Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Axsome Therapeutics, Inc. | a19-1343_1ex99d1.htm |
| 8-K - 8-K - Axsome Therapeutics, Inc. | a19-1343_18k.htm |
NASDAQ: AXSM ASCEND Phase 2 Trial of AXS-05 Topline Results Conference Call January 7, 2019 in MDD © Axsome Therapeutics, Inc.

Overview AXS-05 in MDD ASCEND Phase 2 Trial Topline Results & Medical Affairs © Axsome Therapeutics, Inc. 2 Introduction Mark Jacobson, Senior Vice President, Operations Overview and Summary Herriot Tabuteau, MD, Chief Executive Officer ASCEND Trial Design & Results Cedric O’Gorman, MD, Senior Vice President, Clinical Development Q&A Presenters & Nick Pizzie, Chief Financial Officer Concluding Remarks Herriot Tabuteau, MD, Chief Executive Officer
FLS Forward-Looking Statements & Safe Harbor Certain information contained in this presentation may include “forward-looking statements” within the meaning of the Private Securities Litigation Reform Act of 1995. We may, in some cases, use terms such as “predicts,” “believes,” “potential,” “continue,” “estimates,” “anticipates,” “expects,” “plans,” “intends,” “may,” “could,” “might,” “will,” “should” or other words that convey uncertainty of future events or outcomes to identify these forward-looking statements. In particular, the Company’s statements regarding trends and potential future results are examples of such forward-looking statements. The forward-looking statements include risks and uncertainties, including, but not limited to, the success, timing and cost of our ongoing clinical trials and anticipated clinical trials for our current product candidates, including statements regarding the timing of initiation and completion of the trials, interim analyses and receipt of interim results; the timing of and our ability to obtain and maintain U.S. Food and Drug Administration or other regulatory authority approval of, or other action with respect to, our product candidates; the potential for the ASCEND clinical trial to provide a basis for approval of AXS-05 for the treatment of major depressive disorder and accelerate its development timeline and commercial path to patients; the Company’s ability to obtain additional capital necessary to fund its operations; the Company’s ability to generate revenues in the future; the Company’s ability to successfully defend its intellectual property or obtain the necessary licenses at a cost acceptable to the Company, if at all; the successful implementation of the Company’s research and development programs; the enforceability of the Company’s license agreements; the acceptance by the market of the Company’s product candidates, if approved; and other factors, including general economic conditions and regulatory developments, not within the Company’s control. These factors could cause actual results and developments to be materially different from those expressed in or implied by such statements. Forward-looking statements are not guarantees of future performance, and actual results may differ materially from those projected. The forward-looking statements are made only as of the date of this presentation and the Company undertakes no obligation to publicly update such forward-looking statements to reflect subsequent events or circumstance. This presentation also contains estimates and other statistical data made by independent parties and by us relating to market size and other data about our industry. This data involves a number of assumptions and limitations, and you are cautioned not to give undue weight to such estimates. Neither we nor any other person makes any representation as to the accuracy or completeness of such data or undertakes any obligation to update such data after the date of this presentation. In addition, these projections, assumptions and estimates are necessarily subject to a high degree of uncertainty and risk. © Axsome Therapeutics, Inc. 3

Overview and Summary Herriot Tabuteau, MD Chief Executive Officer Axsome Therapeutics, Inc. © Axsome Therapeutics, Inc. 4

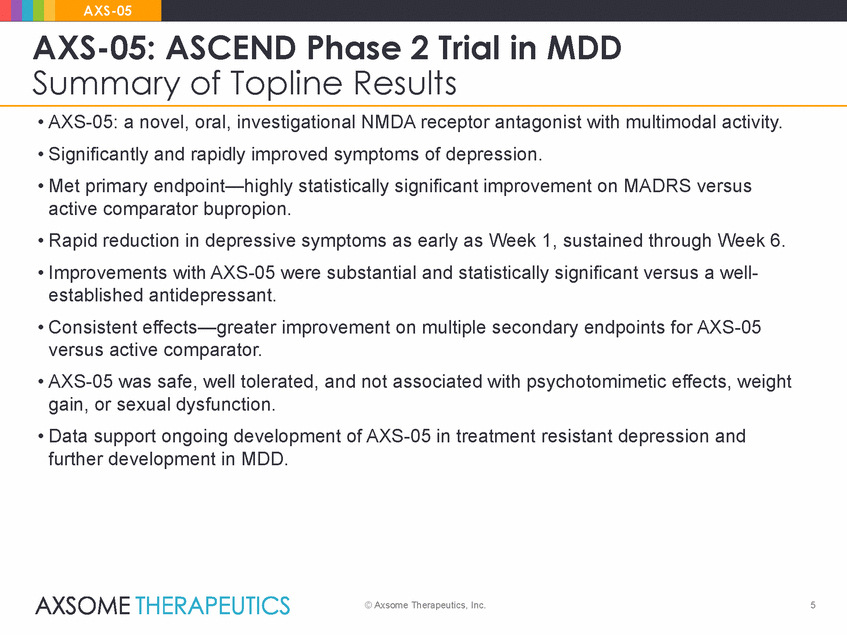
AXS-05 AXS-05: ASCEND Phase 2 Trial in MDD Summary of Topline Results • AXS-05: a novel, oral, investigational NMDA receptor antagonist with multimodal activity. • Significantly and rapidly improved symptoms of depression. • Met primary endpoint—highly statistically significant improvement on MADRS versus active comparator bupropion. • Rapid reduction in depressive symptoms as early as Week 1, sustained through Week 6. • Improvements with AXS-05 were substantial and statistically significant versus a well-established antidepressant. • Consistent effects—greater improvement on multiple secondary endpoints for AXS-05 versus active comparator. • AXS-05 was safe, well tolerated, and not associated with psychotomimetic effects, weight gain, or sexual dysfunction. • Data support ongoing development of AXS-05 in treatment resistant depression and further development in MDD. © Axsome Therapeutics, Inc. 5
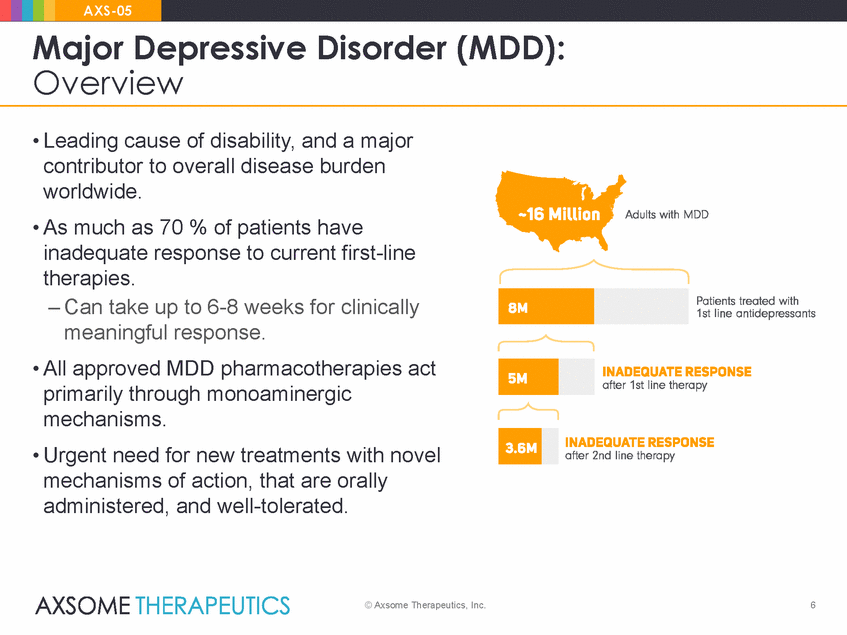
AXS-05 Major Depressive Disorder Overview (MDD): • Leading cause of disability, and a major contributor to overall disease burden worldwide. • As much as 70 % of patients have inadequate response to current first-line therapies. – Can take up to 6-8 weeks for clinically meaningful response. • All approved MDD pharmacotherapies act primarily through monoaminergic mechanisms. • Urgent need for new treatments with novel mechanisms of action, that are orally administered, and well-tolerated. © Axsome Therapeutics, Inc. 6
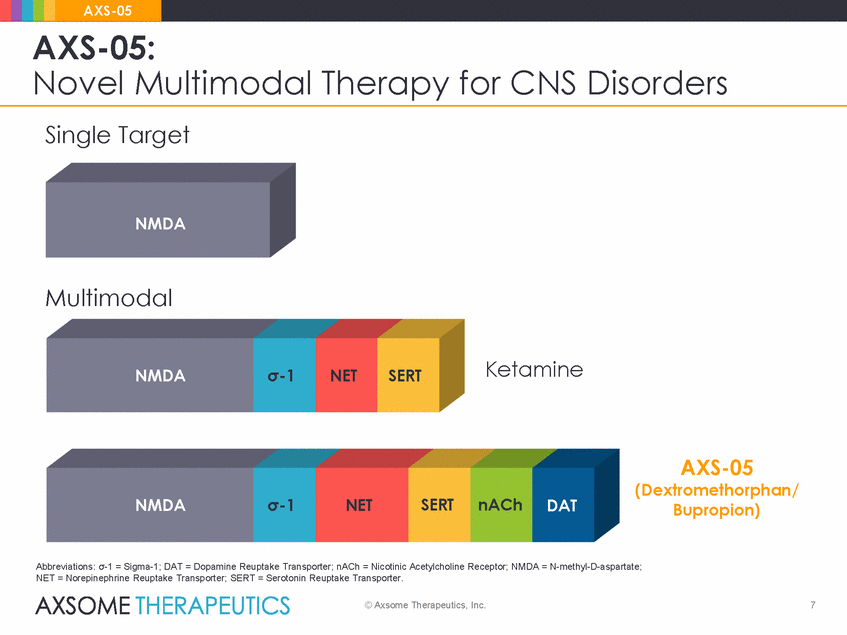
AXS-05 AXS-05: Novel Multimodal Therapy for CNS Disorders Single Target NMDA Multimodal Ketamine NMDA σ-1 NET SERT AXS-05 (Dextromethorphan/ Bupropion) NMDA σ-1 NET SERT nACh DAT Abbreviations: σ-1 = Sigma-1; DAT = Dopamine Reuptake Transporter; nACh = Nicotinic Acetylcholine Receptor; NMDA = N-methyl-D-aspartate; NET = Norepinephrine Reuptake Transporter; SERT = Serotonin Reuptake Transporter. © Axsome Therapeutics, Inc. 7
ASCEND Trial Design & Results Cedric O’Gorman, MD, MBA Senior Vice President, Clinical Development and Medical Affairs Axsome Therapeutics, Inc. © Axsome Therapeutics, Inc. 8
AXS-05 ASCEND: Clinical Trial Design • Phase 2, randomized, double-blind, active-controlled, multi-center, U.S. trial • N=80 adult patients with confirmed diagnosis of moderate to severe MDD • 6-week treatment period – Twice daily dosing • Dose groups (1:1 randomization): – AXS-05 (45 mg dextromethorphan/105 mg bupropion) – Active comparator bupropion (105 mg) • Extensive quality control measures © Axsome Therapeutics, Inc. 9
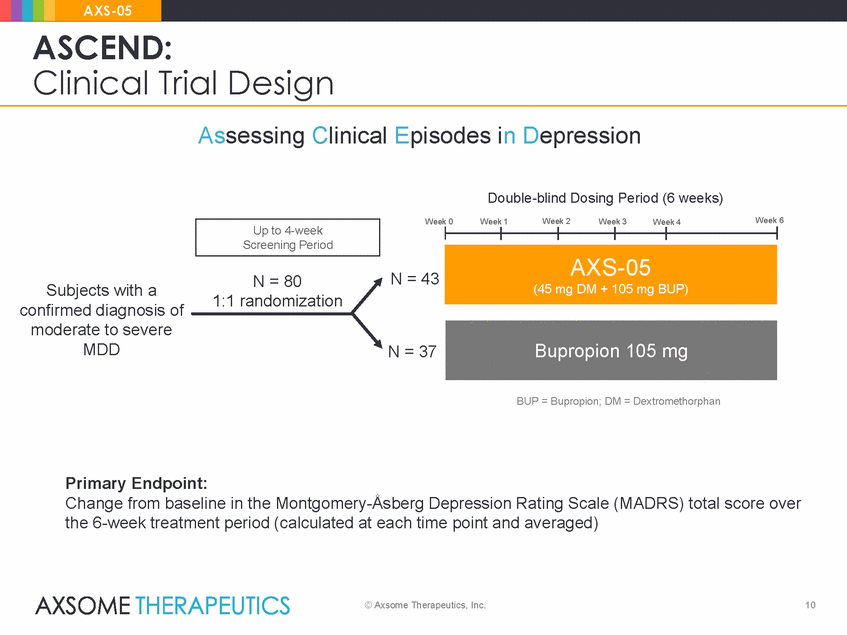
AXS-05 ASCEND: Clinical Trial Design Assessing Clinical Episodes in Depression Double-blind Dosing Period (6 weeks) Week 6 Week 0 Week 1 Week 2 Week 3 Week 4 N = 43 N = 80 1:1 randomization Subjects with a confirmed diagnosis of moderate to severe MDD N = 37 BUP = Bupropion; DM = Dextromethorphan Primary Endpoint: Change from baseline in the Montgomery-Åsberg Depression Rating Scale (MADRS) total score over the 6-week treatment period (calculated at each time point and averaged) © Axsome Therapeutics, Inc. 10 Bupropion 105 mg AXS-05 (45 mg DM + 105 mg BUP) Up to 4-week Screening Period
AXS-05 ASCEND: Demographics and Baseline Characteristics • Mean age (years): 37.3 for AXS-05 group, 37.7 for bupropion group • Mean age at onset of illness (years): 26.5 for AXS-05 group, 26.6 for bupropion group • Mean MADRS total score at baseline: 31.8 for AXS-05 group, 32.2 for bupropion group • 51% of subjects had 3 or more major depressive episodes prior to enrollment • 23% of subjects had received prior first line treatment in their current major depressive episode © Axsome Therapeutics, Inc. 11
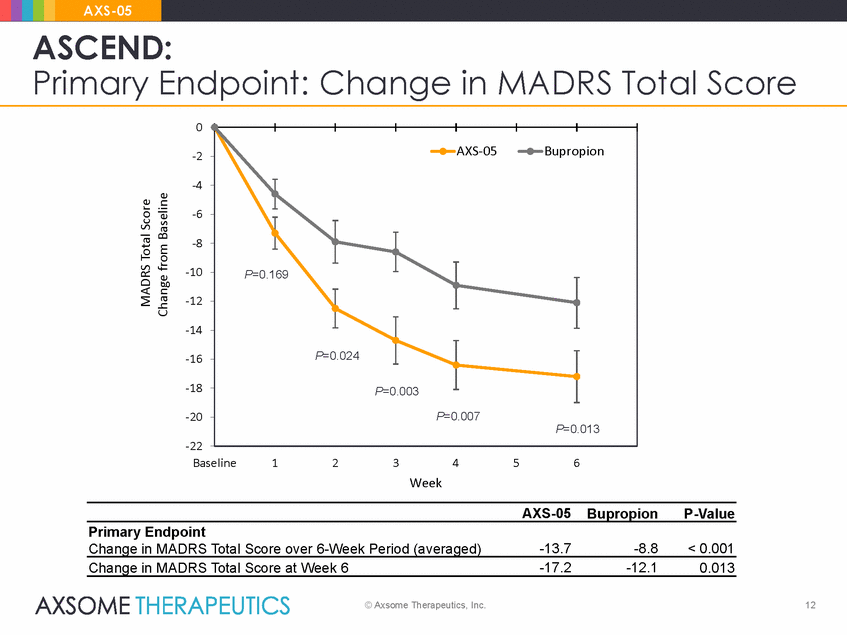
AXS-05 ASCEND: Primary Endpoint: Change in MADRS Total Score 0 -2 -4 -6 -8 -10 -12 -14 -16 -18 -20 -22 Baseline 1 2 3 4 5 6 Week AXS-05 Bupropion P-Value Primary Endpoint Change in MADRS Total Score over 6-Week Period (averaged) -13.7 -8.8 < 0.001 Change in MADRS Total Score at Week 6 -17.2 -12.1 0.013 © Axsome Therapeutics, Inc. 12 MADRS Total Score Change from Baseline AXS-05Bupropion P=0.169 P=0.024 P=0.003 P=0.007 P=0.013
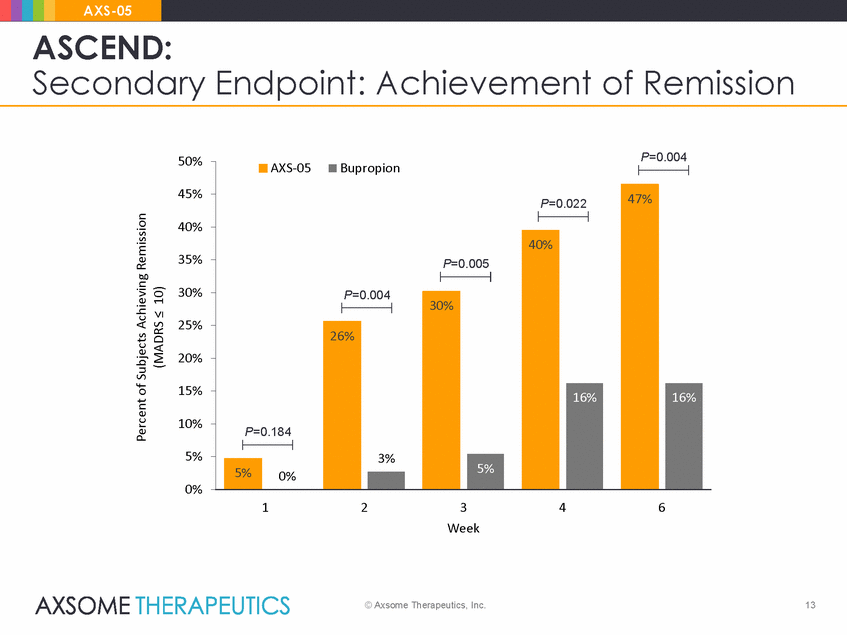
AXS-05 ASCEND: Secondary Endpoint: Achievement of Remission P=0.004 50% AXS-05 Bupropion 45% 40% 35% 30% 25% 20% 15% 10% 5% 0% 1 2 3 Week 4 6 © Axsome Therapeutics, Inc. 13 Percent of Subjects Achieving Remission (MADRS < 10) P=0.022 47% P=0.005 40% P=0.004 30% P=0.184 26% 3% 16% 16% 5% 0% 5%
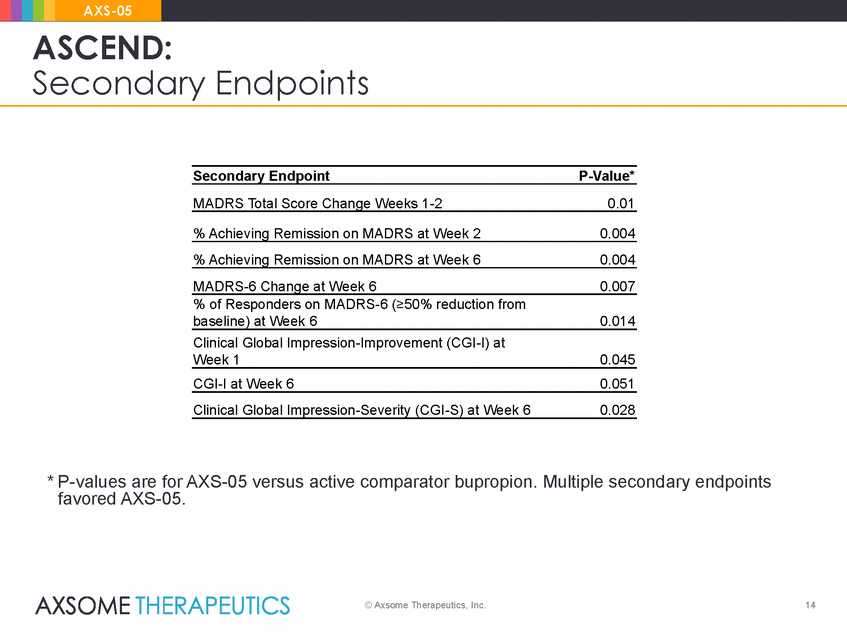
AXS-05 ASCEND: Secondary Endpoints Secondary Endpoint P-Value* MADRS Total Score Change Weeks 1-2 0.01 % Achieving Remission on MADRS at Week 2 0.004 % Achieving Remission on MADRS at Week 6 0.004 MADRS-6 Change at Week 6 0.007 % of Responders on MADRS-6 (>50% reduction from baseline) at Week 6 0.014 Clinical Global Impression-Improvement (CGI-I) at Week 1 0.045 CGI-I at Week 6 0.051 Clinical Global Impression-Severity (CGI-S) at Week 6 0.028 * P-values are for AXS-05 versus active comparator bupropion. Multiple secondary endpoints favored AXS-05. © Axsome Therapeutics, Inc. 14
AXS-05 ASCEND: Safety • AXS-05 was safe and well tolerated with similar rates of adverse events in the AXS-05 and bupropion arms. • No serious adverse events, and no meaningful difference between the two treatment arms in discontinuations due to adverse events. • The most commonly reported adverse events in the AXS-05 arm were nausea, dizziness, dry mouth, decreased appetite, and anxiety. • AXS-05 was not associated with psychotomimetic effects, weight gain, or increased sexual dysfunction. © Axsome Therapeutics, Inc. 15

AXS-05 ASCEND: Summary • Statistically significant improvements on MADRS and secondary efficacy endpoints for AXS-05 in patients with MDD • Early and sustained separation from active comparator bupropion • Safe and well-tolerated with no psychotomimetic effects, weight gain, or increased sexual dysfunction © Axsome Therapeutics, Inc. 16

Q&A © Axsome Therapeutics, Inc. 17

Concluding Remarks Herriot Tabuteau, MD Chief Executive Officer Axsome Therapeutics, Inc. © Axsome Therapeutics, Inc. 18

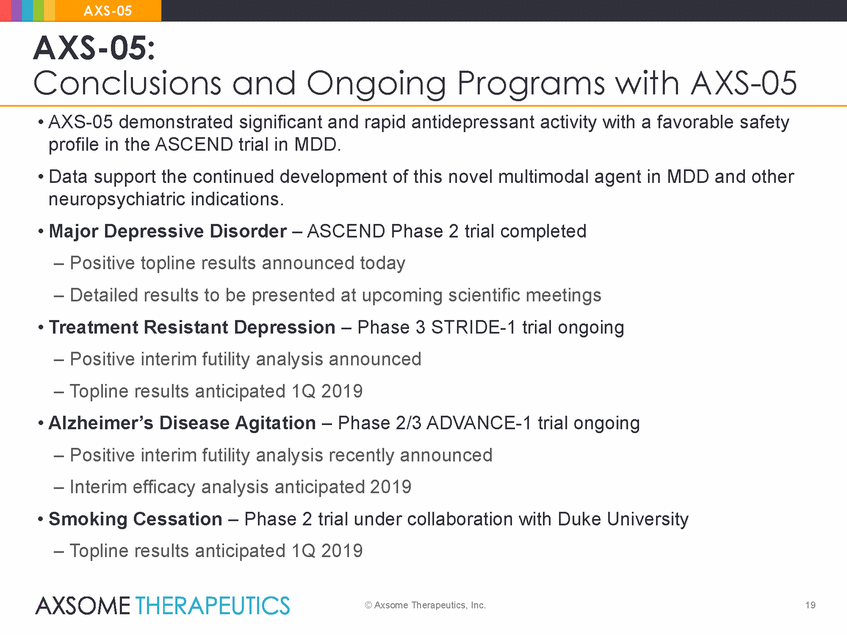
AXS-05 AXS-05: Conclusions and Ongoing Programs with AXS-05 • AXS-05 demonstrated significant and rapid antidepressant activity with a favorable safety profile in the ASCEND trial in MDD. • Data support the continued development of this novel multimodal agent in MDD and other neuropsychiatric indications. • Major Depressive Disorder – ASCEND Phase 2 trial completed – Positive topline results announced today – Detailed results to be presented at upcoming scientific meetings • Treatment Resistant Depression – Phase 3 STRIDE-1 trial ongoing – Positive interim futility analysis announced – Topline results anticipated 1Q 2019 • Alzheimer’s Disease Agitation – Phase 2/3 ADVANCE-1 trial ongoing – Positive interim futility analysis recently announced – Interim efficacy analysis anticipated 2019 • Smoking Cessation – Phase 2 trial under collaboration with Duke University – Topline results anticipated 1Q 2019 © Axsome Therapeutics, Inc. 19
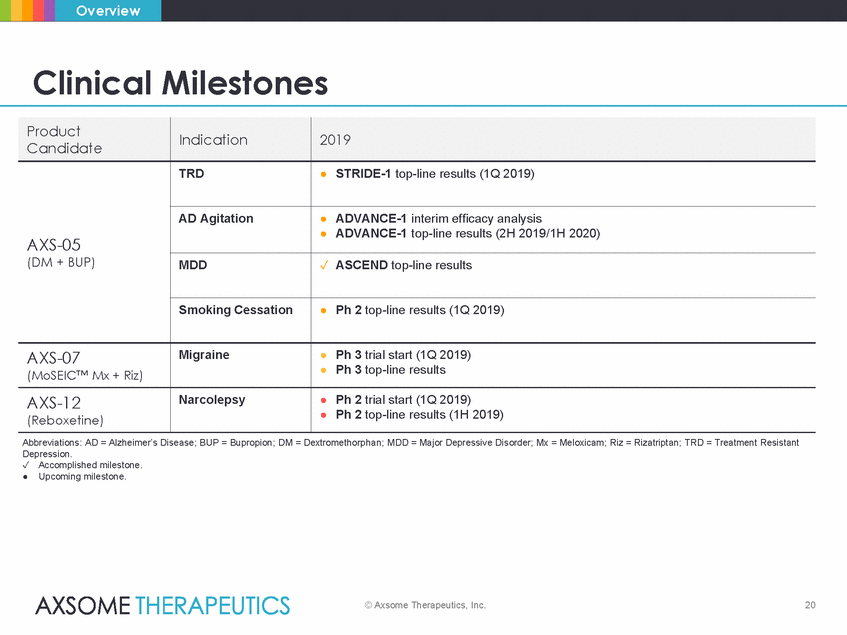
Overview Clinical Milestones • Ph 3 top-line results • Ph 2 top-line results (1H 2019) Abbreviations: AD = Alzheimer’s Disease; BUP = Bupropion; DM = Dextromethorphan; MDD = Major Depressive Disorder; Mx = Meloxicam; Riz = Rizatriptan; TRD = Treatment Resistant Depression. Accomplished milestone. • Upcoming milestone. © Axsome Therapeutics, Inc. 20 Product Candidate Indication 2019 AXS-05 (DM + BUP) TRD • STRIDE-1 top-line results (1Q 2019) AD Agitation • ADVANCE-1 interim efficacy analysis • ADVANCE-1 top-line results (2H 2019/1H 2020) MDD ASCEND top-line results Smoking Cessation • Ph 2 top-line results (1Q 2019) AXS-07 (MoSEIC™ Mx + Riz) Migraine • Ph 3 trial start (1Q 2019) AXS-12 (Reboxetine) Narcolepsy • Ph 2 trial start (1Q 2019)
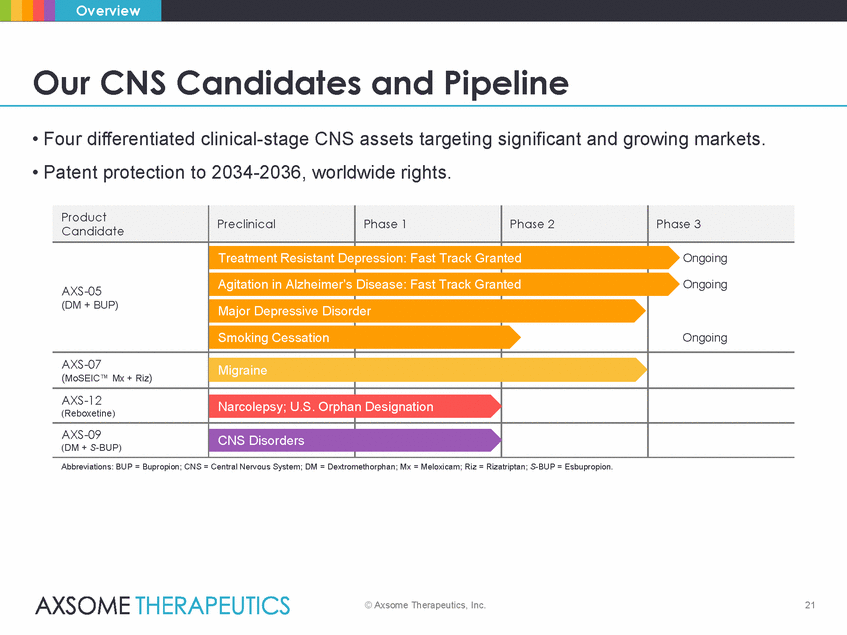
Overview Our CNS Candidates and Pipeline • Four differentiated clinical-stage CNS assets targeting significant and growing markets. • Patent protection to 2034-2036, worldwide rights. epression: Fast Track Granted anted der phan Designation Abbreviations: BUP = Bupropion; CNS = Central Nervous System; DM = Dextromethorphan; Mx = Meloxicam; Riz = Rizatriptan; S-BUP = Esbupropion. © Axsome Therapeutics, Inc. 21 Product Candidate Preclinical Phase 1 Phase 2 Phase 3 AXS-05 (DM + BUP) Treatment Resistant D Agitation in Alzheimer’s Major Depressive Disor Smoking Cessation Disease: Fast Track Gr Ongoing Ongoing Ongoing AXS-07 (MoSEIC™ Mx + Riz) Migraine AXS-12 (Reboxetine) Narcolepsy; U.S. Or AXS-09 (DM + S-BUP) CNS Disorders
axsome.com

