Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Surface Oncology, Inc. | d678400d8k.htm |

Exhibit 99.1 Breaking through for patients with cancer JP MORGAN HEALTHCARE CONFERENCE JANUARY 2019Exhibit 99.1 Breaking through for patients with cancer JP MORGAN HEALTHCARE CONFERENCE JANUARY 2019

Legal Disclaimer This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. All statements contained in this presentation other than statements of historical facts, including statements regarding future results of operations and financial position of Surface Oncology, Inc. (“we,” “us” or “our”) our business strategy and plans, the preclinical and clinical development of our product candidates and our objectives for future operations, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “will” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, clinical development, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward looking statements. These risks and uncertainties include the timing, progress, and results of preclinical studies and clinical trials for NZV930 and SRF231 and our other product candidates, the timing and likelihood of regulatory approvals and those risks identified and discussed in the section titled “Risk Factors,” set forth in, or incorporated by reference in, our Quarterly Report on Form 10-Qs and in our other SEC filings. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, achievements or events and circumstances reflected in the forward-looking statements will occur. We are under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this presentation. By attending or receiving this presentation you acknowledge that you will be solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of our business. 2Legal Disclaimer This presentation includes forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995, as amended. All statements contained in this presentation other than statements of historical facts, including statements regarding future results of operations and financial position of Surface Oncology, Inc. (“we,” “us” or “our”) our business strategy and plans, the preclinical and clinical development of our product candidates and our objectives for future operations, are forward-looking statements. The words “anticipate,” “believe,” “continue,” “estimate,” “expect,” “intend,” “may,” “will” and similar expressions are intended to identify forward-looking statements. We have based these forward-looking statements largely on our current expectations and projections about future events and financial trends that we believe may affect our financial condition, results of operations, business strategy, clinical development, short-term and long-term business operations and objectives and financial needs. These forward-looking statements are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward looking statements. These risks and uncertainties include the timing, progress, and results of preclinical studies and clinical trials for NZV930 and SRF231 and our other product candidates, the timing and likelihood of regulatory approvals and those risks identified and discussed in the section titled “Risk Factors,” set forth in, or incorporated by reference in, our Quarterly Report on Form 10-Qs and in our other SEC filings. Moreover, we operate in a very competitive and rapidly changing environment. New risks emerge from time to time. It is not possible for our management to predict all risks, nor can we assess the impact of all factors on our business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements we may make. Although we believe that the expectations reflected in the forward-looking statements are reasonable, we cannot guarantee future results, levels of activity, performance, achievements or events and circumstances reflected in the forward-looking statements will occur. We are under no duty to update any of these forward-looking statements after the date of this presentation to conform these statements to actual results or revised expectations, except as required by law. You should, therefore, not rely on these forward-looking statements as representing our views as of any date subsequent to the date of this presentation. Moreover, except as required by law, neither we nor any other person assumes responsibility for the accuracy and completeness of the forward-looking statements contained in this presentation. By attending or receiving this presentation you acknowledge that you will be solely responsible for your own assessment of the market and our market position and that you will conduct your own analysis and be solely responsible for forming your own view of the potential future performance of our business. 2

BREAK THROUGH On a mission to dramatically IMPROVE CURE RATES FOR CANCER Proven leadership and UNIQUELY COLLABORATIVE SCIENTIFIC ADVISORS Deep biological expertise across the TUMOR MICROENVIRONMENT Focus on differentiated targets bridging ADAPTIVE AND INNATE IMMUNITY Financial resources and strategic partners provide STRONG FOUNDATION 3BREAK THROUGH On a mission to dramatically IMPROVE CURE RATES FOR CANCER Proven leadership and UNIQUELY COLLABORATIVE SCIENTIFIC ADVISORS Deep biological expertise across the TUMOR MICROENVIRONMENT Focus on differentiated targets bridging ADAPTIVE AND INNATE IMMUNITY Financial resources and strategic partners provide STRONG FOUNDATION 3

Surface Oncology at a glance 4Surface Oncology at a glance 4
Recent Corporate Highlights • Dose escalation ongoing for phase 1 study of NZV930 (CD73) • Multi-arm combination study enrolling up to ~344 patients with solid tumors • SRF231 (CD47) deprioritized • Significant reduction in spend and scope of development • Based upon dose escalation data and competitive landscape • IND-enabling studies for both SRF617 (CD39) and SRF388 (IL-27) are ongoing and both IND filings are anticipated in Q4 2019 • A recent publication in the journal Nature1 highlighted the role of IL-27 in the expression of certain checkpoint proteins • Addition of Stephen Hodi, MD, (Dana-Farber) to Scientific Advisory Board • YE Cash 2018: ~$160M • Following reprioritization of investment in SRF231; cash runway now extended through 2021 5 Chihara et al, Nature 2018Recent Corporate Highlights • Dose escalation ongoing for phase 1 study of NZV930 (CD73) • Multi-arm combination study enrolling up to ~344 patients with solid tumors • SRF231 (CD47) deprioritized • Significant reduction in spend and scope of development • Based upon dose escalation data and competitive landscape • IND-enabling studies for both SRF617 (CD39) and SRF388 (IL-27) are ongoing and both IND filings are anticipated in Q4 2019 • A recent publication in the journal Nature1 highlighted the role of IL-27 in the expression of certain checkpoint proteins • Addition of Stephen Hodi, MD, (Dana-Farber) to Scientific Advisory Board • YE Cash 2018: ~$160M • Following reprioritization of investment in SRF231; cash runway now extended through 2021 5 Chihara et al, Nature 2018

Broad Approach Targeting the Immunosuppressive Tumor Microenvironment Block Suppressive Activate Metabolites Macrophages and Cytokines (CD47) (CD73, CD39, IL-27) Activate Deplete Natural Regulatory Killer Cells T Cells 6Broad Approach Targeting the Immunosuppressive Tumor Microenvironment Block Suppressive Activate Metabolites Macrophages and Cytokines (CD47) (CD73, CD39, IL-27) Activate Deplete Natural Regulatory Killer Cells T Cells 6

Deep Collaboration With Renowned Advisors Deep expertise in: Commitment to: • A break through • Oncology philosophy • Immunology • Transformative SCIENTIFIC SURFACE • Translational ADVISORY impact for TEAM science BOARD patients • Clinical • Collective development learning • Sasha Rudensky, PhD • Carla Rothlin, PhD • John Stagg, PhD • Stephen Hodi, MD • Arlene Sharpe, MD, PhD • David Tuveson, MD, PhD • Christopher Hunter, PhD • Elliott Sigal, MD, PhD • John Wherry, PhD 7Deep Collaboration With Renowned Advisors Deep expertise in: Commitment to: • A break through • Oncology philosophy • Immunology • Transformative SCIENTIFIC SURFACE • Translational ADVISORY impact for TEAM science BOARD patients • Clinical • Collective development learning • Sasha Rudensky, PhD • Carla Rothlin, PhD • John Stagg, PhD • Stephen Hodi, MD • Arlene Sharpe, MD, PhD • David Tuveson, MD, PhD • Christopher Hunter, PhD • Elliott Sigal, MD, PhD • John Wherry, PhD 7

Management Team with Proven Track Record of Success Jeff Goater Vito Palombella, PhD Chief Executive Officer Chief Scientific Officer Bob Steininger Rob Ross, MD Senior Vice President, CMC Chief Medical Officer Liisa Nogelo-Kerr Pam Holland, PhD General Counsel Vice President, Cancer Biology Jessica Fees Lisa McGrath Senior Vice President, Finance and Vice President, Human Resources Business Operations 8Management Team with Proven Track Record of Success Jeff Goater Vito Palombella, PhD Chief Executive Officer Chief Scientific Officer Bob Steininger Rob Ross, MD Senior Vice President, CMC Chief Medical Officer Liisa Nogelo-Kerr Pam Holland, PhD General Counsel Vice President, Cancer Biology Jessica Fees Lisa McGrath Senior Vice President, Finance and Vice President, Human Resources Business Operations 8

Broad Portfolio Targeting the Tumor Microenvironment Four clinical-stage programs anticipated by 2020 1 Novartis Surface 2 Surface Surface Surface Surface 2 Surface (1) Novartis has WW development and commercial rights to this program. (2) Novartis has the right to purchase an option for this program. 9Broad Portfolio Targeting the Tumor Microenvironment Four clinical-stage programs anticipated by 2020 1 Novartis Surface 2 Surface Surface Surface Surface 2 Surface (1) Novartis has WW development and commercial rights to this program. (2) Novartis has the right to purchase an option for this program. 9

High affinity, fully human antibody against human CD73 Potent inhibition of CD73 enzymatic activity Significant reduction of adenosine and increased proliferation of T cells Strong synergistic preclinical in vivo anti-tumor activity in combination with checkpoint inhibitors (PD-1) Phase 1 initiated June 2018 Licensed worldwide rights to Novartis 10High affinity, fully human antibody against human CD73 Potent inhibition of CD73 enzymatic activity Significant reduction of adenosine and increased proliferation of T cells Strong synergistic preclinical in vivo anti-tumor activity in combination with checkpoint inhibitors (PD-1) Phase 1 initiated June 2018 Licensed worldwide rights to Novartis 10

NZV930 Overview Phase I Design and Novartis Economics • Novartis selected NZV930 as part of the of the initial licensing agreement in January 2016 • Over $500M in total milestones associated with the program • >60% of milestones are development and approval related • High single to mid-teen royalties on global sales • $50M in potential milestones associated with NZV930 within the next ~24 months 11NZV930 Overview Phase I Design and Novartis Economics • Novartis selected NZV930 as part of the of the initial licensing agreement in January 2016 • Over $500M in total milestones associated with the program • >60% of milestones are development and approval related • High single to mid-teen royalties on global sales • $50M in potential milestones associated with NZV930 within the next ~24 months 11

High affinity, fully human antibody against human IL-27 Overview of Reduction of IL-27 driven immunosuppression SRF388 Strong translational hypothesis in specific tumor types Potential IND-enabling studies ongoing (Filing Q4 2019) First-in-Class Novartis has right to purchase option (Q1 2019) Antibody Targeting IL-27 12High affinity, fully human antibody against human IL-27 Overview of Reduction of IL-27 driven immunosuppression SRF388 Strong translational hypothesis in specific tumor types Potential IND-enabling studies ongoing (Filing Q4 2019) First-in-Class Novartis has right to purchase option (Q1 2019) Antibody Targeting IL-27 12
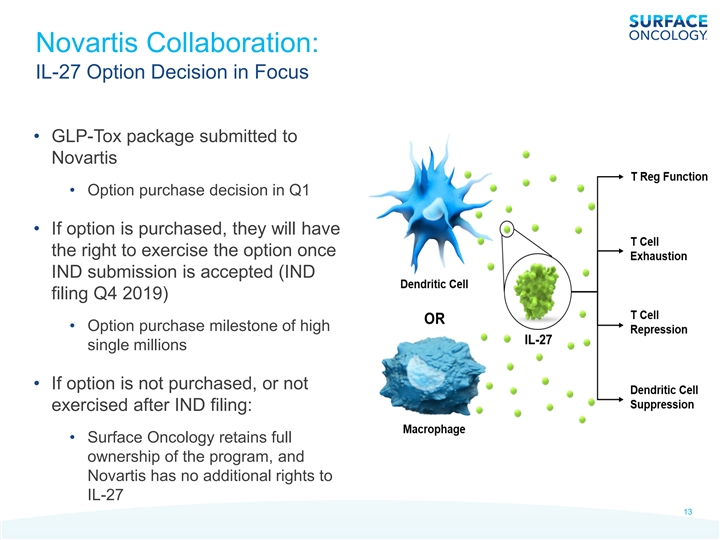
Novartis Collaboration: IL-27 Option Decision in Focus • GLP-Tox package submitted to Novartis • Option purchase decision in Q1 • If option is purchased, they will have the right to exercise the option once IND submission is accepted (IND filing Q4 2019) • Option purchase milestone of high single millions • If option is not purchased, or not exercised after IND filing: • Surface Oncology retains full ownership of the program, and Novartis has no additional rights to IL-27 13Novartis Collaboration: IL-27 Option Decision in Focus • GLP-Tox package submitted to Novartis • Option purchase decision in Q1 • If option is purchased, they will have the right to exercise the option once IND submission is accepted (IND filing Q4 2019) • Option purchase milestone of high single millions • If option is not purchased, or not exercised after IND filing: • Surface Oncology retains full ownership of the program, and Novartis has no additional rights to IL-27 13

High affinity, fully human antibody against human CD39 SRF617 Potent inhibitor of enzymatic activity Significant reduction of adenosine and increased Antibody levels of ATP leads to increased T cell proliferation Targeting and dendritic cell maturation CD39 IND-enabling studies ongoing (Filing Q4 2019) 14High affinity, fully human antibody against human CD39 SRF617 Potent inhibitor of enzymatic activity Significant reduction of adenosine and increased Antibody levels of ATP leads to increased T cell proliferation Targeting and dendritic cell maturation CD39 IND-enabling studies ongoing (Filing Q4 2019) 14

SRF617 Inhibits CD39 Enzymatic Activity & Stimulates Dendritic Cell Maturation Inhibition Of Enzymatic Activity Dendritic Cell Maturation 20000 Donor 1 S R F 6 17 Donor 2 C on t r ol 100 Donor 3 15000 10000 50 5000 0 0 -3 -2 -1 0 1 Log a ntib ody c on c e ntr a tio n (ug /m L ) Assays performed on Human MOLP8 Myeloma Cells Dendritic cell maturation was determined by measuring levels of CD86 15 C ont r ol S R F 617 % In h ib itio n D en d r itic C e ll M atu r atio n (C D 8 6 E x p r es s ion ; gM F I)SRF617 Inhibits CD39 Enzymatic Activity & Stimulates Dendritic Cell Maturation Inhibition Of Enzymatic Activity Dendritic Cell Maturation 20000 Donor 1 S R F 6 17 Donor 2 C on t r ol 100 Donor 3 15000 10000 50 5000 0 0 -3 -2 -1 0 1 Log a ntib ody c on c e ntr a tio n (ug /m L ) Assays performed on Human MOLP8 Myeloma Cells Dendritic cell maturation was determined by measuring levels of CD86 15 C ont r ol S R F 617 % In h ib itio n D en d r itic C e ll M atu r atio n (C D 8 6 E x p r es s ion ; gM F I)

Upcoming Milestones & Summary 16Upcoming Milestones & Summary 16

Upcoming Milestones Four Clinical-Stage Programs Anticipated by 2020 PROGRAM CANDIDATE KEY NEAR TERM MILESTONES • Initial Phase 1 results CD73 NZV930 • Combinations with anti-PD-1 and A2AR • IND-enabling studies underway CD39 SRF617 • Anticipated IND filing Q4 2019 • IND-enabling studies underway IL-27 SRF388 • Novartis option purchase decision (Q1 2019) • Anticipated IND filing Q4 2019 • Dose exploration ongoing CD47 SRF231 • Additional data (H2 2019) 17Upcoming Milestones Four Clinical-Stage Programs Anticipated by 2020 PROGRAM CANDIDATE KEY NEAR TERM MILESTONES • Initial Phase 1 results CD73 NZV930 • Combinations with anti-PD-1 and A2AR • IND-enabling studies underway CD39 SRF617 • Anticipated IND filing Q4 2019 • IND-enabling studies underway IL-27 SRF388 • Novartis option purchase decision (Q1 2019) • Anticipated IND filing Q4 2019 • Dose exploration ongoing CD47 SRF231 • Additional data (H2 2019) 17

Positioned to Break Through Robust Broad Product- Immuno- Generating Oncology Capabilities Pipeline Strong Uniquely Financial Collaborative Position Advisors and and Partners Team 18Positioned to Break Through Robust Broad Product- Immuno- Generating Oncology Capabilities Pipeline Strong Uniquely Financial Collaborative Position Advisors and and Partners Team 18
