Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - INSMED Inc | a19-1332_1ex99d1.htm |
| 8-K - 8-K - INSMED Inc | a19-1332_18k.htm |
This presentation contains forward-looking statements that involve substantial risks and uncertainties. "Forward-looking statements," as that term is defined in the Private Securities Litigation Reform Act of 1995, are statements that are not historical facts and involve a number of risks and uncertainties. Words herein such as "may," "will," "should," "could," "would," "expects," "plans," "anticipates," "believes," "estimates," "projects," "predicts," "intends," "potential," "continues," and similar expressions (as well as other words or expressions referencing future events, conditions or circumstances) may identify forward-looking statements. The forward-looking statements in this press release are based upon the Company’s current expectations and beliefs, and involve known and unknown risks, uncertainties and other factors, which may cause the Company’s actual results, performance and achievements and the timing of certain events to differ materially from the results, performance, achievements or timing discussed, projected, anticipated or indicated in any forward-looking statements. Such risks, uncertainties and other factors include, among others, the following: failure to successfully commercialize or maintain US approval for ARIKAYCE, the Company’s only approved product; uncertainties in the degree of market acceptance of ARIKAYCE by physicians, patients, third-party payers and others in the healthcare community; the Company’s inability to obtain full approval of ARIKAYCE from the FDA, including the risk that the Company will not successfully complete the confirmatory post-marketing study required for full approval; inability of the Company, PARI or the Company’s third party manufacturers to comply with regulatory requirements related to ARIKAYCE or the Lamira™ Nebulizer System; the Company’s inability to obtain adequate reimbursement from government or third-party payers for ARIKAYCE or acceptable prices for ARIKAYCE; development of unexpected safety or efficacy concerns related to ARIKAYCE; inaccuracies in the Company’s estimates of the size of the potential markets for ARIKAYCE; the Company’s inability to create an effective direct sales and marketing infrastructure or to partner with third parties that offer such an infrastructure for distribution of ARIKAYCE; failure to obtain regulatory approval to expand ARIKAYCE’s indication to a broader patient population; failure to successfully conduct future clinical trials for ARIKAYCE and the Company’s product candidates, including due to the Company’s limited experience in conducting preclinical development activities and clinical trials necessary for regulatory approval and the Company’s inability to enroll or retain sufficient patients to complete the trials or generate data necessary for regulatory approval; risks that the Company’s clinical studies will be delayed or that serious side effects will be identified during drug development; failure to obtain regulatory approvals for ARIKAYCE outside the US or for the Company’s product candidates in the US, Europe, Japan or other markets; failure of third parties on which the Company is dependent to manufacture sufficient quantities of ARIKAYCE or the Company’s product candidates for commercial or clinical needs, to conduct the Company’s clinical trials, or to comply with laws and regulations that impact the Company’s business or agreements with the Company; the Company’s inability to attract and retain key personnel or to effectively manage the Company’s growth; the Company’s inability to adapt to its highly competitive and changing environment; the Company’s inability to adequately protect its intellectual property rights or prevent disclosure of its trade secrets and other proprietary information and costs associated with litigation or other proceedings related to such matters; restrictions imposed on the Company by its material license agreements, including its license agreements with PARI and AstraZeneca AB, and failure of the Company to comply with its obligations under such agreements; the cost and potential reputational damage resulting from litigation to which the Company is or may become a party, including product liability claims; limited experience operating internationally; changes in laws and regulations applicable to the Company’s business and failure to comply with such laws and regulations; and inability to repay the Company’s existing indebtedness and uncertainties with respect to the Company’s ability to access future capital. The Company may not actually achieve the results, plans, intentions or expectations indicated by the Company’s forward-looking statements because, by their nature, forward-looking statements involve risks and uncertainties because they relate to events and depend on circumstances that may or may not occur in the future. For additional information about the risks and uncertainties that may affect the Company’s business, please see the factors discussed in Item 1A, "Risk Factors," in the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2018 and any subsequent Company filings with the Securities and Exchange Commission. The Company cautions readers not to place undue reliance on any such forward-looking statements, which speak only as of the date of this press release. The Company disclaims any obligation, except as specifically required by law and the rules of the Securities and Exchange Commission, to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward-looking statements. This presentation includes information related to market opportunity as well as cost and other estimates obtained from various internal analyses and calculations from external sources, including publicly available information, market research and data from a variety of claims databases. The externally sourced information has been obtained from sources we believe to be reliable, but we cannot assure the accuracy and completeness of such information. Similarly, our internal analyses and calculations are based upon management’s understanding of market and industry conditions and has not been verified by independent sources. Forward-looking information presented from each of these sources is subject to the same qualifications and uncertainties as the other forward-looking statements in this presentation. Preliminary Financial Information The fourth quarter 2018 net product sales information and additional launch metrics presented in this presentation are preliminary and subject to revision. The preliminary net product sales information may be revised based on the completion of the Company’s fourth quarter financial close and reporting process and the year-end audit to be performed by Ernst & Young LLP, the Company’s independent registered public accounting firm. During the course of this process, the Company may identify items that would require adjustments to this information. This preliminary net product sales information and additional launch metrics thus constitute forward-looking statements, and the Company cautions you that they are subject to risks and uncertainties. Ernst & Young LLP has not audited, reviewed, compiled or performed any procedures with respect to these preliminary estimates. Accordingly, Ernst & Young LLP does not express an opinion or any other form of assurance with respect to these preliminary estimates. 2 Forward Looking Statements Not for promotional use

To transform the lives of people battling serious rare diseases Our Mission Not for promotional use

4 Insmed: A Global Biopharmaceutical Company Focused on Rare Disease U.S. Commercial Launch of ARIKAYCE 4Q 2018 Global Expansion to Support Potential Commercial Opportunity in EU and Japan ARIKAYCE Confirmatory Clinical Study and Lifecycle Management Pipeline to Support Long-Term Growth Not for promotional use
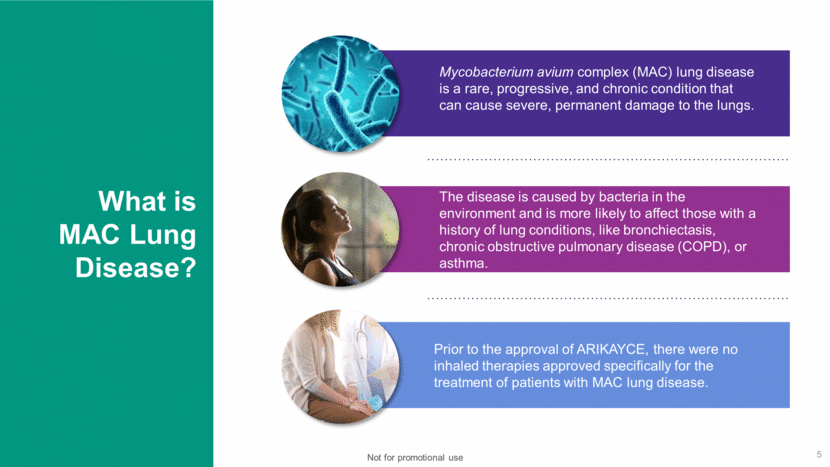
What is MAC Lung Disease? 5 Mycobacterium avium complex (MAC) lung disease is a rare, progressive, and chronic condition that can cause severe, permanent damage to the lungs. The disease is caused by bacteria in the environment and is more likely to affect those with a history of lung conditions, like bronchiectasis, chronic obstructive pulmonary disease (COPD), or asthma. Prior to the approval of ARIKAYCE, there were no inhaled therapies approved specifically for the treatment of patients with MAC lung disease. Not for promotional use
About ARIKAYCE 6 Not for promotional use

7 Now Approved* Not for promotional use * Approved under the U.S. Food and Drug Administration's Limited Population Antibacterial Drug and Accelerated Approval pathways for Adult Patients with MAC Lung Disease who have Limited or No Alternative Treatment Options.

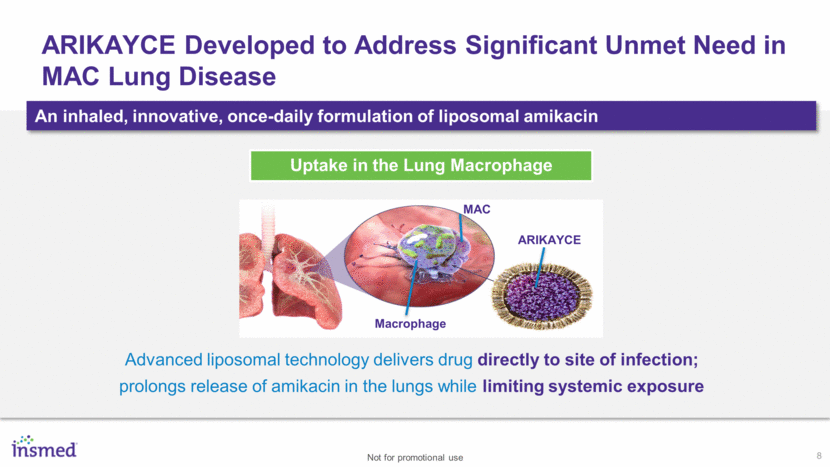
8 ARIKAYCE Developed to Address Significant Unmet Need in MAC Lung Disease An inhaled, innovative, once-daily formulation of liposomal amikacin Advanced liposomal technology delivers drug directly to site of infection; prolongs release of amikacin in the lungs while limiting systemic exposure Uptake in the Lung Macrophage ARIKAYCE MAC Macrophage Not for promotional use
LIMITED POPULATION: ARIKAYCE is indicated in adults, who have limited or no alternative treatment options, for the treatment of Mycobacterium avium complex (MAC) lung disease as part of a combination antibacterial drug regimen in patients who do not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. As only limited clinical safety and effectiveness data for ARIKAYCE are currently available, reserve ARIKAYCE for use in adults who have limited or no alternative treatment options. This drug is indicated for use in a limited and specific population of patients. This indication is approved under accelerated approval based on achieving sputum culture conversion (defined as 3 consecutive negative monthly sputum cultures) by Month 6. Clinical benefit has not yet been established. Continued approval for this indication may be contingent upon verification and description of clinical benefit in confirmatory trials. Limitation of Use: ARIKAYCE has only been studied in patients with refractory MAC lung disease defined as patients who did not achieve negative sputum cultures after a minimum of 6 consecutive months of a multidrug background regimen therapy. The use of ARIKAYCE is not recommended for patients with non-refractory MAC lung disease. 9 ARIKAYCE Indication and Use Not for promotional use
Commercial Approach: Near-Term Focus on U.S. Launch; Expansion to EU and Japan 10 *Source: Internal analysis of published NTM epidemiology, primary market research with treating HCPs, and anonymized patient level claims data in US † EU5 comprised of France, Germany, Italy, Spain and the United Kingdom U.S. 60K - 85K total diagnosed MAC patients (2018E)* 10K - 15K total refractory MAC patients (2018E)* EU5† ~14K total diagnosed NTM patients (2018E)* ~1,400 total refractory MAC patients (2018E)* Japan 125K - 145K total diagnosed NTM patients (2018E)* 15K - 18K total refractory MAC patients (2018E)* Not for promotional use
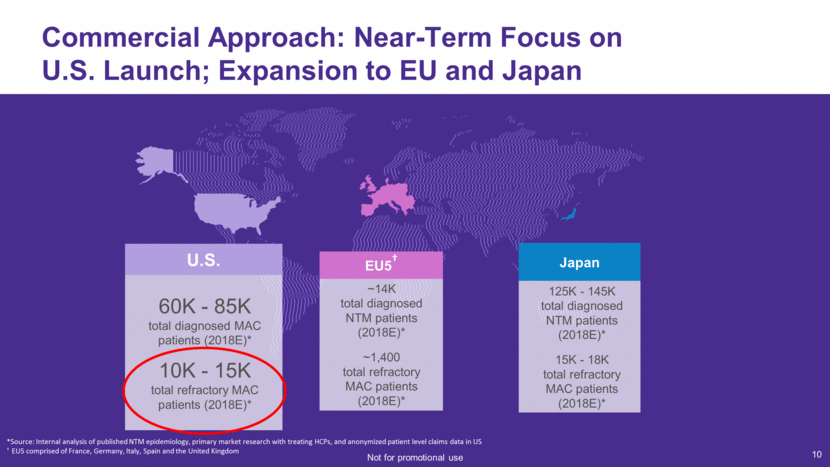
11 72 Therapeutic Specialists Deployed and Covering U.S. Market Based on Concentration of MAC Treatment Providers Source: Symphony Health, PatientSourceTM November 2013 – October 2016 ~5K physicians manage 70% of diagnosed MAC patients 8 in 10 diagnosed MAC patients managed by Pulmonologist or Infectious Disease Specialist Cumulative % of Patients Attributed Cumulative % of Physicians ~2.1K HCPs account for ~50% of potential (Tier 1) ~3K HCPs account for ~20% of potential (Tier 2) ~11.5K HCPs account for ~30% of potential patients Not for promotional use
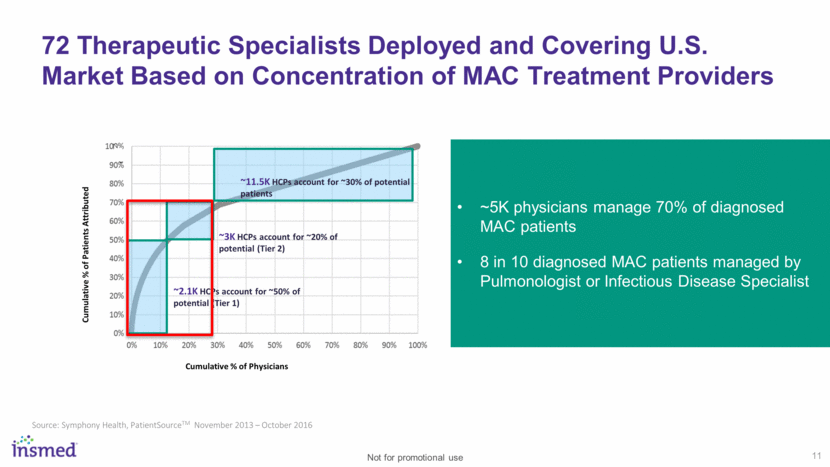
12 Laying the Groundwork for a Successful U.S. Launch Awareness & Education Disease Awareness, HCP engagement, and advocacy group support Patient Identification Ongoing, innovative efforts to identify appropriate patients and enhance disease diagnosis Access Payer engagement to enable efficient patient access Supportive payer environment at launch recognizing significant unmet need of disease state Support Comprehensive patient support program intended to help with treatment introduction, compliance, and navigating the reimbursement landscape Sales Force Deployment 72 therapeutic specialists deployed in U.S. market focused on key geographies with high patient concentrations Not for promotional use
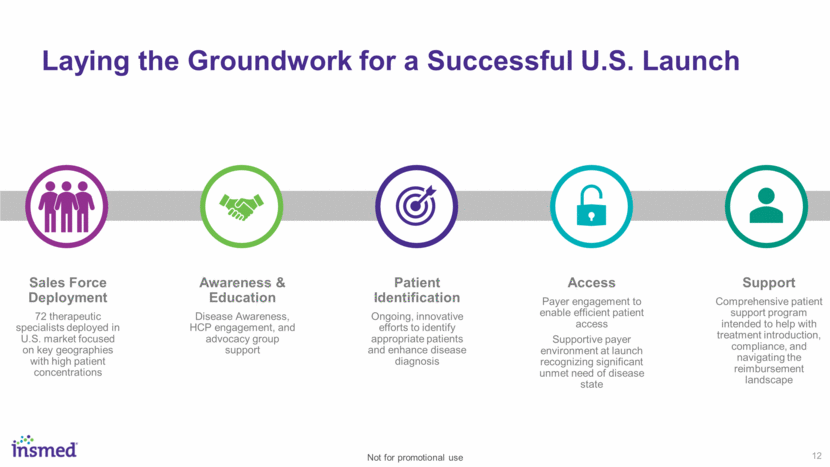
~600 Physicians Prescribed ARIKAYCE 82% of Tier 1 and 65% of Tier 2 Targets Reached U.S. Launch Update As of 12/31/18 13 Not for promotional use Strong Initial Uptake $9.8M in Preliminary, Unaudited 4Q18 Total Net Product Sales $9.2M in U.S. net sales $0.6M in ex-U.S. net sales from French ATU program Over 500 Patients Initiated Treatment Engaged HCP Community Supportive Payer Landscape Strong Reimbursement Trends to Date
Redundant drug supply chain Commercial-scale manufacturing capacity on-line and expansion under way 14 Established Supply Chain 50L 200L * 450L *Patheon long term project initiated 4Q 2017 but not yet completed * Not for promotional use

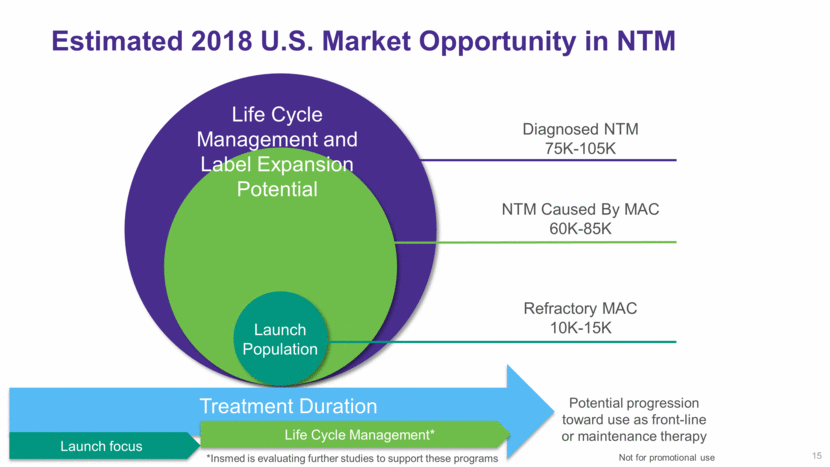
15 Estimated 2018 U.S. Market Opportunity in NTM Refractory MAC 10K-15K NTM Caused By MAC 60K-85K Diagnosed NTM 75K-105K Launch Population Life Cycle Management and Label Expansion Potential Potential progression toward use as front-line or maintenance therapy Life Cycle Management* Launch focus Treatment Duration *Insmed is evaluating further studies to support these programs Not for promotional use
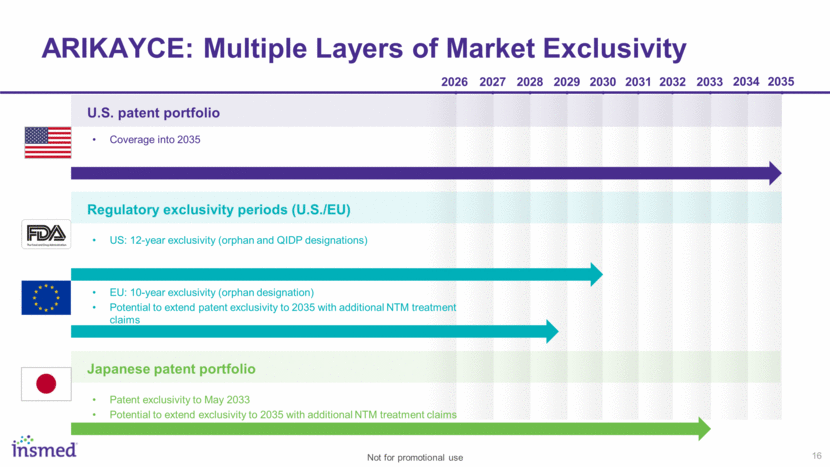
16 ARIKAYCE: Multiple Layers of Market Exclusivity 2033 2027 2030 2028 2029 2026 2031 2032 2034 2035 U.S. patent portfolio Regulatory exclusivity periods (U.S./EU) Japanese patent portfolio Coverage into 2035 US: 12-year exclusivity (orphan and QIDP designations) EU: 10-year exclusivity (orphan designation) Potential to extend patent exclusivity to 2035 with additional NTM treatment claims Patent exclusivity to May 2033 Potential to extend exclusivity to 2035 with additional NTM treatment claims Not for promotional use
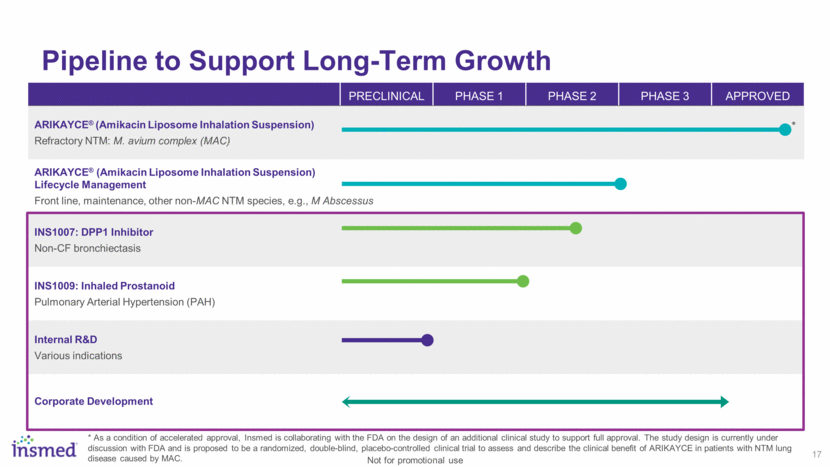
Pipeline to Support Long-Term Growth 17 PRECLINICAL PHASE 1 PHASE 2 PHASE 3 APPROVED ARIKAYCE® (Amikacin Liposome Inhalation Suspension) Refractory NTM: M. avium complex (MAC) ARIKAYCE® (Amikacin Liposome Inhalation Suspension) Lifecycle Management Front line, maintenance, other non-MAC NTM species, e.g., M Abscessus INS1007: DPP1 Inhibitor Non-CF bronchiectasis INS1009: Inhaled Prostanoid Pulmonary Arterial Hypertension (PAH) Internal R&D Various indications Corporate Development * As a condition of accelerated approval, Insmed is collaborating with the FDA on the design of an additional clinical study to support full approval. The study design is currently under discussion with FDA and is proposed to be a randomized, double-blind, placebo-controlled clinical trial to assess and describe the clinical benefit of ARIKAYCE in patients with NTM lung disease caused by MAC. * Not for promotional use
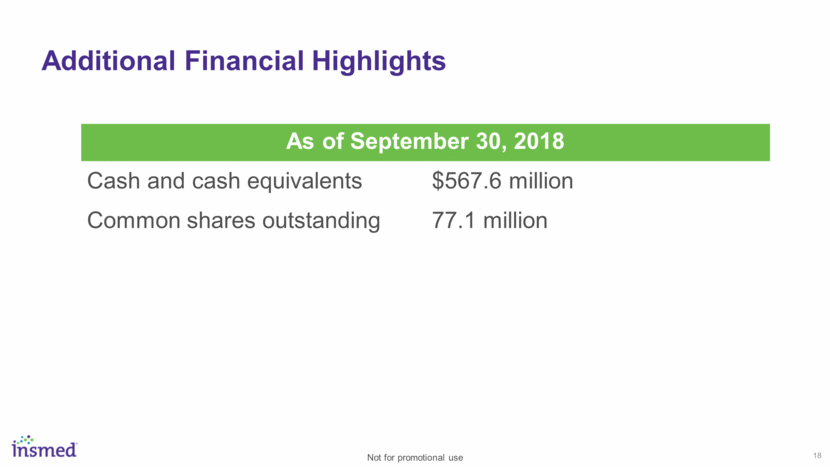
18 Additional Financial Highlights As of September 30, 2018 Cash and cash equivalents $567.6 million Common shares outstanding 77.1 million Not for promotional use
19 Insmed: 2019 Strategic Priorities Complete enrollment in WILLOW, our six-month Phase 2 trial of INS1007 in non-CF bronchiectasis, in mid-2019 Continue to execute successful U.S. launch of ARIKAYCE ARIKAYCE life cycle management through a clinical study in a front-line setting of patients with MAC lung disease Accelerate global expansion to support potential regulatory filings for ARIKAYCE in Europe and Japan Not for promotional use

20 Thank You Not for promotional use


