Attached files
| file | filename |
|---|---|
| 8-K - 8-K - GENOCEA BIOSCIENCES, INC. | gnca201901078k.htm |

Beyond Machines for Antigen Selection: Using ATLAS™ to Enable Novel Cancer Immunotherapies January 2019

Disclaimer This presentation contains “forward-looking” statements that are within the meaning of federal securities laws and are based on our management’s beliefs and assumptions and on information currently available to management. Forward-looking statements include information concerning our possible or assumed future results of operations, business strategies, clinical trials and pre- clinical studies, regulatory approval of our product candidates, liquidity position and capital needs, financing plans, industry environment, potential growth opportunities, potential market opportunities and the effects of competition. Forward-looking statements include all statements that are not historical facts and can be identified by terms such as “anticipates,” “believes,” “expects,” “could,” “seeks,” “estimates,” “intends,” “may,” “plans,” “potential,” “predicts,” “projects,” “should,” “will,” “would” or similar expressions and the negatives of those terms. Forward-looking statements represent our management’s beliefs and assumptions only as of the date of this presentation. Our operations involve risks and uncertainties, many of which are outside our control, and any one of which, or combination of which, could materially affect our results of operations and whether the forward-looking statements ultimately prove to be correct. Factors that may materially affect our results of operations include, among other things, our ability to progress product candidates in preclinical and clinical trials, the ability of ATLAS™ to identify promising oncology vaccine and immunotherapy product candidates, the scope, rate and progress of our preclinical and clinical trials and other research and development activities, anticipated timing of new clinical trails, our estimates regarding the amount of funds we require to conduct our clinical trials for GEN-009, our plans to commercialize GEN-009, the timing of, and ability to, obtain and maintain necessary regulatory approvals for our product candidates, and those listed in our Annual Report on Form 10-K for the fiscal year ended December 31, 2017 and other filings with the Securities and Exchange Commission (“SEC”). Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. You may get copies of our Annual Report on Form 10-K, Quarterly Report on Form 10-Q and our other SEC filings for free by visiting EDGAR on the SEC website at http://www.sec.gov. 2
Selecting the right antigens to enable diverse cancer immunotherapies: POWERFUL NOVEL UNIQUE ADVANTAGE TECHNOLOGY IMMUNOTHERAPIES ATLAS™ We Know, They • Vaccines & T cell Platform Guess therapies; …based on actual patient- • Neoantigen and shared …validated in screening of and antigen-specific ~200 cancer subjects antigen T cell responses 3
Compelling investment opportunity CLINICAL-STAGE cancer immunotherapy company UNIQUE antigen selection platform, ATLAS™ – Personalized tumor, immune repertoire profiling for better antigen selection – Better antigens critical to delivering on promise of neoantigen immunotherapies VALIDATING clinical readout imminent: Phase 1/2a neoantigen vaccine (GEN- 009) immunogenicity late Q2/early Q3 Pioneering Neoantigen Cancer EXPANDING pipeline: GEN-011 (neoantigen cell therapy) IND in 1H 2020 Immunotherapies STRONG team, advisors and investors 4

Next-gen therapies can extend the immuno-oncology revolution FOUNDATIONAL PROMISE VS. OUR OPPORTUNITY THERAPEUTICS REALITY Immune checkpoint inhibitors (ICI) ICI CAR-T Next-generation immunotherapies that direct or deliver T cells to kill tumors • Efficacy limited to • Efficacy limited to CAR-T certain populations blood cancers • Precede/complement ICI • Combinations • Potential for high • Extend cell therapy to solid increase toxicity toxicity tumors 5

T cells directed to the correct targets are integral to successful immunotherapies Vaccines Adoptive cell therapy (ACT) (Neo) Antigen (Neo) Antigen Antigen-specific Phenotype Modality Discovery and and and EXPANSION DELIVERY SELECTION Which antigens can/should the immune system respond to? Most Innovation, $s Industry Weakness is Most Innovation, $s Focused Here Our Core Strength Focused Here 6
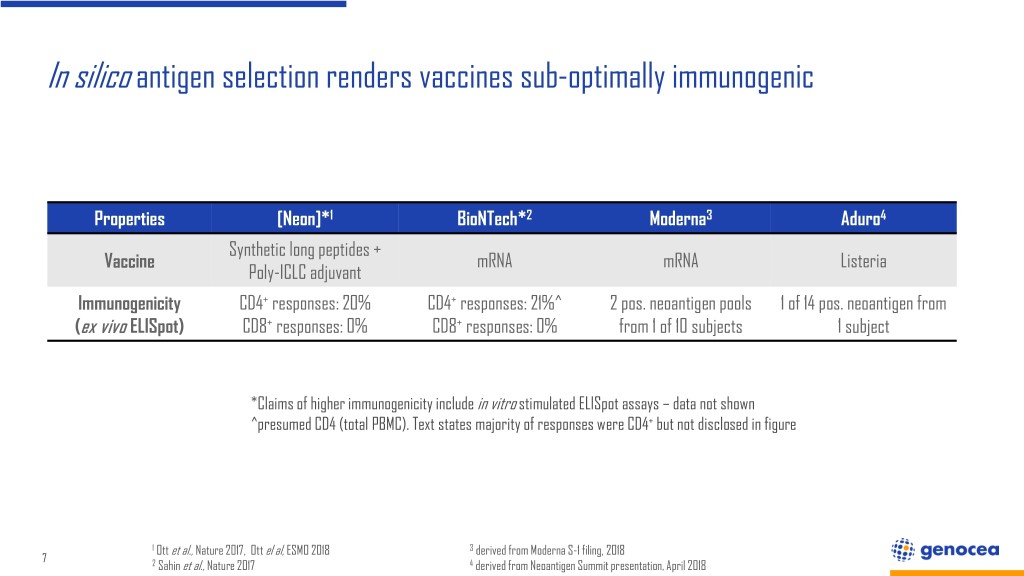
In silico antigen selection renders vaccines sub-optimally immunogenic Properties [Neon]*1 BioNTech*2 Moderna3 Aduro4 Synthetic long peptides + Vaccine mRNA mRNA Listeria Poly-ICLC adjuvant Immunogenicity CD4+ responses: 20% CD4+ responses: 21%^ 2 pos. neoantigen pools 1 of 14 pos. neoantigen from (ex vivo ELISpot) CD8+ responses: 0% CD8+ responses: 0% from 1 of 10 subjects 1 subject *Claims of higher immunogenicity include in vitro stimulated ELISpot assays – data not shown ^presumed CD4 (total PBMC). Text states majority of responses were CD4+ but not disclosed in figure 1 Ott et al., Nature 2017, Ott el al, ESMO 2018 3 derived from Moderna S-1 filing, 2018 7 2 Sahin et al., Nature 2017 4 derived from Neoantigen Summit presentation, April 2018

Only ATLAS™ personalizes profiles of both tumor and T cell responses THE CHALLENGE Identifying true neoantigens: Antigens triggering anti-tumor T cell responses CANCER PATIENTS ATLAS™ T cell Recognized by T cells T cell receptor Neoantigen 0111100 00010110 MHC Molecule IN SILICO PeptidesPeptides Presented presented 1110110110 onon Cell cell Surfacesurface 011 1100 Tumor cell Personalized tumor mutations 8
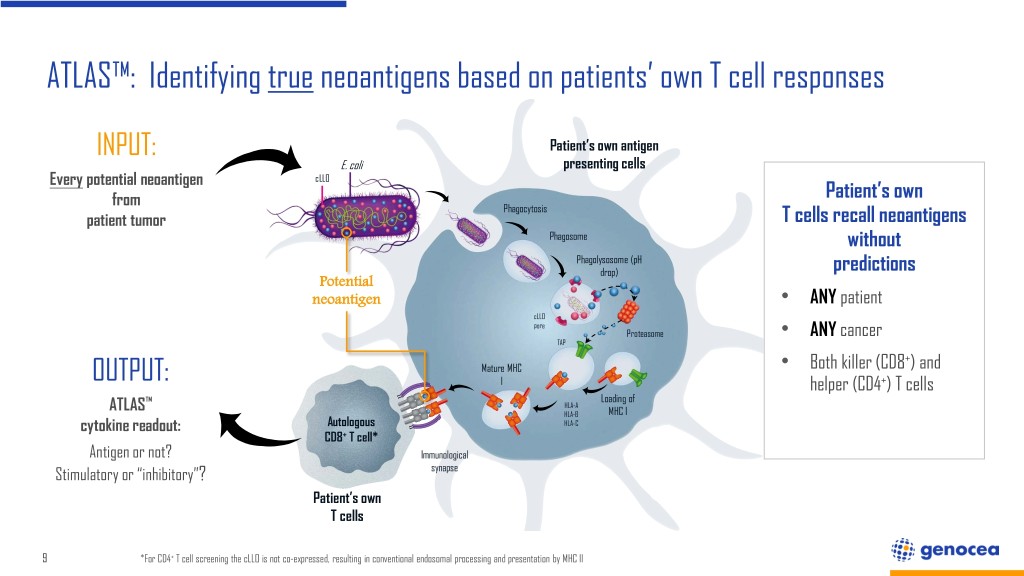
ATLAS™: Identifying true neoantigens based on patients’ own T cell responses INPUT: Patient’s own antigen E. coli presenting cells Every potential neoantigen cLLO from Patient’s own Phagocytosis patient tumor T cells recall neoantigens Phagosome without Phagolysosome (pH drop) predictions Potential neoantigen • ANY patient cLLO pore Proteasome ANY cancer TAP • + Mature MHC • Both killer (CD8 ) and OUTPUT: I helper (CD4+) T cells ™ Loading of ATLAS HLA-A HLA-B MHC I cytokine readout: Autologous HLA-C CD8+ T cell* Antigen or not? Immunological Stimulatory or “inhibitory”? synapse Patient’s own T cells 9 *For CD4+ T cell screening the cLLO is not co-expressed, resulting in conventional endosomal processing and presentation by MHC II

ATLASTM is a rapid, industrialized platform for antigen discovery Neoantigen Targets TAA/Viral Targets ~200 cancer patients’ samples Dendritic Cell screened 10 T cell

ATLAS™ identifies and characterizes all candidates to identify true neoantigens NSCLC PATIENT 52 candidate neoantigens identified & profiled IFNγ responses depicted CD8+ T cells CD4+ T cells True neoantigens – selected for therapy Not antigens “Inhibitory” neoantigens- excluded from therapy Candidate neoantigens Candidate neoantigens ATLAS™ BENEFITS Finds true neoantigens Identifies both CD8+ and CD4+ neoantigens Identifies “inhibitory" antigens (for exclusion) 11

“Inhibitory” neoantigens suppress immune responses, may promote tumor progression mATLAS CD8+ screen Study Design Stimulatory antigen vaccination Vaccine Typical endpoint s.c. >200mm2 D0 D3 D10 D17 B16F10 s.c. Inhibitory antigen vaccination TIL g PBS Inhibitory 8000 6000 4000 CD8 Cell count 2000 DAPI IHC tumor whole slide scan 0 PBS Inhibitory 12

Profiling patient tumor and immune response repertoire with ATLAS yields vastly different antigen sets 0111100 01 10010110 11 0100111 010 1 ININ- SILICOSILICO EX VIVO 11101 01110 10 01111001011 2 011 1100 NetMHCpan Predicted BIOLOGY TOO COMPLEX ATLAS™ FOR THESE APPROACHES “Inhibitory” Stimulatory IDENTIFIES TRUE NEOANTIGENS Antigens Antigens 1128 • MISSES most true neoantigens • IDENTIFIES the true neoantigens • MISCHARACTERIZES “inhibitory” neoantigens • REVEALS the true neoantigens missed by in silico 139 140 132 155 approaches • FAILS to find vital CD4+ antigens prospectively1 • RULES OUT “inhibitory” neoantigens • FINDS CD4+ neoantigens (that rarely overlap with CD8+ neoantigens) CD8+ T cell Screen (2,852 mutations screened across 37 patients with 8 different tumor types) 13 1Melssen and Slingluff, 2017 Curr Opin Immunol 2http://www.cbs.dtu.dk/services/NetMHCpan Note: Epitope predictions cutoff ≤500nM binding affinity

GEN-009: ATLAS™ neoantigen selection for personalized cancer vaccines Collect tumor and blood ATLAS to identify true SYNTHESIZE VACCINE Ship personalized product to samples, sequence exome neoantigens of CD4+ and (synthetic long peptides + Poly clinical site CD8+ ICLC) with T cell responses ATLAS-identified T cell antigens 14

GEN-009 clinical trial designed to demonstrate superiority of ATLAS™ antigen selection in patients PHASE 1/2a STUDY OVERVIEW1 PART A PART B • Patient cohort: No evidence of disease, high risk of • Multiple cohorts: ICI combo with stable disease, relapse partial responses • Multiple tumor types with ICI approval • Objectives: safety, immunogenicity, efficacy PART C RECENT & NEAR-TERM EXPECTED MILESTONES • ICI refractory metastatic disease monotherapy • GEN-009-101 Phase 1/2a first patients dosed • Objectives: safety, immunogenicity, efficacy • Immunogenicity data: late Q2/early Q3 15 1. More information about the study can be found at https://clinicaltrials.gov/ct2/show/NCT03633110?term=genocea&rank=1.

GEN-011 has potential to improve on other ACT approaches TIL therapy GEN-011 TCR transduced T cells CAR-T Remarkable efficacy in some patients Limited to tumors with high TMB and TIL content Emerging for solid tumors Issues with off-tumor on-target effects Hematologic cancers Limited HLA Less success in solid tumors Limited to surface proteins Fail to infiltrate tumor 16 Fousek and Ahmed (2015) Clin Cancer Res

GEN-011 is a first-in class personalized non-engineered ACT targeting multiple neoantigens • Product is autologous, ATLAS-identified neoantigen-expanded peripheral blood T cells (CD4+ and CD8+) – Multiple specificities for broader coverage, reduced potential for escape – Both CD4+ and CD8+ T cells with confirmed neoantigen responsiveness • GEN-011 provides a number of potential advantages over traditional ACT including – Better efficacy – Favorable speed and cost of manufacturing than engineered approaches – Better safety profile than engineered approaches – Applicability to more tumor types 17

GEN-011: ATLAS™ neoantigen selection for personalized T cell therapy ATLAS true neoantigen identification Neoantigen-specific T cell expansion Neoantigen list NGS Mutant antigen library in E. coli ATLAS-defined neoantigens Tumor + + cytokine(s) ATLAS neoantigen identification + (CD4+ and CD8+ Autologous CD4+ and CD8+ T T cell expansion Adoptive transfer of tumor- Leukopak T cell subsets) Autologous monocyte-derived dendritic Autologous CD4+ and cells from peripheral blood specific T cells Cell (MDDC) CD8+ T cells Cryopreserved PBMCs 18

GEN-011: a differentiated ACT approach Neoantigen-specific TCR Features/Benefits GEN-011 TIL ACT TAA-specific ACT ACT Potential for better immunogenicity, efficacy Broad neoantigen coverage Self, not neoantigens CD4+ and CD8+ antigens Single antigen specificity HLA agnostic Rule out inhibitory antigens Potential applicability to more tumor types Requires large, resected Broad indication selection tumors Limited metastatic tumor escape Potential for favorable speed of manufacturing Work from PBMCs 19

ATLAS™ drives deep emerging Genocea neoantigen immunotherapy pipeline1 DISCOVERY PRE-IND PHASE 1/2a PIVOTAL STATUS & ANTICIPATED MILESTONES GEN-009 • Immunogenicity data in 1st Generation late Q2/early Q3 Neoantigen Cancer Vaccine • Clinical efficacy in 2020 GEN-010 Proprietary vaccine 2nd Generation • modality Neoantigen Cancer Vaccine GEN-011 IND in 1H 2020 Adoptive T cell Therapy • Shared Antigen Novel antigens discovered in CRC, NSCLC Cancer Vaccines • Vaccines for Cancers • Novel antigens discovered for Epstein- of Viral Origin Barr Virus 1. Studies as of January, 2019. 20

Team with deep vaccinology, immunology and cancer experience Girish Aakalu, PhD Pamela Carroll, PhD Chief Business Officer SVP, Immuno-oncology Chip Clark Tom Davis, MD President & CEO Chief Medical Officer Jessica Baker Flechtner, PhD Derek Meisner Chief Scientific Officer SVP, General Counsel Narinder Singh SVP Pharmaceutical Sciences & Manufacturing 21

Seasoned SAB and board SCIENTIFIC ADVISORY BOARD BOARD Chuck Drake, MD, PhD Ken Bate (Chair) Former chair, Cubist Ex-Biogen, Millennium Director of Genitourinary Oncology President Ali Behbahani, MD, PhD General partner, NEA Elizabeth Jaffee, MD CEO, Editas Chair Katrine Bosley Deputy Director Former CEO, Avila Ron Cooper CEO, Albireo George Siber, MD, PhD (chair) Vaccines Former President, Europe, BMS Michael Higgins Venture Partner, Polaris Ex-CFO/COO, Ironwood Eric Tran, PhD SVP, CMO, R&D Head, Howard Mayer, MD Neuorsciences, Shire George Siber, MD, PhD Ex-CSO, Wyeth Vaccines Kwok Wong, MD Chief of Hematology and Medical Oncology 22

Compelling investment opportunity CLINICAL-STAGE cancer immunotherapy company UNIQUE antigen selection platform, ATLAS™ – Personalized tumor, immune repertoire profiling for better antigen selection – Better antigens critical to delivering on promise of neoantigen immunotherapies VALIDATING clinical readout imminent: Phase 1/2a neoantigen vaccine (GEN- 009) immunogenicity late Q2/early Q3 Pioneering Neoantigen Cancer EXPANDING pipeline: GEN-011 (neoantigen cell therapy) IND in 1H 2020 Immunotherapies STRONG team, advisors and investors 23

Genocea Biosciences, Inc. NASDAQ: GNCA 100 Acorn Park Drive Cambridge, MA 02140 USA Phone: +1 617.876.8191 www.genocea.com
