Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Inspire Medical Systems, Inc. | a2019-018xkcopresentation.htm |

Inspire Medical Systems, Inc. s January 2019 NYSE: INSP

Disclaimer This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995. All statements other than statements of historical facts contained in this presentation are forward-looking statements. In some cases, you can identify forward-looking statements by terms such as ‘‘may,’’ ‘‘will,’’ ‘‘should,’’ ‘‘expect,’’ ‘‘plan,’’ ‘‘anticipate,’’ ‘‘could,’’ “future,” “outlook,” ‘‘intend,’’ ‘‘target,’’ ‘‘project,’’ ‘‘contemplate,’’ ‘‘believe,’’ ‘‘estimate,’’ ‘‘predict,’’ ‘‘potential,’’ ‘‘continue,’’ or the negative of these terms or other similar expressions, although not all forward-looking statements contain these words. The forward-looking statements in this presentation relate to, among other things, statements regarding our clinical data growth, product development, indication expansion, market development and prior authorization approvals. These forward-looking statements are based on management’s current expectations and involve known and unknown risks and uncertainties that may cause our actual results, performance or achievements to be materially different from any future results, performance or achievements expressed or implied by the forward-looking statements. Such risks and uncertainties include, among others, estimates regarding the annual total addressable market for our Inspire therapy in the U.S. and our market opportunity outside the U.S., future results of operations, financial position, research and development costs, capital requirements and our needs for additional financing; commercial success and market acceptance of our Inspire therapy; our ability to achieve and maintain adequate levels of coverage or reimbursement for our Inspire system or any future products we may seek to commercialize; competitive companies and technologies in our industry; our ability to enhance our Inspire system, expand our indications and develop and commercialize additional products; our business model and strategic plans for our products, technologies and business, including our implementation thereof; our ability to accurately forecast customer demand for our Inspire system and manage our inventory; our ability to expand, manage and maintain our direct sales and marketing organization, and to market and sell our Inspire system in markets outside of the U.S.; our ability to increase the number of active medical centers implanting Inspire therapy; our ability to hire and retain our senior management and other highly qualified personnel; our ability to commercialize or obtain regulatory approvals for our Inspire therapy and system, or the effect of delays in commercializing or obtaining regulatory approvals; FDA or other U.S. or foreign regulatory actions affecting us or the healthcare industry generally, including healthcare reform measures in the U.S. and international markets; the timing or likelihood of regulatory filings and approvals; our ability to establish and maintain intellectual property protection for our Inspire therapy and system or avoid claims of infringement; the volatility of the trading price of our common stock; and our expectations about market trends. Other important factors that could cause actual results, performance or achievements to differ materially from those contemplated in this presentation can be found under the captions “Risk Factors” and "Management's Discussion and Analysis of Financial Condition and Results of Operations“ in our final prospectus filed with the SEC under Rule 424(b) on December 7, 2018, as such factors may be updated from time to time in our other filings with the SEC, which are accessible on the SEC’s website at www.sec.gov. These and other important factors could cause actual results to differ materially from those indicated by the forward-looking statements made in this presentation. Any such forward-looking statements represent management’s estimates as of the date of this presentation. While we may elect to update such forward-looking statements at some point in the future, unless required by applicable law, we disclaim any obligation to do so, even if subsequent events cause our views to change. Thus, one should not assume that our silence over time means that actual events are bearing out as expressed or implied in such forward-looking statements. These forward-looking statements should not be relied upon as representing our views as of any date subsequent to the date of this presentation. This presentation contains trademarks, trade names and service marks of other companies, which are the property of their respective owners. We do not intend our use or display of other parties' trademarks, trade names or service marks to imply, and such use or display should not be construed to imply, a relationship with, or endorsement or sponsorship of us by, these other parties. 2

Inspire is an Innovative Neurostimulation Solution for Patients with Moderate to Severe OSA Our History & Key Milestones First and only FDA-approved neurostimulation technology for OSA 1990s: Medtronic begins early work on the development of Inspire More than 4,000 patients treated with Inspire therapy 2001: Initial clinical results published; Medtronic begins Inspire II development Alternative for the estimated 35 – 65% of non-CPAP compliant patients 2007: Inspire is founded after being spun-out of Medtronic 2011: Initiated Phase III pivotal STAR trial; ~$10bn annual U.S. market opportunity CE mark received in Europe 2014: STAR results published in the New England Innovative, closed-loop, minimally invasive solution Journal of Medicine in January; received PMA approval from the FDA in April 2015: 18-month STAR data published; Safe, comfortable and convenient therapy alternative revenues of $8.0mm 2016: 1,000th implant milestone; revenues of $16.4mm Significant body of clinical evidence involving ~980 patients across 16 studies 2017: Launched Inspire IV in U.S.; announced 5- year STAR results; 2,000th implant milestone; revenues of $28.6mm Strong customer base, growing salesforce and scalable reimbursement infrastructure 2018: Inspire IV CE mark; 5-year STAR results publication; initial public offering on NYSE; Aetna begins covering the Inspire therapy; Proven management team leading our 150 employees 9M18/9M17 revenues of $34.0/$18.6mm, represents 83% growth; 508 patient ADHERE 3 publication

Strong Management Team Other Key Management • Steve Jandrich – Chief Compliance Officer, VP, Human Resources • Kathy Sherwood – VP, Global Market Access • Howard Green – VP, Marketing 3 • Andreas Henke – VP, Commercial Operations, Europe • John Rondoni – VP, Product Development, Tim Herbert Rick Buchholz Randy Ban Operations & QA • Quan Ni – VP, Research President, CEO & Chief Financial Officer SVP, Sales and Marketing Founder 30+ Years of Experience 15+ Years of Experience 20+ Years of Experience 4

Obstructive Sleep Apnea (OSA) is a Serious and Chronic Disease OSA is Caused by a Blocked or Partially Blocked Airway Blockage prevents airflow to the lungs Results in repeated arousals and oxygen de-saturations Severity of sleep apnea is measured by frequency of apnea or hypopnea events per hour, which is referred to as the Apnea-Hypopnea Index (AHI) Normal range: Moderate sleep apnea: AHI < 5 events per hour 15 ≤ AHI < 30 events per hour Airway obstruction Mild sleep apnea: Severe sleep apnea: during breathing 5 ≤ AHI < 15 events per hour AHI ≥ 30 events per hour Inspire’s Focus Most Patients Are Unaware of Their Condition… …and Untreated OSA Multiplies Serious Health Risks High risk patients: obese, male or of advanced age 2x Increased Risk of Mortality (5) Common first indicator: heavy snoring The risk for stroke (1) Other indicators: 2x (2) Lack of energy Memory or concentration The risk for sudden cardiac death Headaches problems 5x (3) % Surviving Depression Excessive daytime The risk for cardiovascular mortality sleepiness Nighttime gasping 57% Increased risk for recurrence of Dry mouth Atrial Fibrillation after ablation (4) Years of Follow-up ____________________ 5 Source: Company Website (4) Li et al, Europace 2014. (1) Redline et al, The Sleep Heart Health Study. Am J Res and Crit Care Med 2010. (5) Prospective Study of Obstructive Sleep Apnea and Incident Coronary Heart Disease and Heart Failure from SHHS and (2) Gami et al, J Am Coll Cardiol 2013. Wisconsin Sleep Cohort Study. (3) Young et al, J Sleep 2008.

Sleep Apnea is a Major Public Health Problem & Awareness is Building Metrics – Week of January 22, 2018 55,823 (+22%) 12,199 (+43%) Web Sessions Doctor Searches 548 (+42%) 202 (+59%) 184 (+25%) Calls Emails CHT Sign-Ups (+) represents change Week over Week 6 ____________________ Source: The New York Times, Science Daily, CBS This Morning, The Washington Post and Pioneer Press.
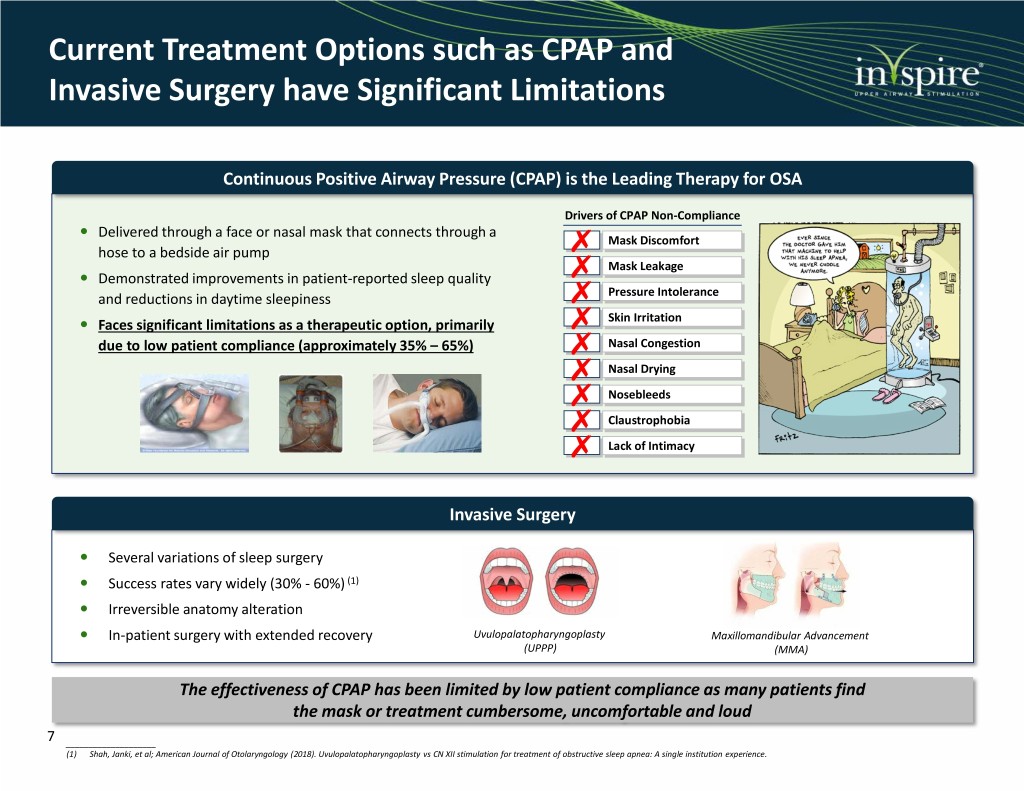
Current Treatment Options such as CPAP and Invasive Surgery have Significant Limitations Continuous Positive Airway Pressure (CPAP) is the Leading Therapy for OSA Drivers of CPAP Non-Compliance Delivered through a face or nasal mask that connects through a Mask Discomfort hose to a bedside air pump Mask Leakage Demonstrated improvements in patient-reported sleep quality and reductions in daytime sleepiness Pressure Intolerance Skin Irritation Faces significant limitations as a therapeutic option, primarily due to low patient compliance (approximately 35% – 65%) Nasal Congestion Nasal Drying Nosebleeds Claustrophobia Lack of Intimacy Invasive Surgery Several variations of sleep surgery Success rates vary widely (30% - 60%) (1) Irreversible anatomy alteration In-patient surgery with extended recovery Uvulopalatopharyngoplasty Maxillomandibular Advancement (UPPP) (MMA) The effectiveness of CPAP has been limited by low patient compliance as many patients find the mask or treatment cumbersome, uncomfortable and loud 7 ____________________ (1) Shah, Janki, et al; American Journal of Otolaryngology (2018). Uvulopalatopharyngoplasty vs CN XII stimulation for treatment of obstructive sleep apnea: A single institution experience.

A Strong Market Opportunity Exists for an Alternative to CPAP that is Effective and Minimally Invasive Prevalence & Economic Costs Our Estimated Annual U.S. Market Opportunity Sleep apnea affects +100 million people worldwide (1) Approximately 17 million individuals in the Adults with Moderate to Severe OSA Prescribed CPAP (2) = U.S. with moderate to severe OSA ~2 million • Annually, ~2 million adult patients are Less: 65% (2) CPAP Published literature prescribed a CPAP device Compliant estimates CPAP non-compliance rates Annual U.S. economic costs of untreated 35% of CPAP Non-Compliant Adults = of 35% - 65% moderate to severe OSA are between ~700,000 (3) $65 - $165 billion Less: 30% Anatomy Challenges OSA economic costs are potentially greater than asthma, heart failure, stroke and hypertensive disease 70% Inspire Anatomy Eligible = ~500,000 OSA is associated with an increase in: Multiplied by: our • Rate & severity of vehicle accidents ASP • Increased healthcare utilization Inspire U.S. Market = • Reduction of work performance ~$10 billion • Occupational injuries ____________________ 8 Note: ASP constitutes abbreviation for average selling price. (1) Source: World Health Organization. (2) Company estimates. (3) Represents moderate to severe OSA. Source: McKinsey & Company, 2010.

Our Inspire Therapy is a Disruptive Solution for Patients with OSA Inspire System Inspire Procedure 3 Stimulation Lead 1 Pressure Sensing Lead Hypoglossal Nerve 2 Neurostimulator Remote control and three implantable components: Approximately a 2-hour outpatient procedure Requires three small incisions (1 in neck and 2 in chest) 1• Pressure sensing lead: detects when the patient is attempting to breathe Patients typically recover quickly and resume normal activities in just a few days 2• Neurostimulator: houses the electronics and battery power for the device System activation occurs 30 days after implantation 3• Stimulation lead: delivers electrical stimulation Patient controls system by turning on the device each to the hypoglossal nerve night with the remote control before going to sleep 9

Inspire Therapy Overcomes Many of These Limitations with a Safe and Effective Solution Mild Stimulation is a Clear Mechanism of Action Inspire Therapy Offers Significant Benefits Strong safety profile Effective and durable treatment Closed-loop system Strong patient compliance High patient satisfaction Minimally invasive outpatient procedure ~11-year battery life (without recharging) Utilizes patient’s natural physiology Short recovery times post surgery Patient controlled therapy Long term outcomes demonstrate that Inspire therapy addresses the shortfalls of current treatments 10

Objective Measures Show the Impact of Our Inspire Therapy on OSA Polysomnogram Before and After Activation of Inspire System Inspire system turned on 12:23:00 AM 12:23:30 AM 12:24:00 AM 12:24:30 AM 12:25:00 AM 12:25:30 AM 12:26:00 AM 12:26:30 AM 12:27:00 AM 12:27:30 AM 12:28:00 AM 12:28:30 AM 12:29:00 AM 12:2 ECG1 N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) N2 (TW RPSGT) m/V F4-M1 - p/V - - - C4-M1 - - - p/V - - - C1-M2 - ousal - ousal Apnea Arousal Apnea Arousal Apnea Arousal Apnea Arousal - p/V - - EMG1 - - - - p/V Nasal PR… - - - - 0 –- - - - - Airflow m/V Airflow Apnea Obstructive (42.40s) Apnea Obstructive (23.80s) Apnea Obstructive (46.86s) Apnea Obstructive (37.93s) - - - 0 – - - - p/V Chest - - 0 –- - p/V Abdomen - Breathing - 0 –- - - - -100 –- p/V SqCG s) 12 Desaturation (38.06s) 8 Desaturation (28.06s) 8 Desaturation (51.94s) 13 Desaturation (36.06s) 8 100 –- - - 97.5 – - - 95 –- - 92.5 –- - - 90 – - - Oxygen 87.5 – Saturation % OSA events No OSA events 11 After activating the Inspire system, the patient exhibited a more regular breathing pattern, higher and more consistent blood oxygen levels, and fewer or no transient arousals

Clinical Evidence

Inspire in ~980 Patients~980 inInspire Studies 16 Across SignificantClinicalof Evidence Body Evaluating 13 (1) ____________________ Independent Company Sponsored Due to the inclusion of certain patients in multiple studies, six studies are not shown in the table because they do notdo they add any because the tablein notare six shown studies studies, multiplein certain Due of patientstheto inclusion Publication Publication Publication 2018 2018 2018 New York- Stimulation TherapyReductionforApnea (STAR) UniversityPittsburgh of MedicalCenter (UPMC) Thomas Jefferson UniversityHospital(TJUH) & TJUH Study of Inspire Therapy and UPPP ClevelandComparisonof Study Clinic Non UniversityAlabama- of Presbyterian MiddlesexHospital & Hospital - PediatricSyndromeDown / German PostMarket Study Academic Hospital in San Diego Inspire Therapy and UPPP ADHERE PatientADHERE Registry TotalPatients Evaluated Clinical Studies Clinical Birmingham incrementalto patientsthe overall total. (1) Number of PatientsNumber of Evaluated 981 508 126 20 27 25 22 90 60 97 6

Overview of the STAR Trial Multi-center, prospective, single-group, Phase III pivotal trial 12 Month Results Publication: Evaluated 126 patients who had difficulty either accepting or New England Journal of Medicine adhering to CPAP 22 medical centers across the United States and Europe Trial Randomized control therapy-withdrawal trial Design 46 patients after 12 months Patients randomized at a 1:1 ratio Withdrawal group had device turned off for at least 5 days until a sleep study or polysomnogram was performed Maintenance group continued nightly use of the device AHI: Reduction in AHI from baseline to 12 months of more than 50% Primary along with final AHI being less than 20 events per hour Endpoints ODI: Reduction in ODI of more than 50% from baseline to 12 months Evaluation of impact to a patient’s quality of life using: Secondary Functional Outcomes of Sleep Questionnaire (FOSQ) Endpoints Epworth Sleepiness Scale (ESS) ____________________ 14 Note: Oxygen desaturation index is abbreviated as “ODI.” It measures the number of times per hour of sleep that the blood’s oxygen level drops by a certain degree below baseline.

STAR Trial Met Both Primary Endpoints & Showed Statistically Significant Reductions in AHI & ODI Significant Reduction in Severity of OSA Meaningful Levels of Compliance Post-Implantation Apnea-Hypopnea Index (Median) Oxygen Desaturation Index (Median) 29.3 25.4 9.0 9.7 7.4 8.6 6.0 6.2 4.8 4.6 Events per Hour Events per Hour Baseline 12 Month 18 Month 3 Year 5 Year Baseline 12 Month 18 Month 3 Year 5 Year N=126 N=124 N=123 N=98 N=71 N=126 N=124 N=123 N=98 N=71 All p values <0.001 vs. baseline All p values <0.001 vs. baseline results in median results in median Withdrawal of Inspire Therapy Resulted in Reversal of Therapeutic Benefit, Further Demonstrating Inspire’s Effectiveness Apnea-Hypopnea Index (Mean) Oxygen Desaturation Index (Mean) 31.3 30.1 26.7 26.8 25.8 23.0 ) Events/hr 8.0 7.2 7.6 8.9 6.3 6.0 Score ( Score (Events/hr) Baseline 1 Year Randomized, Therapy- Baseline 1 Year Randomized, Therapy- withdrawal Trial withdrawal Trial Therapy-maintenance Group (N=23) Therapy-withdrawal Group (N=23) Therapy-maintenance Group (N=23) Therapy-withdrawal Group (N=23) 15

Additional STAR Findings Showed Meaningful Improvement in Compliance and Quality of Life Metrics Inspire has Substantial Positive Implications …and has Resulted in Meaningful Impact to on Patient Compliance… Patients' Quality of Life Functional Outcomes of Sleep Self-Reported Device Use Questionnaire (Median) Epworth Sleepiness Scale (Median) 100% Normalized daytime functioning = 17.9 Normalized daytime sleepiness = 10.0 18.2 18.4 18.8 18.7 11.0 90% 86% 14.6 6.0 6.0 6.0 6.0 81% 81% 80% 80% 70% Baseline 12 Mo. 18 Mo. 3 Yr. 5 Yr. Baseline 12 Mo. 18 Mo. 3 Yr. 5 Yr. 65% CPAP compliance 60% N=126 N=124 N=123 N=98 N=92 N=126 N=124 N=123 N=98 N=92 All p values <0.001 vs. baseline results 50% 40% No or Soft Snoring (Median) Bed Partner Leaves Room (Median) 35% CPAP compliance 30% 86% 87% 90% 81% 30% Patients Reported(%) Nightly Use 20% 10% 17% 5% 4% 3% 0% 1% 12 Month 24 Month 3 Year 5 Year N=124 N=117 N=108 N=92 Baseline 12 Mo. 18 Mo. 3 Yr. 5 Yr. Baseline 12 Mo. 18 Mo. 3 Yr. 5 Yr. N=108 N=103 N=103 N=100 N=80 N=108 N=103 N=103 N=100 N=80 Published literature estimates that only approximately 35% to 65% of patients prescribed a CPAP device are compliant with the therapy 16 After 12 Months, 93% of patients used Inspire Therapy at least 5 days per week
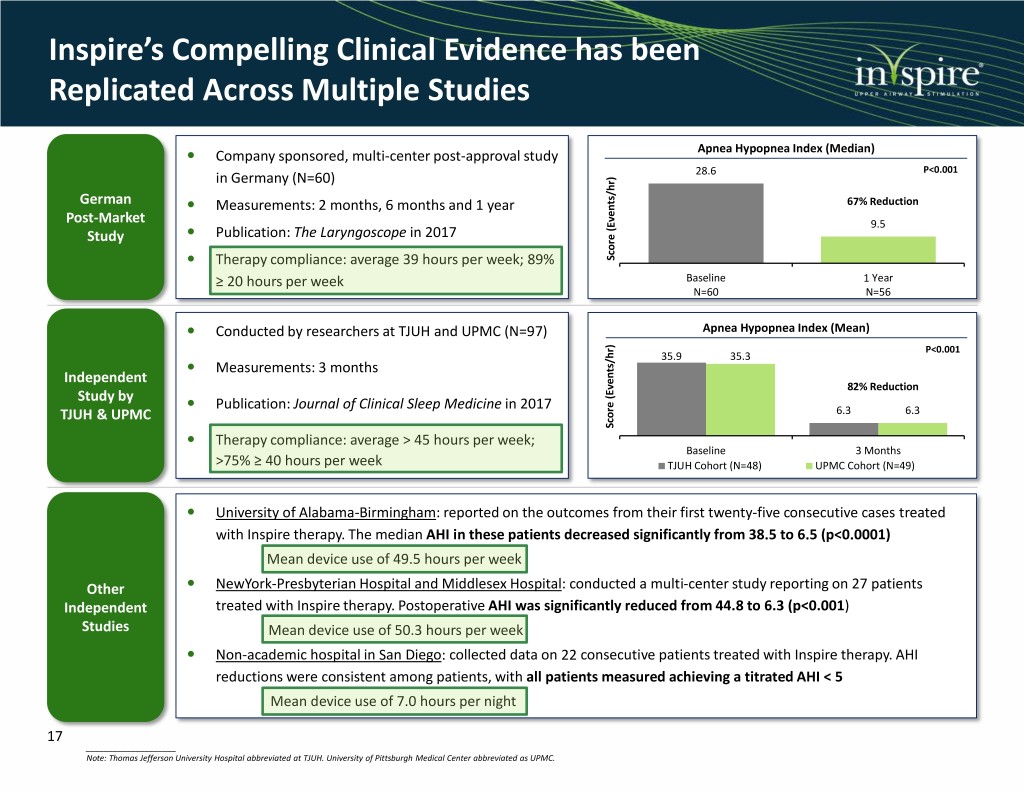
Inspire’s Compelling Clinical Evidence has been Replicated Across Multiple Studies Company sponsored, multi-center post-approval study Apnea Hypopnea Index (Median) P<0.001 in Germany (N=60) 28.6 German Measurements: 2 months, 6 months and 1 year 67% Reduction Post-Market 9.5 Study Publication: The Laryngoscope in 2017 Therapy compliance: average 39 hours per week; 89% Score (Events/hr) ≥ 20 hours per week Baseline 1 Year N=60 N=56 Conducted by researchers at TJUH and UPMC (N=97) Apnea Hypopnea Index (Mean) P<0.001 35.9 35.3 Measurements: 3 months Independent 82% Reduction Study by Publication: Journal of Clinical Sleep Medicine in 2017 TJUH & UPMC 6.3 6.3 Score (Events/hr) Therapy compliance: average > 45 hours per week; Baseline 3 Months >75% ≥ 40 hours per week TJUH Cohort (N=48) UPMC Cohort (N=49) University of Alabama-Birmingham: reported on the outcomes from their first twenty-five consecutive cases treated with Inspire therapy. The median AHI in these patients decreased significantly from 38.5 to 6.5 (p<0.0001) Mean device use of 49.5 hours per week Other NewYork-Presbyterian Hospital and Middlesex Hospital: conducted a multi-center study reporting on 27 patients Independent treated with Inspire therapy. Postoperative AHI was significantly reduced from 44.8 to 6.3 (p<0.001) Studies Mean device use of 50.3 hours per week Non-academic hospital in San Diego: collected data on 22 consecutive patients treated with Inspire therapy. AHI reductions were consistent among patients, with all patients measured achieving a titrated AHI < 5 Mean device use of 7.0 hours per night 17 ____________________ Note: Thomas Jefferson University Hospital abbreviated at TJUH. University of Pittsburgh Medical Center abbreviated as UPMC.

Independent Cleveland Clinic Comparison Study of Inspire vs. UPPP Independent retrospective study conducted by multi- Pre vs Post Operative AHI for UPPP vs HNS Groups (Mean) disciplinary team of physicians from the Cleveland Clinic P= 0.02 P<0.001 (N=40) 40.3 29% Reduction 38.9 28.8 88% Reduction N=20 enrolled in HNS AHI Score N=20 enrolled in UPPP (Events/hr) 4.5 Publication: American Journal of Otolaryngology Mar. 2018 UPPP (N=20) HNS (N=20) Cleveland Clinic Compared outcomes of Inspire Upper Airway Stimulation Pre Operative AHI Post Operative AHI Comparison Study (HNS) vs UPPP in patients with moderate to severe OSA of Inspire Therapy and UPPP HNS: All patients who underwent HNS implantation at a Pre vs Post Operative ESS for UPPP vs HNS Groups (Mean) P=0.001 P<0.001 single institution between Nov. 2015 and Nov. 2016 Normalized daytime sleepiness = 10.0 13.0 (N=20); all patients met STAR trial criteria 11.0 8.0 7.0 UPPP: Utilized a pre-existing database of patients (N=116) who were intolerant to CPAP and underwent UPPP Index Score between 2003-2012. From this data, patients that matched UPPP (N=16) HNS (N=15) STAR trial inclusion criteria were selected (N=20) Pre Operative ESS Post Operative ESS “The present study compares outcomes of the most well established upper airway surgery with outcomes of upper airway stimulation therapy in patients with OSA. Compared to uvulopalatopharyngoplasty, hypoglossal nerve stimulation therapy provides significant objective improvement in outcome measures for select patients with moderate to severe OSA with inability to tolerate CPAP. Although Conclusion traditional upper airway surgery is effective in treating patients with OSA, our study suggests hypoglossal nerve stimulation is curative for many patients as it normalizes the AHI to < 5 and is an excellent option for second line therapy in select patients with OSA who are intolerant to CPAP.” 18 ____________________ Note: Hypoglossal nerve stimulation abbreviated at HNS.

We Intend to Continue to Build the Depth of Our Clinical Data with Our ADHERE Patient Registry ADHERE Patient Registry Our post-implantation study with the goal of collecting data on a group in excess of 2,500 patients Registry Results from 508 Patients Registry study designed to be Apnea Hypopnea Index (Median) Epworth Sleepiness Scale (Median) retrospective and prospective P<0.0001 Normalized daytime sleepiness = 10.0 P<0.0001 14 centers were involved as part of 34.0 12 42% Reduction registry 79% Reduction 7 Registry enrolled 508 patients between October 2016 and January 2018 7.0 For each 1-year increase in age, there was a 4% increase in odds of treatment Baseline 12 Months Baseline 12 Months success; for each 1-unit increase in BMI, N=499 N=227 N=443 N=241 there was 9% reduced odds of treatment Inspire is Better Would Recommend to Overall Patient Patients would success Experience than CPAP Friends / Family Satisfaction Choose Inspire Again 93% of physicians rate improvement of patient symptoms after Inspire implant Adherence Monitoring 96% 96% 94% 94% Average home device use: 5.7 hours / night Early results from the ADHERE registry show Inspire therapy is an effective treatment for OSA in a real world setting 19 ____________________ Note: Latest registry data as of January 2018.

Product and Indication Expansion

We are Committed to Continuous Product Development & Indication Expansion Indication Expansion Product Pipeline Working with the FDA to expand indication in the 5th generation neurostimulator is in the concept U.S. to patients as young as 12 years of age phase of development (including Down syndrome) Inspire Cloud, which is still in development, is being Patients born with Down syndrome have designed to allow physicians to monitor patient higher rates of OSA than the general compliance and therapy efficacy pediatric population Illustrative Inspire Cloud Dashboard 6-patient investigator-initiated trial demonstrated the safety and efficacy of Inspire therapy for treating Down syndrome patients (1) ____________________ Not actual data; for illustrative purposes only. 21 ____________________ (1) Published in JAMA Otolaryngology – Head & Neck Surgery.

Inspire Cloud: Longitudinal Care Longitudinal Care Care team Inspire cloud Referral network Inspire Cloud was launched at the AASM (American Academy of Sleep Medicine) annual meeting June 2018 ____________________ Not actual data; for illustrative purposes only. Monitors Adherence Strengthens the Referral Network Generates Complete Patient Therapy Reports Creates Care Coordination Hub Insight into Center-Wide Therapy Outcomes Fits into existing sleep clinic workflows 22

Reimbursement

Our Team is Focused on All Aspects of Reimbursement, which Include Coding, Payment and Coverage Coding Payment Coverage • Physician • Physician • Medicare • Facility • Facility • Commercial • Neurostimulator and stimulation • National Medicare average payment • Approximately 300 commercial lead: CPT code 64568 for Cranial of approximately $27,000 payors in the U.S. have paid for Nerve Stimulator Inspire procedure, mostly through • Covers the cost of the device and prior authorization • Sensing lead: CPT code 0466T the procedure for implantation (Category III) • Medicare payment in most MACs • Physician society working to convert to a Category I code • Government contract for VA / Military hospitals • AMA meeting in Feb 2019 U.S. Revenue Mix Two-pronged Approach to Reimbursement Part 1: Work with all payers to educate on the therapy during annual and mid-cycle reviews to develop 30% Commercial positive coverage policies (i.e., Aetna was a mid-cycle) 60% Medicare Part 2: Work with physicians and centers to obtain VA prior authorizations from payers as most have 10% negative coverage policies today, yet approve procedures after appeal cycles 24

Developing Positive Coverage Policies – Leveraging the Aetna Policy Decision United Anthem Aetna Healthcare 14 BCBS Plans: 22.2 M California & 45.7 M HCSC Georgia ++ BCBS 38.7 M IL/TX 14.6M BCBS Cigna, Focus on local payments BCBS Others Humana Small Regional Plans 9 BCBS Plans: 17.4MM & others focused in area with 12 Medicare MA, MN, MI, 15MM+ active centers: Medicare MAC ID, WA, FL, TN, • IBC • Medica Nat’l Jurisdictions TN, NC, AL • Horizon • HAP Coverage • Highmark 26.3MM • PreferredOne Decision • Care First 38.7 M • Excellus • Clev Clinic • OSU • Group Health WI Long-term: May look toward national policy • Review Cycles – most major providers have a scheduled annual review for policies • Focus is to provide updated dossier, guidelines, experiences to encourage a deep dive review • Continue to build on already strong clinical dossier and physician support • Leverage internal staff as well as multiple contractors with designated payers assigned to each • Large Payors – most difficult to engage, and more accepting of the appeal (EMR) process • Small Payors – less comfortable with EMR process and more agile to conduct internal reviews 25 Indicates Insurers with Positive Coverage Policies or positive technical assessments

Evidence Street Complete Positive Technical Assessment Report summarized that the evidence is sufficient to determine that the Inspire therapy results in a meaningful improvement in the net health outcome for patients meeting the following selection criteria o Age ≥ 22 years in adults or adolescents with Down’s syndrome age 10 to 21; AND o Diagnosed moderate to severe OSA (with less than 25% central apneas); AND o CPAP failure or inability to tolerate CPAP; AND o Body mass index ≤ 35 kg/m2 in adults; AND o Favorable pattern of palatal collapse NOTE: Adolescents with Down’s syndrome is not FDA approved and is currently in clinical trials What does this mean??? o BCBS Evidence Street conducts many reviews annually (technical assessments) o Does not specifically write coverage policies for each of the 36 BCBS companies o However, it provides BCBS companies with evidence opinions they often use as an input into those coverage policy decisions. Expect many of the regional BCBS payors will write policies in the next 26 few quarters
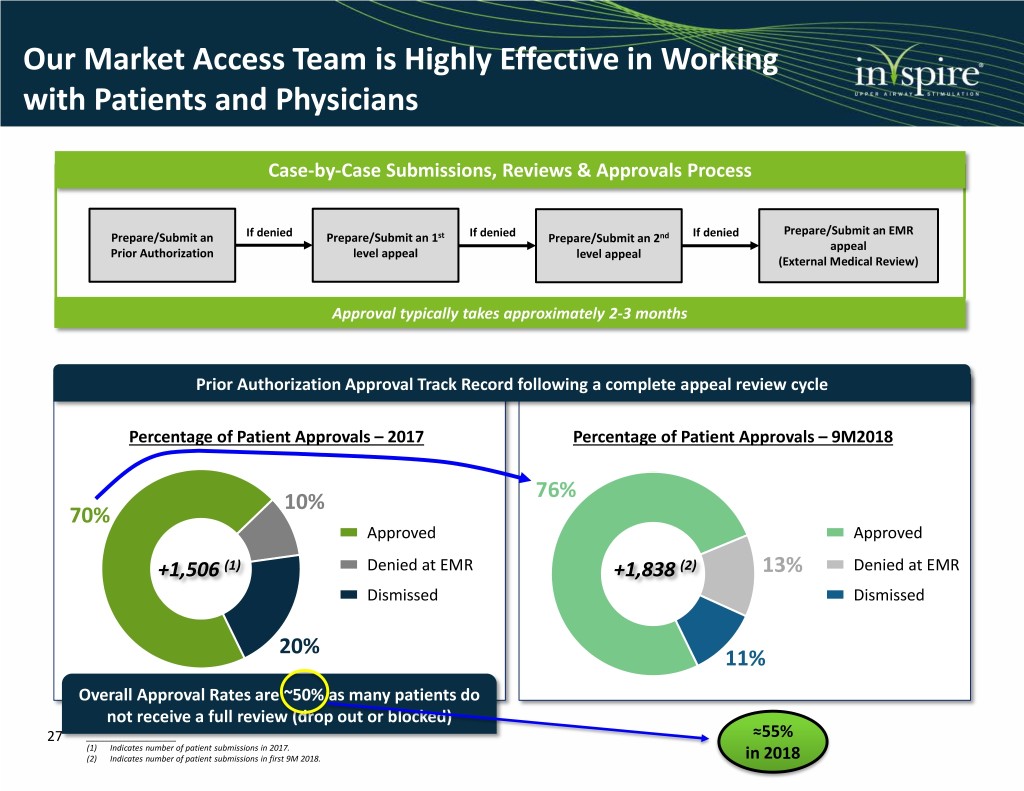
Our Market Access Team is Highly Effective in Working with Patients and Physicians Case-by-Case Submissions, Reviews & Approvals Process Prepare/Submit an EMR Prepare/Submit an If denied Prepare/Submit an 1st If denied Prepare/Submit an 2nd If denied appeal Prior Authorization level appeal level appeal (External Medical Review) Approval typically takes approximately 2-3 months Prior Authorization Approval Track Record following a complete appeal review cycle Percentage of Patient Approvals – 2017 Percentage of Patient Approvals – 9M2018 76% 10% 70% Approved Approved +1,506 (1) Denied at EMR +1,838 (2) 13% Denied at EMR Dismissed Dismissed 20% 11% Overall Approval Rates are ~50% as many patients do not receive a full review (drop out or blocked) 27 ____________________ ≈55% (1) Indicates number of patient submissions in 2017. (2) Indicates number of patient submissions in first 9M 2018. in 2018

U.S. Commercial Prior Authorization Submissions Total Submissions Key focus is to continue to grow individual patient prior 2018YTD: 1,838 authorizations while working with payors to develop 2017A: 1,506 positive coverage policies longer term 2016A: 960 Past Quarter Submission 700 661 60 619 600 558 50 488 500 445 40 400 303 30 300 268 258 270 51 48 209 225 43 38 20 Quarterly TotalSubmissions 200 34 23 QuarterlyAverage Weekly Submissions 21 20 21 10 100 16 17 0 0 Q1'16 Q2'16 Q3'16 Q4'16 Q1'17 Q2'17 Q3'17 Q4'17 Q1'18 Q2'18 Q3'18 Qtrly Weekly Average Submissions Qtrly Total Submissions 28 Process driven by 15 person market access team

U.S. Commercial Prior Authorization Approvals Many patients who are willing to work through the Total Approvals process benefit from an approval; shortly after this 2018YTD: 835 approval, the process shifts to scheduling the placement 2017A: 583 2016A: 330 of the Inspire system Past Quarter Approvals 400 373 40 350 35 300 30 249 250 25 213 190 200 20 133 134 150 126 29 15 Quarterly TotalApprovals 96 103 100 72 19 10 QuarterlyAverage Weekly Approvals 59 15 16 50 10 10 10 5 7 8 5 6 0 0 Q1'16 Q2'16 Q3'16 Q4'16 Q1'17 Q2'17 Q3'17 Q4'17 Q1'18 Q2'18 Q3'18 Qtrly Weekly Average Approvals Qtrly Total Approvals 29

Commercial Development

We have a Targeted Approach to Market Development Inspire Approach to Market Development Direct-to-Patient Outreach Machine Inspire has built a referral network with physicians across the treatment continuum Company Sponsored Public Relations Differentiated marketing engine capable of generating demand through patient channels Physician to Patient Local Radio Direct to Patient Channels / Self-Referred Physician Word of Mouth Cardiac Dental Practice Practice (1) / EP Referrals Referrals Engaged Sessions 1.1 million in 2017 Inspire Program Core Team Dr. Searches 400,000 in 2017 Practice Engagement • Trackable calls (>17,000 in 2017) Sleep Sleep Practice • Emails Practice Refer and • Health Talk Sign Ups Referrals Manage • Visits - MD pages • Clicks - MD news stories ____________________ 31 (1) EP constitutes abbreviation for electrophysiologists.

Our Sales Strategy Engages All Key Stakeholders Across the OSA Treatment Paradigm Holistic Approach to Engagement Across Key Stakeholders in the OSA Treatment Paradigm Sales Organization Sleep 40 Territory Managers (reps) in U.S. and 6 in Europe Centers Managed by Regional Sales Managers (7) Supported by Therapy Awareness Managers and Field Clinical Representatives ENT Patients Physicians Target for each rep to manage 5 – 8 active centers per territory Focus on building long-lasting physician relationships Encourage & sponsor additional publications of clinical data Support physicians through all aspects of a case Increase awareness through training and education Identify new regions with high volume medical centers Continue various direct-to-patient marketing initiatives Summary: Continue to build on capacity to treat patients by adding centers, hiring Territory Managers, and adding support structure including Regional 32 Managers and staff to cover implant cases and activations

Keys To Driving a Strong Territory Territory Manager Strategy New Centers and Territory Framing How TMs Spend their Time Building Regional Manager Team and FCRs to currently have 7 RMs (added 3 in Q3) Support Adding Field Clinical Reps (FCRs) to support implants Attend DISE, Find The Continue adding Territory Managers Cases & Right Centers Ended Q3 with 40 U.S. Territory Managers, Follow up an increase of nine since the IPO Sales Training Drive Manage Prior Inspire University conducted quarterly for Patient Flow Authorization, new employees & Practice Coding & Free up selling time for tenured reps to focus Readiness Payment on driving patient flow Invest in FCRs to cover cases and activations 33

The Great Balancing Act KEEPING CONTROL WHILE GROWING FAST What does it take to start, then grow a business into a successful public company yet maintain total control of product performance? 34

The Inspire Control System Prevalence Pool 17M people in the US with End Goal: Strong OSA of which ≈25% could Incidence Pool be treated by using 500k new cases per Patient Outcomes!!! Inspire year who could be treated using Inspire Question 1: How Question 3: How Question 2: How Question 4: How Question 5: How to find these to direct patients to improve the to improve time to scale the patients and to the correct yield to increase and approval business without connect them to physician to contacts to rates from losing quality in the proper prevent healthcare insurance patient healthcare unnecessary providers? companies? outcomes? provider? visits? Direct-to-Consumer Outreach Program Continuous addition of new sales reps and opening new hospitals Direct-to-Patient Outreach Machine Company Sponsored Public Relations Patient Local Radio Physician to Physician Word of Mouth Physician Appointment Insurance Approvals •≈50% are new sleep •Requires 3-4 review apnea patients and need cycles and averages 3-4 Patient Engaged Sessions to try CPAP first months 1.1 million in 2017 •≈25% of patients are not •≈55% of patients are receives Patients make Patients proceed Patients Dr. Searches proper candidates approved Inspire! 400,000 in 2017 appointment to •≈25% are good Inspire for insurance •≈60% of patients have to scheduled for see doctor approval Inspire Practice Engagement candidates go thru this process • Target 500+ contacts/week 35

Financials
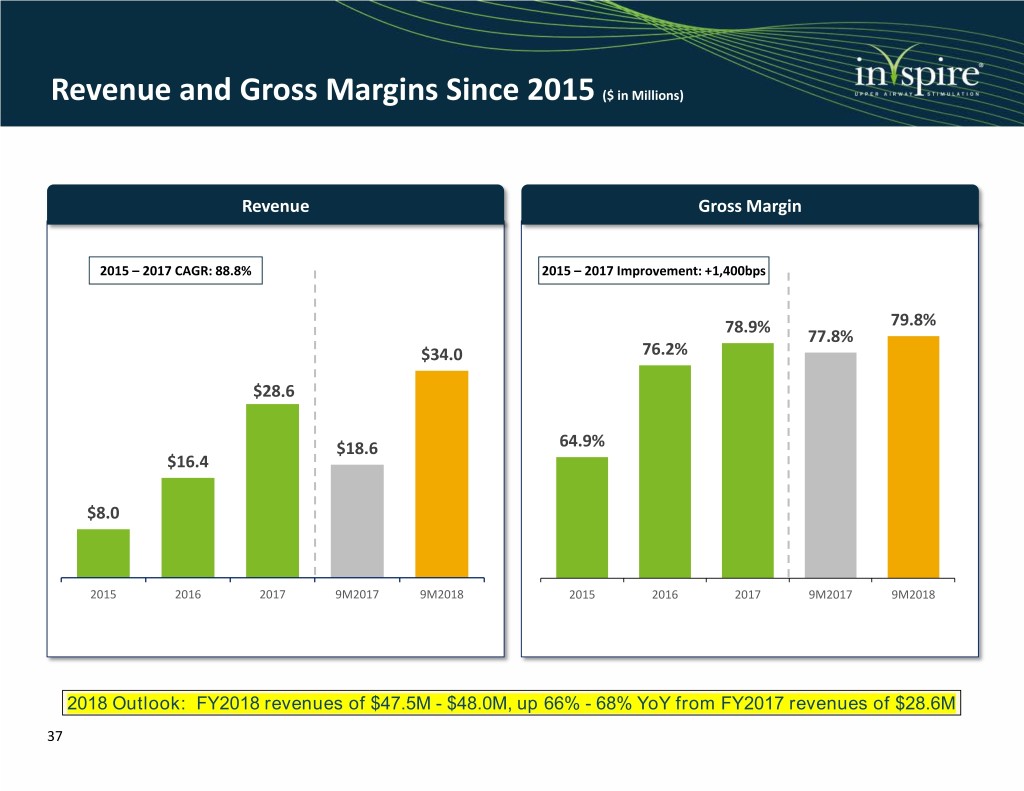
Revenue and Gross Margins Since 2015 ($ in Millions) Revenue Gross Margin 2015 – 2017 CAGR: 88.8% 2015 – 2017 Improvement: +1,400bps 78.9% 79.8% 77.8% $34.0 76.2% $28.6 $18.6 64.9% $16.4 $8.0 2015 2016 2017 9M2017 9M2018 2015 2016 2017 9M2017 9M2018 2018 Outlook: FY2018 revenues of $47.5M - $48.0M, up 66% - 68% YoY from FY2017 revenues of $28.6M 37
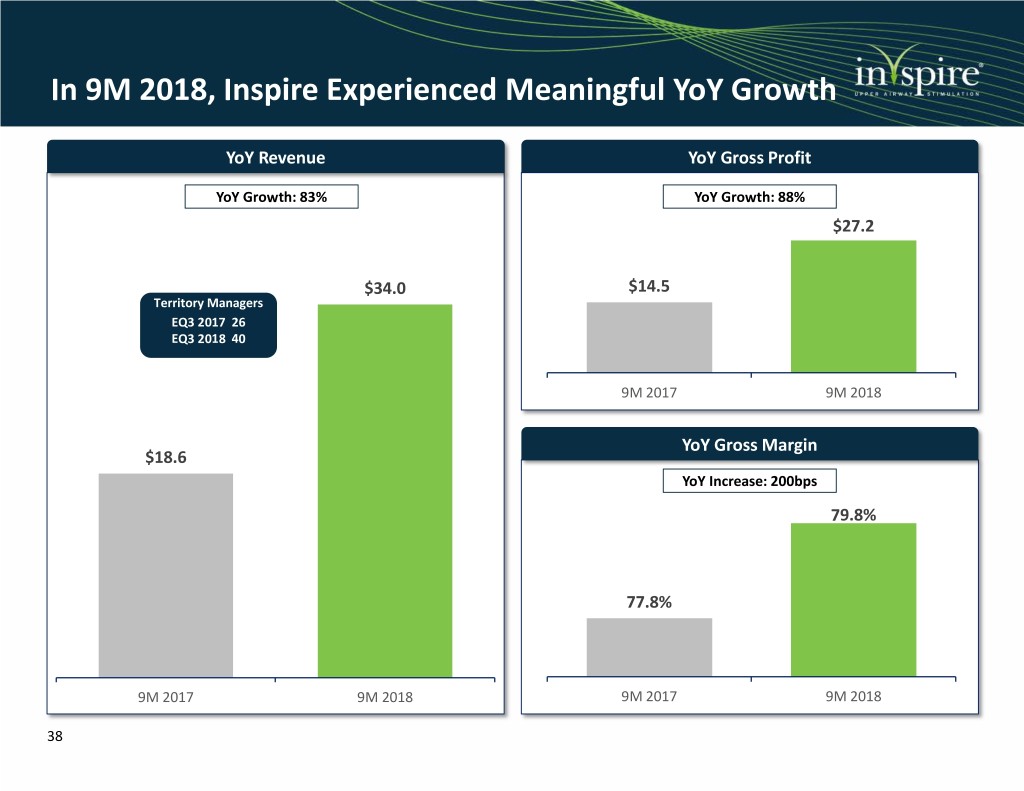
In 9M 2018, Inspire Experienced Meaningful YoY Growth YoY Revenue YoY Gross Profit YoY Growth: 83% YoY Growth: 88% $27.2 $34.0 $14.5 Territory Managers EQ3 2017 26 EQ3 2018 40 9M 2017 9M 2018 YoY Gross Margin $18.6 YoY Increase: 200bps 79.8% 77.8% 9M 2017 9M 2018 9M 2017 9M 2018 38

Our Growth Strategies Ensure strong and consistent patient outcomes globally through deliberate expansion and robust training Promote awareness among patients, ENT physicians, sleep centers and referring physicians Expand U.S. sales and marketing organization to drive adoption of our Inspire therapy Leverage prior authorization model while we work in parallel with payors to broaden coverage Invest in research and development to drive innovation and expand indications Further penetrate and expand into existing and new international markets 39

Our Innovative Inspire Solution has a Significant First Mover Advantage... Inspire Therapy is Strongly Positioned FDA PMA Approval Since 2014 – More than 4,000 patients treated at over 220 medical Compelling Market centers across the U.S. and Europe Opportunity Evidence of Safety and 5-Year Long-Term Sustained Efficacy – Consistent results across four sponsored and more than Large and growing 12 independent clinical studies evaluating ~980 patients prevalence of OSA – Ongoing enrollment of 2,500 patient ADHERE patient registry Physician Society Support Significant economic cost – American Academy of Otolaryngology, American of untreated OSA Academy of Sleep Medicine, Germany S-3 Guidelines and International Sleep Surgery Society Significant Payor Experience Urgent clinical need for an – Focus on broadening payor coverage – Aetna in 2018 effective alternative to CPAP – Highly effective prior authorization model – Approvals from ~300 commercial payors to date – Over 1,500 individual patient submissions in 2017 and over 1,800 in first 9 months of 2018 ~$10bn annual market opportunity in the U.S. Differentiated Product Built on 20 Years of Development – Closed loop system that leverages our pressure sensing lead and proprietary algorithm – Current device represents the 4th generation of our Inspire system, which has an ~11-year battery life and 40 allows for MRI of head and extremities
