Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Axsome Therapeutics, Inc. | tm203196-1_8k.htm |
| EX-99.1 - EXHIBIT 99.1 - Axsome Therapeutics, Inc. | tm203196d1_ex99-1.htm |
| EX-10.1 - EXHIBIT 10.1 - Axsome Therapeutics, Inc. | tm203196d1_ex10-1.htm |
Exhibit 99.2

NASDAQ: AXSM Axsome Exclusive Agreement with Pfizer Conference Call January 13, 2020 © Axsome Therapeutics, Inc.

FLS Forward-Looking Statements & Safe Harbor Certain information contained in this presentation may include “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the success, timing and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation, pace of enrollment and completion of the trials (including our ability to fully fund our disclosed clinical trials, which assumes no material changes to our currently projected expenses), futility analyses and receipt of interim results, which are not necessarily indicative of the final results of our ongoing clinical trials and the number or type of studies or nature of results necessary to support the filing of a new drug application for any of our current product candidates; our ability to fund additional clinical trials to continue the advancement of our product candidates; the timing of and our ability to obtain and maintain U.S. Food and Drug Administration (“FDA”) or other regulatory authority approval of, or other action with respect to, our product candidates (including, but not limited to, FDA’s agreement with the Company’s plan to discontinue the bupropion treatment arm of the ADVANCE-1 study in accordance with the independent data monitoring committee’s recommendations); the Company’s ability to obtain additional capital necessary to fund its operations; the Company’s ability to generate revenues in the future; the potential for the MOMENTUM clinical trial to provide a basis for approval of AXS-07 for the acute treatment of migraine in adults with or without aura, pursuant to our special protocol assessment; the potential for the ASCEND clinical trial, combined with the GEMINI clinical trial results, to provide a basis for approval of AXS-05 for the treatment of major depressive disorder and accelerate its development timeline and commercial path to patients; the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs and collaborations; the enforceability of the Company’s license agreements; the acceptance by the market of the Company’s product candidates, if approved; the Company’s anticipated capital requirements, including the Company’s anticipated cash runway; and other factors, including general economic conditions and regulatory developments, not within the Company’s control. These factors could cause actual results and developments to be materially different from those expressed in or implied by such statements. Forward-looking statements are not guarantees of future performance, and actual results may differ materially from those projected. The forward-looking statements are made only as of the date of this presentation and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, these projections, assumptions and estimates are necessarily subject to a high degree of uncertainty and risk. © Axsome Therapeutics, Inc. 2

Overview Axsome Exclusive Agreement with Pfizer Summary • Exclusive U.S. license to Pfizer’s clinical and nonclinical data, and intellectual property for reboxetine – Axsome is developing reboxetine, the active pharmaceutical ingredient in AXS-12, for the treatment of narcolepsy • Exclusive right from Pfizer to develop and commercialize esreboxetine, a Phase 3-stage product candidate now referred to as AXS-14, in the U.S. for the treatment of fibromyalgia – Met the primary endpoints in completed Pfizer Phase 3 and Phase 2 placebo-controlled clinical trials in fibromyalgia (p<0.001, and p<0.001) • Pfizer to receive $11 million in Axsome stock and upfront cash, and up to $323 million in regulatory and sales milestones • Agreement potentially significantly accelerates the ongoing clinical development of AXS-12 (reboxetine) in narcolepsy – Positive Phase 2 narcolepsy results recently announced; Phase 3 planned in 2020 • Expands Axsome’s pipeline with new AXS-14 (esreboxetine) Phase 3-stage product candidate for fibromyalgia • Axsome has 3 pending U.S. patents covering AXS-14; 2 pending U.S. patents and Orphan Drug designation covering AXS-12 – AXS-12 and AXS-14 are NCEs © Axsome Therapeutics, Inc. 3
Overview Axsome Exclusive Agreement with Pfizer License and Rights Reboxetine (AXS-12) • Axsome receives from Pfizer exclusive U.S. license to Pfizer data from: – Short-term and long-term reboxetine clinical trials – Full range of reboxetine nonclinical studies; other intellectual property • Reboxetine is the active pharmaceutical ingredient in AXS-12 which Axsome is developing for the treatment of narcolepsy Esreboxetine (AXS-14) • Axsome receives from Pfizer exclusive license to develop and commercialize esreboxetine, now referred to as AXS-14, in the U.S. for fibromyalgia and all other indications • License encompasses: – Esreboxetine clinical data, including positive Phase 3 and Phase 2 trials in fibromyalgia conducted by Pfizer – Full range of esreboxetine nonclinical studies; other intellectual property • Esreboxetine is the SS-enantiomer of racemic reboxetine © Axsome Therapeutics, Inc. 4
Overview Axsome Exclusive Agreement with Pfizer Financial Terms Pfizer receives: • Axsome common stock having a value of $8 million, based on the average closing price for the 10 prior trading days • Upfront cash payment of $3 million • Up to $323 million in regulatory and sales milestones, and tiered mid-single to low double-digit royalties on future sales • Right of first negotiation on any potential future strategic transactions involving AXS-12 or AXS-14 © Axsome Therapeutics, Inc. 5
Overview Axsome Exclusive Agreement with Pfizer Benefits of the Transaction • Accelerates AXS-12 development in narcolepsy, and mitigates associated risks and costs – Reduces or eliminates need to conduct certain nonclinical and clinical studies – Licensed clinical data for reboxetine and esreboxetine include trials involving more than 5,000 patients • Expands Axsome’s CNS pipeline with new Phase 3-stage AXS-14 (esreboxetine) product candidate in fibromyalgia – Benefit in fibromyalgia already demonstrated completed efficacy trials – Advances Axsome’s mission to accelerate the development of life-changing medicines for the many people living with difficult-to-treat CNS disorders © Axsome Therapeutics, Inc. 6

AXS-12 Narcolepsy: AXS-12 (reboxetine) Overview • Debilitating sleep disorder characterized by excessive daytime sleepiness (EDS) and cataplexy • Limited treatment options – Only one approved agent for cataplexy – Most currently approved drugs are scheduled • Positive Phase 2 efficacy results with AXS-12 – Significant reduction in cataplexy attacks and EDS – Significant improvement in cognitive function • Phase 3 trial initiation planned in 2020 • U.S. Orphan Drug Designation • Axsome has 2 pending U.S. patents covering AXS-12 Orphan Disease 185,000 patients in the U.S. © Axsome Therapeutics, Inc. 7 Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-12 (Reboxetine) Narcolepsy; U.S. Or phan Designation Phase 3 Planned
AXS-14 Fibromyalgia: AXS-14 (esreboxetine) Overview • Debilitating, chronic, CNS disorder characterized by widespread pain, fatigue, disturbed sleep, depression, and cognitive impairment; ~90% affected are women • Limited treatment options—only 3 approved agents: – Current treatments has variable efficacy and do not address all symptoms • AXS-14 (esreboxetine) is the SS-enantiomer of racemic reboxetine • Positive Phase 3 and Phase 2 efficacy results with AXS-14 in fibromyalgia • Axsome has 3 pending U.S. patents covering AXS-14 5M patients in the U.S.1 1. Decision Resources Group 2019 © Axsome Therapeutics, Inc. 8 Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-14 (Esreboxetine) Fibromyalgia
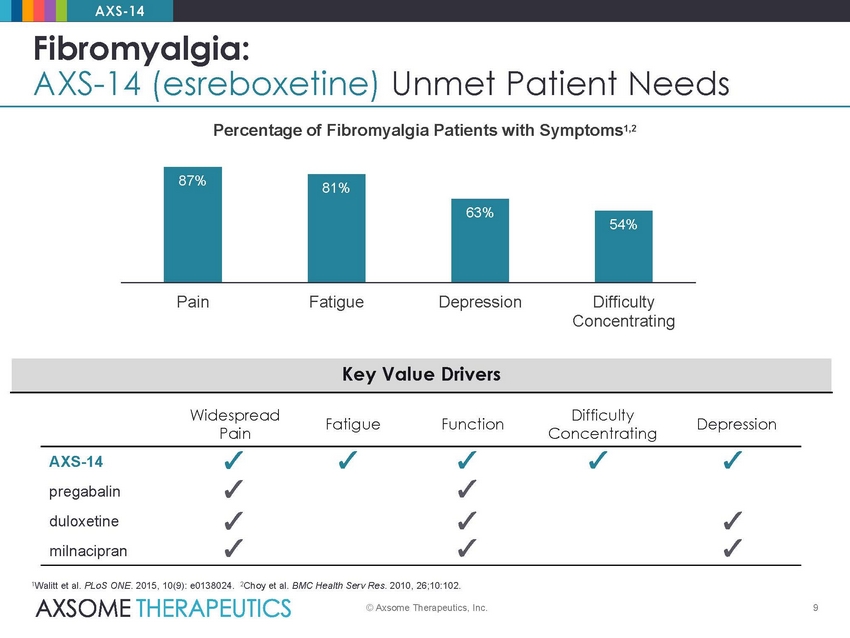
AXS-14 Fibromyalgia: AXS-14 (esreboxetine) Unmet Patient Needs Percentage of Fibromyalgia Patients with Symptoms1,2 Pain Fatigue Depression Difficulty Concentrating Widespread Pain Difficulty Concentrating Fatigue Function Depression AXS-14 pregabalin duloxetine milnacipran 1Walitt et al. PLoS ONE. 2015, 10(9): e0138024. 2Choy et al. BMC Health Serv Res. 2010, 26;10:102. © Axsome Therapeutics, Inc. 9 Key Value Drivers 87% 81% 63% 54%
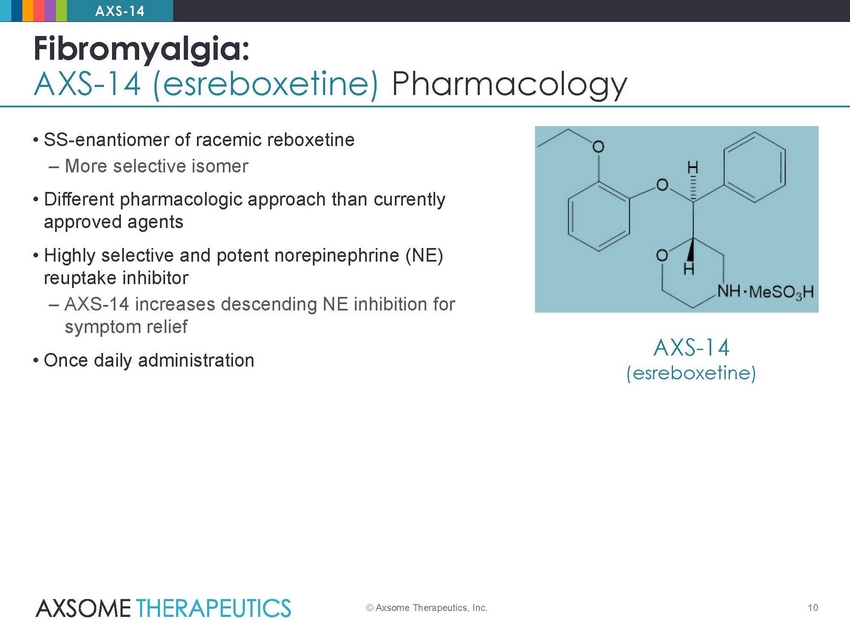
AXS-14 Fibromyalgia: AXS-14 (esreboxetine) Pharmacology • SS-enantiomer of racemic reboxetine – More selective isomer • Different pharmacologic approach than currently approved agents • Highly selective and potent norepinephrine (NE) reuptake inhibitor – AXS-14 increases descending NE inhibition for symptom relief • Once daily administration AXS-14 (esreboxetine) © Axsome Therapeutics, Inc. 10
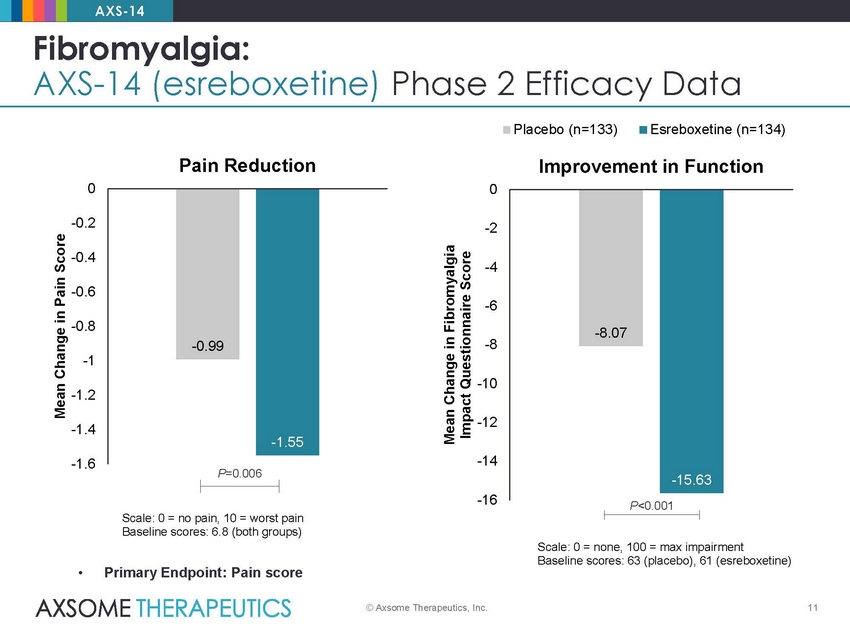
AXS-14 Fibromyalgia: AXS-14 (esreboxetine) Phase 2 Efficacy Data Placebo (n=133) Esreboxetine (n=134) Pain Reduction Improvement in Function 0 0 -0.2 -2 -0.4 -4 -0.6 -6 -0.8 -8 -1 -10 -1.2 -12 -1.4 -14 -1.6 P=0.006 -16 P<0.001 Scale: 0 = no pain, 10 = worst pain Baseline scores: 6.8 (both groups) Scale: 0 = none, 100 = max impairment Baseline scores: 63 (placebo), 61 (esreboxetine) • Primary Endpoint: Pain score © Axsome Therapeutics, Inc. 11 Mean Change in Pain Score Mean Change in Fibromyalgia Impact Questionnaire Score -8.07 -15.63 -0.99 -1.55
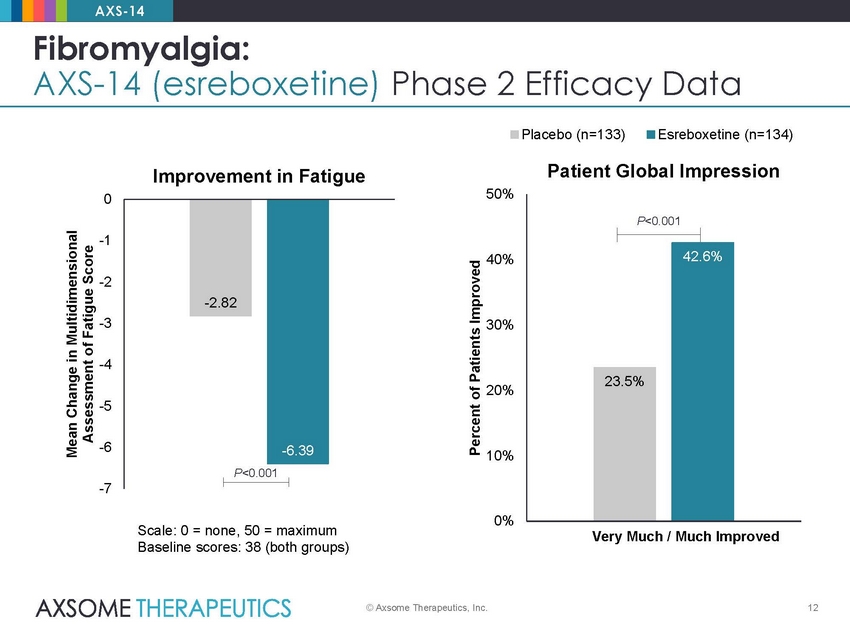
AXS-14 Fibromyalgia: AXS-14 (esreboxetine) Phase 2 Efficacy Data Placebo (n=133) Esreboxetine (n=134) Patient Global Impression Improvement in Fatigue 50% 0 -1 40% -2 -3 30% -4 20% -5 -6 10% -7 0% Scale: 0 = none, 50 = maximum Baseline scores: 38 (both groups) Very Much / Much Improved © Axsome Therapeutics, Inc. 12 Mean Change in Multidimensional Assessment of Fatigue Score Percent of Patients Improved P<0.001 42.6% 23.5% -2.82 -6.39 P<0.001
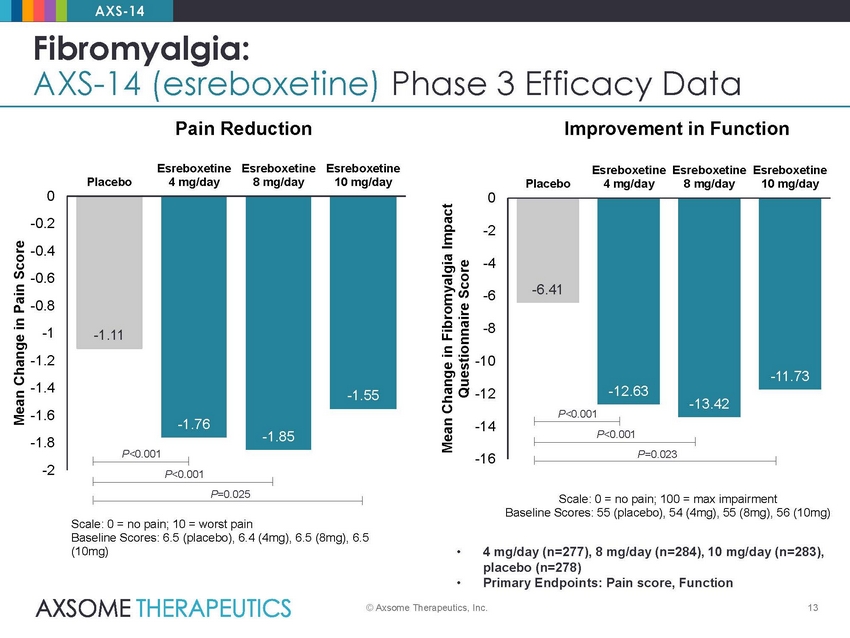
AXS-14 Fibromyalgia: AXS-14 (esreboxetine) Phase 3 Efficacy Data Pain Reduction Improvement in Function Esreboxetine 4 mg/day Esreboxetine 8 mg/day Esreboxetine 10 mg/day Esreboxetine 4 mg/day Esreboxetine 8 mg/day Esreboxetine 10 mg/day Placebo Placebo 0 -0.2 -0.4 -0.6 -0.8 -1 -1.2 -1.4 -1.6 -1.8 -2 0 -2 -4 -6 -8 -10 -12 -14 -16 P<0.001 P=0.025 Scale: 0 = no pain; 100 = max impairment Baseline Scores: 55 (placebo), 54 (4mg), 55 (8mg), 56 (10mg) Scale: 0 = no pain; 10 = worst pain Baseline Scores: 6.5 (placebo), 6.4 (4mg), 6.5 (8mg), 6.5 (10mg) • 4 mg/day (n=277), 8 mg/day (n=284), 10 mg/day (n=283), placebo (n=278) Primary Endpoints: Pain score, Function • © Axsome Therapeutics, Inc. 13 Mean Change in Pain Score Mean Change in Fibromyalgia Impact Questionnaire Score -6.41 -12.63 -13.42 -11.73 P<0.001 P<0.001 P=0.023 -1.11 -1.76 -1.85 -1.55 P<0.001
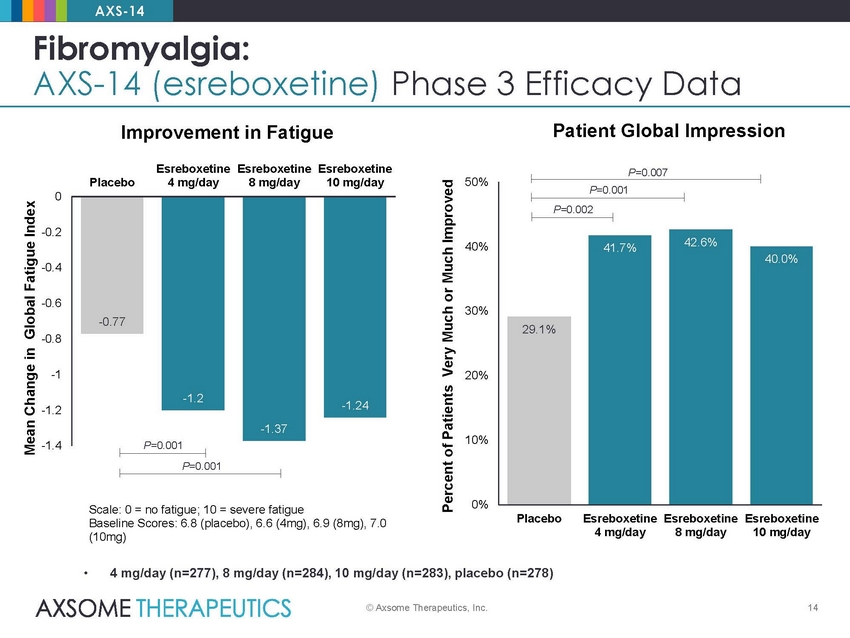
AXS-14 Fibromyalgia: AXS-14 (esreboxetine) Phase 3 Efficacy Data Patient Global Impression Improvement in Fatigue Esreboxetine 4 mg/day Esreboxetine 8 mg/day Esreboxetine 10 mg/day P=0.007 Placebo 50% 0 -0.2 40% -0.4 -0.6 30% -0.8 -1 20% -1.2 10% -1.4 P=0.001 P=0.001 0% Scale: 0 = no fatigue; 10 = severe fatigue Baseline Scores: 6.8 (placebo), 6.6 (4mg), 6.9 (8mg), 7.0 (10mg) Placebo Esreboxetine 4 mg/day Esreboxetine 8 mg/day Esreboxetine 10 mg/day • 4 mg/day (n=277), 8 mg/day (n=284), 10 mg/day (n=283), placebo (n=278) © Axsome Therapeutics, Inc. 14 Mean Change in Global Fatigue Index Percent of Patients Very Much or Much Improved P=0.001 P=0.002 42.6% 41.7% 40.0% 29.1% -0.77 -1.2 -1.37 -1.24
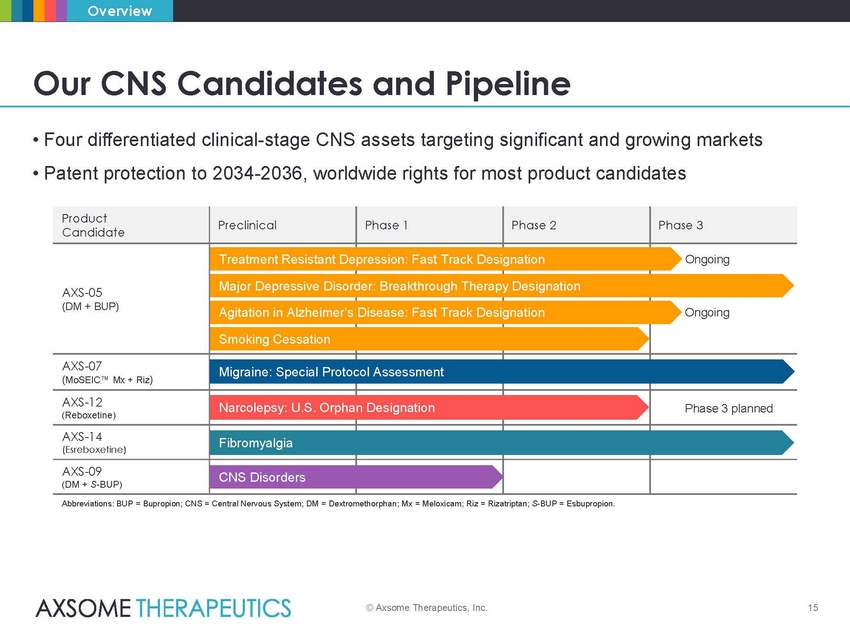
Overview Our CNS Candidates and Pipeline • Four differentiated clinical-stage CNS assets targeting significant and growing markets • Patent protection to 2034-2036, worldwide rights for most product candidates epression: Fast Track Desi der: Breakthrough Therapy Designation otocol Assessment phan Designation Abbreviations: BUP = Bupropion; CNS = Central Nervous System; DM = Dextromethorphan; Mx = Meloxicam; Riz = Rizatriptan; S-BUP = Esbupropion. © Axsome Therapeutics, Inc. 15 Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-05 (DM + BUP) Treatment Resistant D Major Depressive Disor Agitation in Alzheimer’s Smoking Cessation Disease: Fast Track Desi gnation gnation Ongoing Ongoing AXS-07 (MoSEIC™ Mx + Riz) Migraine: Special Pr AXS-12 (Reboxetine) Narcolepsy: U.S. Or Phase 3 planned AXS-14 (Esreboxetine) Fibromyalgia AXS-09 (DM + S-BUP) CNS Disorders

For more information, please contact Mark Jacobson SVP, Operations 212-332-3243 mjacobson@Axsome.com axsome.com
