Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Evofem Biosciences, Inc. | evfm8-kcorporatepresentati.htm |

Exhibit 99.1 Revolutionizing Women’s Sexual and Reproductive Health Saundra Pelletier, CEO January 2020 Nasdaq: EVFM 1 ©2020©2019 Evofem Biosciences, Inc.

Forward-Looking Statements This presentation contains forward looking statements within the meaning of The Private Securities Litigation Reform Act of 1995 and other federal securities laws. In some cases, you can identify forward looking statements by terms such as “may,” ”will,” “should,” “expect,” “plan,” “aim,” “anticipate,” “strategy,” “objective,” “designed,” “suggest,” “currently,” “could,” “intend,” “target,” “project,” “contemplate,” “believe,” “estimate,” “predict,” “potential” or “continue” or the negative of these terms or other similar expressions. Each of these forward-looking statements involves risks and uncertainties. Actual results may differ materially from those, express or implied, in these forward-looking statements. Factors that may cause differences between current expectations and actual results include, but are not limited to, the following: o the outcome or success of Evofem’s clinical trials o Evofem’s ability to obtain the necessary regulatory approvals for its product candidates, including approval from the U.S. Food and Drug Administration for the use of Amphora® as a contraceptive, and the timing of such approvals the rate and degree of market acceptance of Amphora® o ® o Evofem’s ability to successfully commercialize Amphora and its ability to develop sales and marketing capabilities o Evofem’s ability to maintain and protect its intellectual property o Evofem’s ability to raise additional capital when needed and to rely on existing cash reserves to fund its current development plans and operations Evofem’s reliance on third party providers, such as third party manufacturers and clinical research organizations o ® o the absence of any adverse events or side effects relating to the use of Amphora o Evofem’s ability to retain members of its management and other key personnel o and other risk factors detailed in Evofem’s filings from time to time with the U.S. Securities and Exchange Commission including, without limitation, the 10-K filed on March 1, 2019 and subsequent filings The forward looking statements in this presentation represent Evofem’s views only as of the date of this presentation, January 9, 2020, and Evofem expressly disclaims any obligation or undertaking to release publicly any updates or revisions to any forward-looking statements contained herein to reflect any change in Evofem’s expectations with regard thereto or any change in events, conditions or circumstances on which any such statements are based for any reason, except as required by law, even as new information becomes available or other events occur in the future. All forward- looking statements in this presentation are qualified in their entirety by this cautionary statement. 2 ©2020©2019 Evofem Biosciences, Inc.
About Evofem Biosciences A Clinical-Stage Biopharmaceutical Company committed to developing and commercializing innovative products to address unmet needs in women’s sexual and reproductive health First-in-Class Product Experienced Long-term Vision AMPHORA® Management 3 ©2020©2019 Evofem Biosciences, Inc.
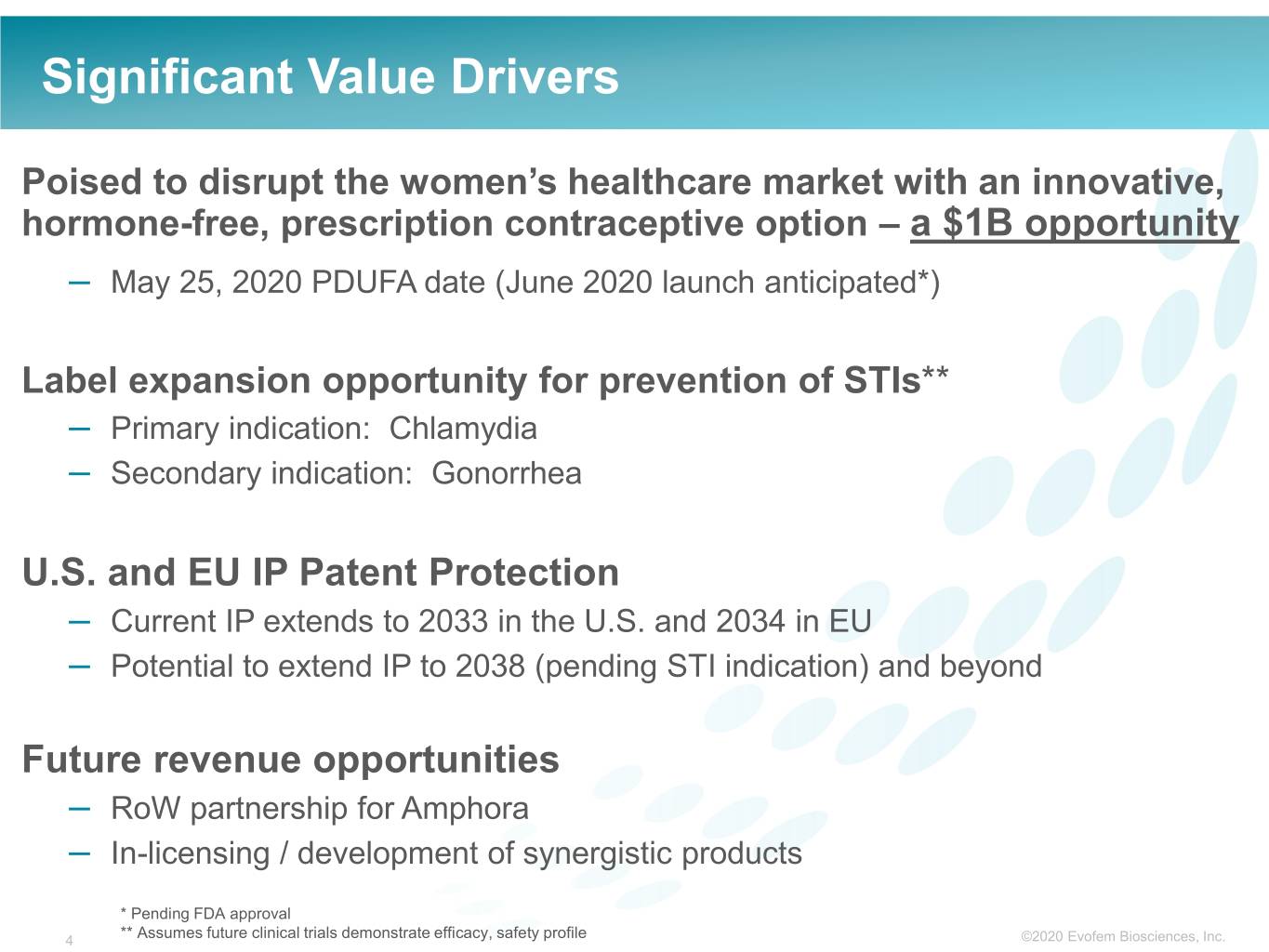
Significant Value Drivers Poised to disrupt the women’s healthcare market with an innovative, hormone-free, prescription contraceptive option – a $1B opportunity – May 25, 2020 PDUFA date (June 2020 launch anticipated*) Label expansion opportunity for prevention of STIs** – Primary indication: Chlamydia – Secondary indication: Gonorrhea U.S. and EU IP Patent Protection – Current IP extends to 2033 in the U.S. and 2034 in EU – Potential to extend IP to 2038 (pending STI indication) and beyond Future revenue opportunities – RoW partnership for Amphora – In-licensing / development of synergistic products * Pending FDA approval 4 ** Assumes future clinical trials demonstrate efficacy, safety profile ©2020©2019 Evofem Biosciences, Inc.
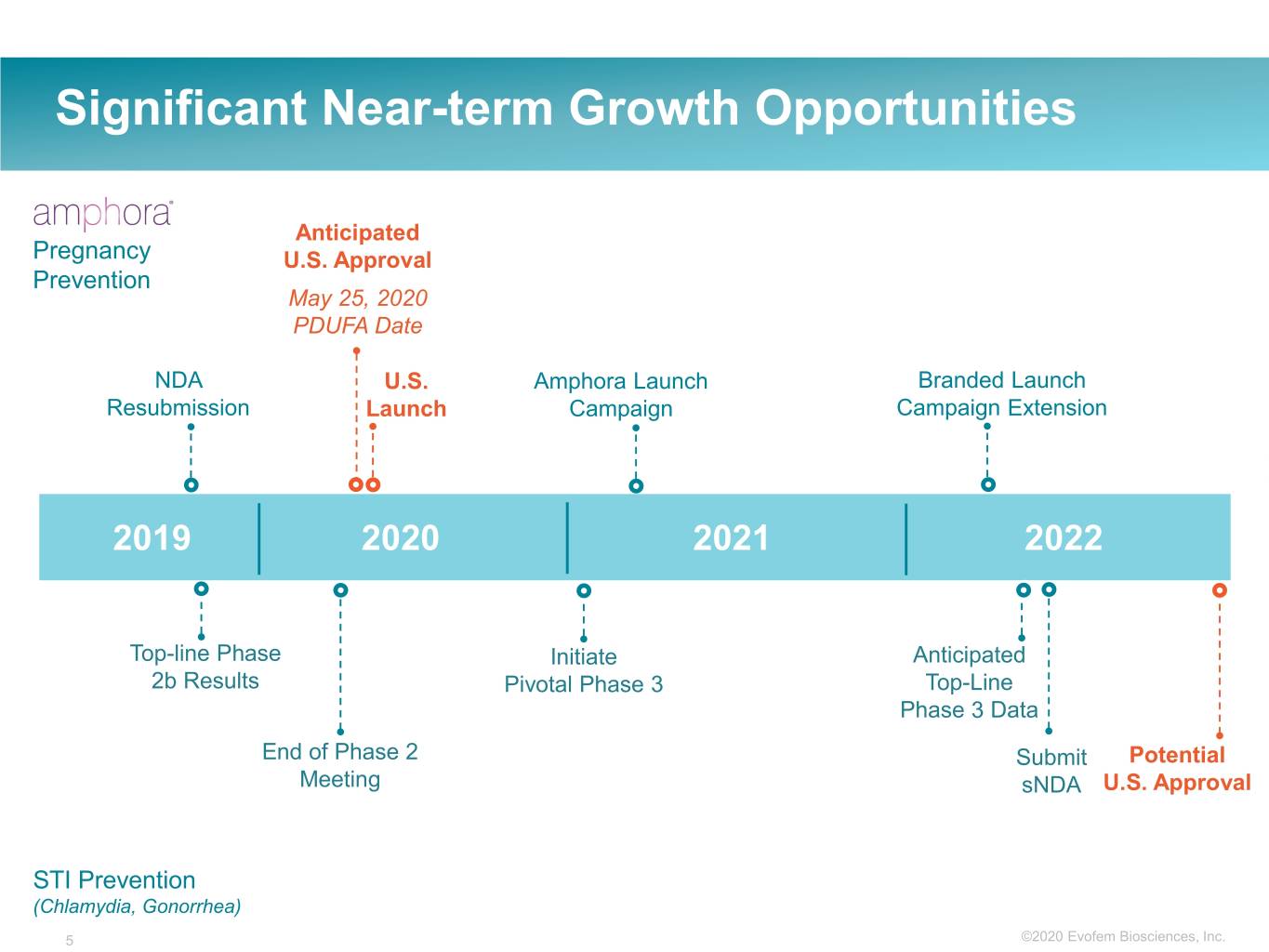
Significant Near-term Growth Opportunities Anticipated Pregnancy U.S. Approval Prevention May 25, 2020 PDUFA Date NDA U.S. Amphora Launch Branded Launch Resubmission Launch Campaign Campaign Extension 2019 2020 2021 2022 Top-line Phase Initiate Anticipated 2b Results Pivotal Phase 3 Top-Line Phase 3 Data End of Phase 2 Submit Potential Meeting sNDA U.S. Approval STI Prevention (Chlamydia, Gonorrhea) 5 ©2020©2019 Evofem Biosciences, Inc.
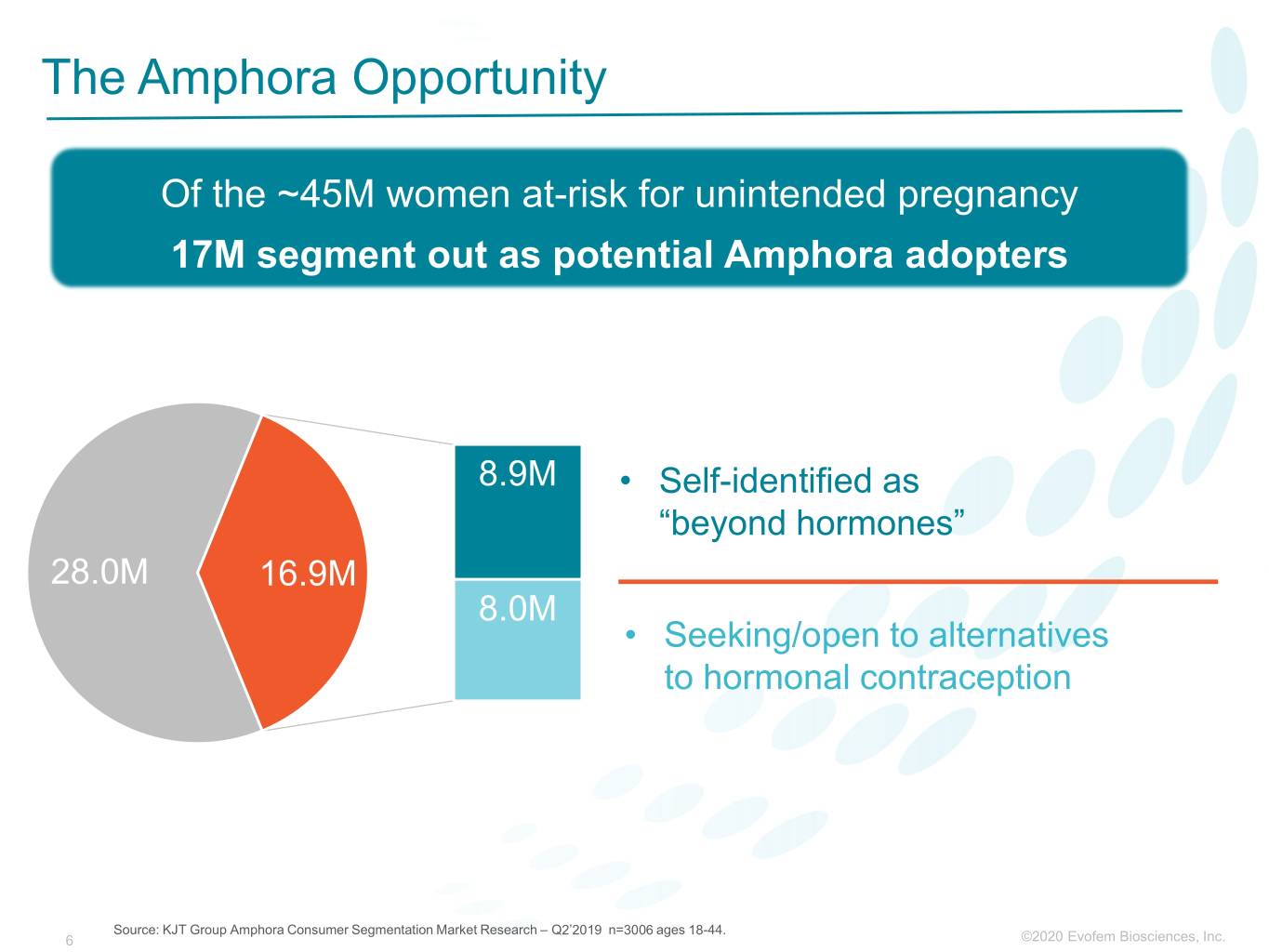
The Amphora Opportunity Of the ~45M women at-risk for unintended pregnancy 17M segment out as potential Amphora adopters 8.9M • Self-identified as “beyond hormones” 28.0M 16.9M 8.0M • Seeking/open to alternatives to hormonal contraception Source: KJT Group Amphora Consumer Segmentation Market Research – Q2’2019 n=3006 ages 18-44. 6 ©2020©2019 Evofem Biosciences, Inc.
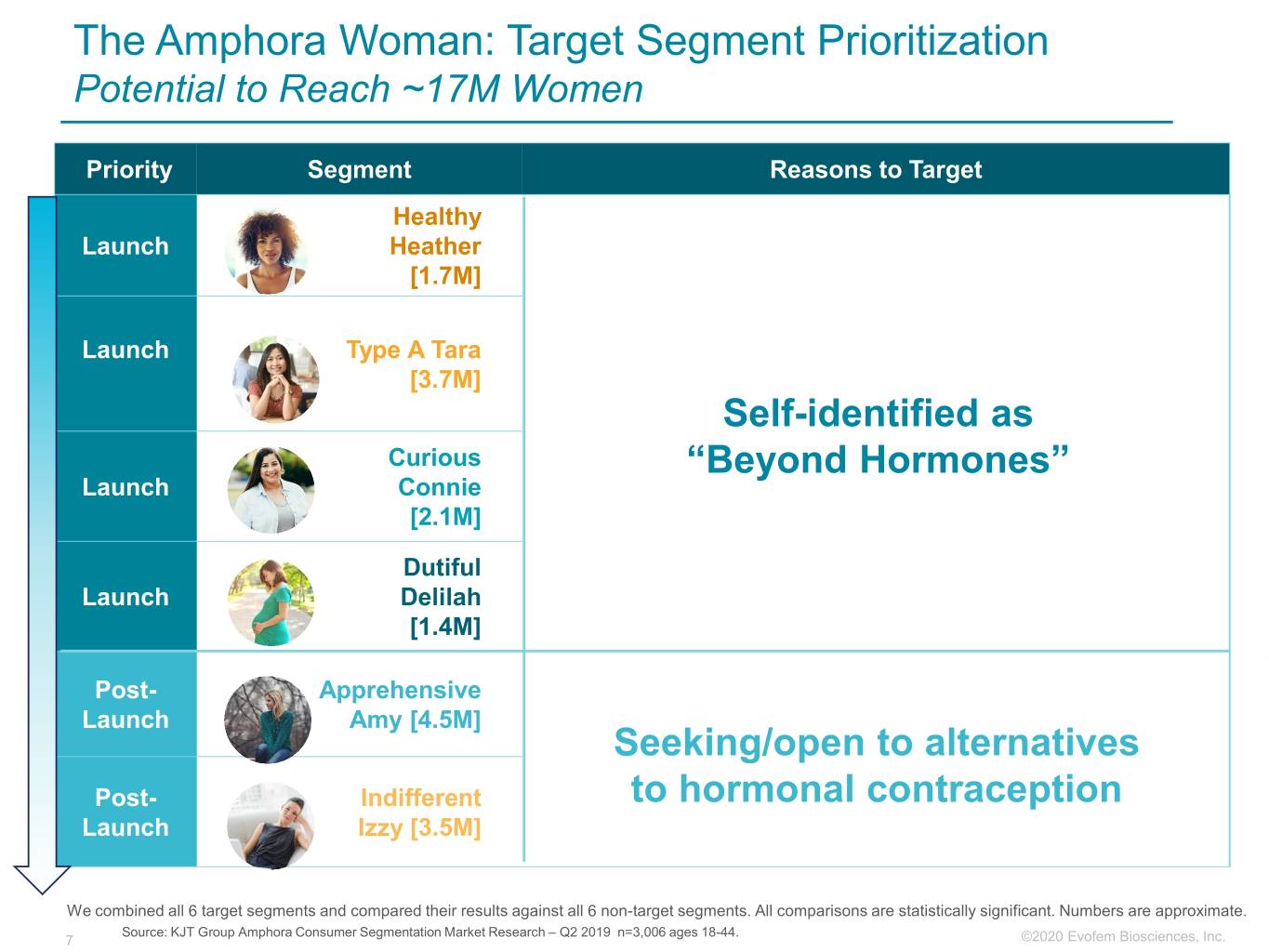
The Amphora Woman: Target Segment Prioritization Potential to Reach ~17M Women Priority Segment Reasons to Target Healthy Launch Heather [1.7M] Launch Type A Tara [3.7M] Self-identified as Curious “Beyond Hormones” Launch Connie [2.1M] Dutiful Launch Delilah [1.4M] Post- Apprehensive Launch Amy [4.5M] Seeking/open to alternatives Post- Indifferent to hormonal contraception Launch Izzy [3.5M] We combined all 6 target segments and compared their results against all 6 non-target segments. All comparisons are statistically significant. Numbers are approximate. Source: KJT Group Amphora Consumer Segmentation Market Research – Q2 2019 n=3,006 ages 18-44. 7 ©2020©2019 Evofem Biosciences, Inc.
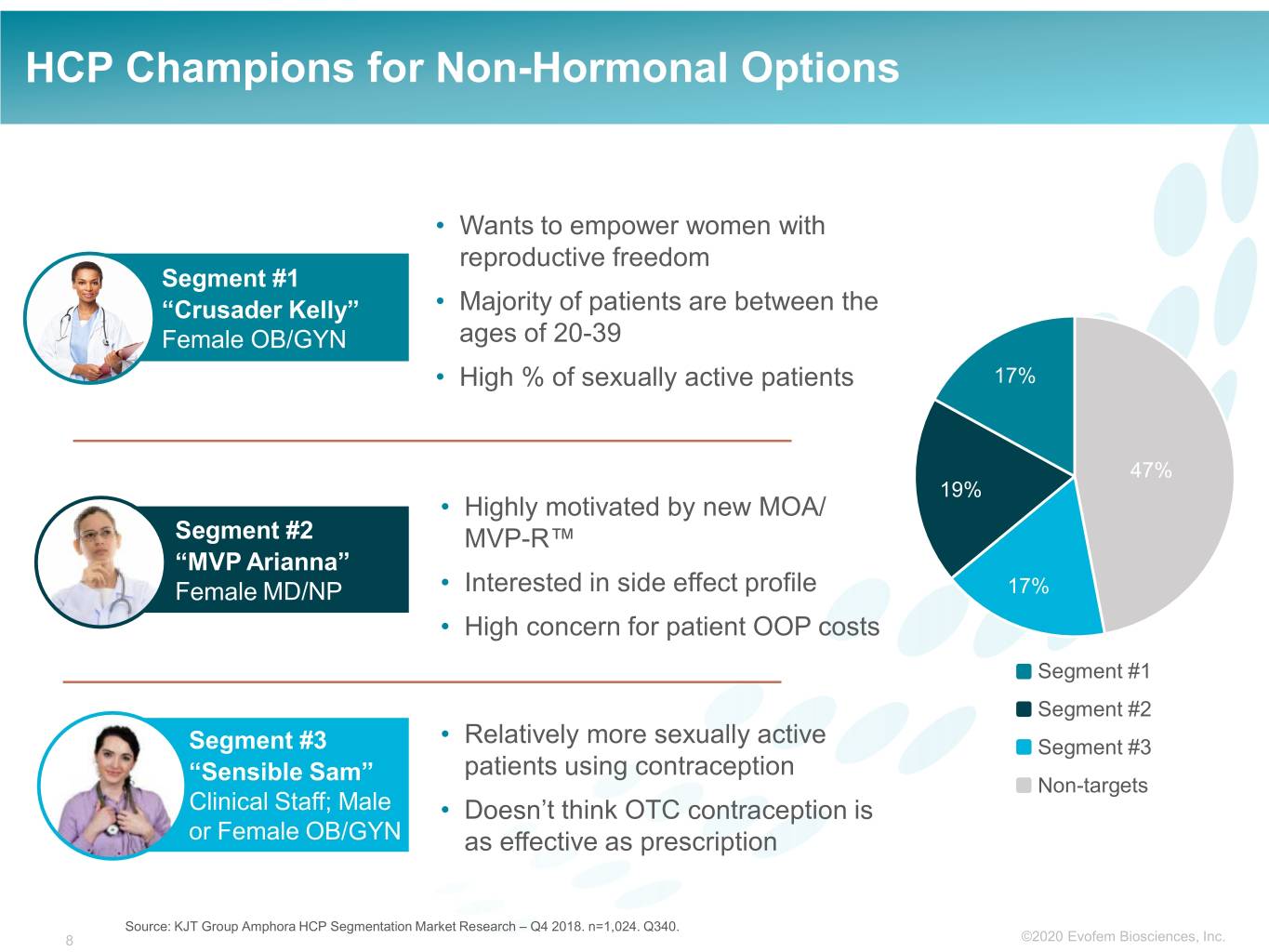
HCP Champions for Non-Hormonal Options • Wants to empower women with reproductive freedom Segment #1 “Crusader Kelly” • Majority of patients are between the Female OB/GYN ages of 20-39 • High % of sexually active patients 17% 47% 19% • Highly motivated by new MOA/ z Segment #2 MVP-R™ “MVP Arianna” Female MD/NP • Interested in side effect profile 17% • High concern for patient OOP costs Segment #1 Segment #2 Segment #3 • Relatively more sexually active Segment #3 patients using contraception “Sensible Sam” Non-targets Clinical Staff; Male • Doesn’t think OTC contraception is or Female OB/GYN as effective as prescription Source: KJT Group Amphora HCP Segmentation Market Research – Q4 2018. n=1,024. Q340. 8 ©2020©2019 Evofem Biosciences, Inc.
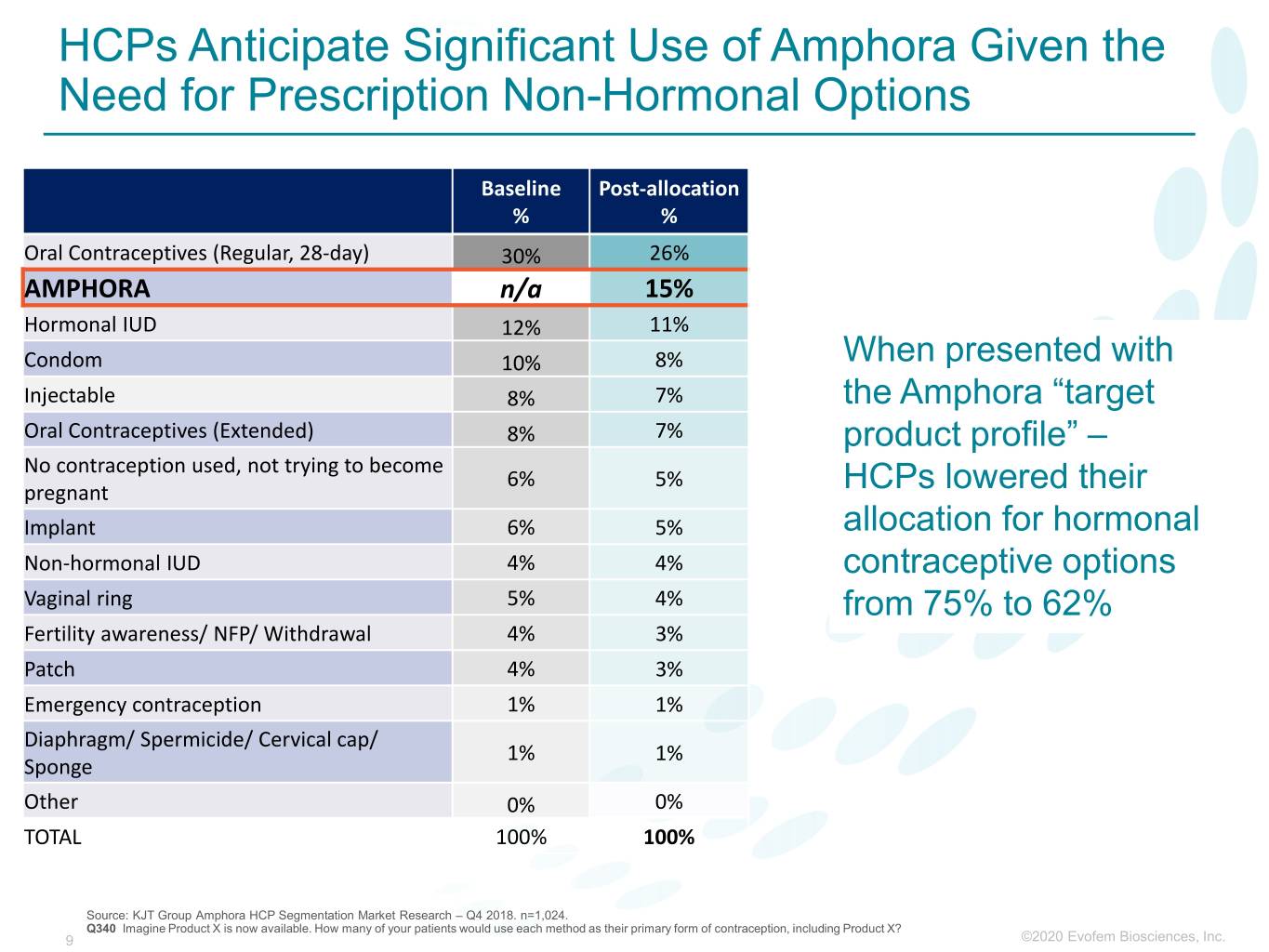
HCPs Anticipate Significant Use of Amphora Given the Need for Prescription Non-Hormonal Options Baseline Post-allocation % % Oral Contraceptives (Regular, 28-day) 30% 26% AMPHORA n/a 15% Hormonal IUD 12% 11% Condom 10% 8% When presented with Injectable 8% 7% the Amphora “target Oral Contraceptives (Extended) 8% 7% product profile” – No contraception used, not trying to become 6% 5% pregnant HCPs lowered their Implant 6% 5% allocation for hormonal Non-hormonal IUD 4% 4% contraceptive options Vaginal ring 5% 4% from 75% to 62% Fertility awareness/ NFP/ Withdrawal 4% 3% Patch 4% 3% Emergency contraception 1% 1% Diaphragm/ Spermicide/ Cervical cap/ 1% 1% Sponge Other 0% 0% TOTAL 100% 100% Source: KJT Group Amphora HCP Segmentation Market Research – Q4 2018. n=1,024. Q340 Imagine Product X is now available. How many of your patients would use each method as their primary form of contraception, including Product X? 9 ©2020©2019 Evofem Biosciences, Inc.
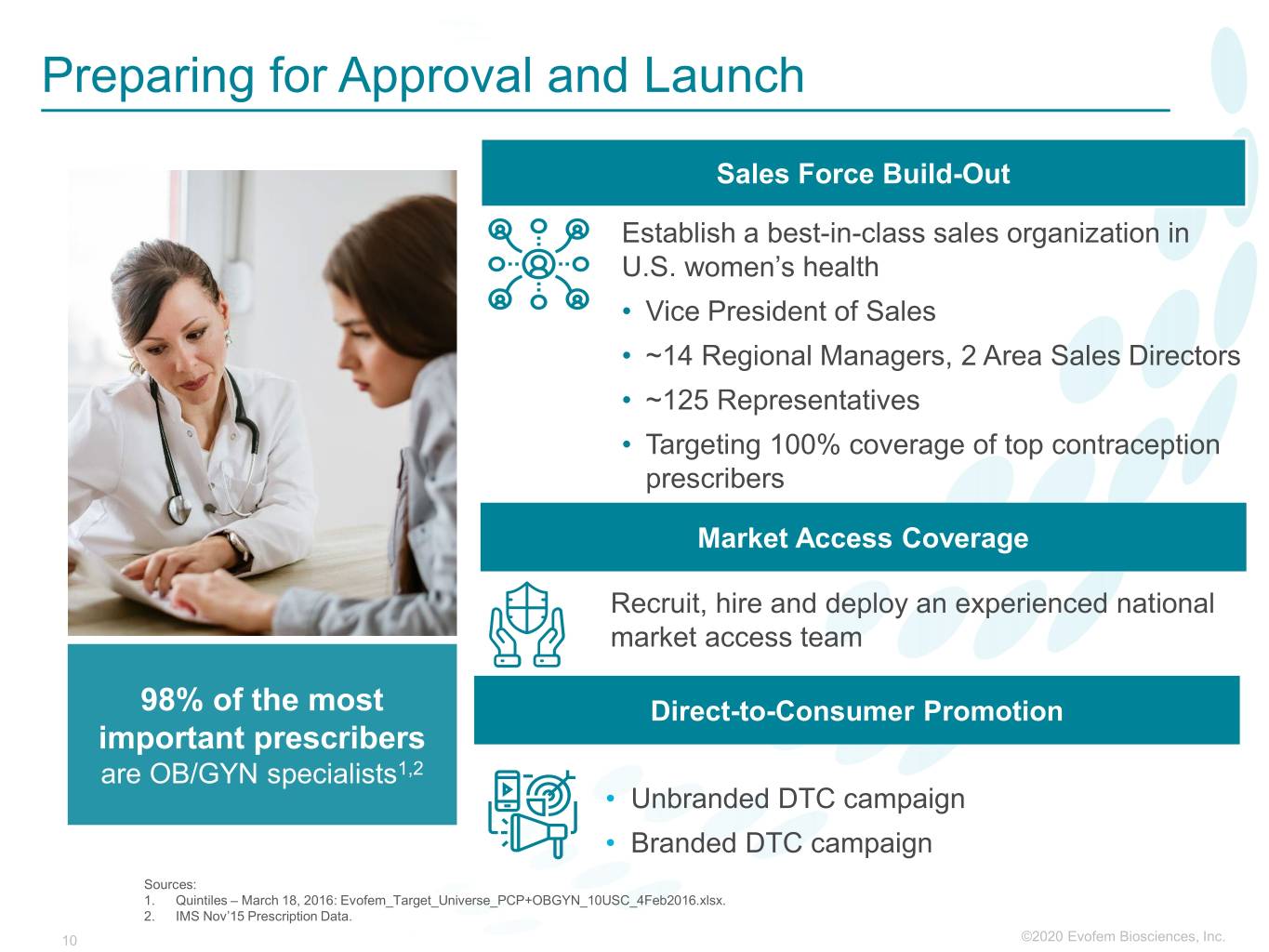
Preparing for Approval and Launch Sales Force Build-Out Establish a best-in-class sales organization in U.S. women’s health • Vice President of Sales • ~14 Regional Managers, 2 Area Sales Directors • ~125 Representatives • Targeting 100% coverage of top contraception prescribers Market Access Coverage Recruit, hire and deploy an experienced national market access team 98% of the most Direct-to-Consumer Promotion important prescribers are OB/GYN specialists1,2 • Unbranded DTC campaign • Branded DTC campaign Sources: 1. Quintiles – March 18, 2016: Evofem_Target_Universe_PCP+OBGYN_10USC_4Feb2016.xlsx. 2. IMS Nov’15 Prescription Data. 10 ©2020©2019 Evofem Biosciences, Inc.
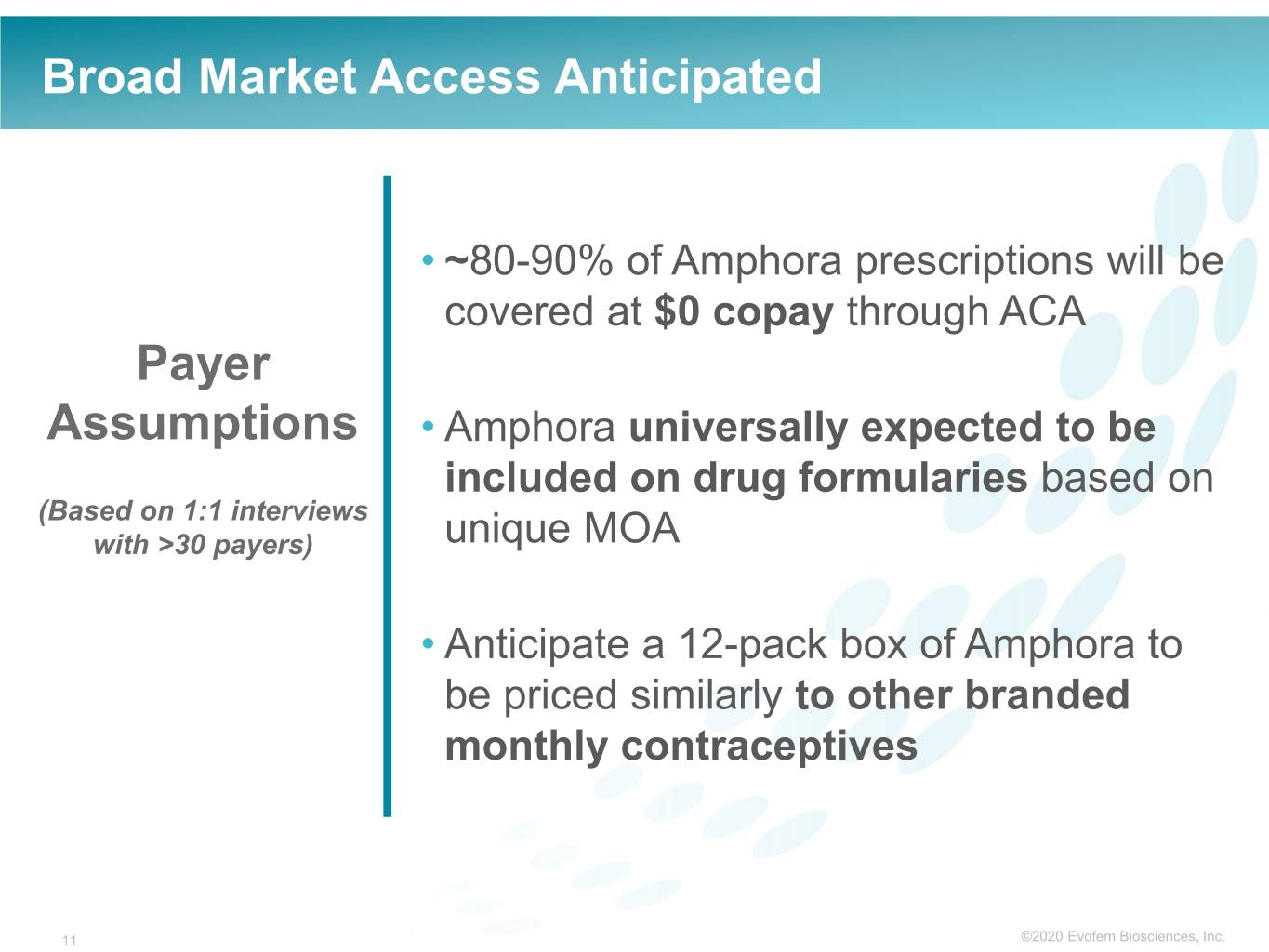
Broad Market Access Anticipated • ~80-90% of Amphora prescriptions will be covered at $0 copay through ACA Payer Assumptions • Amphora universally expected to be included on drug formularies based on (Based on 1:1 interviews with >30 payers) unique MOA • Anticipate a 12-pack box of Amphora to be priced similarly to other branded monthly contraceptives 11 ©2020©2019 Evofem Biosciences, Inc.
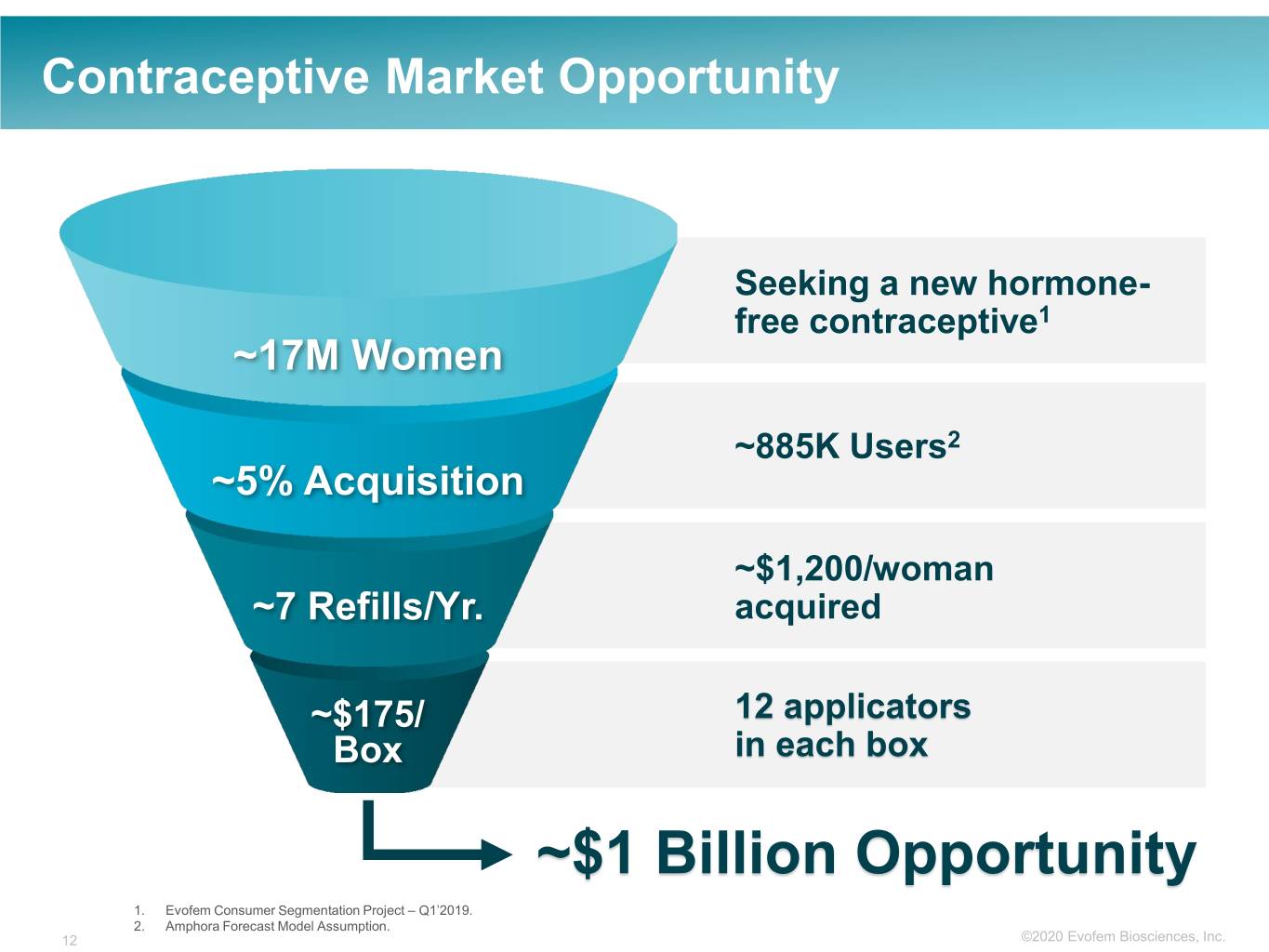
Contraceptive Market Opportunity Seeking a new hormone- free contraceptive1 ~17M Women ~885K Users2 ~5% Acquisition ~$1,200/woman ~7 Refills/Yr. acquired ~$175/ 12 applicators Box in each box ~$1 Billion Opportunity 1. Evofem Consumer Segmentation Project – Q1’2019. 2. Amphora Forecast Model Assumption. 12 ©2020©2019 Evofem Biosciences, Inc.

Amphora for the Prevention of Pregnancy First-in-Class MOA Non-hormonal Female-controlled “On Demand” Multipurpose Vaginal pH Regulator (MVP-R™) 13 ©2020©2019 Evofem Biosciences, Inc.
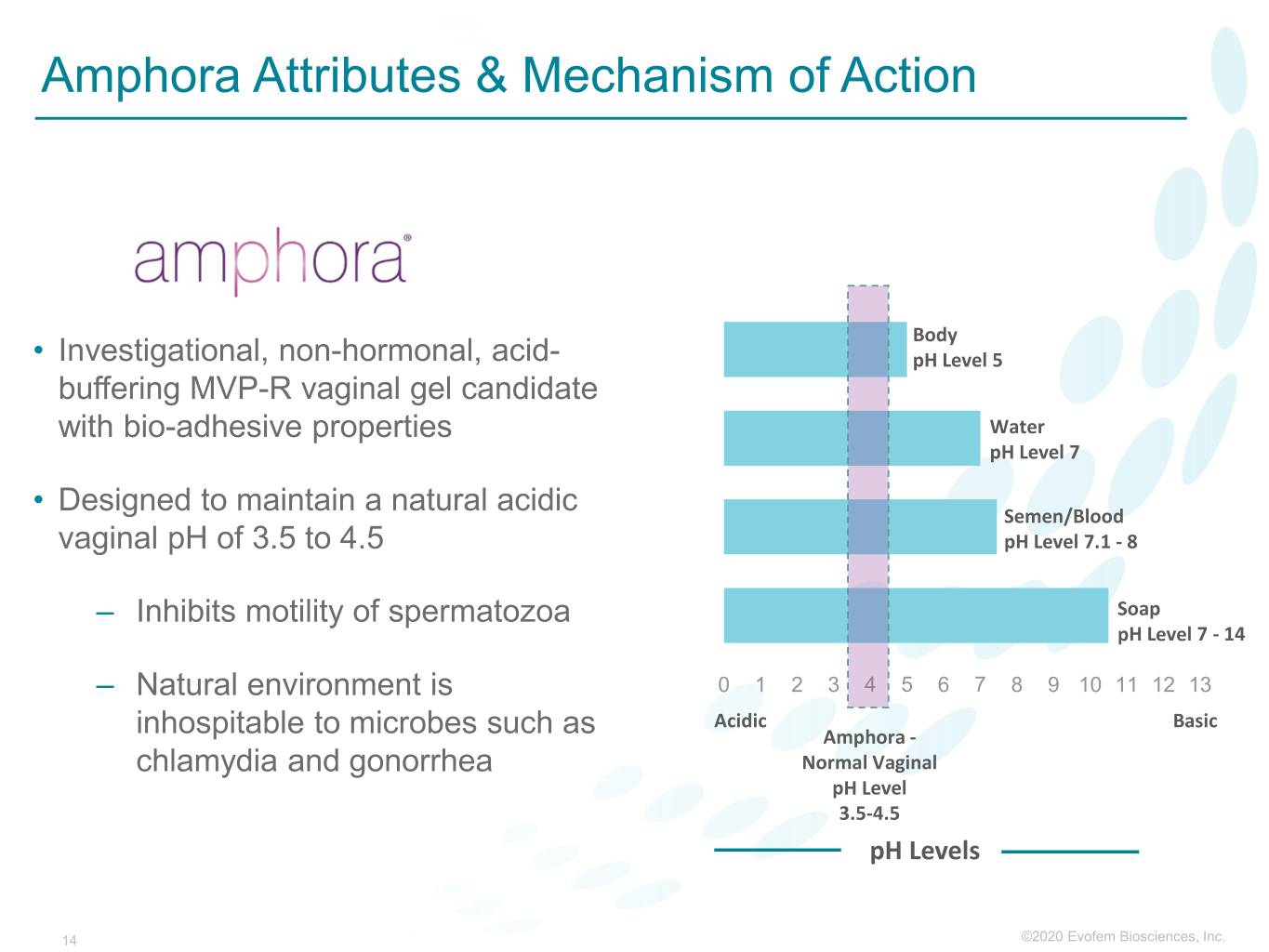
Amphora Attributes & Mechanism of Action Body • Investigational, non-hormonal, acid- pH Level 5 buffering MVP-R vaginal gel candidate with bio-adhesive properties Water pH Level 7 • Designed to maintain a natural acidic Semen/Blood vaginal pH of 3.5 to 4.5 pH Level 7.1 - 8 – Inhibits motility of spermatozoa Soap pH Level 7 - 14 – Natural environment is 0 1 2 3 4 5 6 7 8 9 10 11 12 13 Acidic Basic inhospitable to microbes such as Amphora - chlamydia and gonorrhea Normal Vaginal pH Level 3.5-4.5 pH Levels 14 ©2020©2019 Evofem Biosciences, Inc.

Significant Key Milestones Achieved Resubmitted Amphora NDA to FDA for prevention of pregnancy – First-in-class, hormone-free, female-controlled, on-demand contraceptive – May 25, 2020 PDUFA date – Accelerating pre-commercial activities in anticipation of June launch Reported positive top-line results from AMPREVENCE trial of Amphora for prevention of chlamydia & gonorrhea – Met primary and secondary endpoints with high statistical significance – End-of-Phase 2 meeting expected 1H 2020 – Initiation of single, pivotal Phase 3 trial anticipated in Q1 2021 – Potential for U.S. FDA approval in 2022 15 ©2020©2019 Evofem Biosciences, Inc.
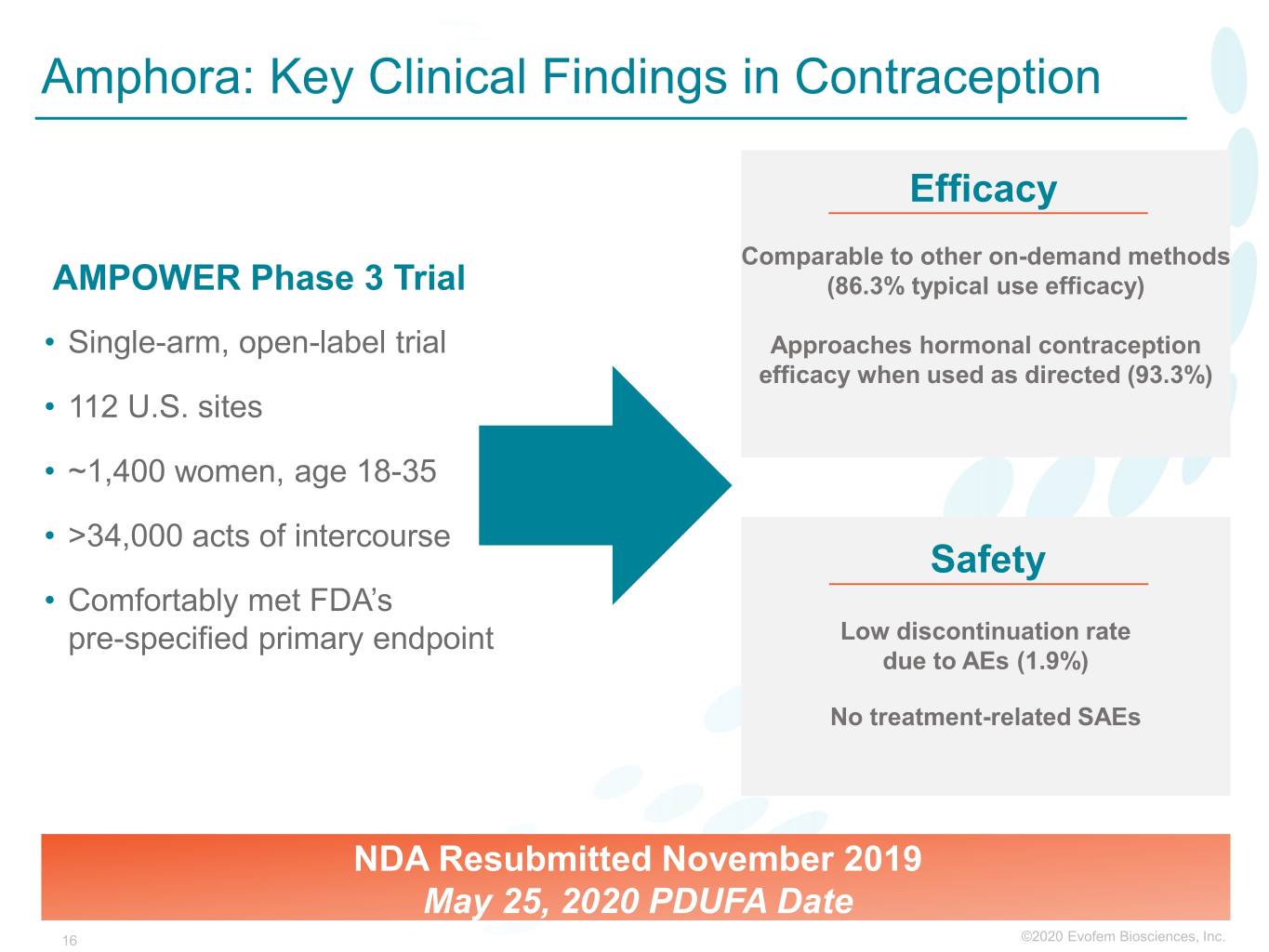
Amphora: Key Clinical Findings in Contraception Efficacy Comparable to other on-demand methods AMPOWER Phase 3 Trial (86.3% typical use efficacy) • Single-arm, open-label trial Approaches hormonal contraception efficacy when used as directed (93.3%) • 112 U.S. sites • ~1,400 women, age 18-35 • >34,000 acts of intercourse Safety • Comfortably met FDA’s pre-specified primary endpoint Low discontinuation rate due to AEs (1.9%) No treatment-related SAEs NDA Resubmitted November 2019 May 25, 2020 PDUFA Date 16 ©2020©2019 Evofem Biosciences, Inc.
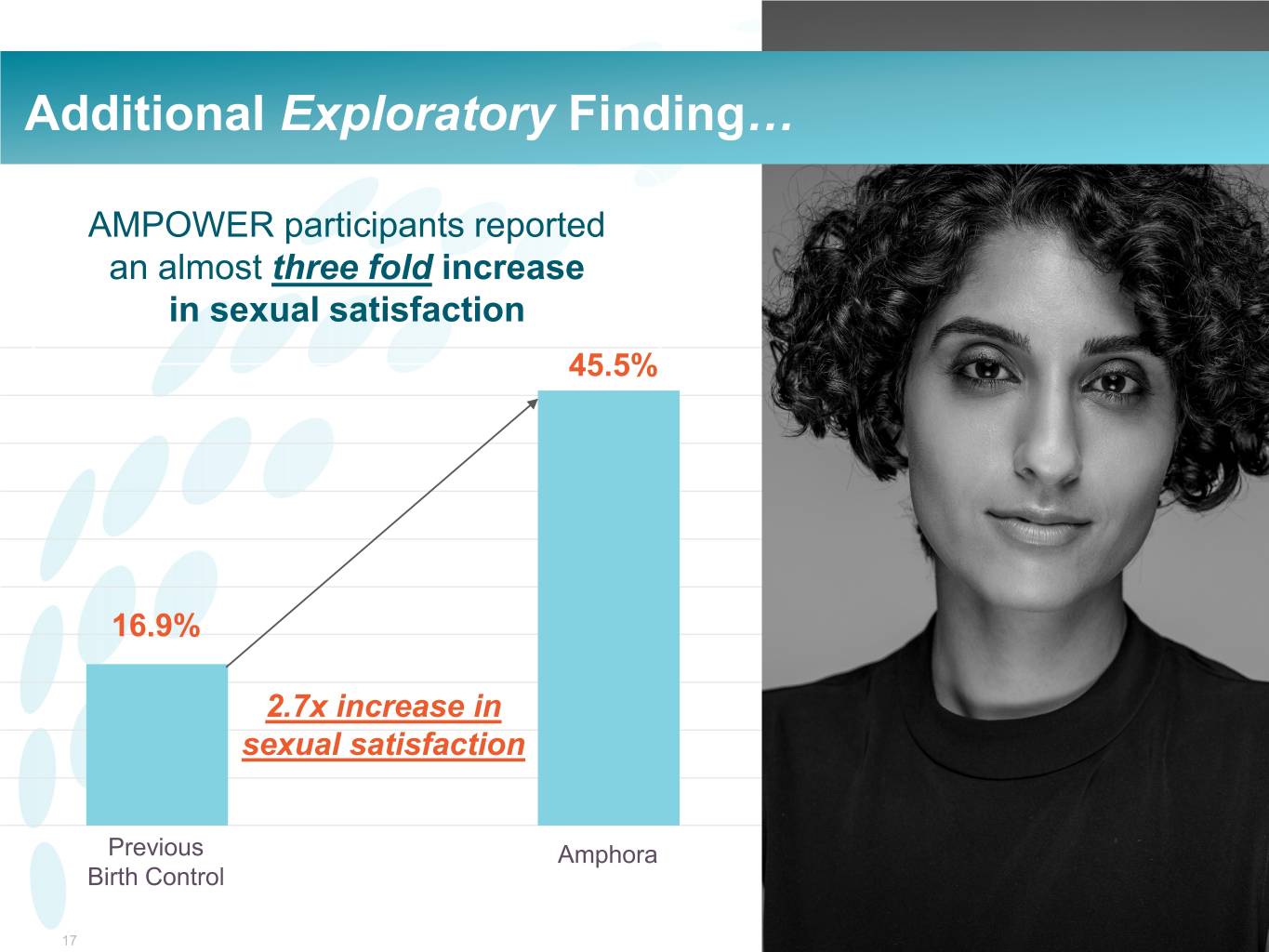
Additional Exploratory Finding… AMPOWER participants reported an almost three fold increase in sexual satisfaction 45.5% 16.9% 2.7x increase in sexual satisfaction Previous Amphora Birth Control 17 ©2020©2019 Evofem Biosciences, Inc.
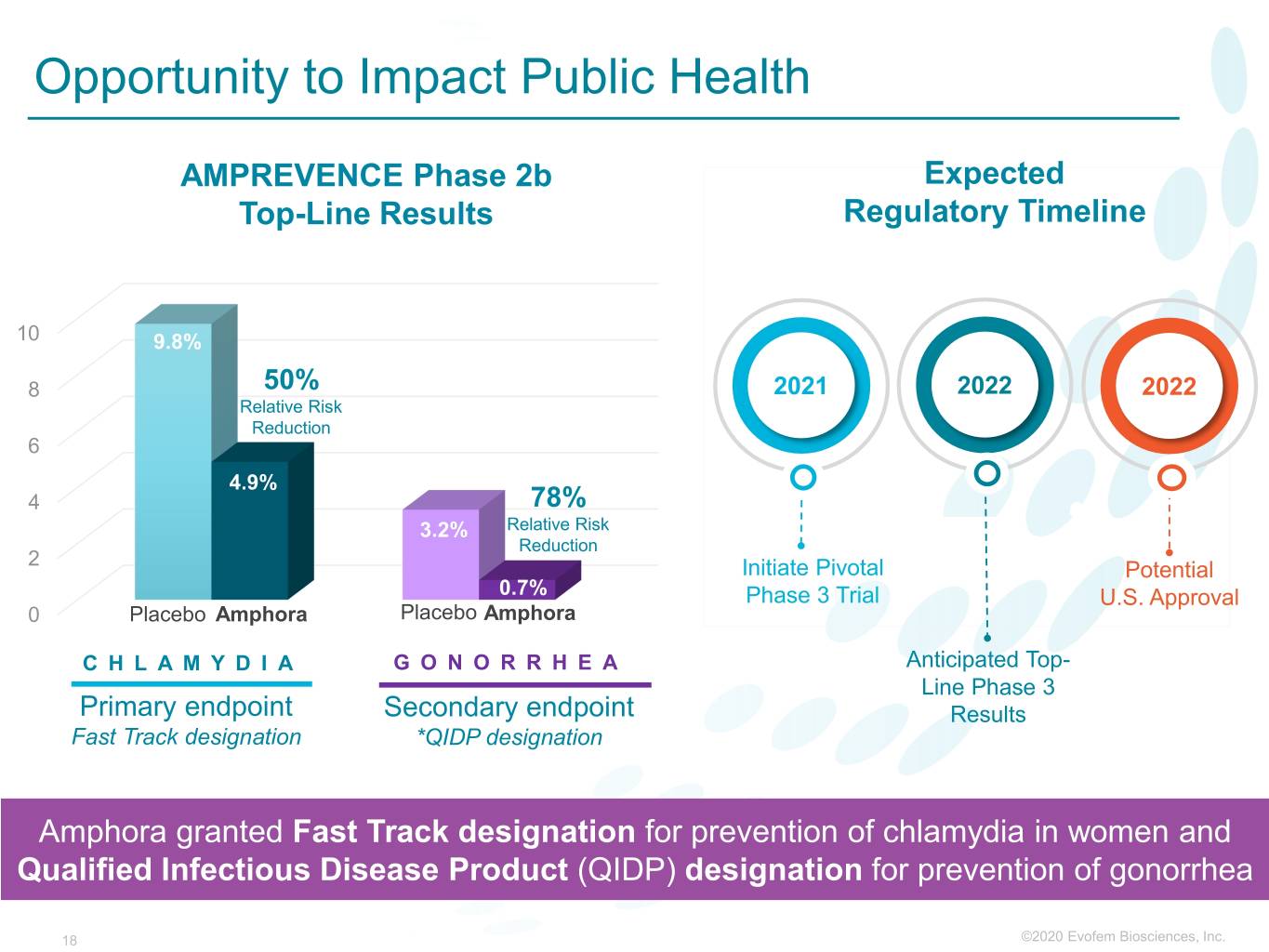
Opportunity to Impact Public Health Expected AMPREVENCE Phase 2b 1.7M Annually1 Top-Line Results AddressableRegulatory Population Timeline 10 9.8% 8 50% 2021 2022 2022 Relative Risk Reduction 6 4.9% 4 78% 3.2% Relative Risk Reduction 2 Initiate Pivotal Potential 0.7% Phase 3 Trial U.S. Approval 0 Placebo Amphora Placebo Amphora CHLAMYDIA GONORRHEA Anticipated Top- Line Phase 3 Primary endpoint Secondary endpoint Results Fast Track designation *QIDP designation Amphora granted Fast Track designation for prevention of chlamydia in women and Qualified Infectious Disease Product (QIDP) designation for prevention of gonorrhea 18 ©2020©2019 Evofem Biosciences, Inc.

OUR TIME IS NOW! There exists a significant unmet need in a market starved for innovation, and we have… An innovative, first-in-class product Compelling data The experience and the expertise And…we have the VISION 1919 ©2020©2019 Evofem Biosciences, Inc.

® 20 ©2020©2019 Evofem Biosciences, Inc.
