. Published October 12, 2007. Accessed September 9, 2019.

ABUSE POTENTIAL Controlled substance schedule pending CNS DEPRESSION, SEROTONIN SYNDROME & MEDICATION OVERUSE HEADACHES (detoxification may be necessary) DRIVING IMPAIRMENT Advise patients not to drive for 8 hours REYVOW™ ( Lasmiditan ): Serotonin (5 - HT) 1F Receptor Agonist JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 26 Driving Impairment REYVOW may cause significant driving impairment. In a driving study, administration of single 50 mg, 100 mg, or 200 mg doses of REYVOW significantly impaired subjects’ ability to drive. Patient who cannot follow this advice should not take REYVOW. Prescribers and patients should be aware that patients may not be able to assess their own driving competence and the degree of impairment caused by REYVOW. 5.2 Central Nervous System Depression REYVOW may cause central nervous system (CNS) depression, including dizziness and sedation. Because of the potential for REYVOW to cause sedation, other cognitive and/or neuropsychiatric adverse reactions, and driving impairment, REYVOW should be used with caution if used in combination with alcohol or other CNS depressants. Serotonin Syndrome In clinical trials, reactions consistent with serotonin syndrome were reported in patients treated with REYVOW who were not taking any other drugs associated with serotonin syndrome. Serotonin syndrome may also occur with REYVOW during coadministration with serotonergic drugs… Medication Overuse Headache Overuse of acute migraine drugs (e.g., ergotamines, triptans, opioids, or a combination of these drugs for 10 or more days per month) may lead to exacerbation of headache… “The recommended controlled substance classification for REYVOW is currently under review by the the Drug Enforcement Administration (DEA) and is expected within 90 days of today’s FDA Approval, after which REYVOW will be available to patients in retail pharmacies.” — Lilly press release, October 11, 2019 Label Indication: Acute Treatment of Migraine

RECRUIT TOP TALENT STRATEGICALLY INVEST BUILD LAUNCH CAPABILITIES ESTABLISH CULTURE OF ACCOUNTABILITY EFFICIENT, INNOVATIVE, MODERN DAY LAUNCH Rimegepant Launch Readiness Objectives JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 27

Rimegepant Launch Readiness Marketing Across Relevant Channels JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 28 Social Search Cross Screen V ideo Disease State Awareness Campaign Branded Launch Campaign

ALL PRIORITY ACCOUNTS BY LIVES Rimegepant Launch Readiness: Clinical Presentation Penetration JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 29 All Targeted Accounts Total Lives % Total All Accounts Completed 182,000,082 75.4% Scheduled 39,321,353 16.3% Not Yet Scheduled 19,923,412 8.3% Total 241,244,847 100.0% A Priority Accounts Lives % Total A Accounts Completed 180,126,400 82.3% Scheduled 34,023,593 15.5% Not Yet Scheduled 4,751,116 2.2% Total 218,901,109 100.0% B Priority Accounts Lives % Total B Accounts Completed 1,873,682 8.4% Scheduled 5,297,760 23.7% Not Yet Scheduled 15,172,296 67.9% Total 22,343,738 100.0% Completed or Scheduled Pie / Clinical Presentations 91.7% 8.3% Not Yet Scheduled 16.3% Scheduled 75.4% Completed 91.7% 97.8% 32.1% Total

GLUTAMATE PLATFORM Therapies for Neurologic and Neuropsychiatric Indications

NORMAL FUNCTION Healthy State JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 31 The Role of Glutamate: Present in 90% of Brain Synapses EXCITOTOXICITY Diseased State AMYOTROPHIC LATERAL SCLEROSIS SPINOCEREBELLAR ATAXIA DEMENTIA NEURODEGENERATION NEUROTOXICITY SEIZURES DEPRESSION ANXIETY STRESS CANCER PAIN STROKE MELANOMA ABNORMAL CELL GROWTH RETT SYNDROME NEURO - TRANSMISSION MEMORY CELL SURVIVAL SYNAPTO - PLASTICITY LEARNING NEUROTROPHIC STRESS RESILIENCE ACTION POTENTIAL COGNITION MOOD NEURONAL CONNECTIONS Biohaven is focused on normalizing glutamate to treat disease

JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 32 Glutamate Mechanisms of Action in CNS Glutamate Transporter Modulation • Troriluzole Glutamate NMDA Receptor Antagonism • BHV - 5000 Third - party clinical trials provide basis for exploration of troriluzole and BHV - 5000 in multiple neurologic and neuropsychiatric disorders 1 2 1 2

UNMET MEDICAL NEED IN ALS Riluzole ( Rilutek ® ) is approved for the treatment of patients with amyotrophic lateral sclerosis (ALS) and proven to extend survival • Originally marketed by Sanofi, received FDA approval in 1995 • In 2013, the FDA approved the first generic versions of riluzole • Doses above 100 mg for efficacy not approved due to dose - dependent liver effects Riluzole ( Rilutek ® ): Use and Limitations BENEFITS x Mechanism of action well understood x Neuroprotective, survival benefit in ALS x Well tolerated, safe in clinical settings at approved dose LIMITATIONS ✘ Twice daily dosing, low bioavailability ✘ Fasting required for 6 hours/day, can’t be taken with meals ✘ Dose dependent LFT liability 1 ✘ Marked PK variability ✘ High drug burden relative to efficacy 2 ✘ Only one approved indication (ALS) 1. LFT = liver function test 2. Poor oral bioavailability results in a high liver burden relative to efficacy as ~40% is either not absorbed or is metabol ize d in the liver JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 33
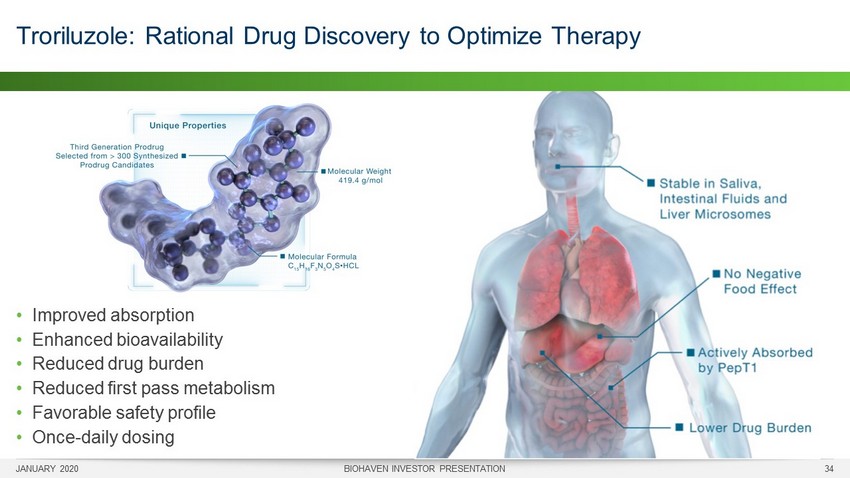
JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 34 Troriluzole: Rational Drug Discovery to Optimize Therapy • Improved absorption • Enhanced bioavailability • Reduced drug burden • Reduced first pass metabolism • Favorable safety profile • Once - daily dosing
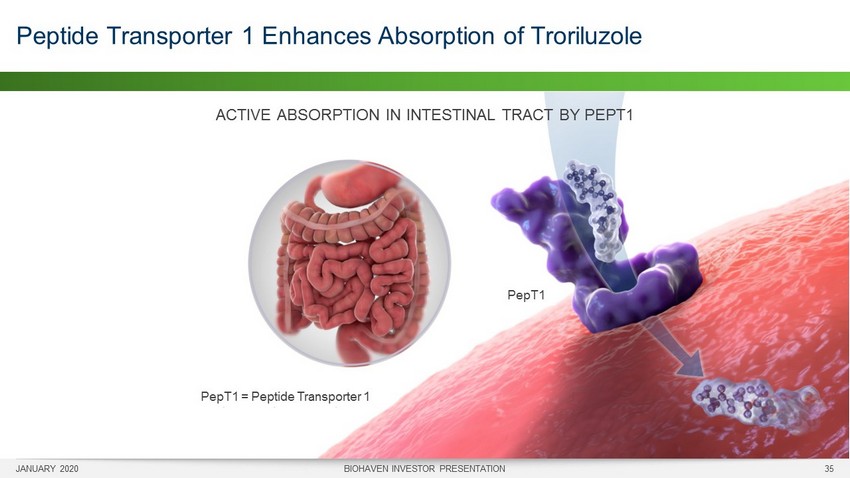
JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 35 Peptide Transporter 1 Enhances Absorption of Troriluzole PepT1 PepT1 = Peptide Transporter 1 ACTIVE ABSORPTION IN INTESTINAL TRACT BY PEPT1

Social Anxiety Disorder Generalized Anxiety Disorder Bipolar Depression JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 36 Troriluzole: Targeted Lead - Indication Development Strategy * = Third - party study / collaboration ongoing or planned (ADCS Collaboration for AD; ET Study Group Collaboration for ET) Lead indications across an array of potential neurologic and neuropsychiatric indications Obsessive - Compulsive & Related Disorders Neurodegenerative Disorders Mild - to - Moderate Alzheimer’s Disease* Prodromal Alzheimer’s Disease ALS (High Dose) Affective Disorders Cerebellar Disorders Spinocerebellar Ataxia (SCA) Friedreich’s Ataxia Sporadic Ataxia Other Ataxias Essential Tremor* Obsessive - Compulsive Disorder Trichotillomania Hoarding Disorder

• Lifetime prevalence approximately 4% in the US 1 • Typically chronic 2 • Low remission rate ~50% 3 • Symptoms characterized by • Excessive anxiety and worry • Associated with restlessness, easy fatigue, difficulty concentrating, irritability, tension, and sleep disturbance • Unmet medical need • Overuse of benzodiazepines • Associated with abuse, dependence and dangerous withdrawal syndromes • 30% of overdose deaths involve benzodiazepine 4 • Limited alternatives • Remission rates of SSRIs/SNRIs of ~30% Generalized Anxiety Disorder: Disease Overview 1. Ruscio et al 2017; 2. Bruce et al 2005. 3. Rickels et al 2006;4 McClure et al 2017; NBC New, 28 - JUL - 18 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 37

RILUZOLE PRECLINICAL MODELS • Riluzole efficacious in multiple preclinical models (elevated plus maze, the light - dark test, open field test) 1 • Riluzole enhances recognition memory and facilitates extinction of fear response 2 • Benzodiazepine - like efficacy without cognitive impairment 3 Glutamate Implicated in Anxiety Pathways Riluzole Demonstrates Efficacy in Preclinical Models 1 Sugiyama et al 2012; 2 Sugiyama 2015; 3 Sasaki - Hamada 2013 Treatment with riluzole increases amount of time spent in open arms and number of entries 1 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 38

• Single center, open - label, fixed dose study • 18 subjects with GAD • Administered riluzole 50 mg BID for 8 weeks • Primary endpoint HAM - A (Hamilton Anxiety Scale) • Results • 15 patients completed trial • 12 responders • 8 remitters • Mean HAM - A decreased from 20.0 at baseline to 7.5 at week 8 • Improvement on Secondary Outcomes • Anxiety Sensitivity Index • Sheehan Disability Scale (SDS) Riluzole Open - Label Trial in Generalized Anxiety Disorder 0 10 20 30 40 50 60 70 80 90 Intent-to-treat (n=18) Completer (n=15) Proportion of Treated Sample (%) Efficacy After 8 Weeks Responders (≥50% decrease in HAM - A) Remitters (HAM - A ≤ 7) Mathew SJ et al Am J Psychiatry 2005 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 39

JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 40 • Improvement in anxiety strongly correlated with increased hippocampal NAA (marker for neuronal function) 1,2 • Increases precede therapeutic benefit • Increases in hippocampus volume observed in remitters 3 — that correlate with improvement Objective Biomarkers Correlate with Improvement 1. Mathew et al, 2008; 2. Abdullah et al, 2012; 3. Abdullah et al 2013; MRS, Magnetic Resonance Spectroscopy; NAA, n - acetylaspartate ; PSWQ, Penn State Worry Questionnaire Hippocampal NAA ( mM ) Normalized Neuronal Function in Treatment Responders Increases in Hippocampus Volume Correlate with Improvement in Anxiety Measures

BHV - 0223 DESIGN • Double - blind, placebo controlled crossover trial with two Impromptu Speech Task sessions • Subjects diagnosed with Social Anxiety Disorder • N=22 randomized (21 completed) • BHV - 0223 vs PBO, dosed 1 hour prior to public speaking stress task; separated by two to ten days to allow for medication washout Yale POC Study: Anti - Anxiety Effects of BHV - 0223 Primary outcome: trial powered at 80%, to detect an effect size of 0.58, at an alpha of p=0.10; prespecified analysis showed p=0.056 and likelihood - based analysis showed p=0.0259 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 41
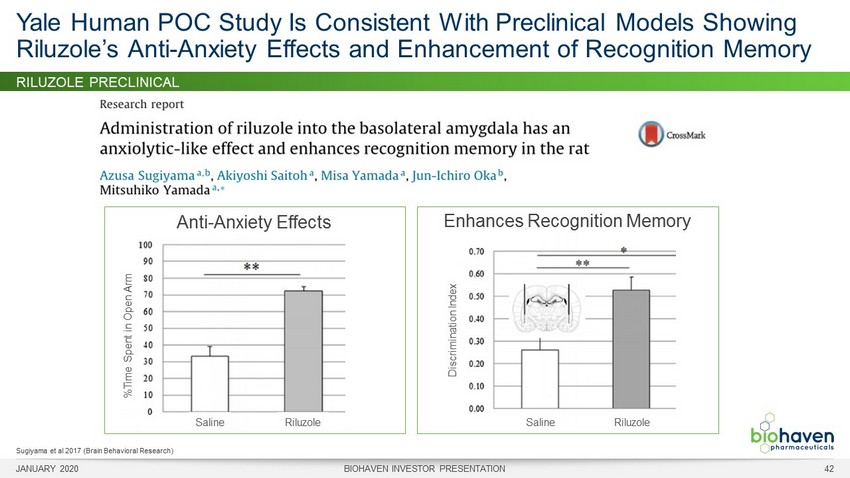
RILUZOLE PRECLINICAL Yale Human POC Study Is Consistent With Preclinical Models Showing Riluzole’s Anti - Anxiety Effects and Enhancement of Recognition Memory Sugiyama et al 2017 (Brain Behavioral Research) Anti - Anxiety Effects %Time Spent in Open Arm Saline Riluzole Enhances Recognition Memory Discrimination Index Saline Riluzole JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 42

• Multicenter (US only), randomized, double - blind, placebo - controlled trial in outpatients with GAD • Troriluzole 100 mg vs Placebo BID*, N=372 • Primary Outcome: Change on HAM - A from baseline to Week 8 TRORILUZOLE (BHV - 4157) Phase 2/3 GAD TRIAL DESIGN * BID: twice daily Troriluzole 100 mg BID Placebo BID Screening Phase 42 days Randomized Phase 8 weeks R JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 43

• Brain atrophy • Neuronal cell death • Amyloid and tau pathology • Synapse loss (most highly correlated with clinical symptoms) • Glial cell dysfunction Pathological Features of Alzheimer’s Disease Congdon Nat Rev Neurol. 2018;14(7):399 - 415 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 44

JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 45 Glutamate Transporter (GLT - 1) Is Reduced in Human AD Brains Human autopsy observations from AD and controls (n=13) GLT - 1 immunoreactivity in temporal neocortex area in AD is significantly weaker than that in control A. Hosi et al. Neuropathology and Applied Neurobiology (2018) HEALTHY HUMAN BRAIN ALZHEIMER'S DISEASE
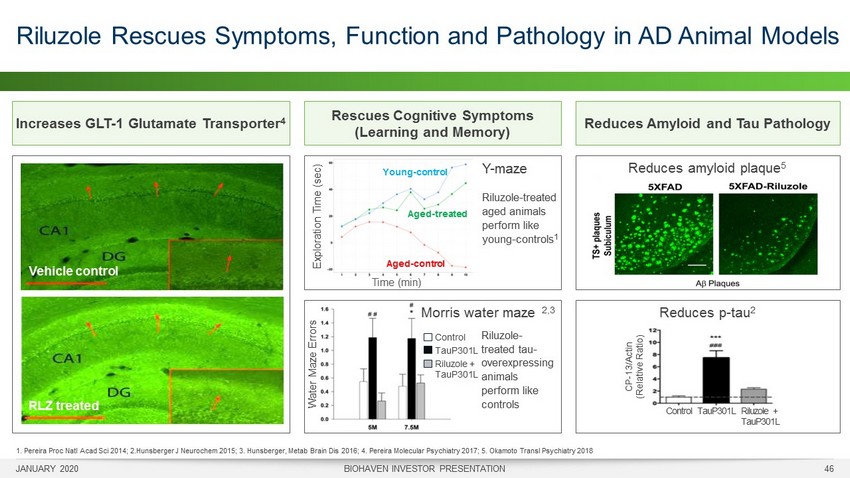
Increases GLT - 1 Glutamate Transporter 4 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 46 Riluzole Rescues Symptoms, Function and Pathology in AD Animal Models 1. Pereira Proc Natl Acad Sci 2014; 2.Hunsberger J Neurochem 2015; 3. Hunsberger, Metab Brain Dis 2016; 4. Pereira Molecular Psychiatry 2017; 5. Okamoto Transl Psychiatry 2018 Reduces Amyloid and Tau Pathology Reduces amyloid plaque 5 Reduces p - tau 2 Control TauP301L Riluzole + TauP301L CP - 13/Actin (Relative Ratio) Rescues Cognitive Symptoms (Learning and Memory) Water Maze Errors Control TauP301L Riluzole + TauP301L Morris water maze 2,3 Time (min) Exploration Time (sec) Aged - control Young - control Aged - treated Y - maze Riluzole - treated aged animals perform like young - controls 1 Riluzole - treated tau - overexpressing animals perform like controls Vehicle control RLZ treated

TRORILUZOLE (BHV - 4157) PHASE 2/3 • Key entry criteria • Diagnosis of mild to moderate Alzheimer’s disease with Mini - Mental State Exam (MMSE) score of 14 – 24 • Co - primary efficacy endpoints • Alzheimer's Disease Assessment Scale (ADAS) - Cog11 • Clinical Dementia Rating - Sum of Boxes (CDR - SB) • Secondary efficacy endpoints • Brain volumes: MRI imaging • Activities of daily living: ADCS - ADL • Neuropsychiatric: NPI • Neuropsychological: NTB • Cognitive: MMSE/ MoCA • Sample size: 292 subjects • Randomization: 1:1 • Collaborator: Alzheimer’s Disease Cooperative Study Troriluzole Phase 2/3 Clinical Trial Design in AD Troriluzole 280 mg QD Placebo QD Screening Phase 42 days Randomized Phase 48 weeks R Passed interim Futility Analysis December 2019 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 47

Cerebellar Ataxias JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 48 • Characterized by: • Poor balance with falls • Dysarthria / dysphagia • Incoordination of limbs • Cognitive impairment • Postural or kinetic tremor • Oculomotor dysfunction • Spinocerebellar ataxia (SCA; >40 subtypes) • Affects 1 – 5.6 per 100,000 (estimated 3,200 – 18,000 in US) • Neurodegeneration of cerebellum and input/output tracts • Relentlessly progressive, often fatal (aspiration) • Increasing disability over time (requiring wheelchair, assistance with activities of daily living) • The 6 most common genotypes caused by triplet repeat expansion mutations, sharing many phenotypic features • Genetic anticipation in some: subsequent generations affected at earlier ages and with greater severity • Other ataxias have similar core features • Can be recessive, dominant, immune, mitochondrial, post - stroke, e.g., Friedreich’s ataxia, ataxia telangiectasia, multiple system atrophy — cerebellar type No FDA - Approved Medications for SCA Spinocerebellar Ataxia type 2 Cerebellar and brainstem volume loss

JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 49 • Post - hoc analysis of patients enrolled in long - term extension of Phase 2b/3 troriluzole SCA trial • Primary efficacy endpoint: change from baseline in the Total SARA Score after 48 weeks • Patients from BHV4157 - 201 trial versus eligibility criteria matched Ashizawa Natural History cohort: • SCA Genotype • SCA1, SCA2, SCA3, SCA6 • Age at baseline • 18 to 75 years of age • Gender • SARA Score at baseline • ≥ 8 and ≤ 30, and • Initial SARA gait item score ≥ 2 Troriluzole Treated SCA Patients Treated for 1 Year Compared to Matched Ashizawa Natural History Cohort Troriluzole Study (BHV4157 - 206) in Spinocerebellar Ataxia (SCA) Achieved Phase 2/3 start in 1Q19 Least Squares Mean 2 Change in Total SARA Score (from baseline ± SE) Difference: - 1.41 ± 0.411 (95% confidence interval of - 2.22 to - 0.60) suggesting therapeutic benefits of troriluzole (p=0.0007) SARA: Scale for the Assessment and Rating of Ataxia SCA Patients on Troriluzole vs. Natural History Cohort 1. Matched on eligibility criteria 2. ANCOVA model with fixed effects for cohort, sex, & SCA genotype with age and baseline SARA scores as covariates

Key Entry Criteria • SCA genotypes ( SCA1, SCA2, SCA3, SCA6, SCA7, SCA8, SCA10) Design • Sample size: 230 subjects • Randomization: 1:1 • Stratification: by SCA genotype • Dose: Troriluzole 200 mg QD vs. Placebo QD • Primary Outcome: Modified SARA Scale • FDA aligned outcome measure Status • Phase 3 (initiated 1Q19) Troriluzole Study BHV4157 - 206 Randomized Controlled Trial in SCA Troriluzole 200 mg QD Placebo QD Screening Phase 6 weeks Randomization Phase 48 weeks R Extension Phase 48 weeks Troriluzole 200 mg QD SARA: Scale for the Assessment and Rating of Ataxia JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 50

JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 51 • Few FDA - approved treatments • Serotonin reuptake inhibitors (SSRIs and clomipramine) are the only approved monotherapy • No new mechanisms for 30 years • With limited efficacy • <50% of patients respond well • 25 – 30% are refractory • Many of those categorized as ‘responders’ continue to suffer • Leading many patients to seek invasive treatments • Deep brain stimulation • Ablative neurosurgery OCD Treatments Are Limited and Need Is Vast 1992 1997 1994 1996

JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 52 Accumulating Evidence for Glutamatergic Dysfunction in OCD GENES ANIMAL MODELS NEURAL NETWORKS PATIENTS IN THE CLINIC Genetic association studies: Glutamate transporter gene (SLC1A1 on chromosome 9p24) associated w/OCD 1 GWAS studies are focusing attention on DLGAP1, a post - synaptic scaffolding molecule at glutamate synapse ↑Glutamatergic activity worsens OCD behaviors SAPAP3 knockout mice ( - / - ) exhibit OCD - like behaviors 2 SAPAP3 is an ortholog of DLGAP3, in the same family as a gene implicated by OCD GWAS studies Multiple neuroimaging studies: Increased activity in cortico - striatal - thalamic (CST) pathway 3 MRS studies: Glutamate dysfunction in OCD suggested by some studies 4,5 Spinal fluid studies: Increased glutamate in OCD patients 6 Preliminary efficacy evidence: Glutamate modulating agents show promise in OCD and anxiety disorder patients 1. Arnold et al 2006; 2. Aida et a; 2015; 3. Baxter et al 1987, 1988, 1992; Nordhal et al 1989; Swedo et al 1989; Sawle et al 1991; Rubin et al 1992, 1995; Adams et al 1993; Perani et al 1995; Adams et al 1993; Perani et al 1995; McGuire et al 1994; Breiter et al 1996; Rausch et al 1996; 4. Rosenberg et al 2000; 5. Bolton et al 2001; 6. Chakrabarty et al 2005

JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 53 Neurochemical Evidence for Glutamate Dysregulation in OCD Rosenberg et al. 2000 Consistent with pathologic hyperactivity in cortico - striatal circuit Suggests clinical testing of agents that enhance glutamate uptake Healthy Control Subjects n = 11 Treatment Naïve OCD Patients n = 11 Caudate Glutamatergic Concentration ( x10 4 /water)

JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 54 Neurochemical Evidence for Glutamate Dysregulation in OCD Consistent with pathologic hyperactivity in cortico - striatal circuit Suggests clinical testing of agents that enhance glutamate uptake CSF Glutamate Concentrations Bhattacharyya et al. 2009

• Outpatients with moderate - to - severe OCD severity (Y - BOCS score ≥ 21) and free of medication treatment for at least six weeks • Randomized to receive fluvoxamine (up to 200 mg/day) plus either placebo or riluzole (50 mg twice daily) • Robust benefit on primary outcome measure (change in Y - BOCS scores) Randomized Controlled Trial with Riluzole Augmentation *, p<0.05; Cohen’s d 0.59 * Placebo (n=25) Riluzole (n=25) Y - BOCS Total Score YBOCS, Yale - Brown Obsessive Compulsive Scale Emamzadehfard et al. 2016 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 55

KEY ENTRY CRITERIA • Moderate - to - severe OCD and inadequate response to standard of care DESIGN • Sample size: 226 subjects • Randomization: 1:1 • Dose: Troriluzole 200 mg QD vs. Placebo QD (in patients on standard of care) • Primary Outcome: Y - BOCS, a precedented outcome measure accepted by FDA STATUS • Enrollment active • Expect to complete enrollment in 1Q2020 Troriluzole Study BHV4157 - 202 in Randomized Controlled Trial in OCD SOC + Troriluzole 200 mg QD SOC + Placebo QD Screening Phase 42 days Randomization Phase 12 weeks R Extension Phase 48 weeks SOC + Troriluzole 200 mg QD Subjects (n=226) with Moderate to severe OCD and inadequate response to standard of care SOC, standard of care YBOCS, Yale - Brown Obsessive Compulsive Scale JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 56

MPO PLATFORM Therapies for Neuroinflammation
VERDIPERSTAT (BHV - 3241) FOR NEUROINFLAMMATION • Rare, rapidly progressive and fatal neurodegenerative disease • Prevalence: 2 – 5 per 100,000 • Clinical symptoms • Parkinsonism: characteristic tremor (not responsive to L - DOPA), rigidity, dysarthria, falls • Cerebellar ataxia • Autonomic failure: orthostatic hypotension, urinary dysfunction, erectile dysfunction • Prognosis: more rapidly progressive than Parkinson’s disease • Time to loss of ambulation: 3.5 – 5 years • Mean survival from symptom onset: 6 – 10 years • Pathology: glial cytoplasmic inclusions (GCIs) containing alpha - synuclein • Disease mechanisms: oxidative stress and neuroinflammation • No disease modifying treatments • Symptomatic and palliative management Multiple System Atrophy (MSA) JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 58

BHV - 3241 FOR NEUROINFLAMMATION • Myeloperoxidase (MPO) enzyme • Key mediator of oxidative and inflammatory processes that lead to neurodegeneration • Promotes alpha - synuclein aggregation • Increased in human MSA brains in areas of neurodegeneration 1 • Verdiperstat • Potent, first - in - class, brain - penetrant MPO inhibitor • Developed by AstraZeneca (formerly AZ3241) Verdiperstat (BHV - 3241) is a myeloperoxidase inhibitor 1. Neurotox Res 2012;21(4):393 - 404 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 59

BHV - 3241 FOR NEUROINFLAMMATION Verdiperstat Neuroprotective Effects in MSA Animal Model 1 Reduces GCIs of alpha - synuclein Promotes functional (motor) improvements Suppresses microglial activation RLZ treated Rescues neurodegeneration 1. Neurotox Res 2012;21(4):393 - 404 JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 60
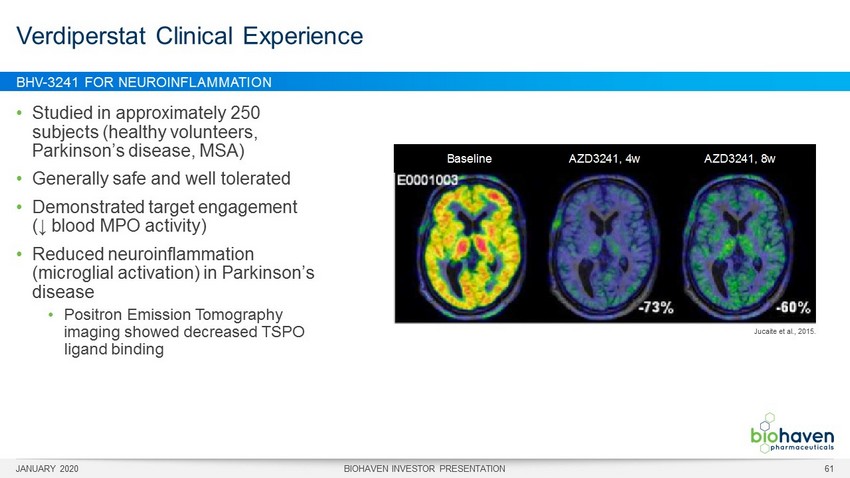
BHV - 3241 FOR NEUROINFLAMMATION • Studied in approximately 250 subjects (healthy volunteers, Parkinson’s disease, MSA) • Generally safe and well tolerated • Demonstrated target engagement (↓ blood MPO activity) • Reduced neuroinflammation (microglial activation) in Parkinson’s disease • Positron Emission Tomography imaging showed decreased TSPO ligand binding Verdiperstat Clinical Experience Jucaite et al., 2015. Baseline AZD3241, 4w AZD3241, 8w JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 61

BHV - 3241 FOR NEUROINFLAMMATION • Phase 2 study: randomized double - blind controlled trial • N=61 subjects (MSA - C, 34; MSA - P, 24) • Randomized to 12 weeks treatment • Placebo BID vs BHV - 3241 300 mg BID vs BHV - 3241 600 mg BID • Outcome measures: UMSARS*, PET, safety (labs, AE’s, ECGs, vitals) • Generally safe and well tolerated • Emerging efficacy signals warrant further study in MSA • Dose proportional benefit on mean UMSARS decline • Dose proportional rates of clinically meaningful improvement • 600 mg dose with statistical trends (p<0.10) for superiority over placebo on multiple UMSARS items • 600 mg dose shows numerical improvement on MSA Quality of life scale Verdiperstat Phase 2 Study in MSA Completed by AstraZeneca * UMSARS, Unified MSA Rating Scale: primary efficacy measure for the study Mean Change on UMSARS Total Score UMSARS Total Change from Baseline JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 62
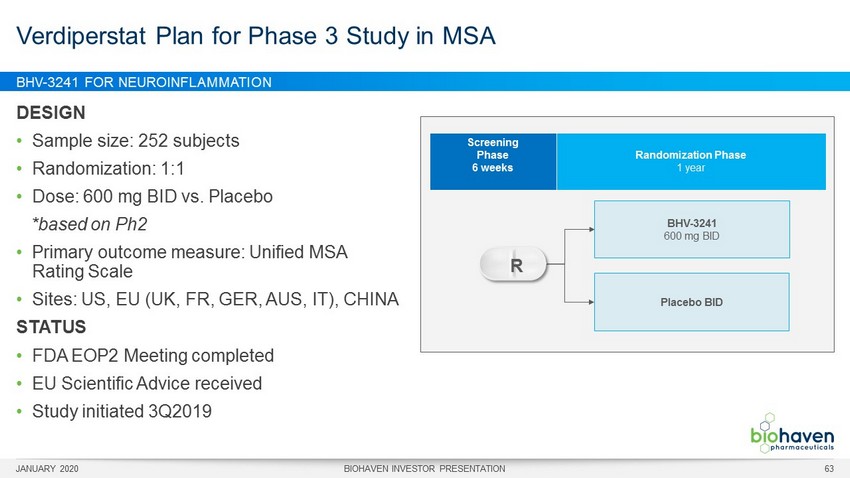
BHV - 3241 FOR NEUROINFLAMMATION DESIGN • Sample size: 252 subjects • Randomization: 1:1 • Dose: 600 mg BID vs. Placebo *based on Ph2 • Primary outcome measure: Unified MSA Rating Scale • Sites: US, EU (UK, FR, GER, AUS, IT), CHINA STATUS • FDA EOP2 Meeting completed • EU Scientific Advice received • Study initiated 3Q2019 Verdiperstat Plan for Phase 3 Study in MSA BHV - 3241 600 mg BID Placebo BID Screening Phase 6 weeks Randomization Phase 1 year R JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 63

METALLOTHIONEIN BLOCKADE A Novel Anti - Inflammatory Therapeutic

Metallothionein Can Alter Immune Response by Influencing Cellular Trafficking Healthy tissue Stress - causing cell damage Metallothionein production and release as a “danger signal” Extracellular MT recruits inflammatory cell Disease propagation cycle: more stress and more inflammation JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 65

Metallothionein: A Novel Target in the Immune Cascade • Metallothionein (MT) is an intracellular protein in non - stressed conditions • MT is released from cells/tissues that are stressed, damaged, or killed • Extracellular MT can act as a pro - inflammatory agent • MT acts as an agent of disease progression in multiple inflammatory models • MT elevations demonstrated in patients with Inflammatory Bowel Disease (IBD) • MT is elevated in diabetes, multiple brain disorders, inflammatory liver disease, inflammatory skin disease, pulmonary arterial hypertension, neoplasia, etc. • Anti - MT monoclonal antibody (UC1MT) significantly reduces disease severity in multiple models tested Inflammatory stress MT induction and release Pro - inflammatory effects of released MT Progressive inflammation and MT synthesis UC1MT blockade JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 66

UC1MT Reduces Inflammation in the Adoptive T Cell Transfer Model of IBD Anti - MT performance exceeded conventional anti - TNF therapeutic JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 67

UC1MT: First in Class Metallothionein Antagonist for a Novel Pro - Inflammatory Target • UC1MT is a high affinity monoclonal antibody that recognizes both mouse and human MT1 and MT2 • Confirmed the role of MT involvement in multiple disease models with better activity than anti - TNF • Evidence of molecular mechanism • Demonstrated the ability reduce markers of inflammation by UC1MT • Strong global patent protection of Anti - MT antibody therapeutic in several disease areas JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 68

2020 Milestones/Commercial Transformation

2020 Milestones & Next Steps JANUARY 2020 BIOHAVEN INVESTOR PRESENTATION 70 4Q20/ 1Q21 Phase 2/3 trial data of troriluzole in Alzheimer’s Disease 1Q20 Phase 2/3 topline data of troriluzole in OCD 1Q20 Phase 2/3 topline data of troriluzole in GAD ALS: amyotrophic lateral sclerosis, AD: Alzheimer’s Disease, OCD: Obsessive - Compulsive Disorder, GAD: Generalized Anxiety Disorder, SCA: Spinocerebellar Ataxia 1Q20 Phase 3 topline data for rimegepant in preventive treatment of migraine 1Q20 PDUFA for rimegepant in acute treatment of migraine Phase 3 clinical trial data for verdiperstat for the treatment of MSA 2021

NEURO INNOVATION WWW.BIOHAVENPHARMA.COM Zydis ® is a registered trademark of Catalent. Rilutek ® is a registered trademark of Covis Pharma B.V. Reyvow is a trademark of Eli Lilly and Company NYSE: BHVN © 2019 Biohaven Pharmaceuticals Inc. All rights reserved.
