Attached files
| file | filename |
|---|---|
| 8-K - 8-K - IDERA PHARMACEUTICALS, INC. | a19-1337_18k.htm |
This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements, other than statements of historical fact, included or incorporated in this presentation, including statements regarding the Company's strategy, future operations, collaborations, intellectual property, cash resources, financial position, future revenues, projected costs, prospects, plans, and objectives of management, are forward-looking statements. The words "believes," "anticipates," "estimates," "plans," "expects," "intends," "may," "could," "should," "potential," "likely," "projects," "continue," "will," and "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Idera cannot guarantee that it will actually achieve the plans, intentions or expectations disclosed in its forward-looking statements and you should not place undue reliance on the Company's forward-looking statements. There are a number of important factors that could cause Idera's actual results to differ materially from those indicated or implied by its forward-looking statements. Factors that may cause such a difference include: whether interim results from a clinical trial will be predictive of the final results of the trial, whether results obtained in preclinical studies and clinical trials will be indicative of the results that will be generated in future clinical trials, including in clinical trials in different disease indications; whether products based on Idera's technology will advance into or through the clinical trial process on a timely basis or at all and receive approval from the United States Food and Drug Administration or equivalent foreign regulatory agencies; whether, if the Company's products receive approval, they will be successfully distributed and marketed; and such other important factors as are set forth under the caption "Risk Factors" in the Company's Annual Report filed on Form 10-K for the period ended December 31, 2017 and Quarterly Report filed on Form 10-Q for the period ended June 30, 2018. Although Idera may elect to do so at some point in the future, the Company does not assume any obligation to update any forward-looking statements and it disclaims any intention or obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. Forward Looking Statements and Other Important Cautions

Creating the Long-term Value of Idera Tilsotolimod Business Development 2 Priorities Drug Development Know-How Commercial Expertise Proven BD Acumen
Injecting a New Solution to Advance Cancer Immunotherapy 4 Near Term Value Growth Led by Tilsotolimod Pursuit of Orphan Indications Compelling Clinical Outcomes Clinical Results and Expansion Pathway Bolstered by Translational Data

Tilsotolimod Strategic Development Program 5 Anti-PD-1 Relapsed / Refractory Metastatic Melanoma Explore Pre-clinical Studies ILLUMINATE 101 – Multiple Solid Tumor Types Translational Research – ILLUMINATE 101 and 204 Confirm ILLUMINATE 204 ILLUMINATE 301 Expand ILLUMINATE 206 – Additional Unmet Solid Tumor Types Investigator Sponsored Trials Clinical Collaborations / Partnerships

6 Pre-clinical Studies ILLUMINATE 101 – Multiple Solid Tumor Types Translational Research – ILLUMINATE 101 and 204 Explore

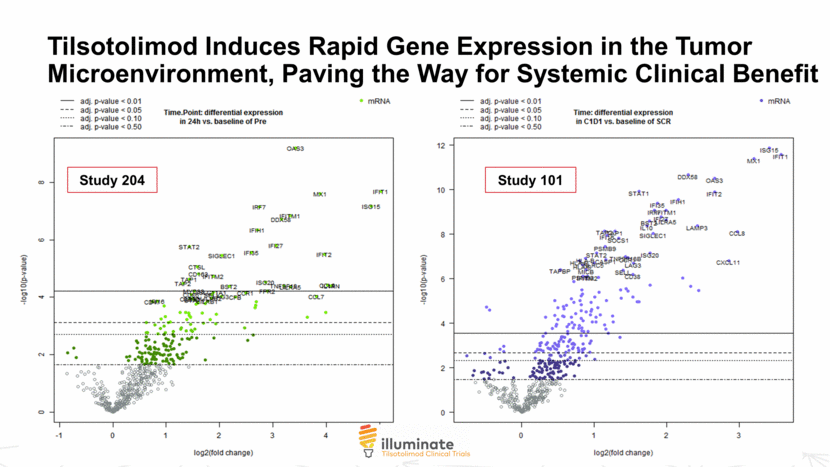
Tilsotolimod Induces Rapid Gene Expression in the Tumor Microenvironment, Paving the Way for Systemic Clinical Benefit 7 © 2018 Idera N = 15 pairs Study 204 Study 101
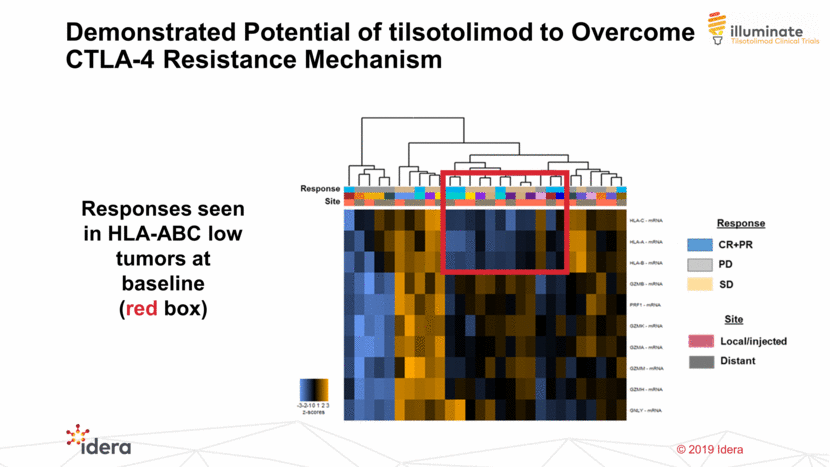
28 Responses seen in HLA-ABC low tumors at baseline (red box) Demonstrated Potential of tilsotolimod to Overcome CTLA-4 Resistance Mechanism
ILLUMINATE 101 Monotherapy Trial Demonstrating Tumor Priming beyond Melanoma Site status US: 10 sites active Ex-US: 4 sites active in Israel Dose escalation in refractory solid tumors, N= 39 Cancer types included: ocular, esophageal, colorectal, pancreatic, sarcoma, NSCLC, breast with skin met, urothelial Majority of subjects being dosed via administration into visceral lesions – no safety concerns Translational data confirms robust Type I IFN pathway activation in 24 hours 9
Demonstrating Tumor Priming beyond Melanoma Site status US: 10 sites active Ex-US: 4 sites active in Israel Dose escalation in refractory solid tumors, N=39 Cancer types included: ocular, esophageal, colorectal, pancreatic, sarcoma, NSCLC, breast with skin met, urothelial Majority of subjects being dosed via administration into visceral lesions – safety consistent with other studies Translational data confirms robust Type I IFN pathway activation in 24 hours ILLUMINATE 101 Monotherapy Trial 10
11 ILLUMINATE 204 ILLUMINATE 301 Anti-PD-1 Relapsed / Refractory Metastatic Melanoma Confirm

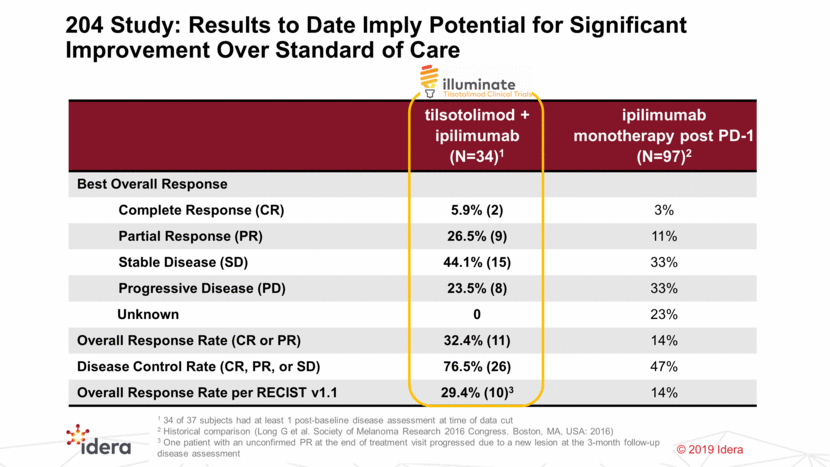
204 Study: Results to Date Imply Potential for Significant Improvement Over Standard of Care 12 tilsotolimod + ipilimumab (N=34)1 ipilimumab monotherapy post PD-1 (N=97)2 Best Overall Response Complete Response (CR) 5.9% (2) 3% Partial Response (PR) 26.5% (9) 11% Stable Disease (SD) 44.1% (15) 33% Progressive Disease (PD) 23.5% (8) 33% Unknown 0 23% Overall Response Rate (CR or PR) 32.4% (11) 14% Disease Control Rate (CR, PR, or SD) 76.5% (26) 47% Overall Response Rate per RECIST v1.1 29.4% (10)3 14% 1 34 of 37 subjects had at least 1 post-baseline disease assessment at time of data cut 2 Historical comparison (Long G et al. Society of Melanoma Research 2016 Congress. Boston, MA, USA: 2016) 3 One patient with an unconfirmed PR at the end of treatment visit progressed due to a new lesion at the 3-month follow-up disease assessment
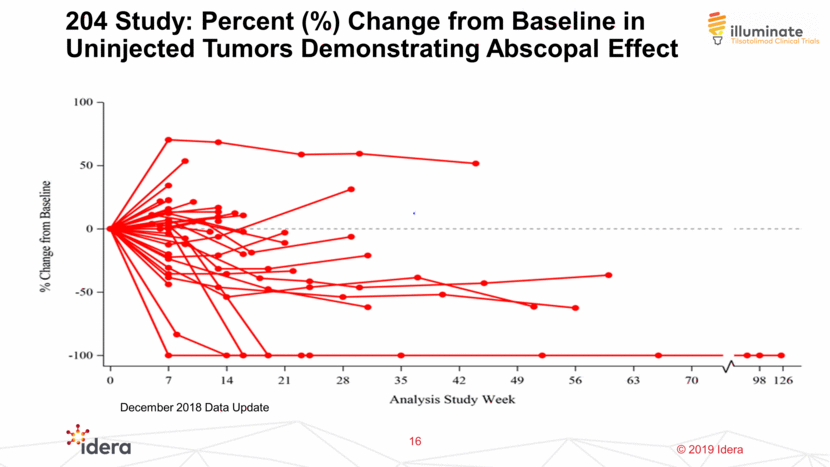
204 Study: Time To and Duration of Disease Control ORR of 32.4% with DCR of 76.5% 13 Stable diseases have evolved to responses over time for some patients December 2018 Data Update

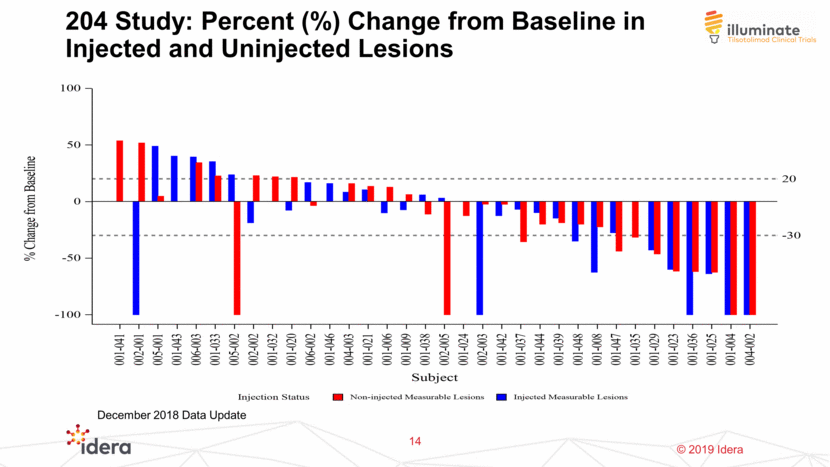
204 Study: Percent (%) Change from Baseline in Injected and Uninjected Lesions 14 December 2018 Data Update
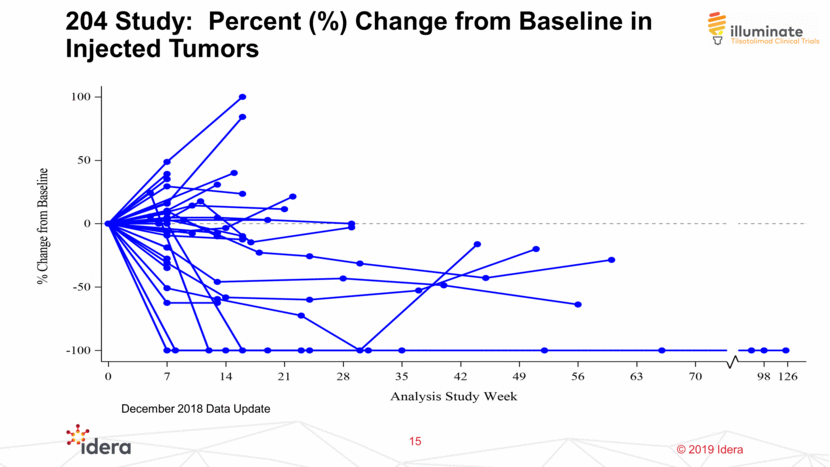
204 Study: Percent (%) Change from Baseline in Injected Tumors 15 December 2018 Data Update
204 Study: Percent (%) Change from Baseline in Uninjected Tumors Demonstrating Abscopal Effect 16 December 2018 Data Update
Illuminate 204 Trial Goals Achieved Established the recommended Phase 2 dose (RP2D) of 8mg tilsotolimod in combination with ipilimumab and pembrolizumab Provided proof of mechanism for tilso based on translational work from Phase 1 Rapid, within 24 hours, induction of IFN Responses in tumors not expected to respond to ipilimumab alone based on HLA-ABC low baseline expression Provided clinical proof of concept with ORR ~30% vs historic control of 10-16% Final data expected 2nd half 2019 17 Illuminate 204 Trial to be closed to enrollment end of January 2019. 42 patients currently enrolled.
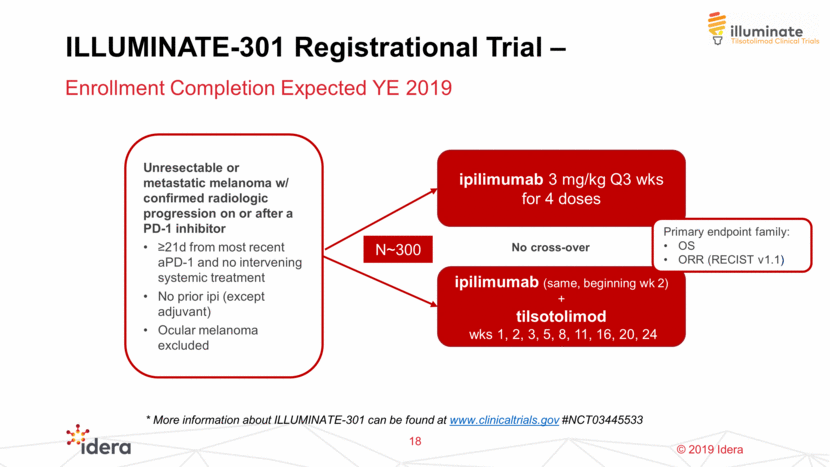
ILLUMINATE-301 Registrational Trial – Enrollment Completion Expected YE 2019 18 ipilimumab 3 mg/kg Q3 wks for 4 doses ipilimumab (same, beginning wk 2) + tilsotolimod wks 1, 2, 3, 5, 8, 11, 16, 20, 24 Unresectable or metastatic melanoma w/ confirmed radiologic progression on or after a PD-1 inhibitor >21d from most recent aPD-1 and no intervening systemic treatment No prior ipi (except adjuvant) Ocular melanoma excluded Primary endpoint family: OS ORR (RECIST v1.1) No cross-over N~300 * More information about ILLUMINATE-301 can be found at www.clinicaltrials.gov #NCT03445533
19 ILLUMINATE 206 – Additional Unmet Solid Tumor Types Investigator Sponsored Trials Clinical Collaborations / Partnerships Expand

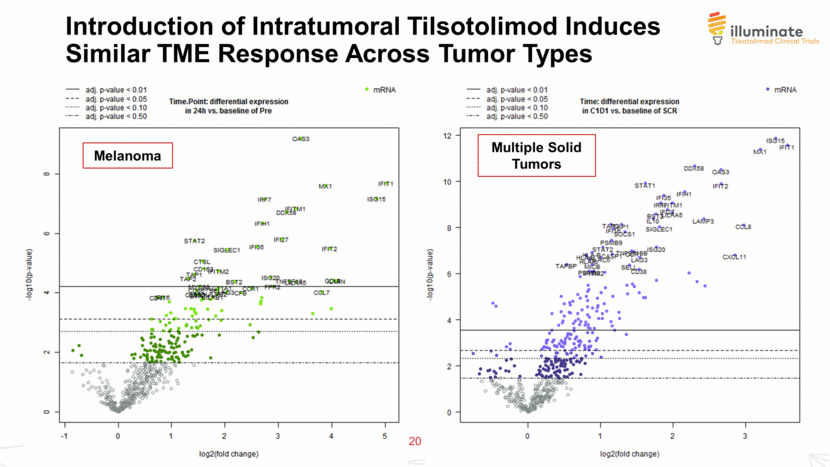
Introduction of Intratumoral Tilsotolimod Induces Similar TME Response Across Tumor Types 20 © 2018 Idera N = 15 pairs Melanoma Multiple Solid Tumors
ILLUMINATE 206 – Master Protocol Basket Design Individual sub-studies for each tumor type and combination Efficacy evaluation designed with 2 parts Part 1: signal finding, Simon’s Minimax 2-stage Part 2: randomized, controlled expansion of Part 1 indications Mandatory sequential tumor biopsies collected in Part 1 Safety, Blood biomarkers, PK, Immunogenicity Evaluation of tilsotolimod combined with one or more immunotherapy agents for the treatment of solid tumors 21
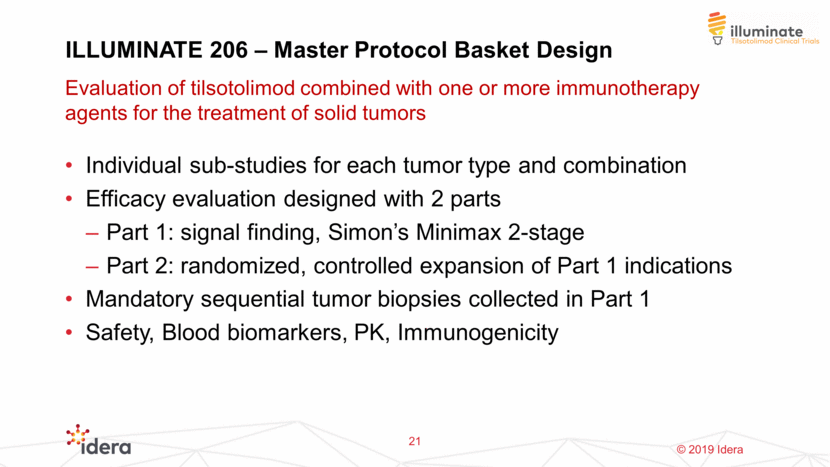
2125-MST-206 Trial Design adult subjects age >18 Histologically confirmed solid tumor Part 1: Simon's Minimax Two-Stage Design Enroll n1 subjects in Stage 1: if >r1 responses observed, enroll n2 subjects in stage 2 Sub-study 1 Stage 1 Stage 2 Sub-study Stage 1 Stage 2 Sub-study 3 Stage 1 Stage 2 Safety Review: For each sub-study, safety data from first 6 subjects are reviewed after 4 weeks of treatment Stage 2 Pass Criteria: Of the total enrolled subjects in both stages (n1+n2), if>r responses observed, the study expands to Part 2 Part 2: Expansion Sub-study 1 Sub-study 2 Sub-study 3
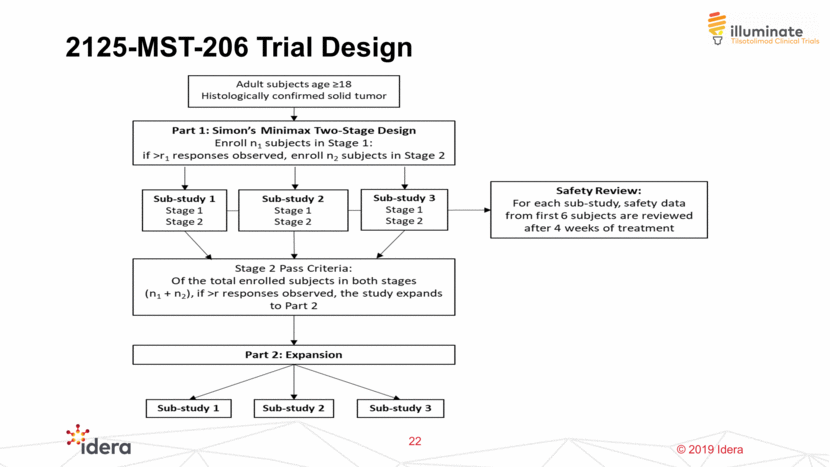
ILLUMINATE 206 Initial Expansion Beyond Melanoma Squamous Cell Carcinoma of the Head and Neck (SCCHN) Broad Effort to Determine Appropriate First Tumor Types for Expansion 23 Colorectal Cancer (MSS-CRC) SCCHN & MSS-CRC Competitive Analysis Translational Data KOL Feedback Potential Partner Feedback
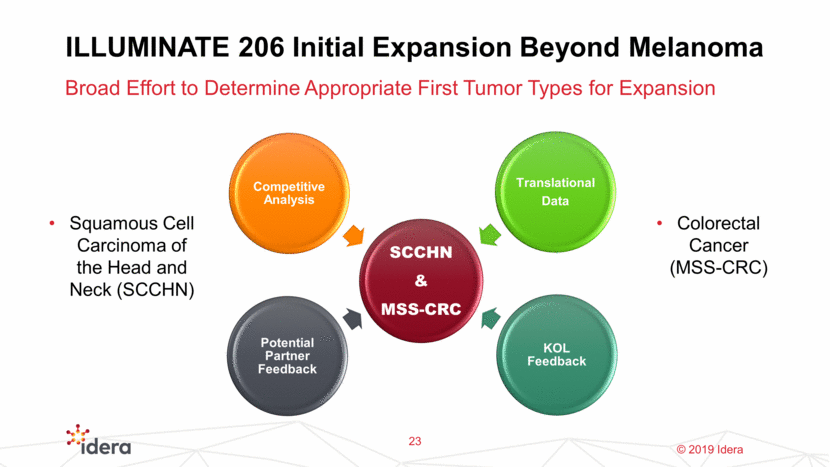
ILLUMINATE 206: Initial Expansion Beyond Melanoma ~55,000 new cases with 12,000 deaths in the US annually Immunotherapy naïve SCCHN Immunotherapy progressing SCCHN Triple Combination Therapy in Orphan Indications of Significant Unmet Need 24 ~135,500 new cases with ~50,000 deaths. Of total CRC cases, MSS represents 80-85% (and a higher proportion of deaths) MSS-CRC, Chemo refractory, immunotherapy naïve Additional indications/I-O combinations can be added Squamous Cell Carcinoma of the Head and Neck (SCCHN) Colorectal Cancer (MSS-CRC)
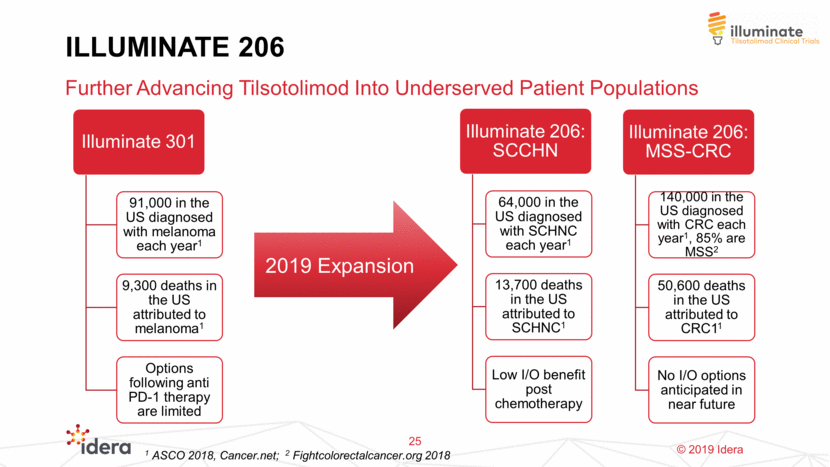
ILLUMINATE 206 Further Advancing Tilsotolimod Into Underserved Patient Populations 25 2019 Expansion 1 ASCO 2018, Cancer.net; 2 Fightcolorectalcancer.org 2018 Illuminate 301 91,000 in the US diagnosed with melanoma each year1 9,300 deaths in the US attributed to melanoma1 Options following anti PD-1 therapy are limited Illuminate 206: SCCHN 64,000 in the US diagnosed with SCHNC each year1 13,700 deaths in the US attributed to SCHNC1 Low I/O benefit post chemotherapy Illuminate 206: MSS-CRC 140,000 in the US diagnosed with CRC each year1, 85% are MSS2 50,600 deaths in the US attributed to CRC11 No I/O options anticipated in near future
Building for Sustainable Growth Leveraging Business Development to Generate Additional Growth Management Track Record Focused Screening

Leveraging Management’s Track Record and Expertise Built ViroPharma, an international rare disease company with over $500 million in annual sales at time of being acquired Successfully commercialized products in the US in areas not initially well-appreciated by the investment community Built a multi-product European business Completed numerous deals, both commercial and pipeline, that shaped the foundation and the future of the company Demonstrated a strong track record of resilience 27

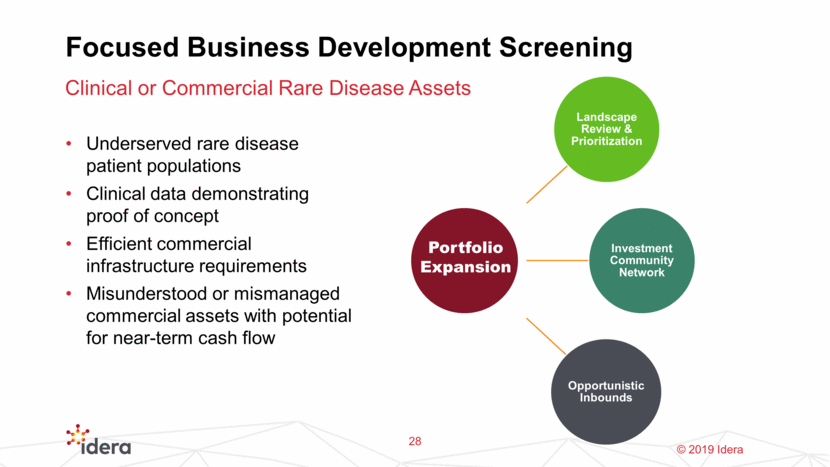
Focused Business Development Screening Underserved rare disease patient populations Clinical data demonstrating proof of concept Efficient commercial infrastructure requirements Misunderstood or mismanaged commercial assets with potential for near-term cash flow Clinical or Commercial Rare Disease Assets 28 Portfolio Expansion
Financials and Capital Structure Updates Completed Q3 2018 with $82.5M cash Expected cash runway into Q1 2020 Approximately 27M shares outstanding ATM in place to raise up to $50M 29

Critical Growth Catalysts in 2019 ILLUMINATE 204 Final Data ILLUMINATE 206 Initiation and Execution ILLUMINATE 101 Translational Data ILLUMINATE 301 Completion of Enrollment Tilsotolimod 30 Corporate Potential Execution of Business Development Deal Potential Partnerships/Collaborations - tilsotolimod


