Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - EYENOVIA, INC. | tv511916_ex99-1.htm |
| 8-K - FORM 8-K - EYENOVIA, INC. | tv511916_8k.htm |
Exhibit 99.2

Making it Possible MIST - 1 Topline Results January 30 th , 2019
Except for historical information, all of the statements, expectations, and assumptions contained in this presentation are forward - looking statements . Forward - looking statements include, but are not limited to, statements that express our intentions, beliefs, expectations, strategies, predictions or any other statements relating to our future activities or other future events or conditions . These statements are based on current expectations, estimates and projections about our business based, in part, on assumptions made by management . These statements are not guarantees of future performance and involve risks, uncertainties and assumptions that are difficult to predict . Therefore, actual outcomes and results may, and are likely to, differ materially from what is expressed or forecasted in the forward - looking statements due to numerous factors discussed from time to time in documents which we file with the SEC . In addition, such statements could be affected by risks and uncertainties related to, among other things : risks involved in clinical trials, including, but not limited to, the initiation, timing, progress and results of such trials ; the timing and our ability to submit applications for, and obtain and maintain regulatory approvals for, our product candidates and to raise money, including in light of U . S . government shut - downs ; our ability to develop and implement commercialization, marketing and manufacturing capabilities and strategies ; the potential advantages of our product candidates ; the rate and degree of market acceptance and clinical utility of our product candidates ; our estimates regarding the potential market opportunity for our product candidates ; intellectual property risks ; the impact of government laws and regulations ; and our competitive position . Any forward - looking statements speak only as of the date on which they are made, and except as may be required under applicable securities laws, we do not undertake any obligation to update any forward - looking statements . 1 Forward - Looking Statements

• Pharmacologic mydriasis, or dilation of the pupil of the eye with drugs, is the cornerstone of today’s ophthalmic/optometric care • Indispensable part of: – Comprehensive Eye Exam – Diabetic Retinal Exam – Macular Degeneration Retinal Exam – Retinopathy of Prematurity Screening • Current eyedropper paradigm can use two medications which can overdose the eye (100µL vs 7 µL) 1 – Phenylephrine 2.5% 1 - 2 drops – Tropicamide 1% 1 - 2 drops • Can be inefficient and result in patient discomfort…and increased systemic/ocular exposure 2 Pharmacologic Mydriasis: ~80M office administrations & ~4M surgical per year in the United States 1 Bhurayanontachai, P., Saengkaew, S., & Apiromruck, P. (2016). Efficacy of an eye drop mixture for pupillary dilatation: A randomized comparative study. Journal of optometry, 10(2), 111 - 116.
Why should we be concerned about overdosing with eye drops? • Many eye drugs are compounds that were designed for cardiovascular or other systemic effects (beta blockers, phenylephrine and others) • When these drugs are overdosed to eye, they can seep into systemic circulation through periocular absorption and via nasolacrimal duct (bypassing liver metabolism, similar to IV delivery) • The result can be changes in blood pressure, heart rate and lung function 3 “Mean blood pressure increased significantly in infants given standard dilating drops..” 1 “Cardiovascular effects of ophthalmic Timolol” 2 1 Acta Ophthalmol Scand. 1997 2 Ann Intern Med. 1986;104(2):197 – 198

Designed for the estimated 80 million mydriatic exams performed every year in the United States and the estimated 4 million pharmacologic mydriasis applications for cataract surgery • Current eyedropper paradigm is approximately 100 year old • Legacy technology can be inefficient, wasteful and imprecise • Eyedroppers overdose the ocular surface by more than 300% 1 - 3 • Overdosing of drug (30 - 50µL vs physiologic 7 µL tear film capacity) 1 - 3 • Overdosing of preservative and excipients which are known in higher quantities to be toxic to the ocular surface (>90% of ophthalmic formulations) 4 4 MicroStat 1 Washington N, Washington C, Wilson CG. Ocular drug delivery. In: Physiological Pharmaceutics: Barriers to Drug Absorption. 2n d ed. Boca Raton, FL: CRC Press; 2001:249 – 270. 2 Mishima S, Gasset A, Klyce SD, Baum JL. Determination of tear volume and tear flow. Invest Ophthalmol. 1966;5(3):264 – 276. 3 Scherz W, Doane MG, Dohlman CH. Tear volume in normal eyes and keratoconjunctivitis sicca. Albrecht Von Graefes Arch Klin Exp Op hthalmol. 1974;192(2):141 – 150. 4 Epstein et al, J Ocul Pharmacol Ther, 2009
• Eyenovia’s OpteJet ™ technology is designed to revolutionize ophthalmic drug delivery • We believe our piezo - print micro - dosing can increase efficiency, precision and reduce waste • The Optejet aims to eliminate ocular overdose to the ocular surface 5 MicroStat Smart … Precise … Brilliant

6 Eyenovia’s Optejet: 21st century platform for smarter eyecare…

• Comparable clinical efficacy with ¼ the dose of traditional eye drops • Significant improvement in tolerability and systemic exposure for same formulation microdose versus eye drops 7 EYE 102: Phase II Trial demonstrated the advantages Eyenovia’s microdosing approach 4x PE Eye Drop 1x PE µD ( p=0.009 ) Adverse Event PE 10% EYN - 1601 Ocular blurriness 1 0 Ocular burning/stinging/irritation 4 1 Ocular dryness 2 0 Subtotal by Treatment Group 7 1 Ocular Adverse Events by Treatment

• Double - masked, active - controlled, cross - over design • MIST - 1 (N64 randomized):µD phenylephrine - tropicamide vs µD tropicamide vs µD phenylephrine • MIST - 2 (N70 randomized):µD phenylephrine - tropicamide VS µD placebo 8 MicroStat Registration Program: Two Phase 3 Superiority Studies (MIST - 1 and MIST - 2) • Primary endpoint: Mean change in pupil diameter at 35 minutes vs baseline • Hypothesis: MicroStat superior to Phen, Trop (MIST - 1) and placebo eye wash (MIST - 2) • Power at 90% for each study, assuming 54 evaluable subjects 35’ 20’ MIST - 1 MIST - 2 35’ 20’

1. 64 subjects randomized 2. MIST - 1 met primary efficacy endpoint: pupil dilation change from Baseline at 35 minutes 3. Statistically larger 35 minute dilation for MicroStat vs components 4. Additional outcomes: • 94% of eyes achieved 6 mm or greater pupil dilation at 35 minutes compared with 78% and 1.6% for the tropicamide - only and phenylephrine - only groups, respectively • 57% of the MicroStat - treated eyes achieved 6 mm dilation or greater at 20 minutes versus 38% of the tropicamide treated eyes and none in the phenylephrine treated eyes 9 MIST - 1 Results: Primary Endpoint Analysis
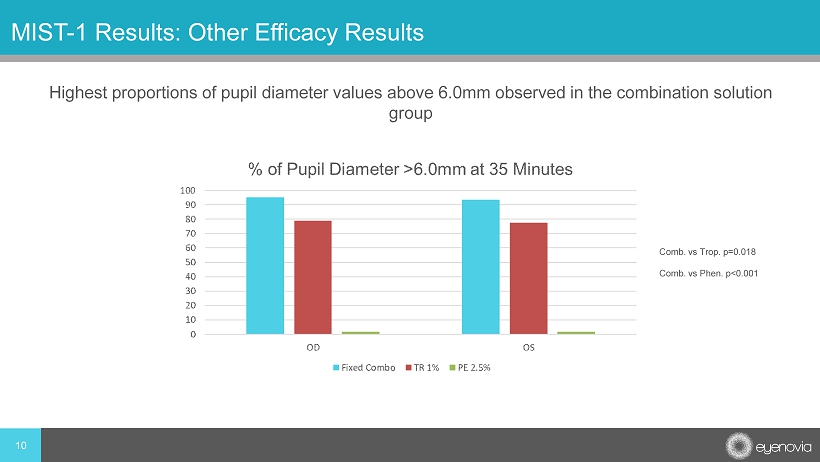
Highest proportions of pupil diameter values above 6.0mm observed in the combination solution group 10 MIST - 1 Results: Other Efficacy Results Comb. vs Trop. p=0.018 Comb. vs Phen . p<0.001

• Treatment emergent adverse events were ocular, related to mydriasis, mild and transient • No non - ocular adverse events 11 MIST - 1 Results: Safety Results

Making it Possible MIST - 1 Topline Results January 30 th , 2019
