Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Intellia Therapeutics, Inc. | d683843d8k.htm |

Corporate Overview January 2019 Exhibit 99.1

Intellia Therapeutics’ Legal Disclaimer This presentation contains “forward-looking statements” of Intellia Therapeutics, Inc. (“Intellia”) within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements include, but are not limited to, express or implied statements regarding Intellia’s ability to advance and expand the CRISPR/Cas9 technology to develop human therapeutic products, as well as our CRISPR/Cas9 intellectual property portfolio; our ability to achieve stable or effective genome editing; our ability to effectively administer one dose or multiple doses of our CRISPR/Cas9 product candidates; the potential timing and advancement of our preclinical studies, including continuing non-human primate studies for our Transthyretin Amyloidosis (“ATTR”) program and other studies for our other programs (such as, alpha-1 antitrypsin deficiency (“AATD”)), and human clinical trials; the timing and potential achievement of milestones to advance our pipeline including initiation of investigational new drug (“IND”)-enabling studies and filing INDs; our ability to replicate results achieved in our preclinical studies, including those in our ATTR, AATD, primary hyperoxaluria type 1 (“PH1”) and Wilms’ Tumor 1 (“WT1")/acute myeloid leukemia programs, as well as central nervous system-related efforts, in any future studies, including human clinical trials; our ability to generate data and replicate results relating to enhancements to our proprietary lipid nanoparticle (“LNP”) technology, including its formulation and components, in preclinical or clinical studies, or that any enhancements will result in an improved product candidate profile; the potential development of our proprietary LNP- adeno-associated virus (“AAV”) hybrid delivery system to advance our complex genome editing capabilities; the potential development of other in vivo or ex vivo cell therapeutics of all types, and those targeting WT1 in particular, using CRISPR/Cas9 technology; our ability to conduct successful IND-enabling studies of a lead ATTR development candidate and subsequently submitting an IND application that will be accepted by the regulatory agencies; our intent to generate and present additional data for organs beyond the liver, additional insertion/repair data, and preclinical data in support of our first ex vivo programs on immuno-oncology during 2019 or thereafter; the intellectual property position and strategy of Intellia’s licensors or other parties from which it derives rights, as well as third-parties and competitors; actions by government agencies; our growth as a company and the anticipated contribution of the members of our board of directors and our executives to our operations and progress; the impact of our collaborations on our research and development programs; the potential timing of regulatory filings regarding our development programs; the potential commercialization opportunities, including value and market, for our product candidates; our expectations regarding our uses of capital, expenses, future accumulated deficit and other 2018 financial results; and our ability to fund operations into 2021. Any forward-looking statements in this presentation are based on management’s current expectations and beliefs of future events, and are subject to a number of risks and uncertainties that could cause actual results to differ materially and adversely from those set forth in or implied by such forward-looking statements. These risks and uncertainties include, but are not limited to: uncertainties related to the initiation and conduct of studies and other development requirements for our product candidates; the risk that any one or more of Intellia’s product candidates will not be successfully developed and commercialized; the risk that the results of preclinical studies will not be predictive of future results in connection with future studies; and the risk that Intellia’s collaborations with Novartis or Regeneron or its other ex vivo collaborations will not continue or will not be successful; and risks related to Intellia’s ability to protect and maintain our intellectual property position; risks related to the ability of our licensors to protect and maintain their intellectual property position. For a discussion of these and other risks and uncertainties, and other important factors, any of which could cause Intellia’s actual results to differ from those contained in the forward-looking statements, see the section entitled “Risk Factors” in Intellia’s most recent annual report on Form 10-K and quarterly reports on Form 10-Q filed with the Securities and Exchange Commission, as well as discussions of potential risks, uncertainties, and other important factors in Intellia’s other filings with the Securities and Exchange Commission. All information in this presentation is as of the date of the release, and Intellia Therapeutics undertakes no duty to update this information unless required by law.

Our Mission Developing curative genome editing treatments that can positively transform the lives of people living with severe and life-threatening diseases
Elements for Our Success Develop Curative CRISPR-Based Medicines Advance Our Science Be the Best Place to Make Therapies Build for Long-Term Sustainability
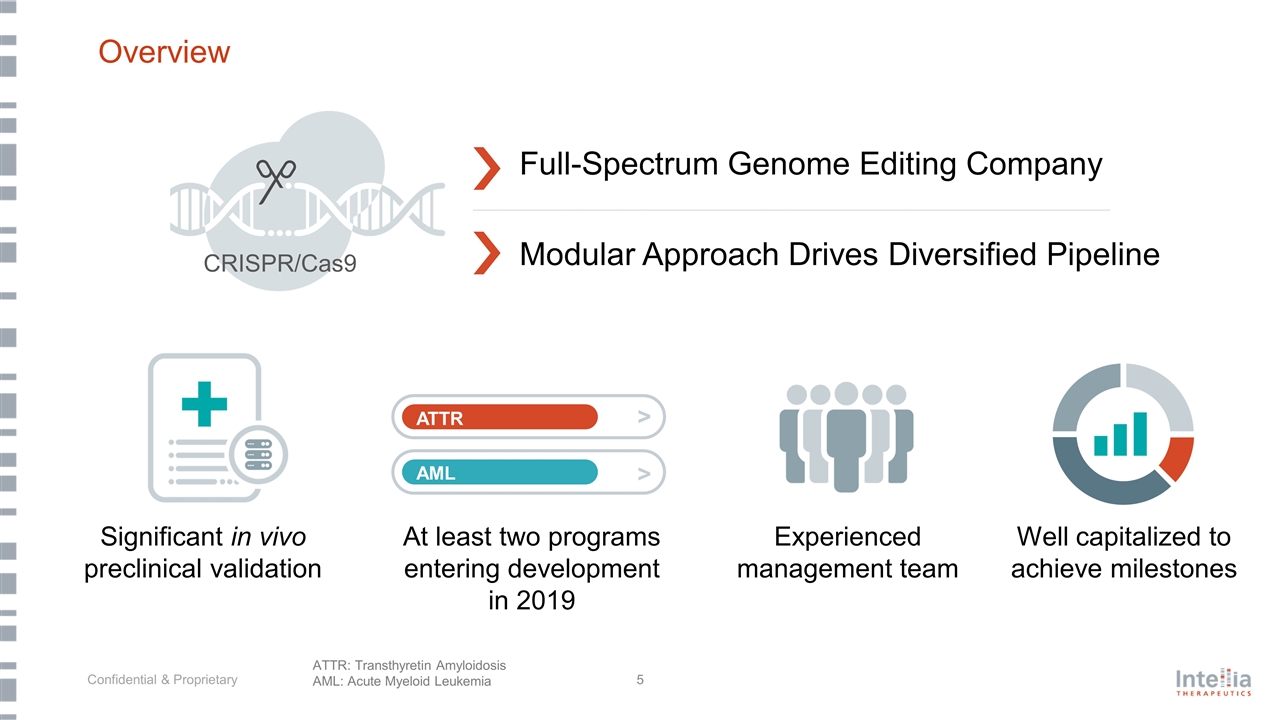
Overview Modular Approach Drives Diversified Pipeline Full-Spectrum Genome Editing Company Significant in vivo preclinical validation Experienced management team At least two programs entering development in 2019 Well capitalized to achieve milestones ATTR AML > > CRISPR/Cas9 ATTR: Transthyretin Amyloidosis AML: Acute Myeloid Leukemia
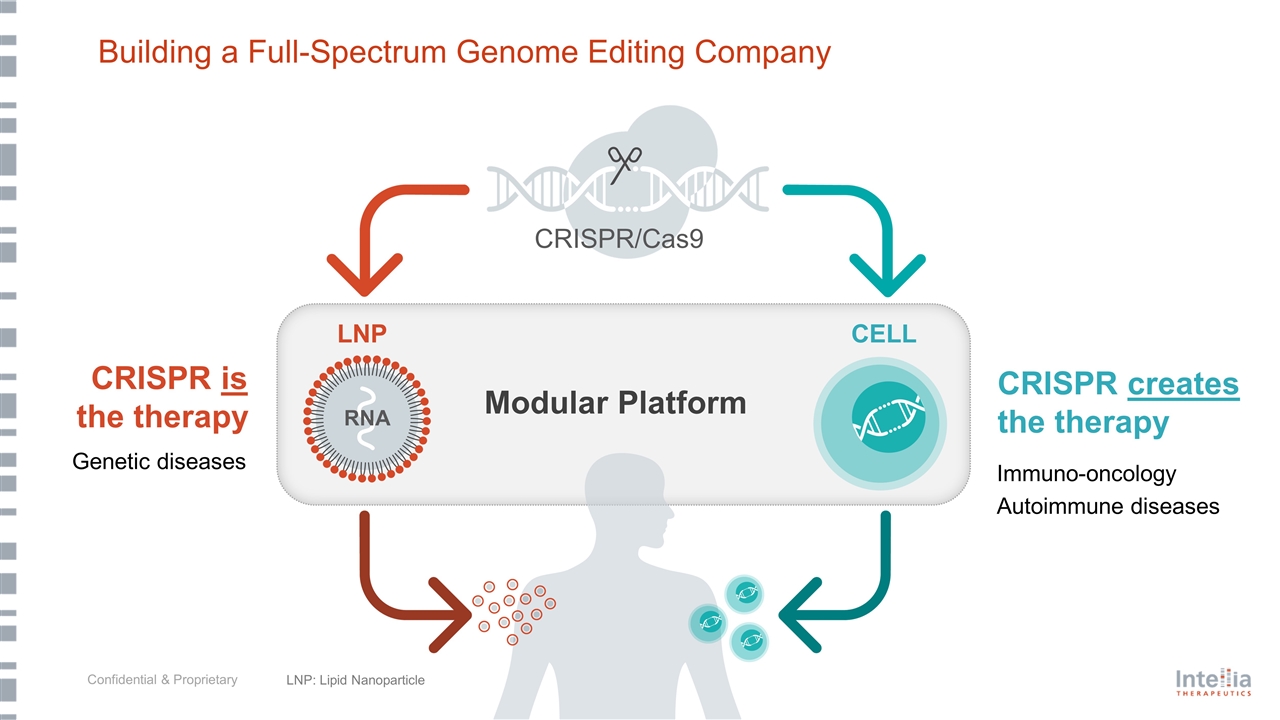
Building a Full-Spectrum Genome Editing Company CRISPR creates the therapy CRISPR is the therapy Immuno-oncology Autoimmune diseases LNP: Lipid Nanoparticle Genetic diseases Modular Platform LNP CELL RNA CRISPR/Cas9
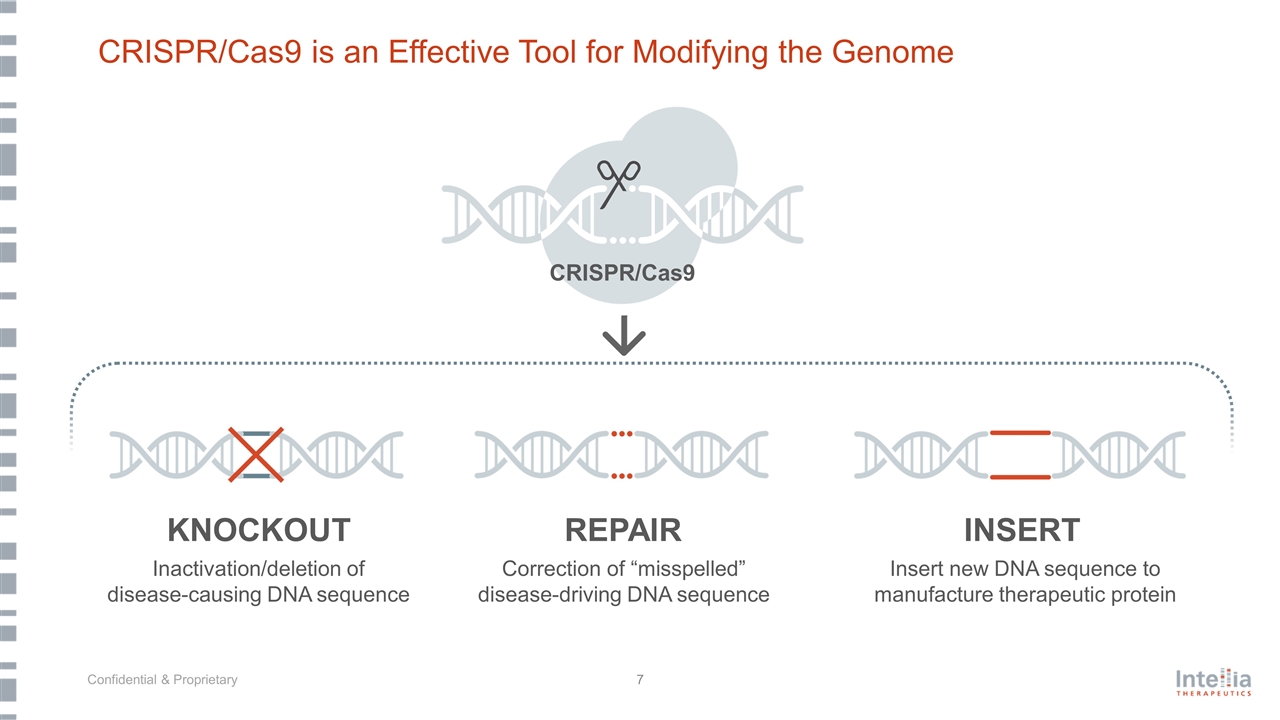
CRISPR/Cas9 is an Effective Tool for Modifying the Genome KNOCKOUT Inactivation/deletion of disease-causing DNA sequence Insert new DNA sequence to manufacture therapeutic protein Correction of “misspelled” disease-driving DNA sequence REPAIR INSERT CRISPR/Cas9
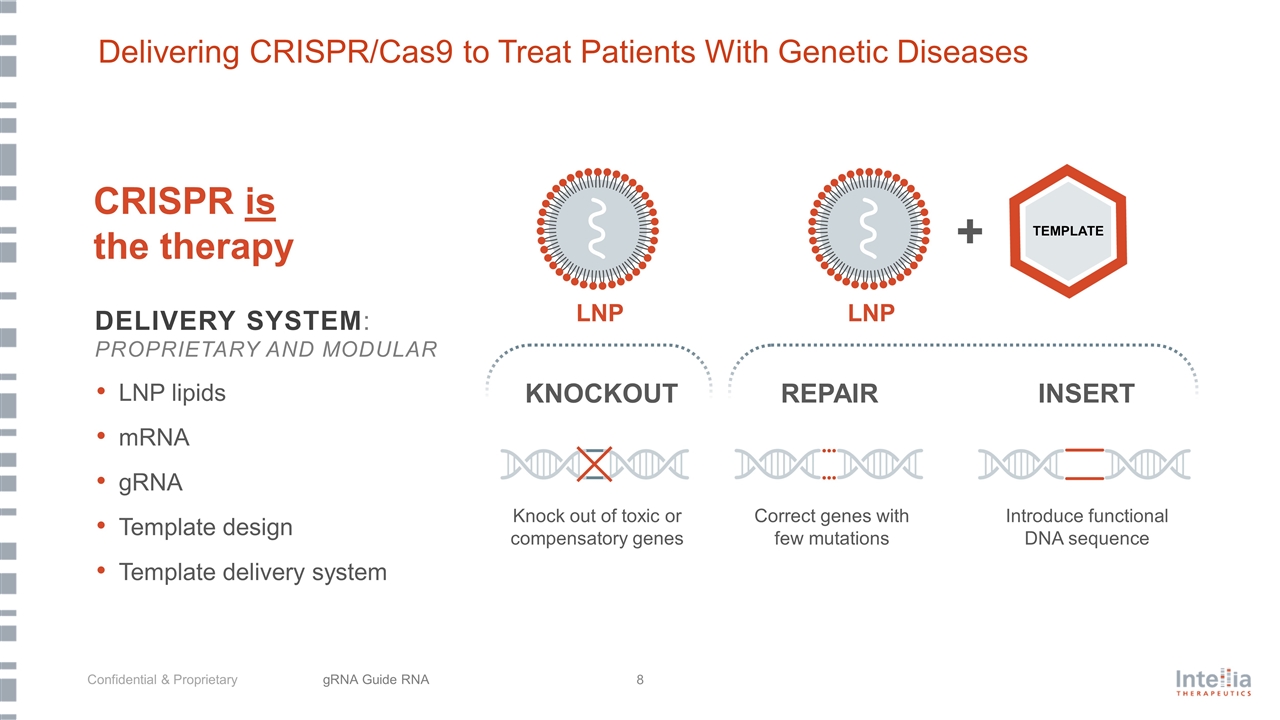
CRISPR is the therapy KNOCKOUT REPAIR INSERT + Knock out of toxic or compensatory genes Introduce functional DNA sequence Correct genes with few mutations DELIVERY SYSTEM: PROPRIETARY AND MODULAR LNP lipids mRNA gRNA Template design Template delivery system TEMPLATE Delivering CRISPR/Cas9 to Treat Patients With Genetic Diseases gRNA Guide RNA LNP LNP
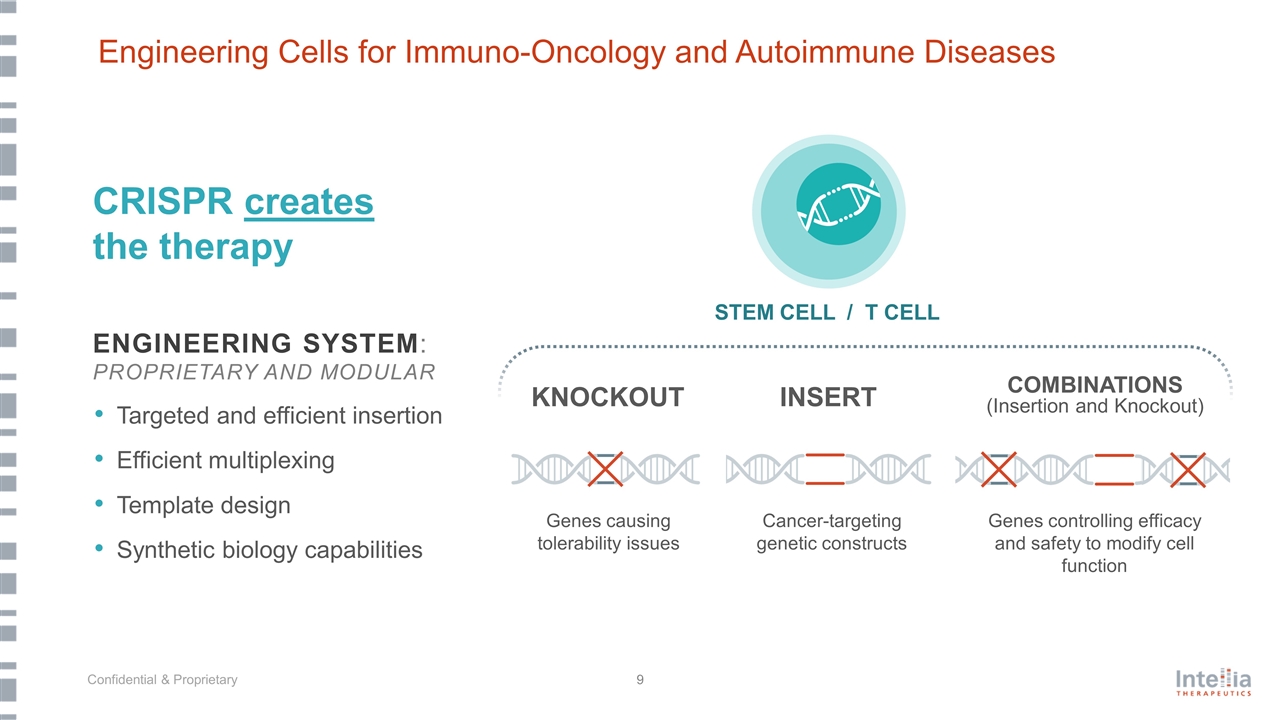
STEM CELL / T CELL INSERT COMBINATIONS (Insertion and Knockout) CRISPR creates the therapy KNOCKOUT Genes causing tolerability issues Genes controlling efficacy and safety to modify cell function Cancer-targeting genetic constructs ENGINEERING SYSTEM: PROPRIETARY AND MODULAR Targeted and efficient insertion Efficient multiplexing Template design Synthetic biology capabilities Engineering Cells for Immuno-Oncology and Autoimmune Diseases
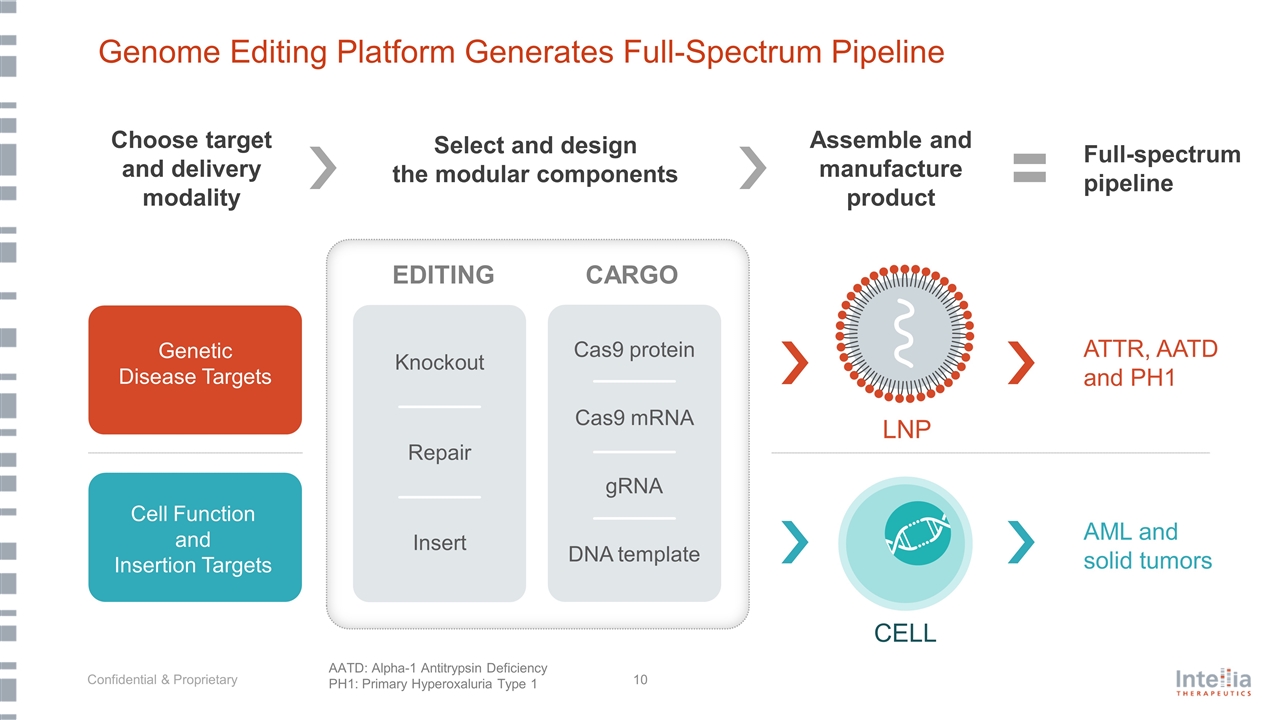
Genome Editing Platform Generates Full-Spectrum Pipeline LNP Select and design the modular components Genetic Disease Targets CELL Cell Function and Insertion Targets ATTR, AATD and PH1 AML and solid tumors AATD: Alpha-1 Antitrypsin Deficiency PH1: Primary Hyperoxaluria Type 1 Full-spectrum pipeline Choose target and delivery modality Assemble and manufacture product CARGO gRNA Cas9 mRNA Cas9 protein DNA template EDITING Knockout Insert Repair
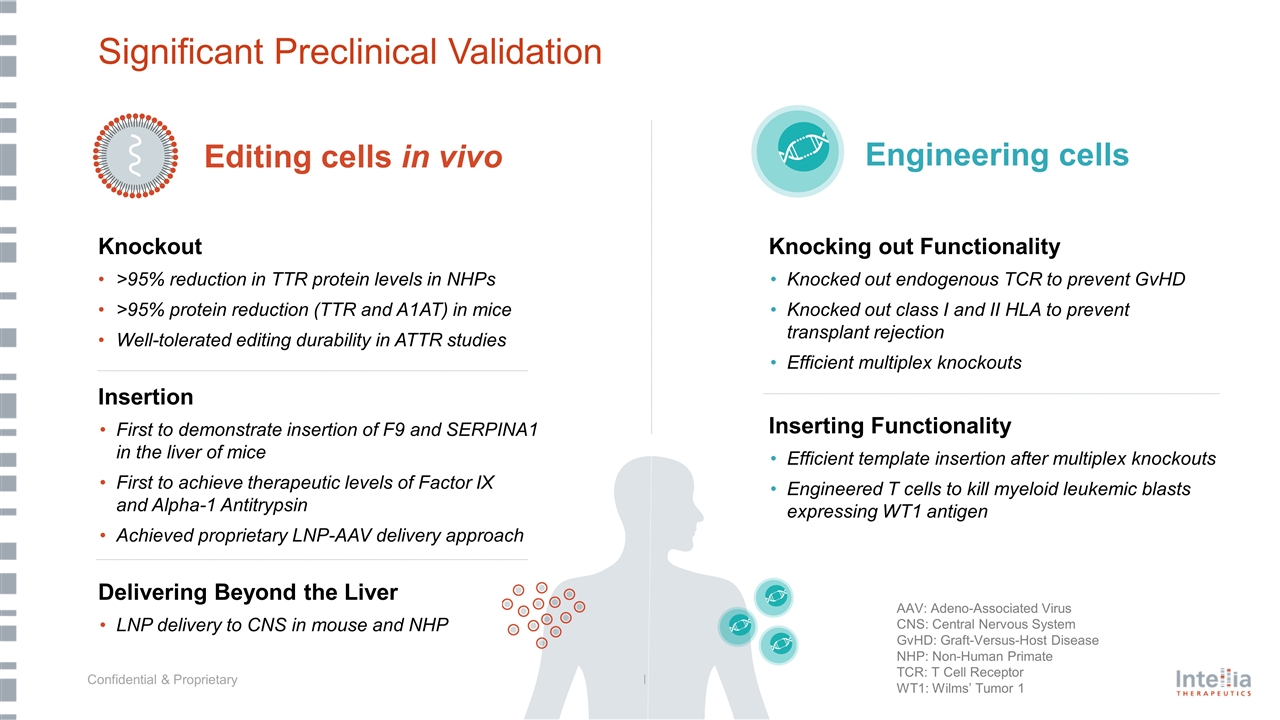
Significant Preclinical Validation Knockout >95% reduction in TTR protein levels in NHPs >95% protein reduction (TTR and A1AT) in mice Well-tolerated editing durability in ATTR studies Insertion First to demonstrate insertion of F9 and SERPINA1 in the liver of mice First to achieve therapeutic levels of Factor IX and Alpha-1 Antitrypsin Achieved proprietary LNP-AAV delivery approach Delivering Beyond the Liver LNP delivery to CNS in mouse and NHP Knocking out Functionality Knocked out endogenous TCR to prevent GvHD Knocked out class I and II HLA to prevent transplant rejection Efficient multiplex knockouts Inserting Functionality Efficient template insertion after multiplex knockouts Engineered T cells to kill myeloid leukemic blasts expressing WT1 antigen Editing cells in vivo Engineering cells AAV: Adeno-Associated Virus CNS: Central Nervous System GvHD: Graft-Versus-Host Disease NHP: Non-Human Primate TCR: T Cell Receptor WT1: Wilms’ Tumor 1
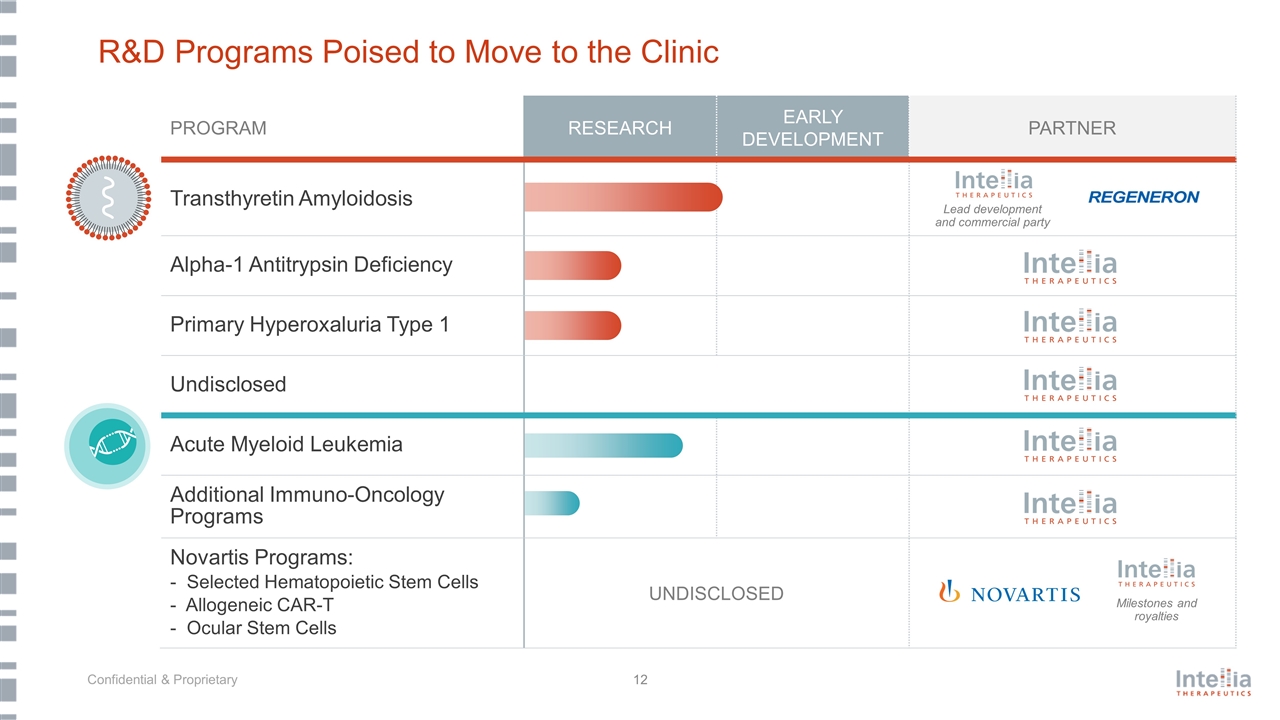
R&D Programs Poised to Move to the Clinic PROGRAM RESEARCH EARLY DEVELOPMENT PARTNER Transthyretin Amyloidosis Alpha-1 Antitrypsin Deficiency Primary Hyperoxaluria Type 1 Undisclosed Acute Myeloid Leukemia Additional Immuno-Oncology Programs Novartis Programs: - Selected Hematopoietic Stem Cells - Allogeneic CAR-T - Ocular Stem Cells UNDISCLOSED Lead development and commercial party Milestones and royalties
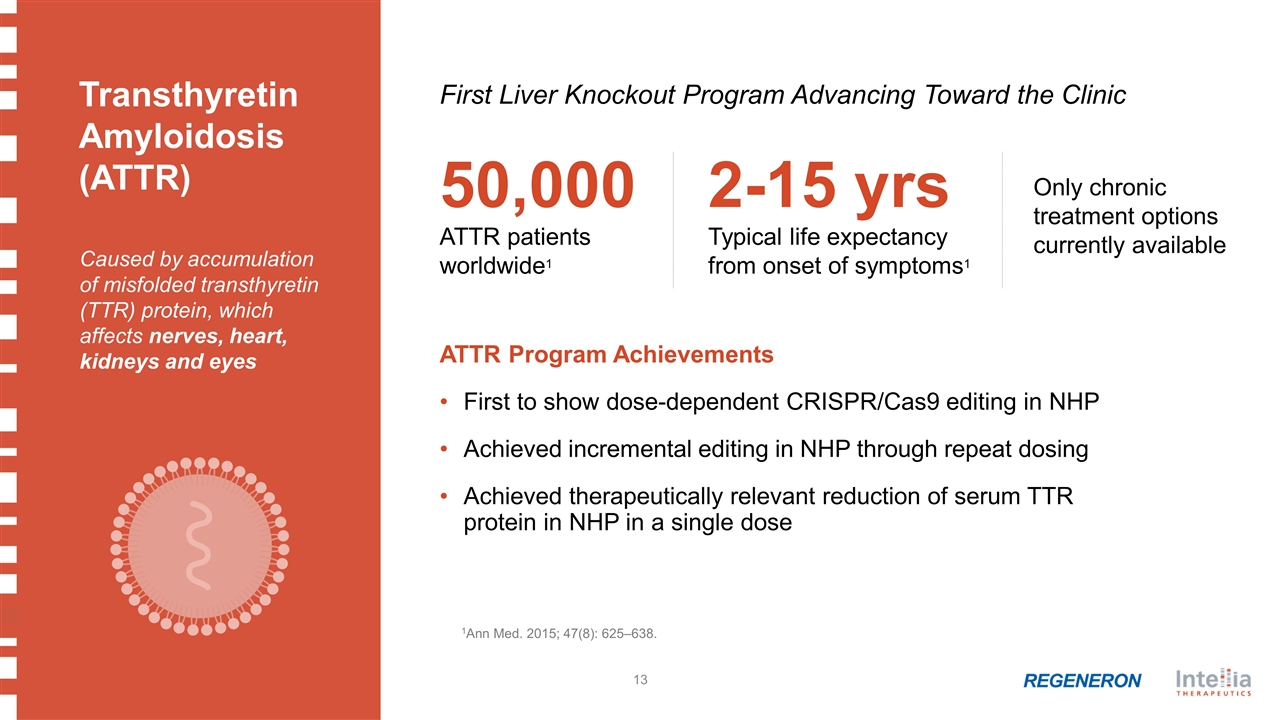
1Ann Med. 2015; 47(8): 625–638. 50,000 ATTR patients worldwide1 Caused by accumulation of misfolded transthyretin (TTR) protein, which affects nerves, heart, kidneys and eyes 2-15 yrs Typical life expectancy from onset of symptoms1 Only chronic treatment options currently available ATTR Program Achievements First to show dose-dependent CRISPR/Cas9 editing in NHP Achieved incremental editing in NHP through repeat dosing Achieved therapeutically relevant reduction of serum TTR protein in NHP in a single dose First Liver Knockout Program Advancing Toward the Clinic Transthyretin Amyloidosis (ATTR)
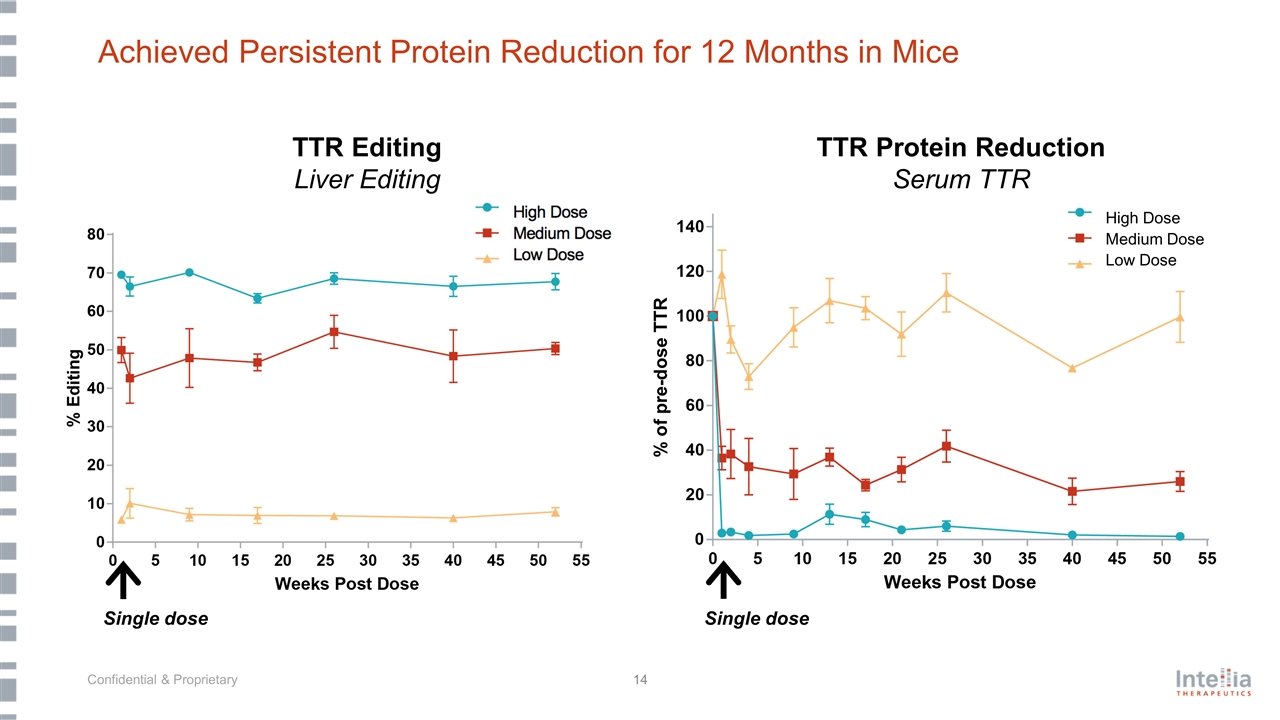
Achieved Persistent Protein Reduction for 12 Months in Mice TTR Editing TTR Protein Reduction Serum TTR High Dose Medium Dose Low Dose Single dose TTR Editing Liver Editing Single dose
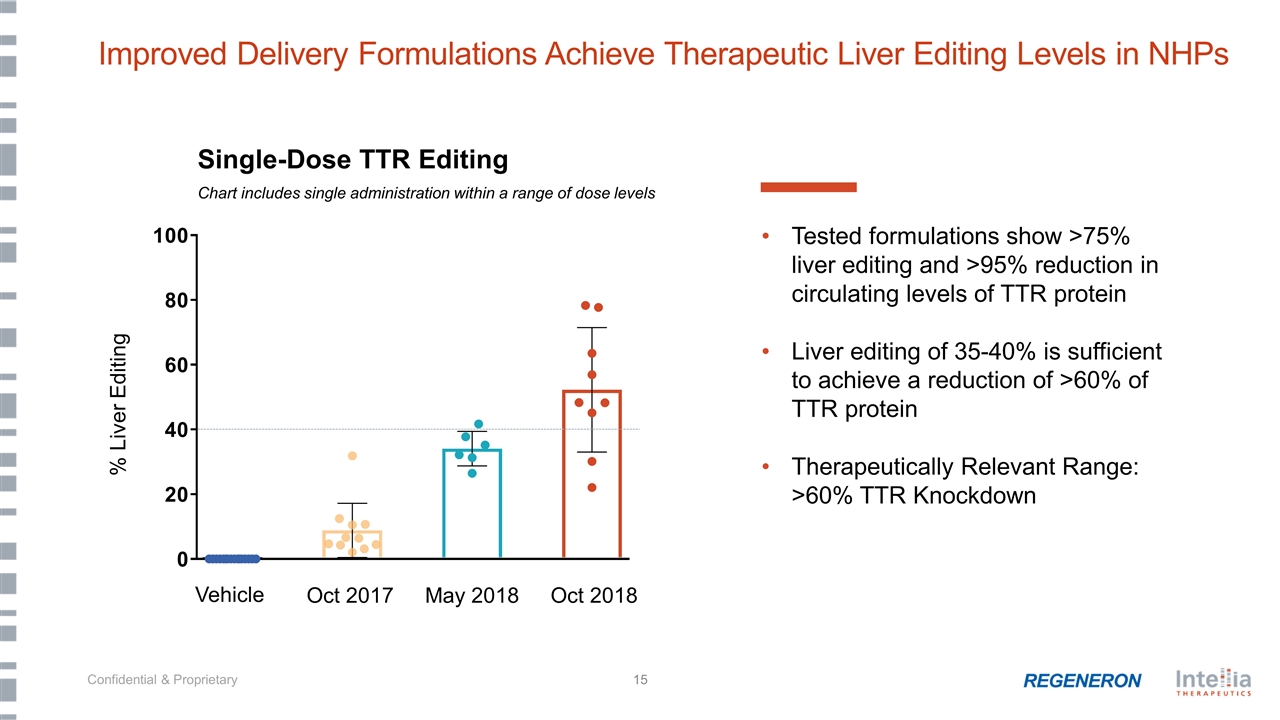
Tested formulations show >75% liver editing and >95% reduction in circulating levels of TTR protein Liver editing of 35-40% is sufficient to achieve a reduction of >60% of TTR protein Therapeutically Relevant Range: >60% TTR Knockdown Improved Delivery Formulations Achieve Therapeutic Liver Editing Levels in NHPs % Liver Editing Single-Dose TTR Editing Chart includes single administration within a range of dose levels Vehicle Oct 2017 May 2018 Oct 2018
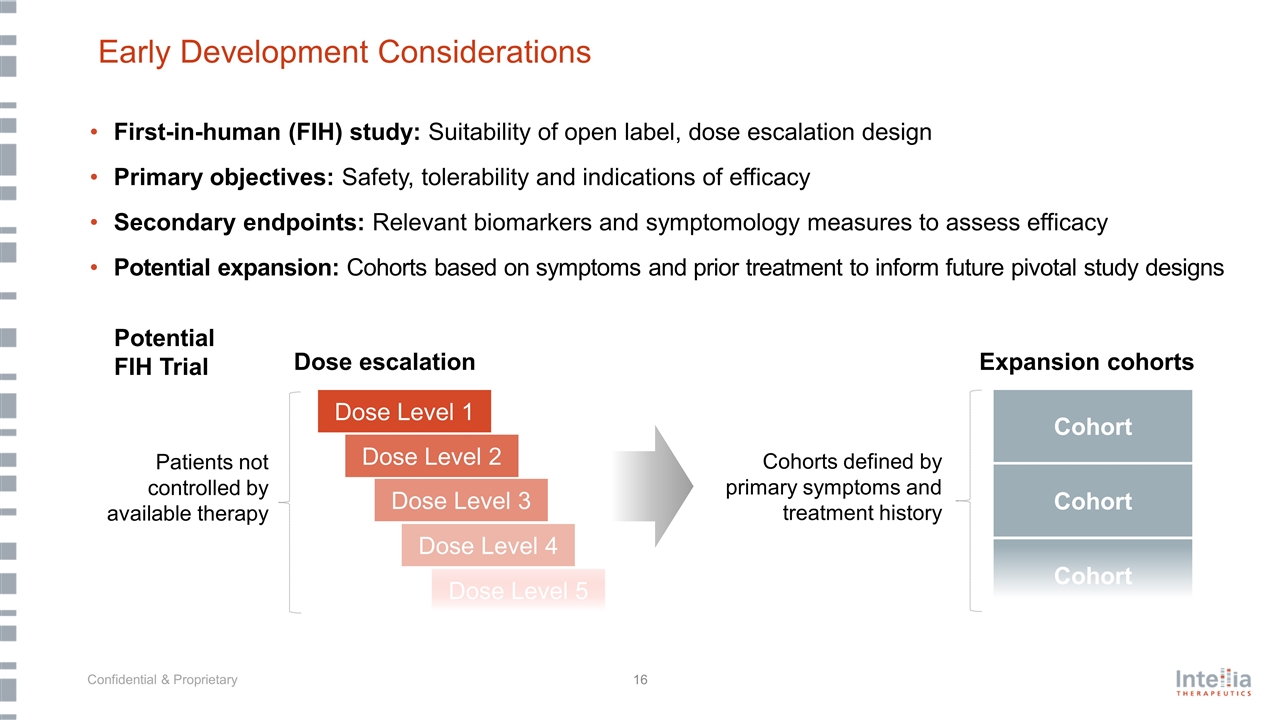
Early Development Considerations First-in-human (FIH) study: Suitability of open label, dose escalation design Primary objectives: Safety, tolerability and indications of efficacy Secondary endpoints: Relevant biomarkers and symptomology measures to assess efficacy Potential expansion: Cohorts based on symptoms and prior treatment to inform future pivotal study designs Dose Level 1 Dose Level 3 Dose Level 4 Dose Level 5 Dose Level 2 Cohort Cohort Cohort Cohorts defined by primary symptoms and treatment history Patients not controlled by available therapy Dose escalation Expansion cohorts Potential FIH Trial
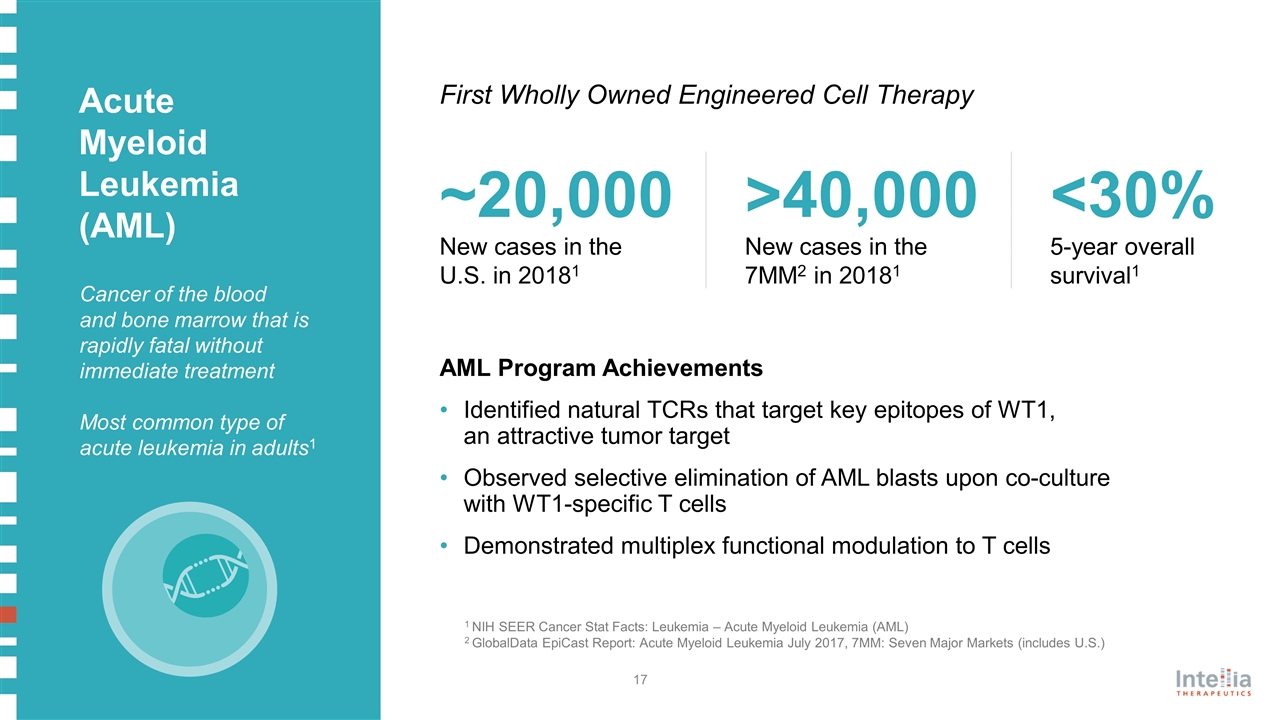
1 NIH SEER Cancer Stat Facts: Leukemia – Acute Myeloid Leukemia (AML) 2 GlobalData EpiCast Report: Acute Myeloid Leukemia July 2017, 7MM: Seven Major Markets (includes U.S.) ~20,000 New cases in the U.S. in 20181 Cancer of the blood and bone marrow that is rapidly fatal without immediate treatment Most common type of acute leukemia in adults1 First Wholly Owned Engineered Cell Therapy Acute Myeloid Leukemia (AML) <30% 5-year overall survival1 >40,000 New cases in the 7MM2 in 20181 AML Program Achievements Identified natural TCRs that target key epitopes of WT1, an attractive tumor target Observed selective elimination of AML blasts upon co-culture with WT1-specific T cells Demonstrated multiplex functional modulation to T cells
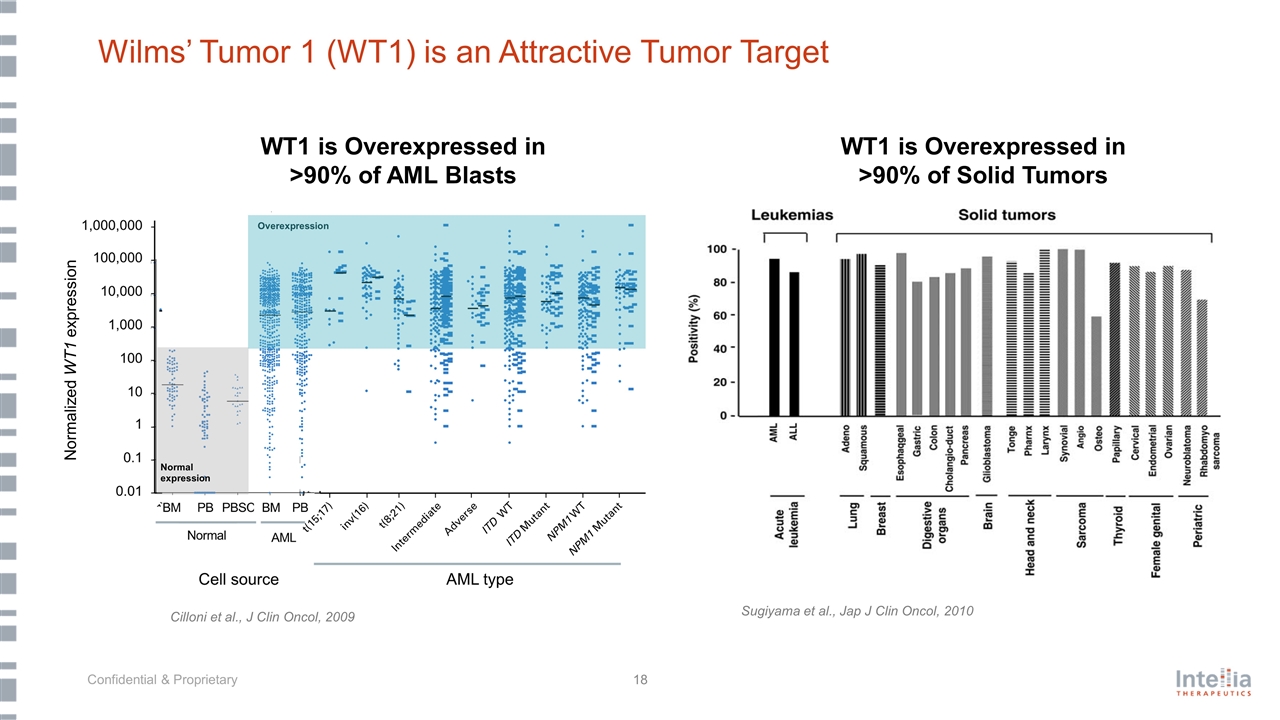
Wilms’ Tumor 1 (WT1) is an Attractive Tumor Target Sugiyama et al., Jap J Clin Oncol, 2010 WT1 is Overexpressed in >90% of AML Blasts Cilloni et al., J Clin Oncol, 2009 WT1 is Overexpressed in >90% of Solid Tumors Normalized WT1 expression Normal expression Overexpression
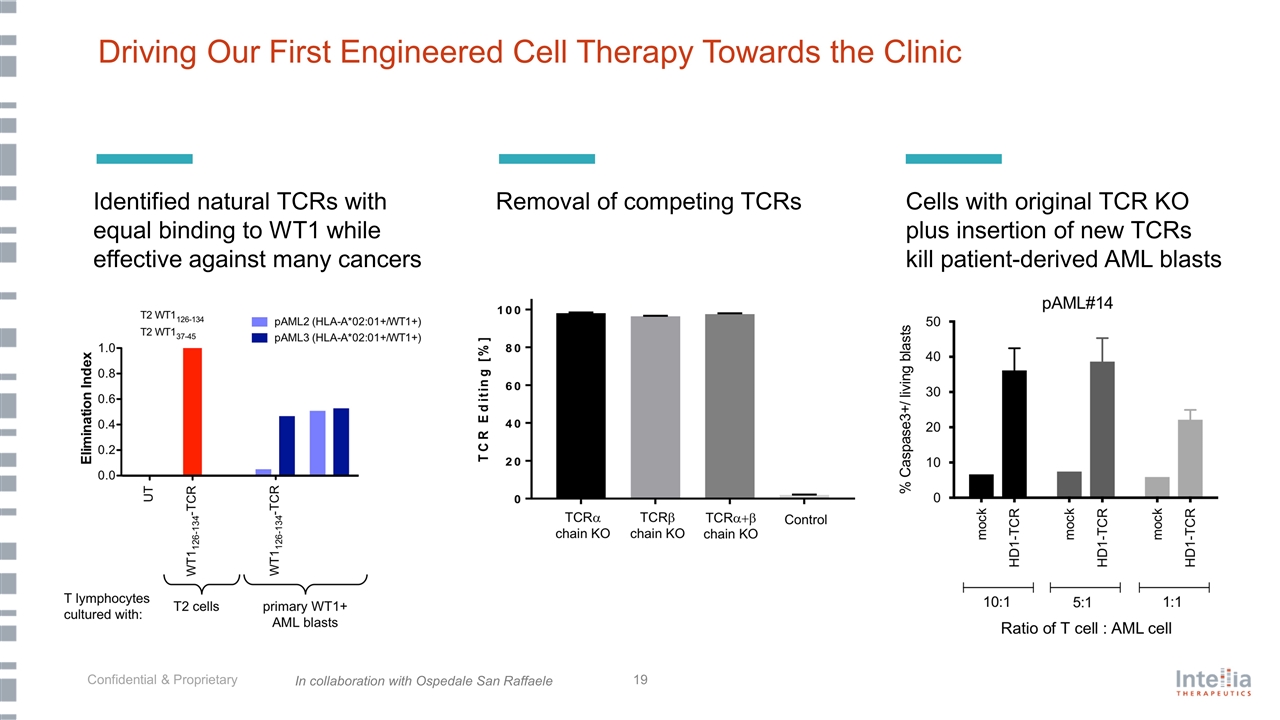
Driving Our First Engineered Cell Therapy Towards the Clinic Cells with original TCR KO plus insertion of new TCRs kill patient-derived AML blasts Identified natural TCRs with equal binding to WT1 while effective against many cancers Removal of competing TCRs In collaboration with Ospedale San Raffaele
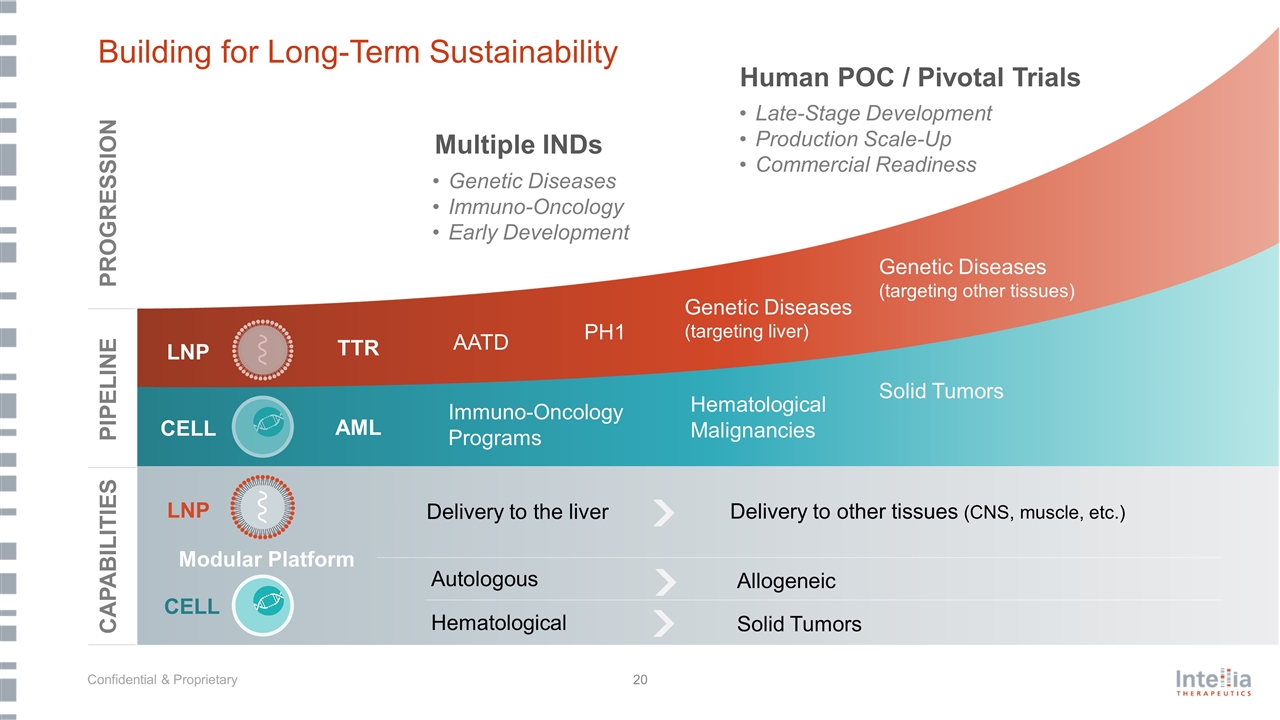
Building for Long-Term Sustainability Modular Platform Delivery to the liver CAPABILITIES PIPELINE PROGRESSION Multiple INDs Human POC / Pivotal Trials LNP CELL Autologous Hematological Delivery to other tissues (CNS, muscle, etc.) Allogeneic Solid Tumors LNP CELL TTR AML AATD PH1 Immuno-Oncology Programs Hematological Malignancies Solid Tumors Genetic Diseases (targeting liver) Genetic Diseases (targeting other tissues) Genetic Diseases Immuno-Oncology Early Development Late-Stage Development Production Scale-Up Commercial Readiness

Experienced Management Team Driving Intellia to Deliver Innovative Therapies John Leonard, M.D. President and Chief Executive Officer Jennifer King, Ph.D. Senior Vice President, Business Development Nishla Keiser, Ph.D. Senior Vice President, Deputy General Counsel José Rivera Executive Vice President and General Counsel Jennifer Mound Smoter Senior Vice President, External Affairs and Communications Thomas Barnes, Ph.D. Senior Vice President, Innovation Sciences Lindsey Trickett Vice President, Investor Relations Glenn Goddard Executive Vice President, Chief Financial Officer Andrew D. Schiermeier, Ph.D. Executive Vice President, Corporate Strategy
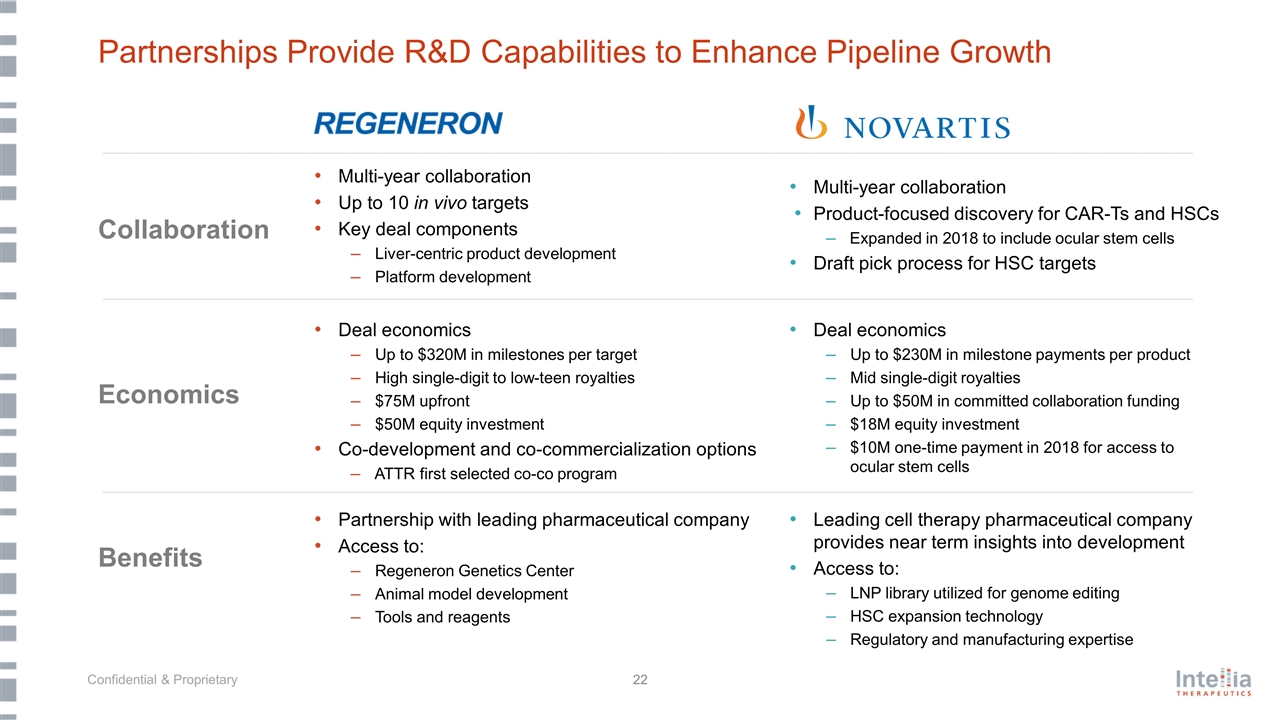
Partnerships Provide R&D Capabilities to Enhance Pipeline Growth Multi-year collaboration Up to 10 in vivo targets Key deal components Liver-centric product development Platform development Partnership with leading pharmaceutical company Access to: Regeneron Genetics Center Animal model development Tools and reagents Deal economics Up to $320M in milestones per target High single-digit to low-teen royalties $75M upfront $50M equity investment Co-development and co-commercialization options ATTR first selected co-co program Multi-year collaboration Product-focused discovery for CAR-Ts and HSCs Expanded in 2018 to include ocular stem cells Draft pick process for HSC targets Leading cell therapy pharmaceutical company provides near term insights into development Access to: LNP library utilized for genome editing HSC expansion technology Regulatory and manufacturing expertise Deal economics Up to $230M in milestone payments per product Mid single-digit royalties Up to $50M in committed collaboration funding $18M equity investment $10M one-time payment in 2018 for access to ocular stem cells Collaboration Economics Benefits
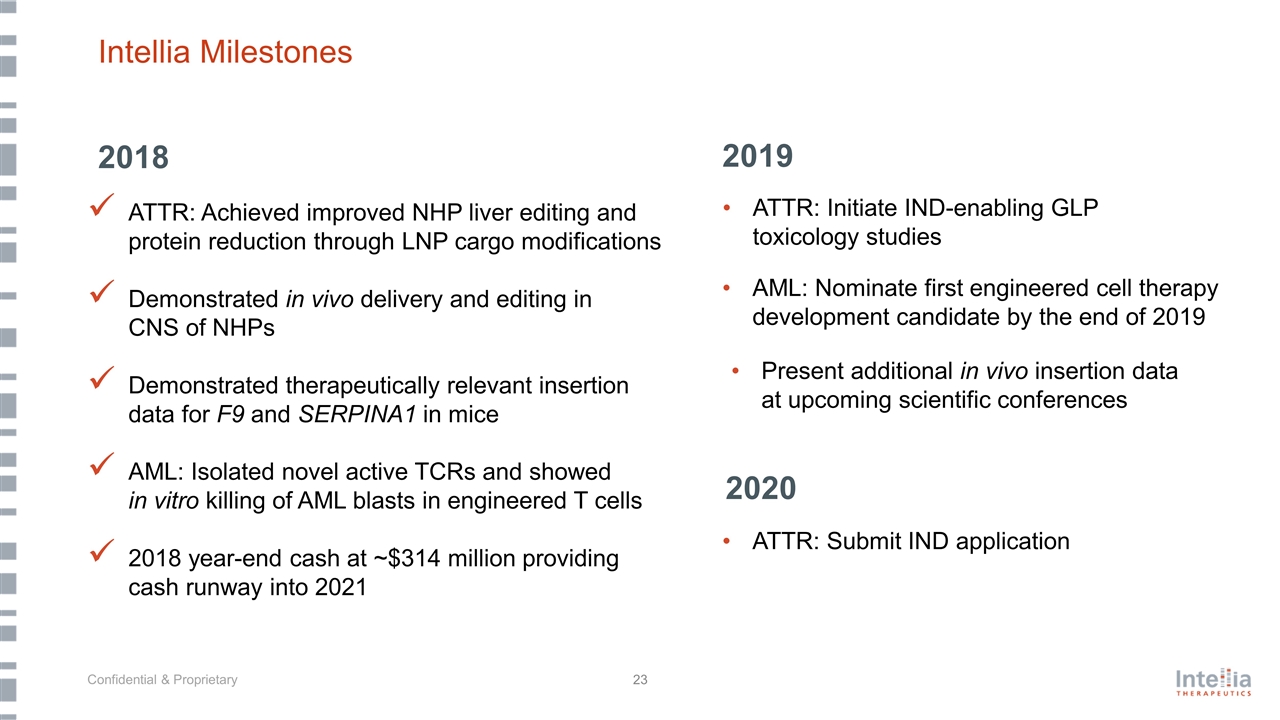
Intellia Milestones 2019 ATTR: Initiate IND-enabling GLP toxicology studies AML: Nominate first engineered cell therapy development candidate by the end of 2019 Present additional in vivo insertion data at upcoming scientific conferences ATTR: Submit IND application 2020 ATTR: Achieved improved NHP liver editing and protein reduction through LNP cargo modifications Demonstrated in vivo delivery and editing in CNS of NHPs Demonstrated therapeutically relevant insertion data for F9 and SERPINA1 in mice AML: Isolated novel active TCRs and showed in vitro killing of AML blasts in engineered T cells 2018 year-end cash at ~$314 million providing cash runway into 2021 2018
Elements for Our Success Develop Curative CRISPR-Based Medicines Advance Our Science Be the Best Place to Make Therapies Build for Long-Term Sustainability

