UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the
Securities Exchange Act of 1934
Date of Report (Date of earliest event reported)
December 30, 2019
Aridis Pharmaceuticals, Inc.
(Exact name of registrant as specified in its charter)
| Delaware | 001-38630 | 47-2641188 | ||
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (I. R. S. Employer Identification No.) |
5941 Optical Ct.
San Jose, California 95138
(Address of principal executive offices, including ZIP code)
(408) 385-1742
(Registrant’s telephone number, including area code)
(Former name or former address, if changed since last report)
Securities registered pursuant to Section 12(b) of the Act:
| Title of each class: | Trading Symbol(s) | Name of each exchange on which registered: | ||
| Common Stock | ARDS | Nasdaq Capital Market |
Check the appropriate box below if the Form 8-K filing is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
o Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425)
o Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12)
o Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b))
¨ Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c))
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter).
Emerging growth company x
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
| Item 7.01 | Regulation FD Disclosure |
On December 30, 2019, Aridis Pharmaceuticals, Inc. (the “Company”) announced the development of a novel monoclonal antibody (“mAb”) discovery, antibody production and yield maximizing technology called APEXTM and a collaboration with Mapp Biopharmaceutical, Inc, a company incorporated in the U.S. (“MappBio”).
APEXTM is a technology suite comprising of a monoclonal antibody discovery platform technology that enables the screening and single cell cloning of antibody producing B-cells using microarrays (‘APEXTM NanoArrays’), a proprietary mAb cell line technology called BREATHTM CHO that enables gene editing-assisted gene swapping of the candidate antibody genes with endogenous versions to generate a high mAb productivity cell line, and a gene editing-based technology to activate endogenous genes in antibody production cell lines to increase mAb production. A schematic representation of APEXTM is shown in figure below.
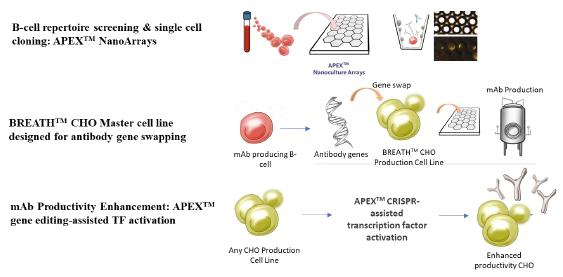
APEXTM builds upon the Company’s original hybridoma-based technology platform, MabIgX®, which allows for rapid fusion and production of mAbs directly from patients antibody-producing B-cells.
MappBio agreed to enter into research collaborations with the Company whereby the Company will apply its APEXTM technology to develop mAb productivity enhanced versions of an undisclosed number of CHO cell lines that were originally developed by MappBio.
The information in this Item 7.01 shall not be deemed “filed” for purposes of Section 18 of the Securities Exchange Act of 1934, as amended (the “Exchange Act”), or otherwise subject to the liabilities of that section, nor shall it be deemed incorporated by reference in any filing under the Securities Act of 1933, as amended, or the Exchange Act, except as expressly set forth by specific reference in such a filing.
-2-
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| Date: December 30, 2019 | ARIDIS PHARMACEUTICALS, INC. |
| /s/ Vu Truong | |
| Vu Truong | |
| Chief Executive Officer |
-3-
