Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - TYME TECHNOLOGIES, INC. | d678702dex991.htm |
| 8-K - FORM 8-K - TYME TECHNOLOGIES, INC. | d678702d8k.htm |

January 2019 NASDAQ: TYME Exhibit 99.2
TYME IS AN EMERGING BIOTECHNOLOGY COMPANY COMMITTED TO THE DISCOVERY, DEVELOPMENT AND DELIVERY OF INNOVATIVE METABOLIC-BASED THERAPIES ADVANCING MEDICAL INNOVATION THAT HELPS CANCER PATIENTS LIVE LONGER BETTER LIVES AND REDUCING THE BURDEN ON HEALTHCARE


Overview Steve Hoffman, Chairman & CEO Ben R. Taylor, President & CFO

Benchmarking 3rd Line Pancreatic Cancer Michele Korfin, Chief Commercial Officer
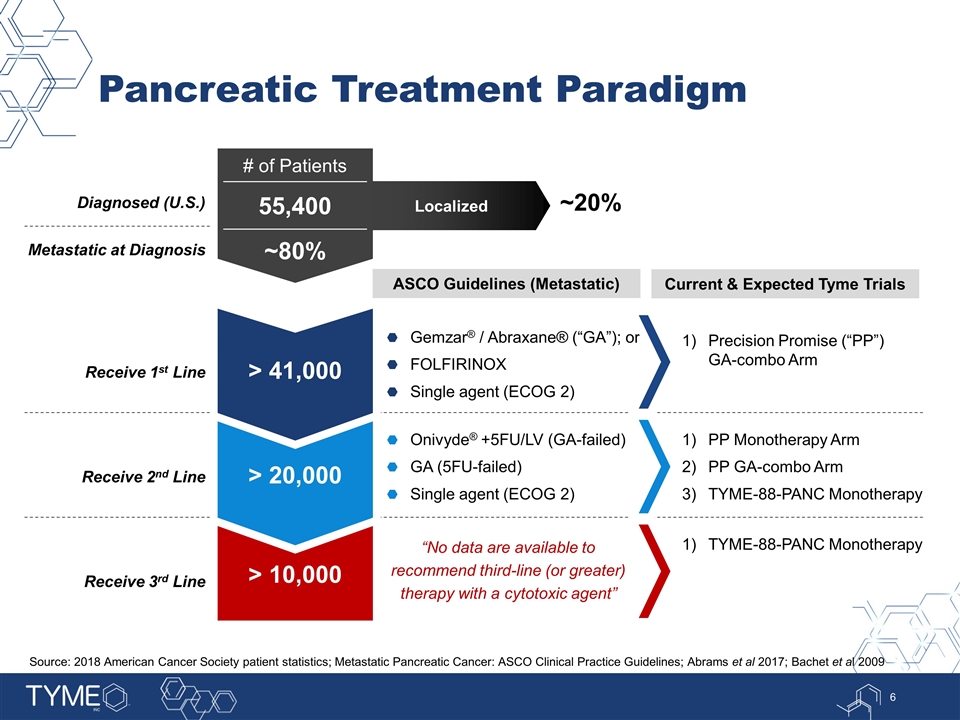
> 10,000 Receive 3rd Line TYME-88-PANC Monotherapy > 20,000 Receive 2nd Line Onivyde® +5FU/LV (GA-failed) GA (5FU-failed) Single agent (ECOG 2) PP Monotherapy Arm PP GA-combo Arm TYME-88-PANC Monotherapy > 41,000 Receive 1st Line Gemzar® / Abraxane® (“GA”); or FOLFIRINOX Single agent (ECOG 2) Precision Promise (“PP”) GA-combo Arm Pancreatic Treatment Paradigm Diagnosed (U.S.) ASCO Guidelines (Metastatic) Metastatic at Diagnosis # of Patients 55,400 ~80% Localized ~20% “No data are available to recommend third-line (or greater) therapy with a cytotoxic agent” Source: 2018 American Cancer Society patient statistics; Metastatic Pancreatic Cancer: ASCO Clinical Practice Guidelines; Abrams et al 2017; Bachet et al 2009 Current & Expected Tyme Trials
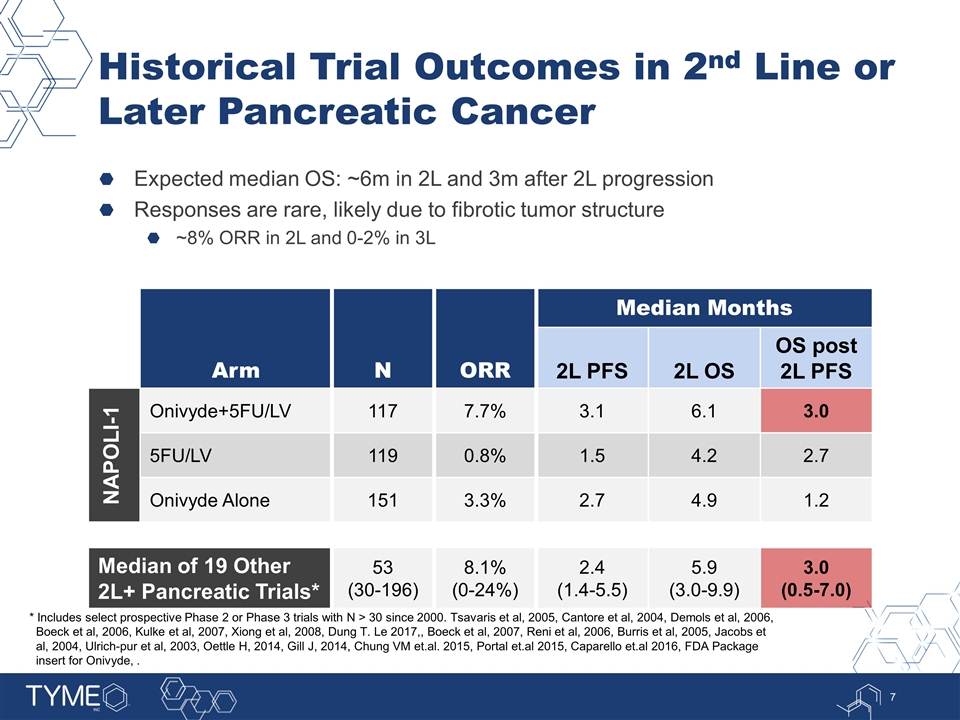
Historical Trial Outcomes in 2nd Line or Later Pancreatic Cancer Expected median OS: ~6m in 2L and 3m after 2L progression Responses are rare, likely due to fibrotic tumor structure ~8% ORR in 2L and 0-2% in 3L Arm N ORR Median Months 2L PFS 2L OS OS post 2L PFS NAPOLI-1 Onivyde+5FU/LV 117 7.7% 3.1 6.1 3.0 5FU/LV 119 0.8% 1.5 4.2 2.7 Onivyde Alone 151 3.3% 2.7 4.9 1.2 Median of 19 Other 2L+ Pancreatic Trials* 53 (30-196) 8.1% (0-24%) 2.4 (1.4-5.5) 5.9 (3.0-9.9) 3.0 (0.5-7.0) * Includes select prospective Phase 2 or Phase 3 trials with N > 30 since 2000. Tsavaris et al, 2005, Cantore et al, 2004, Demols et al, 2006, Boeck et al, 2006, Kulke et al, 2007, Xiong et al, 2008, Dung T. Le 2017,, Boeck et al, 2007, Reni et al, 2006, Burris et al, 2005, Jacobs et al, 2004, Ulrich-pur et al, 2003, Oettle H, 2014, Gill J, 2014, Chung VM et.al. 2015, Portal et.al 2015, Caparello et.al 2016, FDA Package insert for Onivyde, .
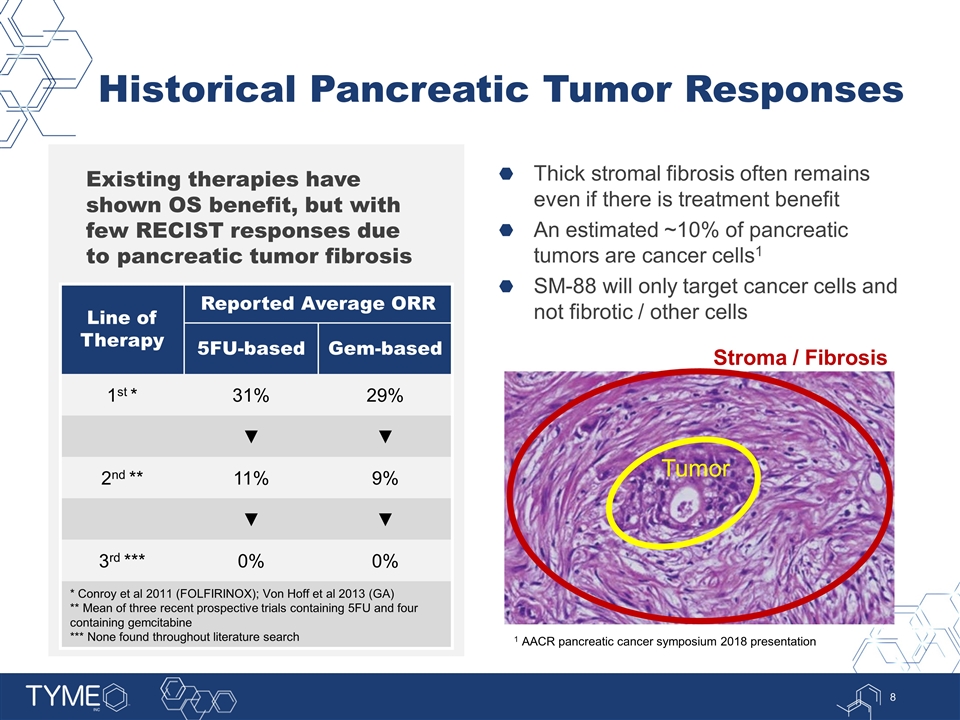
Historical Pancreatic Tumor Responses Existing therapies have shown OS benefit, but with few RECIST responses due to pancreatic tumor fibrosis Thick stromal fibrosis often remains even if there is treatment benefit An estimated ~10% of pancreatic tumors are cancer cells1 SM-88 will only target cancer cells and not fibrotic / other cells Line of Therapy Reported Average ORR 5FU-based Gem-based 1st * 31% 29% ▼ ▼ 2nd ** 11% 9% ▼ ▼ 3rd *** 0% 0% * Conroy et al 2011 (FOLFIRINOX); Von Hoff et al 2013 (GA) ** Mean of three recent prospective trials containing 5FU and four containing gemcitabine *** None found throughout literature search Tumor Stroma / Fibrosis 1 AACR pancreatic cancer symposium 2018 presentation

88-Panc preliminary results Dr. Vincent Picozzi, Director of the Pancreas Center of Excellence at Virginia Mason Medical Center Dr. Giuseppe Del Priore, Chief Medical Officer
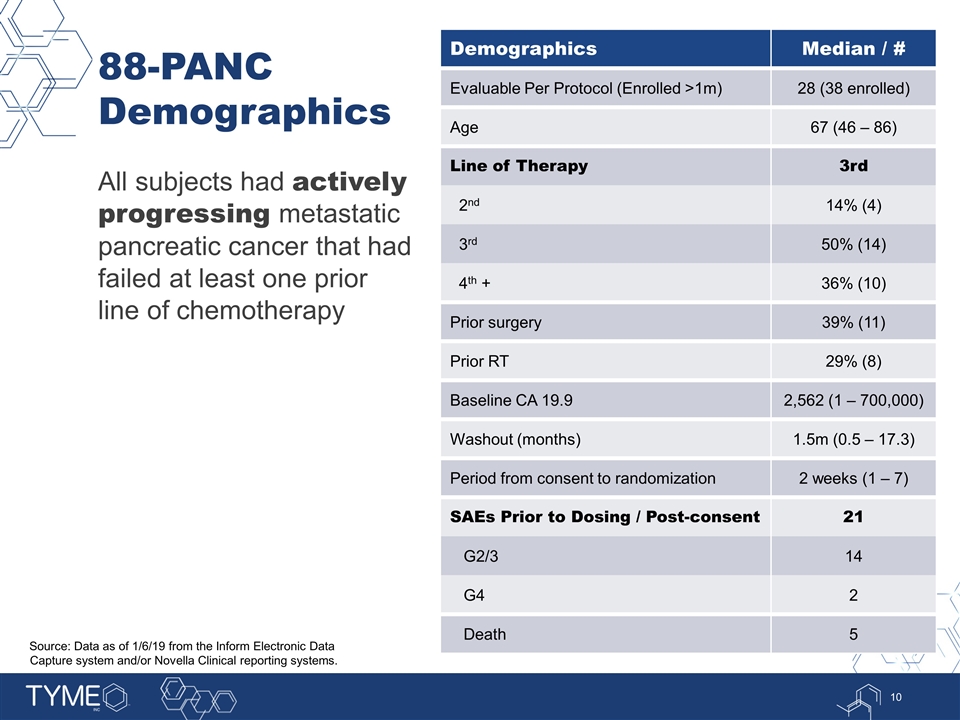
88-PANC Demographics All subjects had actively progressing metastatic pancreatic cancer that had failed at least one prior line of chemotherapy Demographics Median / # Evaluable Per Protocol (Enrolled >1m) 28 (38 enrolled) Age 67 (46 – 86) Line of Therapy 3rd 2nd 14% (4) 3rd 50% (14) 4th + 36% (10) Prior surgery 39% (11) Prior RT 29% (8) Baseline CA 19.9 2,562 (1 – 700,000) Washout (months) 1.5m (0.5 – 17.3) Period from consent to randomization 2 weeks (1 – 7) SAEs Prior to Dosing / Post-consent 21 G2/3 14 G4 2 Death 5 Source: Data as of 1/6/19 from the Inform Electronic Data Capture system and/or Novella Clinical reporting systems.
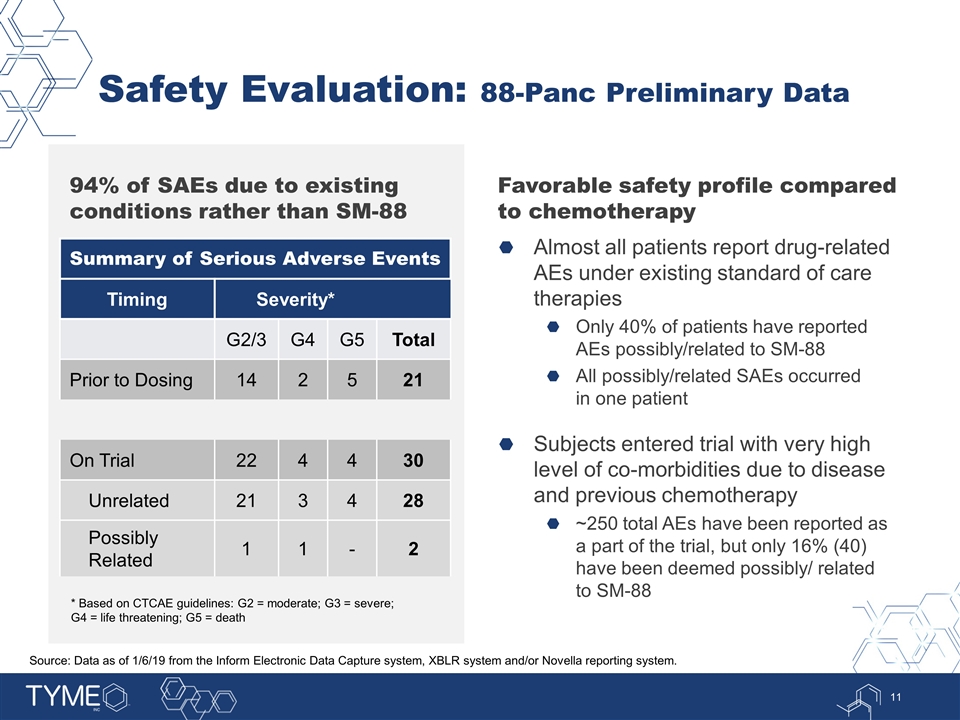
Safety Evaluation: 88-Panc Preliminary Data Favorable safety profile compared to chemotherapy Almost all patients report drug-related AEs under existing standard of care therapies Only 40% of patients have reported AEs possibly/related to SM-88 All possibly/related SAEs occurred in one patient Subjects entered trial with very high level of co-morbidities due to disease and previous chemotherapy ~250 total AEs have been reported as a part of the trial, but only 16% (40) have been deemed possibly/ related to SM-88 Summary of Serious Adverse Events Timing Severity* G2/3 G4 G5 Total Prior to Dosing 14 2 5 21 On Trial 22 4 4 30 Unrelated 21 3 4 28 Possibly Related 1 1 - 2 * Based on CTCAE guidelines: G2 = moderate; G3 = severe; G4 = life threatening; G5 = death 94% of SAEs due to existing conditions rather than SM-88 Source: Data as of 1/6/19 from the Inform Electronic Data Capture system, XBLR system and/or Novella reporting system.
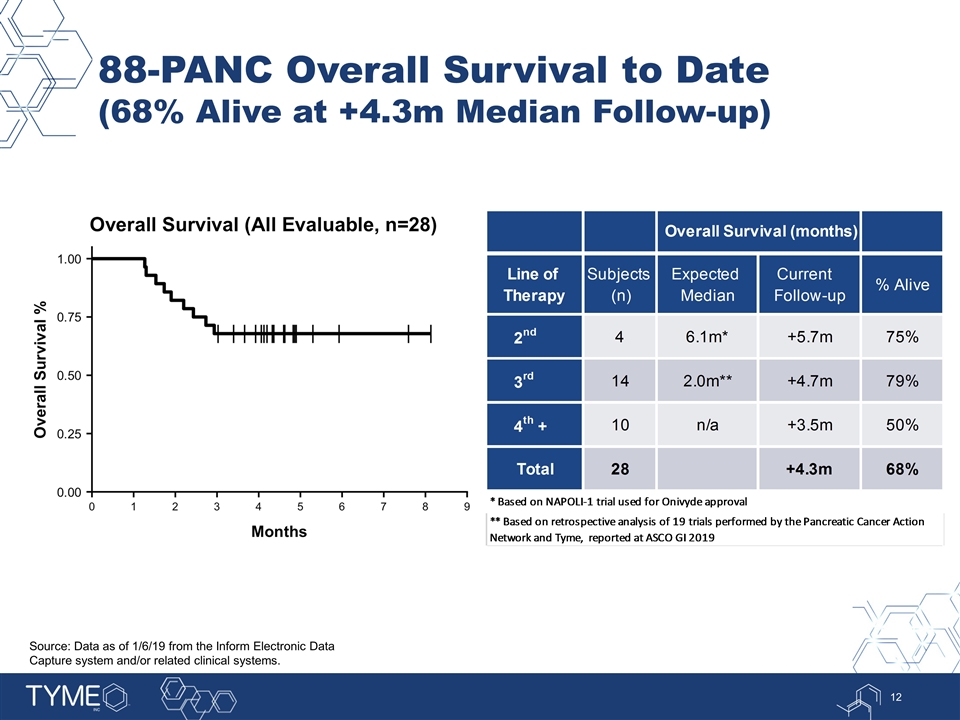
88-PANC Overall Survival to Date (68% Alive at +4.3m Median Follow-up) Source: Data as of 1/6/19 from the Inform Electronic Data Capture system and/or related clinical systems. Overall Survival (months) Line of Therapy Subjects (n) Expected Median Current Follow-up % Alive 2nd 4 6.1m* +5.7m 0.75 3rd 14 2.0m** +4.7m 0.79 4th + 10 n/a +3.5m 0.5 Total 28 +4.3m 0.68 * Based on NAPOLI-1 trial used for Onivyde approval ** Based on retrospective analysis of 19 trials performed by the Pancreatic Cancer Action Network and Tyme, reported at ASCO GI 2019
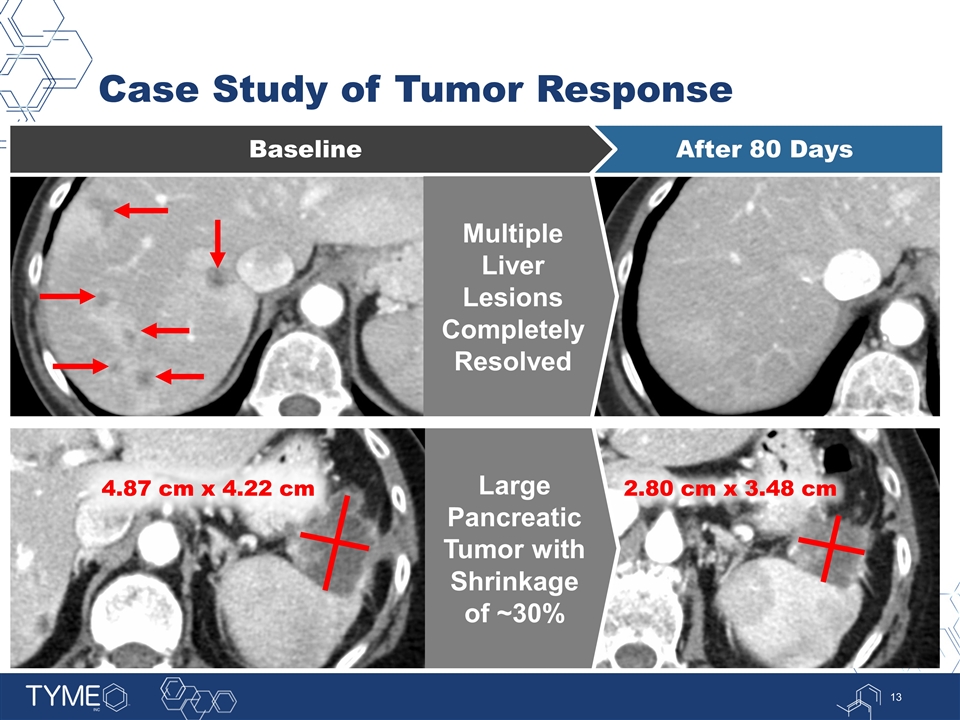
Case Study of Tumor Response After 80 Days Baseline Multiple Liver Lesions Completely Resolved 4.87 cm x 4.22 cm 2.80 cm x 3.48 cm Large Pancreatic Tumor with Shrinkage of ~30%
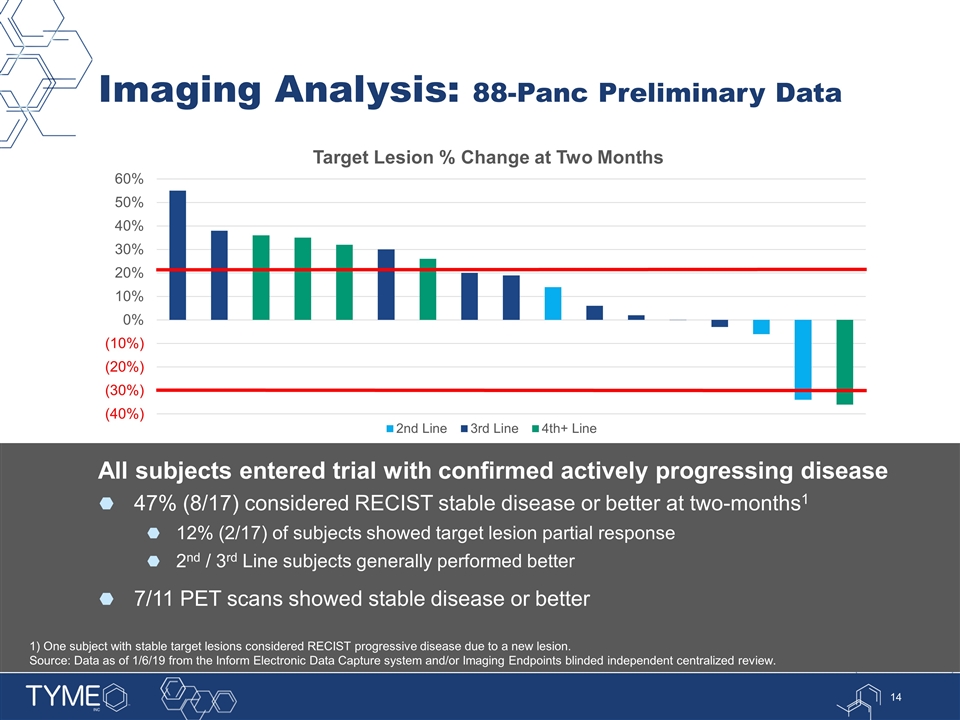
Imaging Analysis: 88-Panc Preliminary Data All subjects entered trial with confirmed actively progressing disease 47% (8/17) considered RECIST stable disease or better at two-months1 12% (2/17) of subjects showed target lesion partial response 2nd / 3rd Line subjects generally performed better 7/11 PET scans showed stable disease or better 1) One subject with stable target lesions considered RECIST progressive disease due to a new lesion. Source: Data as of 1/6/19 from the Inform Electronic Data Capture system and/or Imaging Endpoints blinded independent centralized review.
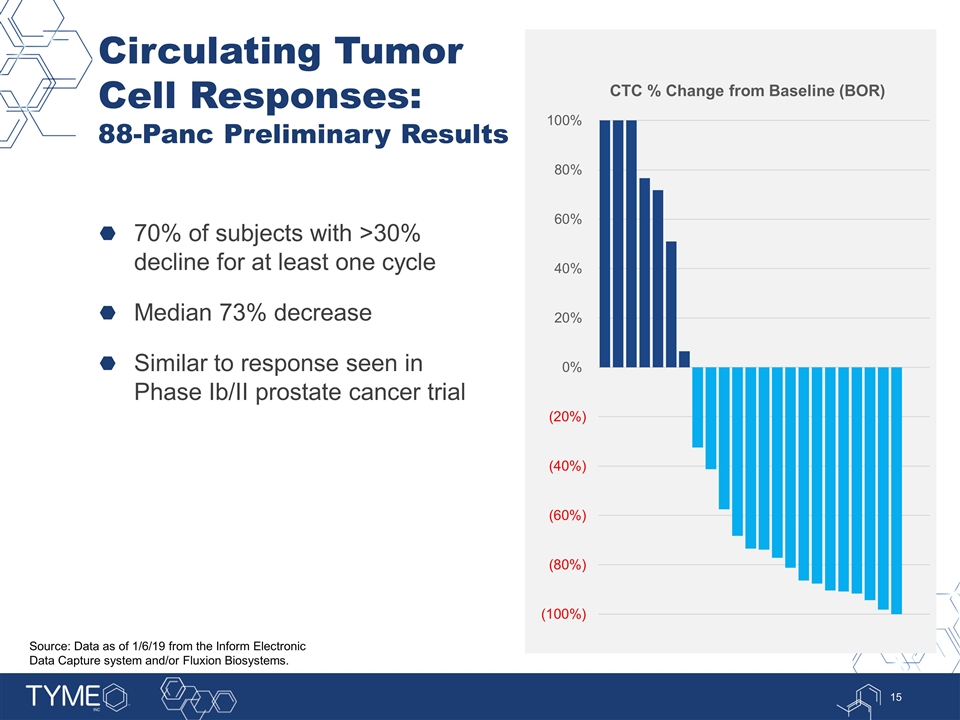
Circulating Tumor Cell Responses: 88-Panc Preliminary Results 70% of subjects with >30% decline for at least one cycle Median 73% decrease Similar to response seen in Phase Ib/II prostate cancer trial Source: Data as of 1/6/19 from the Inform Electronic Data Capture system and/or Fluxion Biosystems.
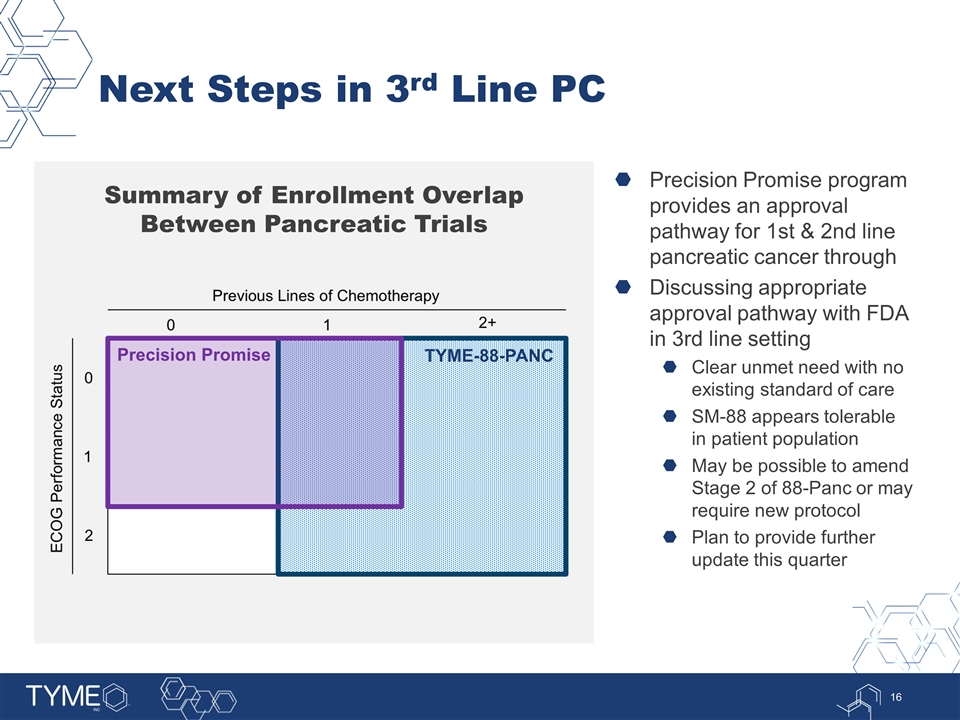
Next Steps in 3rd Line PC Summary of Enrollment Overlap Between Pancreatic Trials Precision Promise program provides an approval pathway for 1st & 2nd line pancreatic cancer through Discussing appropriate approval pathway with FDA in 3rd line setting Clear unmet need with no existing standard of care SM-88 appears tolerable in patient population May be possible to amend Stage 2 of 88-Panc or may require new protocol Plan to provide further update this quarter

Conclusion Ben Taylor, President & CFO Steve Hoffman, Chairman & CEO
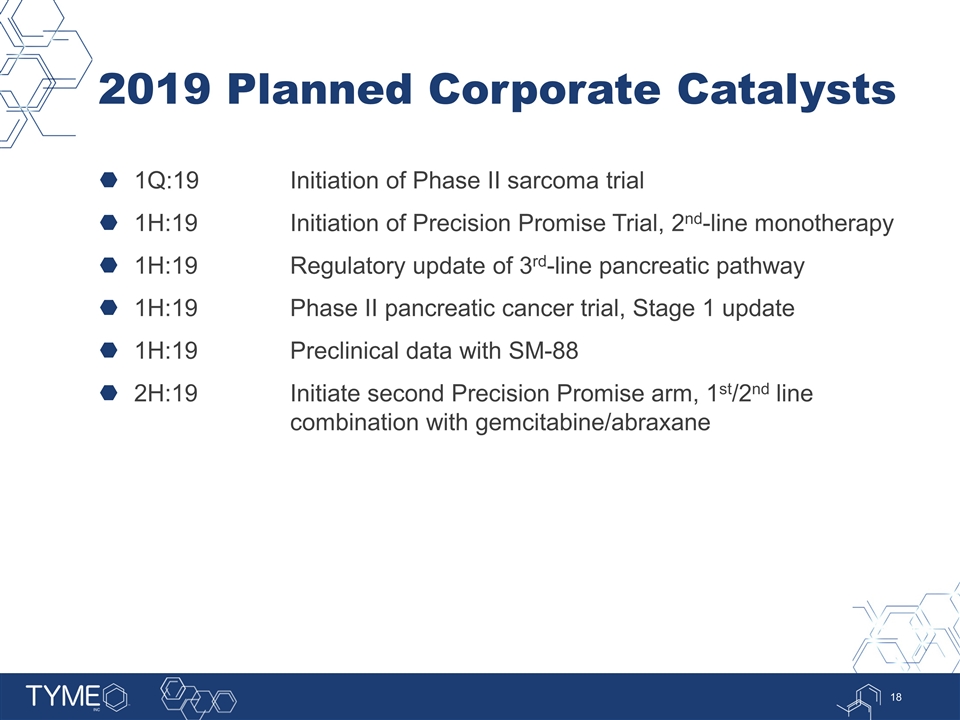
2019 Planned Corporate Catalysts 1Q:19Initiation of Phase II sarcoma trial 1H:19Initiation of Precision Promise Trial, 2nd-line monotherapy 1H:19Regulatory update of 3rd-line pancreatic pathway 1H:19Phase II pancreatic cancer trial, Stage 1 update 1H:19Preclinical data with SM-88 2H:19Initiate second Precision Promise arm, 1st/2nd line combination with gemcitabine/abraxane
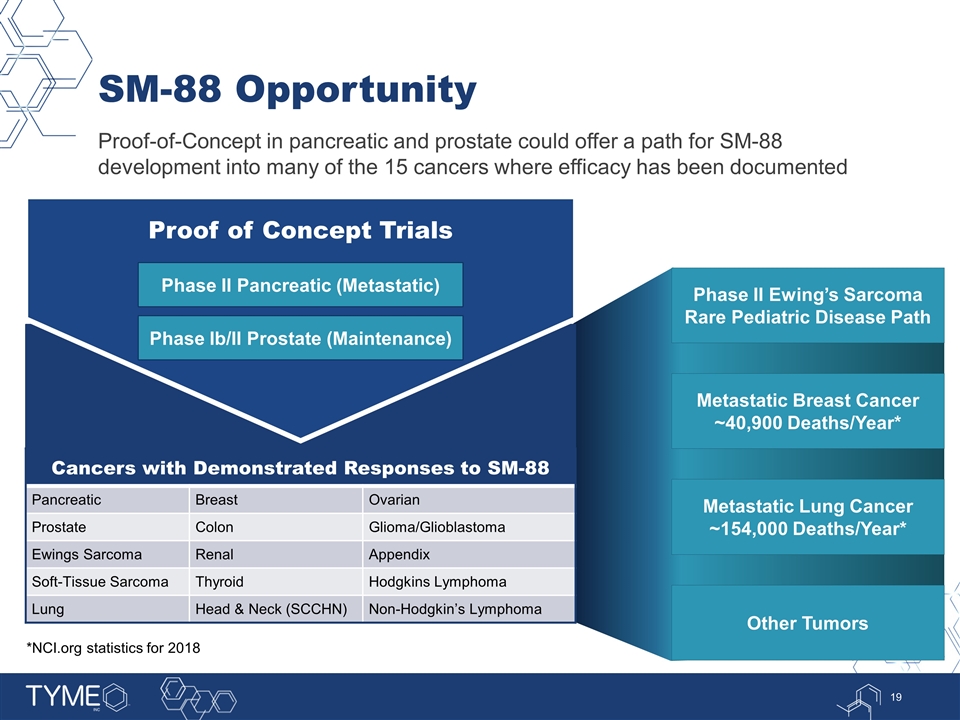
SM-88 Opportunity Proof-of-Concept in pancreatic and prostate could offer a path for SM-88 development into many of the 15 cancers where efficacy has been documented Proof of Concept Trials Cancers with Demonstrated Responses to SM-88 Pancreatic Breast Ovarian Prostate Colon Glioma/Glioblastoma Ewings Sarcoma Renal Appendix Soft-Tissue Sarcoma Thyroid Hodgkins Lymphoma Lung Head & Neck (SCCHN) Non-Hodgkin’s Lymphoma Phase II Ewing’s Sarcoma Rare Pediatric Disease Path Metastatic Breast Cancer ~40,900 Deaths/Year* Metastatic Lung Cancer ~154,000 Deaths/Year* Other Tumors Phase Ib/II Prostate (Maintenance) Phase II Pancreatic (Metastatic) *NCI.org statistics for 2018

Questions
