Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Syros Pharmaceuticals, Inc. | a19-1318_18k.htm |
| EX-99.2 - EX-99.2 - Syros Pharmaceuticals, Inc. | a19-1318_1ex99d2.htm |
Forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding our strategy, research and clinical development plans, collaborations, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including whether or when Incyte will exercise any of its options or any option exercise fees, milestone payments or royalties under the Incyte collaboration will ever be paid, our ability to: advance the development of our programs, including SY-1425 and SY-1365, under the timelines we project in current and future clinical trials; demonstrate in any current and future clinical trials the requisite safety, efficacy and combinability of our drug candidates; successfully progress SY-5609 through IND-enabling preclinical and toxicology studies; replicate scientific and non-clinical data in clinical trials; successfully develop a companion diagnostic test to identify patients with the RARA and IRF8 biomarkers; obtain and maintain patent protection for our drug candidates and the freedom to operate under third party intellectual property; obtain and maintain necessary regulatory approvals; identify, enter into and maintain collaboration agreements with third parties, including our ability to perform under the collaboration agreement with Incyte; manage competition; manage expenses; raise the substantial additional capital needed to achieve our business objectives; attract and retain qualified personnel; and successfully execute on our business strategies and long-term vision; risks described under the caption “Risk Factors” in our Annual Report on Form 10-K for the year ended December 31, 2017, as updated in our Quarterly Report on Form 10-Q for the quarters ended March 31, June 30 and September 30, 2018, each of which is on file with the Securities and Exchange Commission (SEC); and risks described in other filings that we may make with the SEC in the future. Any forward-looking statements contained in this presentation speak only as of the date this presentation is made, and we expressly disclaim any obligation to update any forward-looking statements, whether because of new information, future events or otherwise. 2

[LOGO]

Syros today: Clear vision, growing pipeline, pioneering platform and disciplined execution Two first-in-class clinical-stage programs Clinical trials in 5 cancer patient populations 4 Multiple potential near-term catalysts Leading gene control platform Experienced leadership team
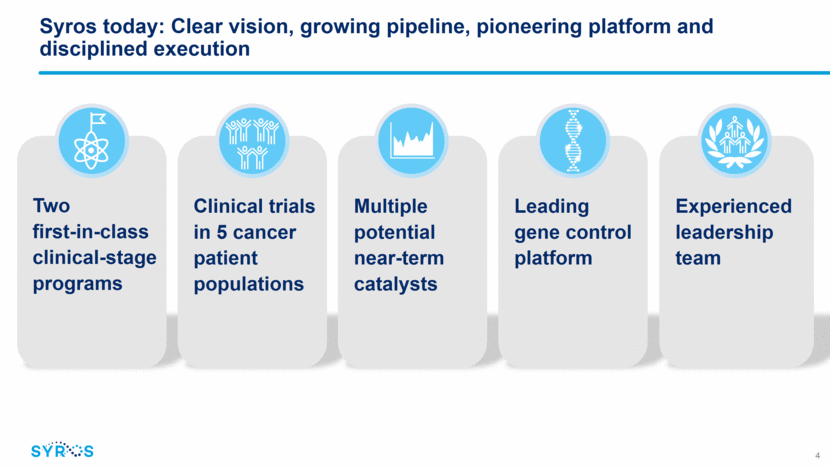
Multiple clinical milestones in 2019 and beyond SY-1425 SY-1365 SY-5609 Complete IND-enabling studies to support initiation of Phase 1 oncology trial in early 2020 5 Report initial data from ongoing Phase 1 expansion cohorts in Q4 2019 Complete enrollment in azacitidine combination cohort in biomarker-positive patients in mid-2019 Report updated azacitidine combination data in 2H 2019
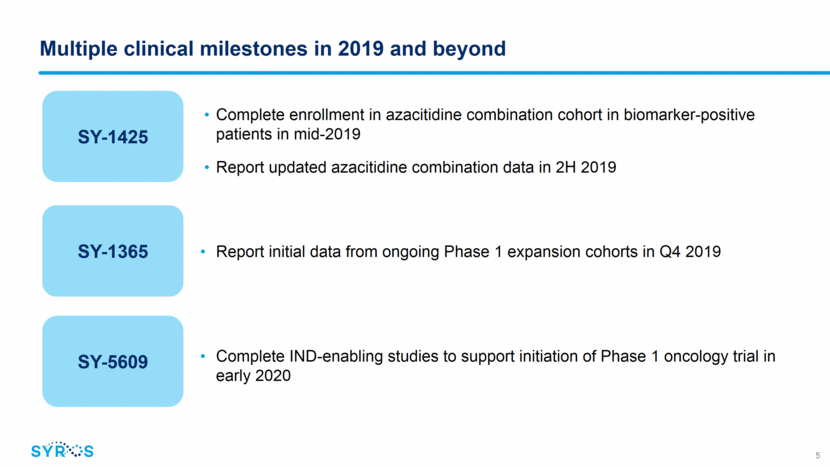
6 98% Previously unexplored regulatory regions of the genome control the expression of genes determining cell function; majority of disease variation found in these regions Patient Impact Medicines that control the expression of genes to provide a profound benefit for patients with severe diseases Pioneering a new approach: Medicines that control the expression of genes Our leading gene control platform Regulatory Genomics Disease Biology Transcriptional Chemistry !
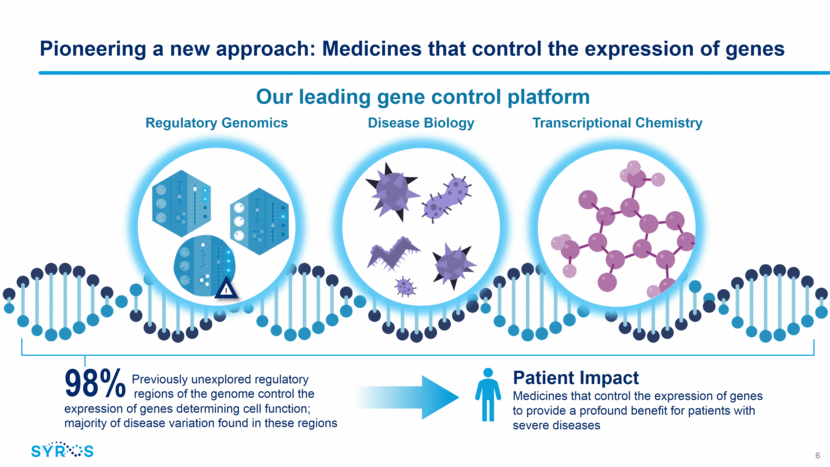
Deep and growing pipeline with multiple potential first-in-class programs 7 SY-1425 is approved in Japan as Amnolake® (tamibarotene) for patients with relapsed/refractory APL Program Indication Drug Discovery IND- Enabling Early Clinical Mid- Clinical Pivotal Commercial Rights RAR agonism SY-1425 (RAR agonist) AML Syros (North America and Europe) CDK Inhibition SY-1365 (CDK7 inhibitor) Ovarian cancer Syros (Global) HR+ breast cancer SY-5609 (Oral CDK7 inhibitor) Cancer CDK12/13 Inhibitor Cancer Cancer Macrophage target Immuno-oncology Cancer/Immuno-oncology Monogenic Disease Undisclosed target Sickle cell disease Monogenic diseases MPNs Myeloproliferative neoplasms Incyte (Global)
SY-1425: First-in-class selective, oral RAR agonist supported by promising initial clinical data Approximately one-third of AML and higher-risk MDS patients with novel biomarkers identified using our platform In Phase 2 trial in combination with azacitidine Initial data shows high response rates, rapid onset of action and no increased neutropenia in biomarker-positive patients Additional clinical data expected in 2H 2019 Significant opportunity in multi-billion dollar AML and HR MDS market Ongoing need for well-tolerated combination therapies to extend survival and improve quality of life Acute Myelogenous Leukemia (AML) and Myelodysplastic Syndromes (MDS) 8
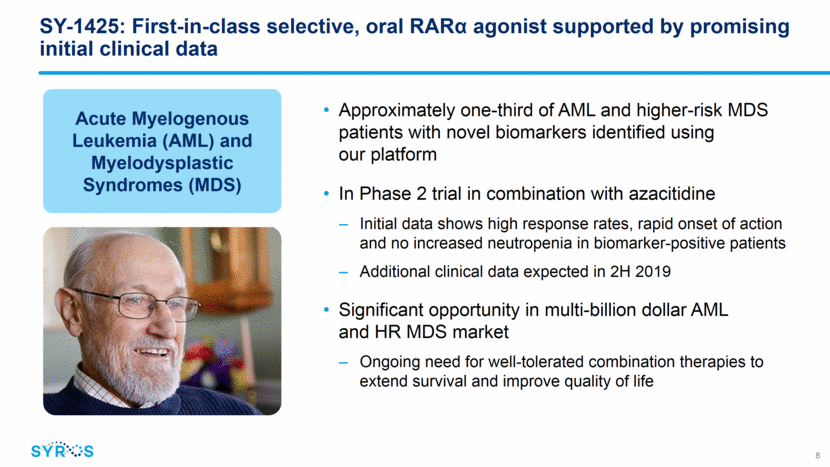
1425 SY-1425 binds to RAR and activates differentiation genes SY-1425 has broad combination potential across RARA and IRF8 biomarker-positive AML and HR MDS patients RARA Aza cross links to DNA SY-1425 enhances apoptosis preclinically Apoptosis DNA damage Gene control platform identifies novel patient subsets Immature blood cells Mature blood cells Discovery: RARA and IRF8 SEs 9 Known: Mutations RARA Leukemia SY-1425 shows synergy with a range of AML therapies, including chemotherapy and targeted agents Data published in October 2018 in Haematologica Data published in October 2017 in Cancer Discovery
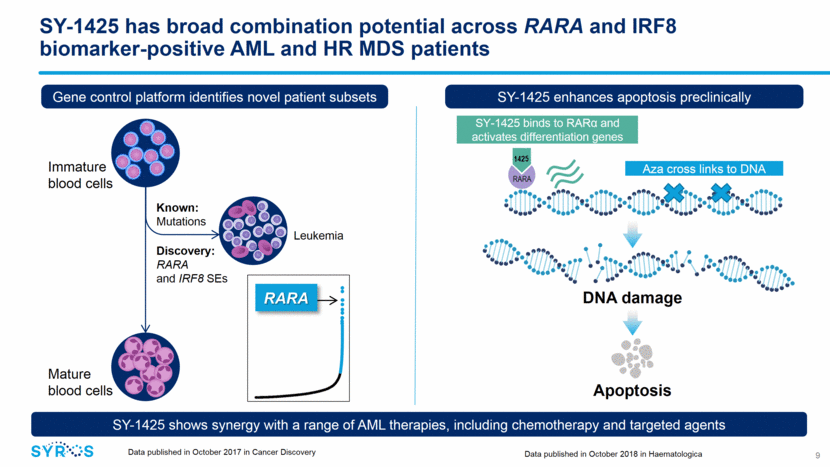
Significant need for well-tolerated oral therapies that extend survival and improve quality of life 10 Fast-growing AML and HR MDS market is projected to be > $1 billion market this year ~40,000 AML and HR MDS patients Unmet need across populations ~35% RARA and IRF8 biomarker-positive Targeted patient population AML >50% of newly diagnosed patients are unfit for intensive chemo HMAs are backbone of therapy with modest efficacy as single agents Survival of 9 mos for newly diagnosed, unfit and < 6 mos for R/R pts Despite recent approvals, high unmet medical need still exists HR MDS Vast majority of newly diagnosed patients receive HMAs/less intensive therapy with modest efficacy Survival of < 1.6 years for newly diagnosed and < 6 months for R/R patients No new drugs approved since 2006 2018 incidence in the U.S. and the EU 5 (UK, Germany, France, Spain and Italy) from Decision Resources Group. Annual sales forecast from Decision Resources Group. Sources: NCCN guidelines AML (Feb 2018); Clinical Lymphoma, Myeloma & Leukemia, 16:625-36 (2016); Blood 120:2454-2465 (2012)
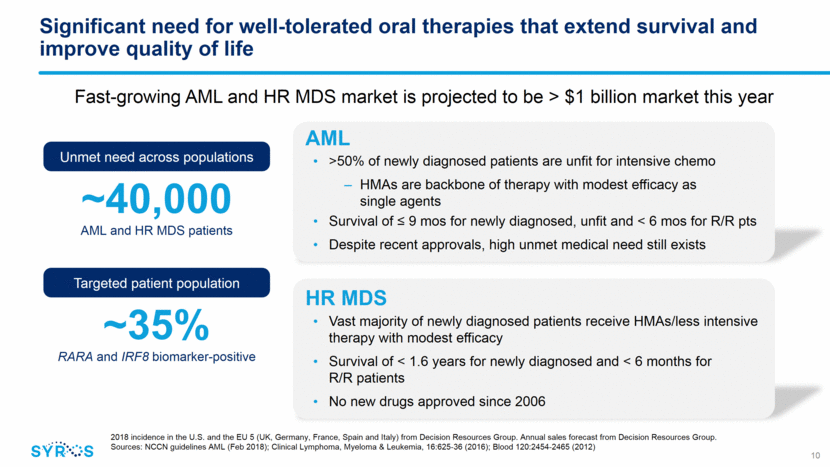
Ongoing Phase 2 trial evaluating SY-1425 in combination with azacitidine in newly diagnosed, unfit AML patients Promising initial data presented at ASH 2018 Additional data expected in second half of 2019 11 Biomarker-positive newly diagnosed, unfit AML (25 patients) Assess safety and efficacy Phase 2 clinical trial design Biomarker-negative newly diagnosed, unfit AML (25 patients) Primary purpose Support development of commercial companion diagnostic azacitidine Combo agent azacitidine Patient population
Initial data shows SY-1425 in combination with azacitidine has high response rates and rapid onset of action in biomarker-positive AML patients 50% 1 month CR/CRi Rate (n=8) Time to response 1 Fenaux et al, JCO 2010; Dombret et al, Blood 2015; Vidaza® (azacitidine) Prescribing Information, Celgene Revision 09/2018. 2 Thepot et al, AJH 2014. Responses seen in biomarker-positive newly diagnosed, unfit AML patients Elderly, high-risk population with median age of 76 and more than half having poor-risk cytogenetics Generally well-tolerated with no increased neutropenia Initial data compare favorably to single-agent azacitidine, which shows a response rate of 18-29%1 in unfit AML patients with initial response generally occurring after four cycles2 Initial data support RARA and IRF8 biomarkers for patient selection, with 17% ORR in biomarker-negative cohort (n=6) 12 63% ORR (n=8) Data as of Oct. 29, 2018 snapshot presented in December 2018 at ASH Annual Meeting 0 3 6 9 12 15 Treatment Duration (Months) Best Response CR MR CRm PRi CR PRi CRi MLFS On treatment Discontinued
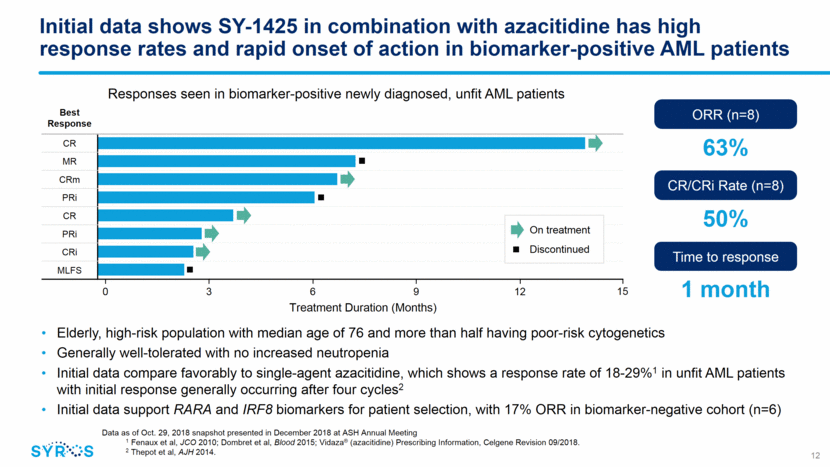
Building an industry-leading franchise in selective CDK7 inhibition Apoptosis Selectively inhibiting CDK7 has been shown preclinically to decrease expression of oncogenic transcription factors and anti-apoptotic proteins Selectively inhibiting CDK7 is thought to interfere with cancer-driving adaptations at multiple points in the cell cycle, promoting the induction of apoptosis MYC MYB MCL1 MCL1 Cell cycle Transcription CDK7 Apoptosis RB signaling pathway Synthesis Growth Mitosis preparation Mitosis CDK2 CDK1 13
SY-1365: First-in-class selective CDK7 inhibitor with broad potential in a range of difficult-to-treat solid tumors and blood cancers Potent, covalent and highly selective Preferentially kills cancer cells over non-cancerous cells in preclinical models Currently in Phase 1 clinical trial as single and combination agent in ovarian and breast cancers Dose escalation data demonstrated proof-of-mechanism at tolerable doses and early signs of clinical activity Initial data from expansion cohorts expected in Q4 2019 Broad potential in additional solid tumors and blood cancers Difficult-to-treat solid tumors and blood cancers 14
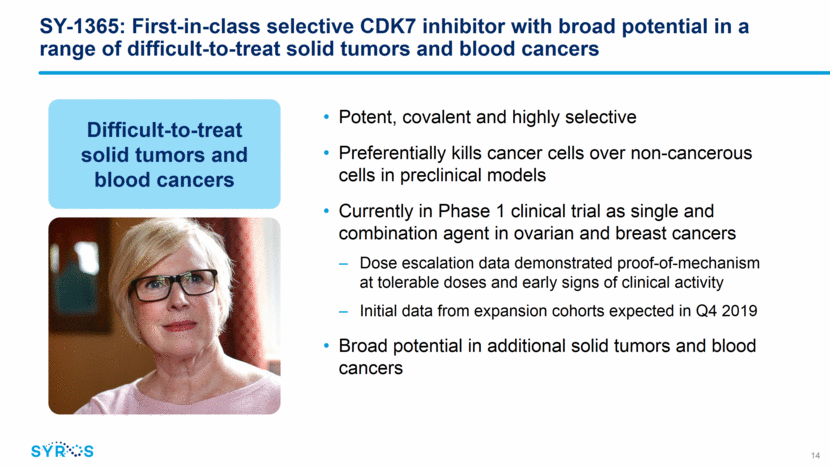
Expansion cohorts in Phase 1 trial explore SY-1365 as single agent and in combination in multiple ovarian and breast cancer patient populations 15 Completed dose escalation in advanced solid tumor patients and opened expansion cohorts in fall 2018 Primary objectives of expansion are efficacy and further evaluation of safety, dose and schedule Initiated expansion cohorts at 53 and 80 mg/m2; exploring once and twice weekly regimens Initial data from expansion cohorts expected in Q4 2019 Ongoing expansion cohorts Relapsed ovarian cancer, 3+ prior lines Relapsed ovarian cancer, 1+ prior lines (platinum sensitive) Primary platinum refractory ovarian cancer Solid tumors accessible for biopsy HR+ metastatic breast cancer, CDK4/6 inhibitor resistant Single agent Combination with carboplatin Single agent Single agent Combination with fulvestrant N=24 N=24 N=12 N=30 N=12 Target enrollment Single/combo agent Patient population
Significant need for new therapies in ovarian cancer and HR-positive metastatic breast cancer 16 Ovarian and HR-positive breast cancers represent > $8 billion fast-growing market >60,000 Ovarian cancer patients ~58,000 HR+ metastatic breast cancer patients Ovarian cancer Most patients present with advanced disease at initial diagnosis Platinum-based therapy is foundation of care Majority of patients, even those who initially respond, relapse within a year Continued unmet need for improved initial treatment for patients with recurrent disease and for non-BRCA mutated patients HR-positive metastatic breast cancer Standard of care includes CDK4/6 inhibitor plus an aromatase inhibitor Half of patients relapse within ~2 years Second-line hormone-based therapies have limited efficacy Emerging therapies limited to targeted patient subsets 2018 incidence in the U.S., Japan and the EU 5 (UK, Germany, France, Spain and Italy) from Decision Resources Group. Annual sales forecast from Decision Resources Group. Sources: NCCN Guidelines Ovarian Cancer (Mar 2018). Gabra H. EJC Suppl. 2014 Dec;12(2):2-6. and Herzog TJ and Monk BJ. Onitilo AA et al., Clin Med Res 2009; 7(1-2):4-13. Rugo HS et al., JCO 2016; 34: 3069-3103. Finn RS et al, N Engl J Med 2016; 375(20): 1925-1936. Faslodex USPI
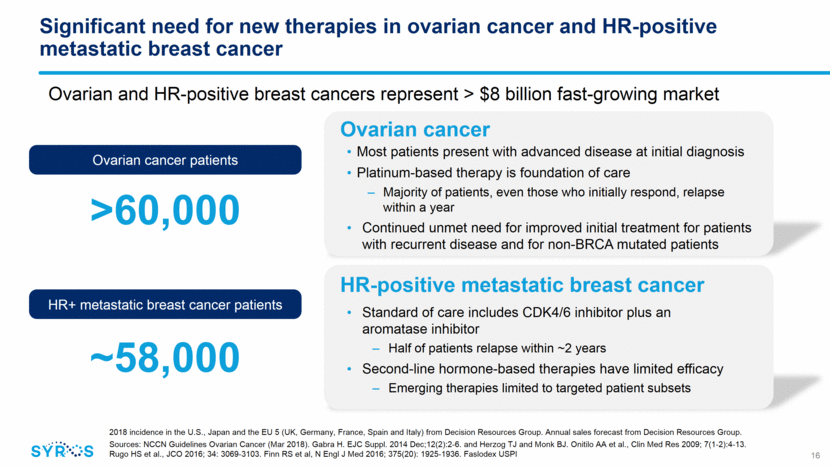
SY-1365 demonstrated proof-of-mechanism at tolerable doses in dose escalation portion of ongoing Phase 1 trial 17 Relative Expression (Log2) 3h post-dose vs. pre-dose SY-1365 Dose (mg/m2) 0 -1 -2 -3 1 4 8 16 32 53 64 80 107 PBMC Gene Expression Signature PBMC CDK7 Occupancy Exceeded desired target occupancy levels at doses 32 mg/m2 Most frequent related AEs include headache, nausea, vomiting and fatigue No reports of neutropenia Demonstrated dose-dependent downstream changes in gene expression Adverse events (AEs) were predominantly low grade, reversible and generally manageable Data presented in November 2018 at EORTC-NCI-AACR Symposium 0 10 20 30 40 50 60 70 80 90 100 0 10 20 30 40 50 60 70 80 90 100 110 120 % CDK7 occupancy SY - 1365 Dose (mg/m 2 )
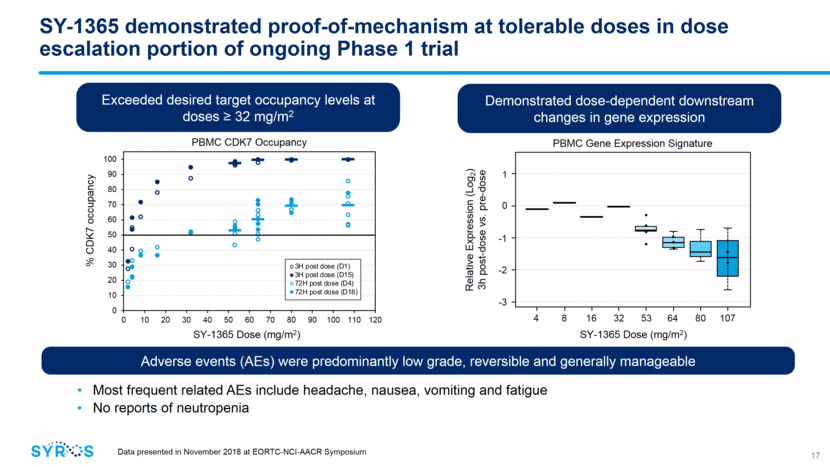
SY-1365 demonstrated early evidence of clinical activity Clinical activity per RECIST 1.1 criteria observed in 7 of 19 evaluable patients 1 confirmed PR (clear cell ovarian cancer patient) observed at 80 mg/m2 BIW 6 stable disease (2 ovarian, 2 breast and 2 endometrial cancer patients), mostly at doses 32 mg/m2 BIW Duration of treatment ranged from 50 - 127 days 18 Data presented in November 2018 at EORTC-NCI-AACR Symposium Before treatment After 2 months After 6 months CT images of heavily pretreated stage IV clear cell ovarian cancer patient SY-1365 demonstrated 37% disease control rate (CR+PR+SD) Confirmed PR after 2 cycles (31.8% reduction at C3D1) Remained on study in PR in 7th month of SY-1365 treatment as of data snapshot (49% decrease at C7D1) Best response to prior therapies was stable disease
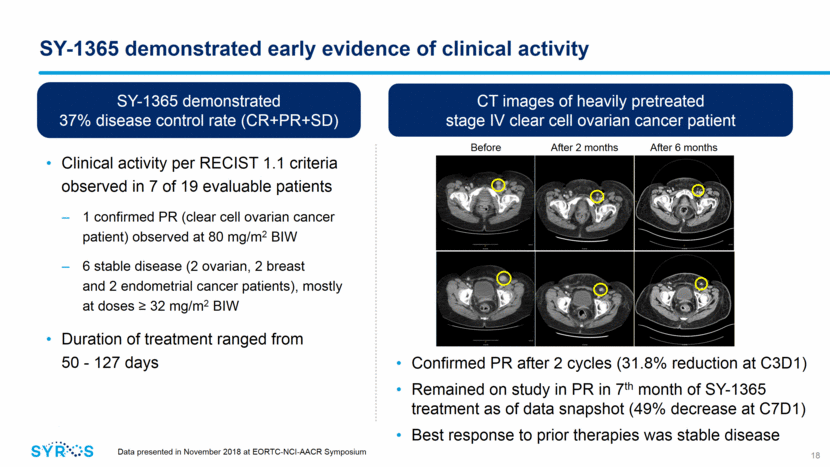
SY-1365 shows anti-tumor activity as single agent and in combination with standard-of-care in ovarian models, supporting ongoing clinical investigation Weekly SY-1365 in combination with carboplatin enhances activity in ovarian cancer xenograft models SY-1365 induces tumor growth inhibition, including complete regressions, in heavily pretreated ovarian cancer models Tumor Vol. (mm3) Days Historical/untreated Treated 40mg/kg BIW 30mg/kg BIW 19 Data presented in April 2018 at the American Association of Cancer Research (AACR) Annual Meeting 1TCGA Ovarian Cancer Integrated Analysis, Nature 2011 Data presented in November 2018 at EORTC-NCI-AACR Symposium Vehicle QW*3 30mpk SY-1365 QW 50mpk Carboplatin QW 50mpk Carboplatin + 30mpk SY-1365 QW 0 10 20 0 300 600 900 1200 1500 Days Dosing Tumor Vol. (mm3) SY-1365 inhibited DNA repair and transcription of HRR genes in preclinical models, inducing an HRD-like state that may increase sensitivity to DNA-damaging agents and DNA repair inhibitors Responses observed in 10/17 (59%) models, irrespective of BRCA status or PARP inhibitor sensitivity Sensitivity to SY-1365 was associated with low expression of BCLXL and RB pathway alterations Approximately 2/3 of high-grade serous ovarian cancer patients have RB alterations1
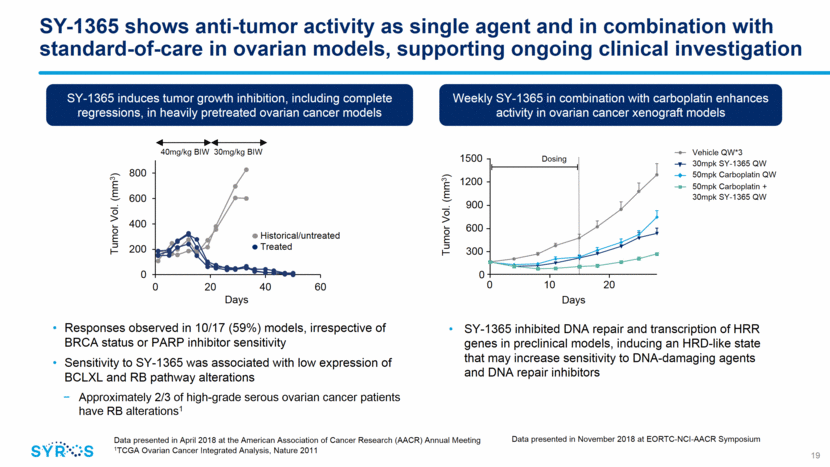
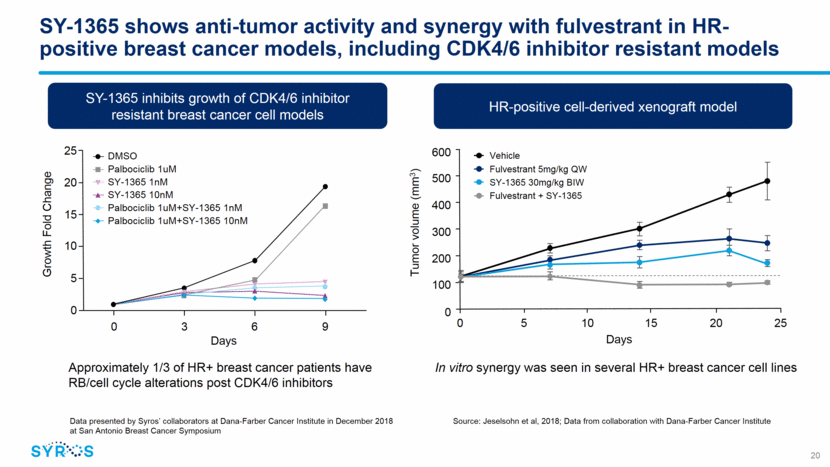
SY-1365 shows anti-tumor activity and synergy with fulvestrant in HR-positive breast cancer models, including CDK4/6 inhibitor resistant models 20 Data presented by Syros’ collaborators at Dana-Farber Cancer Institute in December 2018 at San Antonio Breast Cancer Symposium SY-1365 inhibits growth of CDK4/6 inhibitor resistant breast cancer cell models Approximately 1/3 of HR+ breast cancer patients have RB/cell cycle alterations post CDK4/6 inhibitors Growth Fold Change 25 20 15 10 5 0 0 3 6 9 Days DMSO Palbociclib 1uM SY-1365 1nM SY-1365 10nM Palbociclib 1uM+SY-1365 1nM Palbociclib 1uM+SY-1365 10nM In vitro synergy was seen in several HR+ breast cancer cell lines HR-positive cell-derived xenograft model 600 500 400 300 200 100 0 0 5 10 15 20 25 Tumor volume (mm3) Days Vehicle Fulvestrant 5mg/kg QW SY-1365 30mg/kg BIW Fulvestrant + SY-1365 Source: Jeselsohn et al, 2018; Data from collaboration with Dana-Farber Cancer Institute
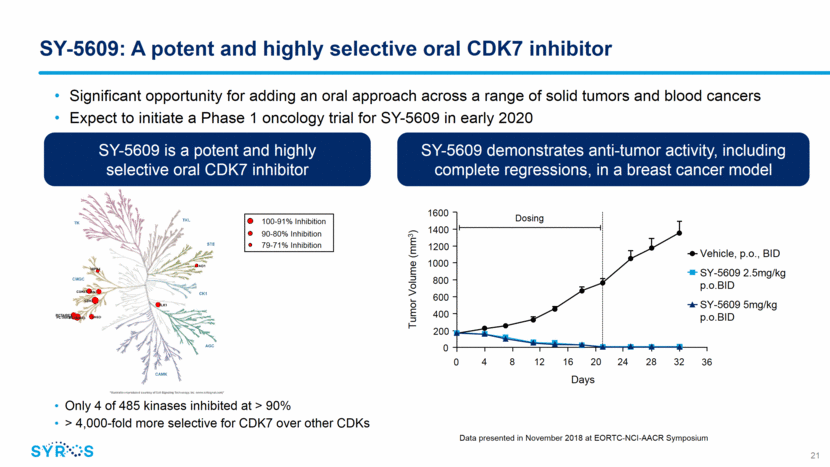
SY-5609: A potent and highly selective oral CDK7 inhibitor 21 SY-5609 is a potent and highly selective oral CDK7 inhibitor SY-5609 demonstrates anti-tumor activity, including complete regressions, in a breast cancer model Tumor Volume (mm3) Days 1600 4 8 12 16 20 24 28 32 36 0 1400 1200 1000 800 600 400 200 0 Dosing Significant opportunity for adding an oral approach across a range of solid tumors and blood cancers Expect to initiate a Phase 1 oncology trial for SY-5609 in early 2020 Only 4 of 485 kinases inhibited at > 90% > 4,000-fold more selective for CDK7 over other CDKs 100-91% Inhibition 90-80% Inhibition 79-71% Inhibition Data presented in November 2018 at EORTC-NCI-AACR Symposium Vehicle, p.o., BID SY-5609 2.5mg/kg p.o.BID SY-5609 5mg/kg p.o.BID
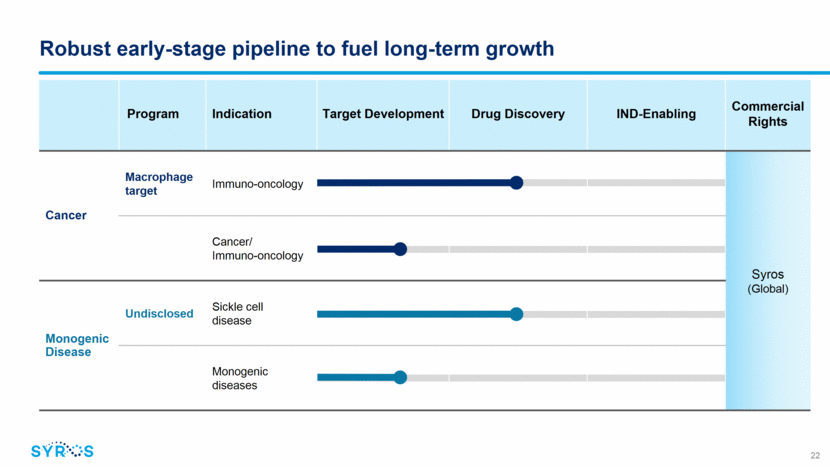
Program Indication Target Development Drug Discovery IND-Enabling Commercial Rights Cancer Macrophage target Immuno-oncology Syros (Global) Cancer/ Immuno-oncology Monogenic Disease Undisclosed Sickle cell disease Monogenic diseases Robust early-stage pipeline to fuel long-term growth 22
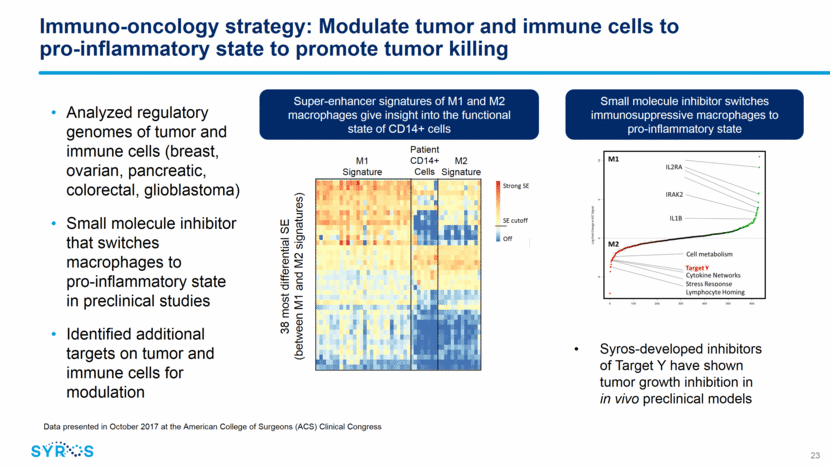
Immuno-oncology strategy: Modulate tumor and immune cells to pro-inflammatory state to promote tumor killing Analyzed regulatory genomes of tumor and immune cells (breast, ovarian, pancreatic, colorectal, glioblastoma) Small molecule inhibitor that switches macrophages to pro-inflammatory state in preclinical studies Identified additional targets on tumor and immune cells for modulation M1 Signature M2 Signature Patient CD14+ Cells Small molecule inhibitor switches immunosuppressive macrophages to pro-inflammatory state Super-enhancer signatures of M1 and M2 macrophages give insight into the functional state of CD14+ cells 38 most differential SE (between M1 and M2 signatures) 23 Data presented in October 2017 at the American College of Surgeons (ACS) Clinical Congress Syros-developed inhibitors of Target Y have shown tumor growth inhibition in in vivo preclinical models
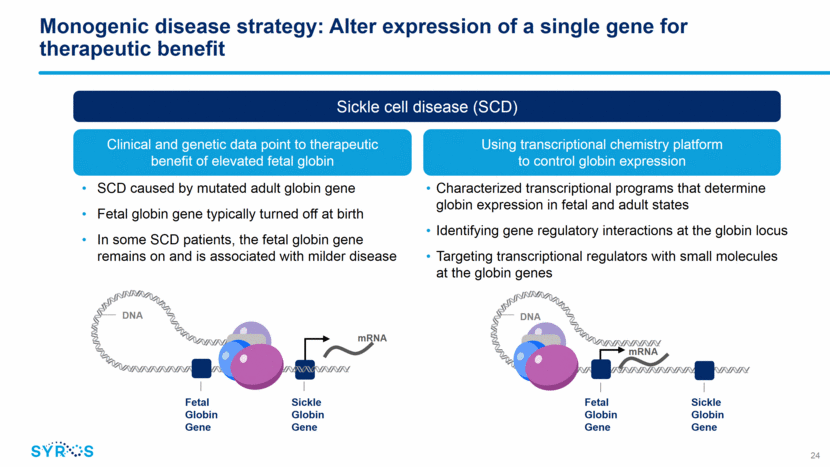
Monogenic disease strategy: Alter expression of a single gene for therapeutic benefit SCD caused by mutated adult globin gene Fetal globin gene typically turned off at birth In some SCD patients, the fetal globin gene remains on and is associated with milder disease Sickle cell disease (SCD) Clinical and genetic data point to therapeutic benefit of elevated fetal globin Using transcriptional chemistry platform to control globin expression mRNA Sickle Globin Gene DNA mRNA Fetal Globin Gene Sickle Globin Gene DNA Fetal Globin Gene Characterized transcriptional programs that determine globin expression in fetal and adult states Identifying gene regulatory interactions at the globin locus Targeting transcriptional regulators with small molecules at the globin genes 24
Rapidly advancing toward our vision 25 Progressing to pivotal development Advancing multiple programs in clinic Preparing for commercial launch Continued investment in discovery Fully integrated company with medicines that provide a profound benefit for patients Driving SY-1425 and SY-1365 to key milestones Advancing SY-5609 toward clinical development Investing in discovery to support goal of one IND every other year Capital to fund planned operations into 2020 Now Next Vision
[LOGO]


