Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Assertio Therapeutics, Inc | a19-1305_18k.htm |
About this Presentation The statements that are not historical facts contained in this presentation are forward-looking statements including, but not limited to, statements relating to the commercialization of Gralise, CAMBIA, and Zipsor, royalties associated with Collegium’s commercialization of NUCYNTA and NUCYNTA ER, regulatory approval and clinical development of cosyntropin depot, our loan agreements, including our senior secured debt facility, and expectations regarding financial results and potential business and investment opportunities. These forward-looking statements involve significant risks and uncertainties, including risks detailed in the Company’s Securities and Exchange Commission filings, including the Company’s most recent Annual Report on Form 10-K and most recent Quarterly Report on Form 10-Q. The inclusion of forward-looking statements should not be regarded as a representation that any of the Company’s plans or objectives will be achieved. You are cautioned not to place undue reliance on these forward-looking statements, which speak only as of the date hereof. Assertio undertakes no duty or obligation to update any forward-looking statements contained in this presentation as a result of new information, future events or changes in its expectations except as may be required by law. This presentation contains non-GAAP financial measures. Please refer to the appendix to this presentation for an explanation of these non-GAAP financial measures and for tables that reconcile the non-GAAP figures to their GAAP equivalent. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy any securities, nor shall there be any sale of these securities in any state or jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or jurisdiction. 2

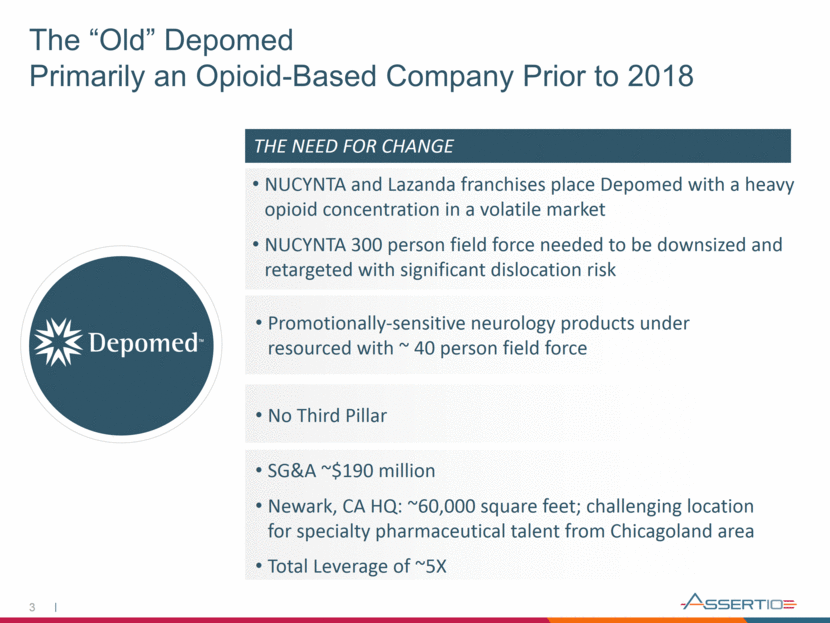
Past 3 The “Old” Depomed Primarily an Opioid-Based Company Prior to 2018 NUCYNTA and Lazanda franchises place Depomed with a heavy opioid concentration in a volatile market NUCYNTA 300 person field force needed to be downsized and retargeted with significant dislocation risk Promotionally-sensitive neurology products under resourced with ~ 40 person field force No Third Pillar SG&A ~$190 million Newark, CA HQ: ~60,000 square feet; challenging location for specialty pharmaceutical talent from Chicagoland area Total Leverage of ~5X THE NEED FOR CHANGE
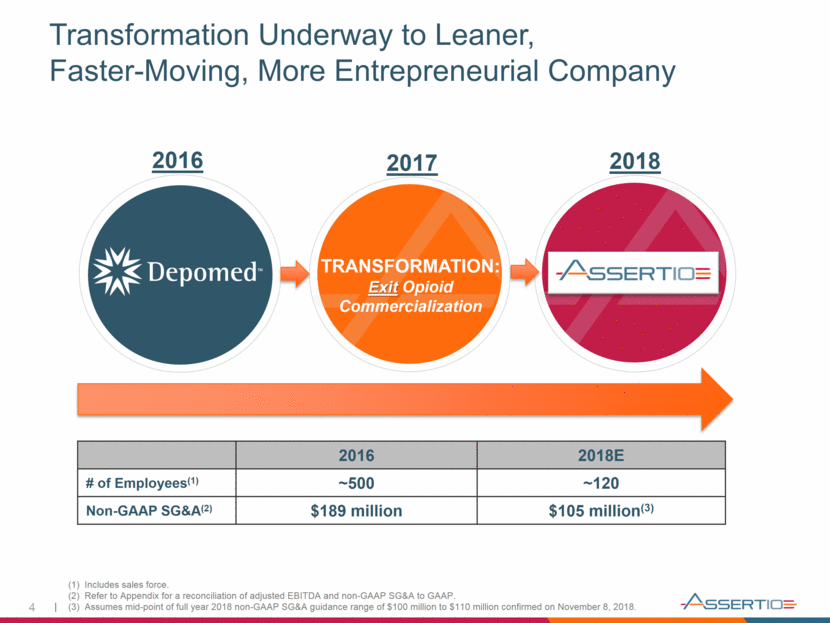
4 Transformation Underway to Leaner, Faster-Moving, More Entrepreneurial Company TRANSFORMATION: Exit Opioid Commercialization 2016 2018E # of Employees(1) ~500 ~120 Non-GAAP SG&A(2) $189 million $105 million(3) 2016 2017 2018 (1) Includes sales force. (2) Refer to Appendix for a reconciliation of adjusted EBITDA and non-GAAP SG&A to GAAP. (3) Assumes mid-point of full year 2018 non-GAAP SG&A guidance range of $100 million to $110 million confirmed on November 8, 2018.
New Name, Renewed Mission: Advance Patient Care in the Company’s Core Areas of Neurology, Orphan & Specialty Medicines 5 Assertio Therapeutics, Inc. (Assertio) is a new name that reflects a new business strategy, mission, vision and values A Company committed to putting patients first and creating sustainable shareholder value Established new headquarters in Lake Forest, IL Re-incorporated from California to Delaware The Company’s common stock trades under a new NASDAQ ticker symbol “ASRT”

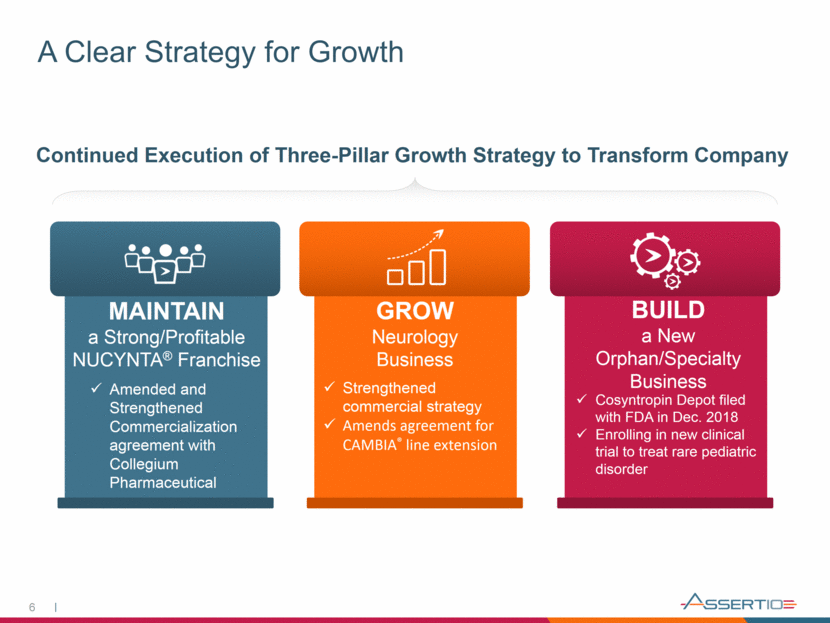
A Clear Strategy for Growth 6 Continued Execution of Three-Pillar Growth Strategy to Transform Company MAINTAIN a Strong/Profitable NUCYNTA® Franchise GROW Neurology Business BUILD a New Orphan/Specialty Business Amended and Strengthened Commercialization agreement with Collegium Pharmaceutical Strengthened commercial strategy Amends agreement for CAMBIA® line extension Cosyntropin Depot filed with FDA in Dec. 2018 Enrolling in new clinical trial to treat rare pediatric disorder
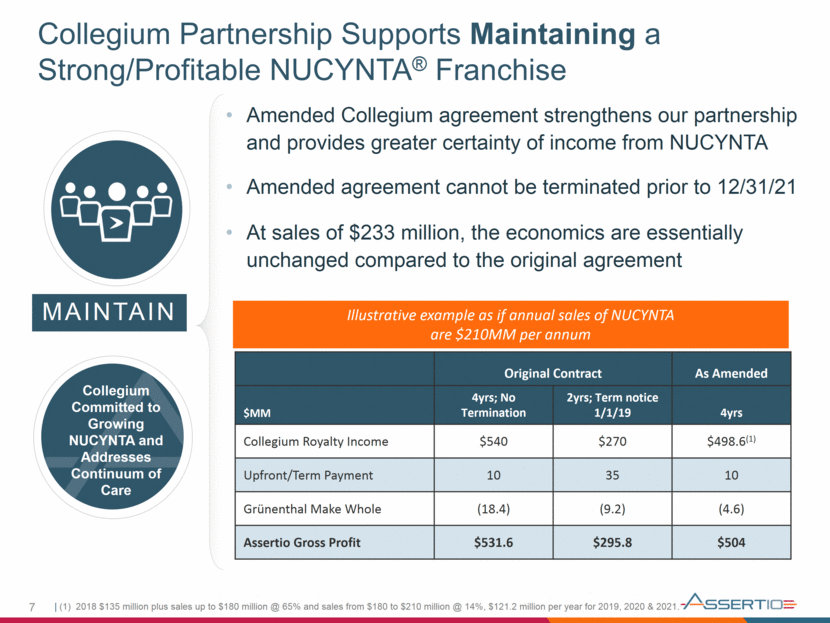
Collegium Partnership Supports Maintaining a Strong/Profitable NUCYNTA® Franchise 7 MAINTAIN Original Contract As Amended $MM 4yrs; No Termination 2yrs; Term notice 1/1/19 4yrs Collegium Royalty Income $540 $270 $498.6(1) Upfront/Term Payment 10 35 10 Grünenthal Make Whole (18.4) (9.2) (4.6) Assertio Gross Profit $531.6 $295.8 $504 Collegium Committed to Growing NUCYNTA and Addresses Continuum of Care (1) 2018 $135 million plus sales up to $180 million @ 65% and sales from $180 to $210 million @ 14%, $121.2 million per year for 2019, 2020 & 2021. Illustrative example as if annual sales of NUCYNTA are $210MM per annum Amended Collegium agreement strengthens our partnership and provides greater certainty of income from NUCYNTA Amended agreement cannot be terminated prior to 12/31/21 At sales of $233 million, the economics are essentially unchanged compared to the original agreement
Growing our Neurology Business A Portfolio with Extended Patent Exclusivity *Amended existing license agreement for a new presentation of Cambia which, if approved by FDA, may provide patent protection through at least 2026 ASSERTIO LAUNCH Q4 2011 Q2 2012 Q1 2014 INDICATIONS Management of postherpetic neuralgia (PHN) Mild to moderate pain in adults 18 years of age and older Acute treatment of migraine attacks with or without aura in adults 18 years of age or older KEY MESSAGES Once-daily PHN pain relief with a well-defined safety profile (less dizziness and somnolence) Provides rapid relief via unique ProSorb Dispersion Technology that results in peak plasma levels in 27 minutes The only FDA-approved single-agent prescription NSAID that provides rapid onset of action and proven efficacy Q3 2018 YTD NET REVENUES $43.3M $13.2M $24.9M ANTICIPATED EXCLUSIVITY UNTIL JANUARY 2024 (ANDA settled in 2015) March 2022 (ANDA settled in 2015) January 2023* (ANDA settled in 2013) (1)Source: SHA PHAST National Data. Dates Included: 01/01/2017 – 12/31/2017. Gralise Branded Market: Gralise, Horizant, Lyrica, and Neurontin. Zipsor Branded Market: Duexis, Felctor Tivorbex, Vimovo, Vivlodex, Zipsor, and Zorvolex. Cambia Branded Market: Cambia, Frova, Onzetra Xsail, Relpax, Sumavel Dosepro, Treximet, Zembrace Symtouch, and Zomig 8
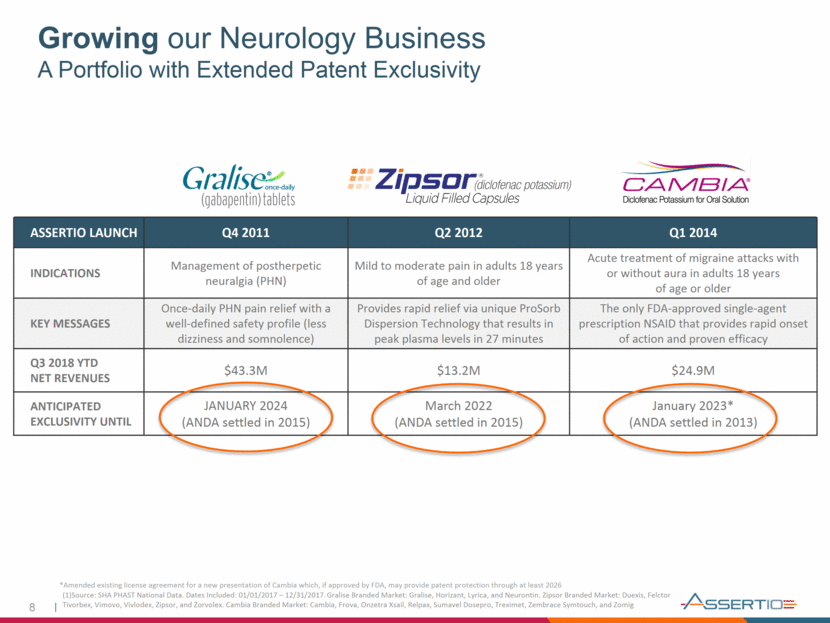
Growing Our Neurology Business 9 GROW Q3 ’18 stabilized core neurology brands with positive sequential total prescription growth for the franchise; but more work to do with Gralise® Q3 ’18 neurology product net sales increased $29.4 million, up 14 percent sequentially Q3 ‘18 began roll-out of new commercial initiatives designed to return neurology franchise, including Gralise, to growth in 2019 Q1 ’18 amended existing license agreement for CAMBIA® line extension; potential to expand exclusivity through 2026 Three Marketed Products with Growth Potential and Extended Exclusivity
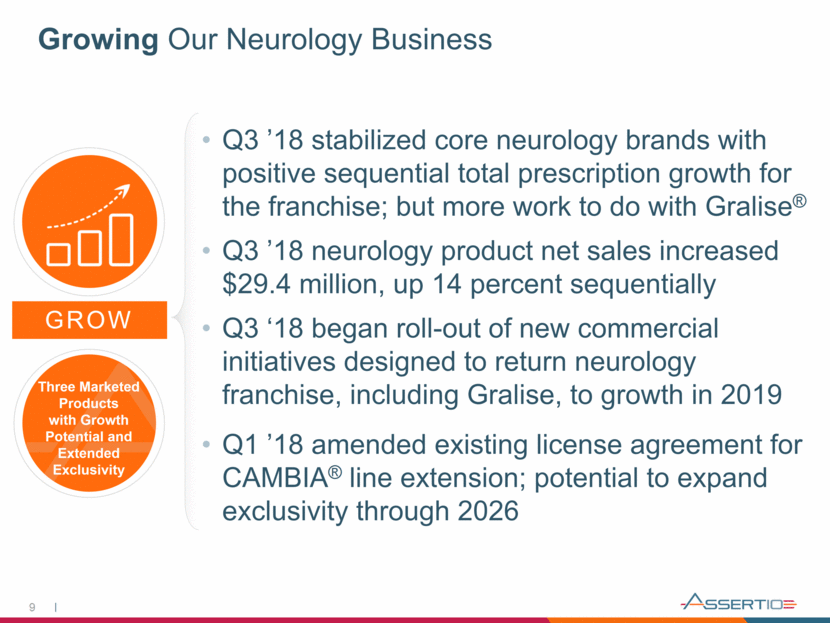
Building a Specialty / Orphan Business Cosyntropin (synthetic ACTH depot) is First in a Portfolio of High-Value, High-Touch Orphan/Specialty Medicine Positioned to Address the Needs of Patients, Physicians and Payors 10 BUILD Filed for FDA approval of cosyntropin depot in Dec. 2018 1st Indication (diagnostic - for suspected adrenocortical insufficiency) Endocrinologists commonly use exogenous ACTH to trigger the body’s cortisol response Helps determine if a patient’s adrenal glands are functioning properly Goal is to demonstrate that the diagnostic performance of cosyntropin depot is comparable to the reference product (Cortrosyn) 2nd Indication (infantile spasms) investigational new drug trial ongoing Foundation for New Business Unit
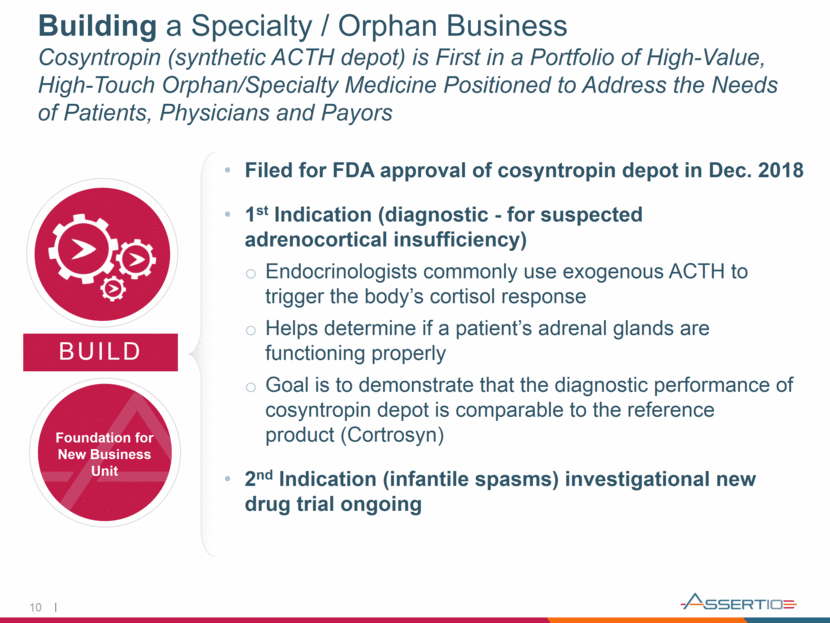
Improving Capital Structure Current Secured Debt Payment Schedule Significantly De-levers Company 11 (1) Assumes mid-point of trailing 12-month adjusted EBITDA of $125 to $135; excludes PDL. $97 million in non-dilutive cash secured through transactions in 2018 combined with future operating cash flow is sufficient to cover senior debt scheduled payments 2.9 x 2.4 x 1.3 x 4.7 x 4.1 x 0.0 x 0.5 x 1.0 x 1.5 x 2.0 x 2.5 x 3.0 x 3.5 x 4.0 x 4.5 x 5.0 x $100 $150 $200 $250 $300 $350 $400 Debt / EBITDA Leverage (1) Outstanding Sr. Debt Balance ($MM) Sr. Debt Balance Payments Sr. Leverage Total Leverage, Net
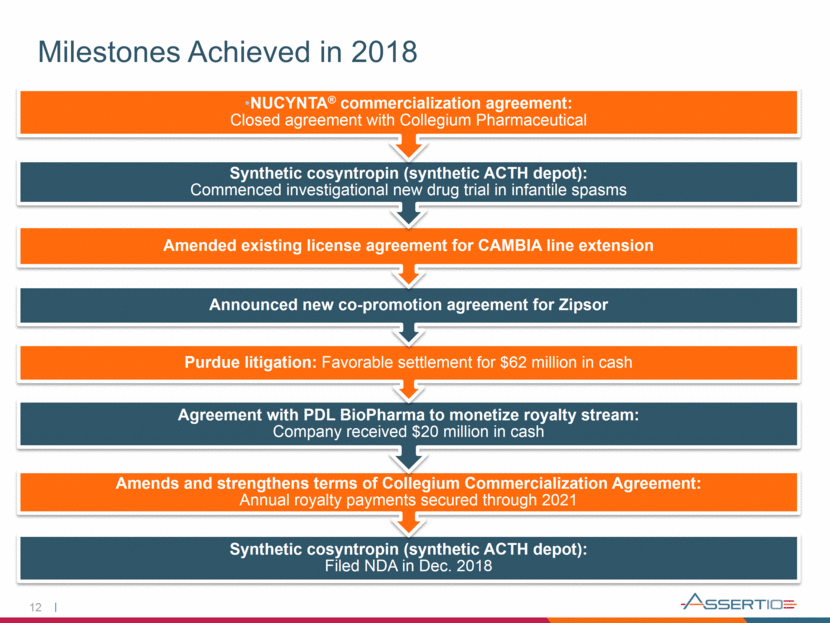
Milestones Achieved in 2018 12 Synthetic cosyntropin (synthetic ACTH depot): Filed NDA in Dec. 2018 Amends and strengthens terms of Collegium Commercialization Agreement: Annual royalty payments secured through 2021 Agreement with PDL BioPharma to monetize royalty stream: Company received $20 million in cash Purdue litigation: Favorable settlement for $62 million in cash Announced new co-promotion agreement for Zipsor Amended existing license agreement for CAMBIA line extension Synthetic cosyntropin (synthetic ACTH depot): Commenced investigational new drug trial in infantile spasms NUCYNTA® commercialization agreement: Closed agreement with Collegium Pharmaceutical
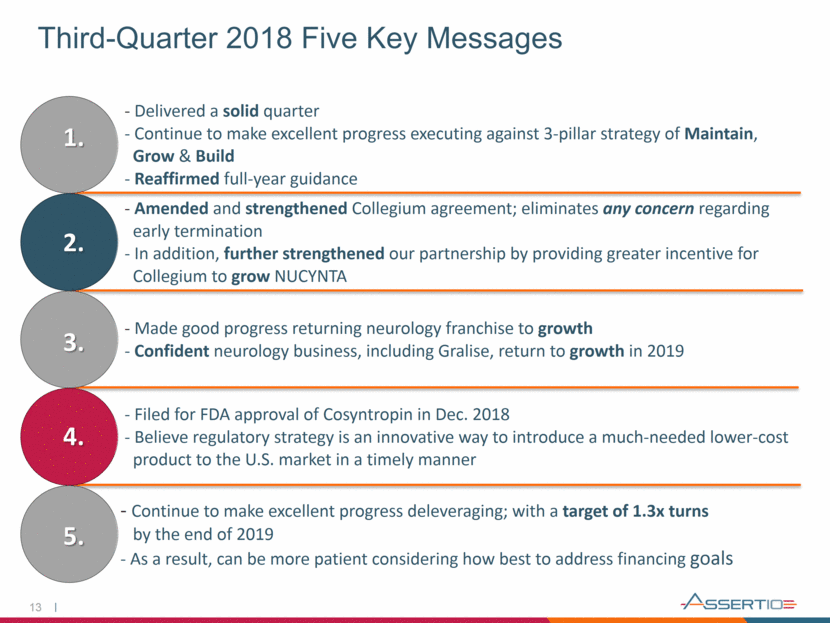
13 Third-Quarter 2018 Five Key Messages 1. 2. 3. 4. 5. - Delivered a solid quarter - Continue to make excellent progress executing against 3-pillar strategy of Maintain, Grow & Build - Reaffirmed full-year guidance - Amended and strengthened Collegium agreement; eliminates any concern regarding early termination - In addition, further strengthened our partnership by providing greater incentive for Collegium to grow NUCYNTA - Made good progress returning neurology franchise to growth - Confident neurology business, including Gralise, return to growth in 2019 - Filed for FDA approval of Cosyntropin in Dec. 2018 - Believe regulatory strategy is an innovative way to introduce a much-needed lower-cost product to the U.S. market in a timely manner - Continue to make excellent progress deleveraging; with a target of 1.3x turns by the end of 2019 - As a result, can be more patient considering how best to address financing goals
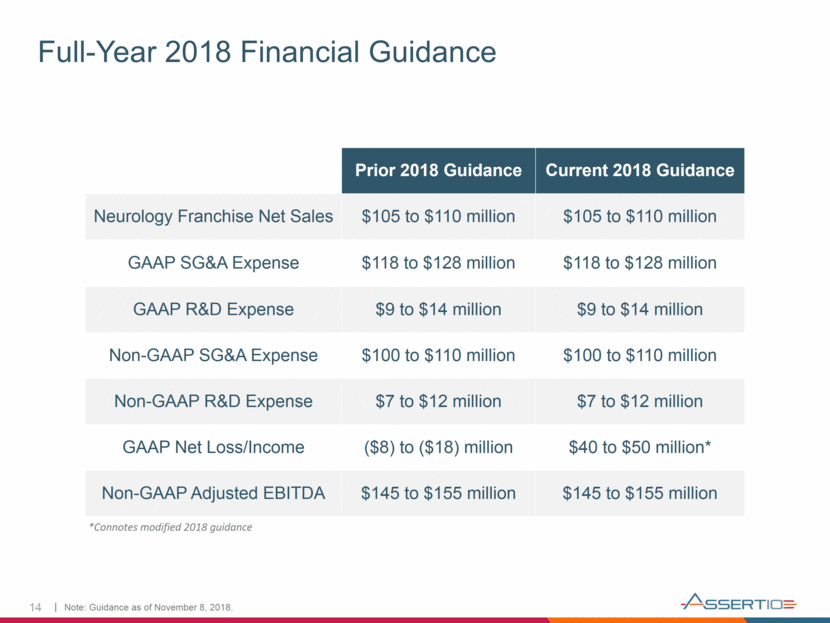
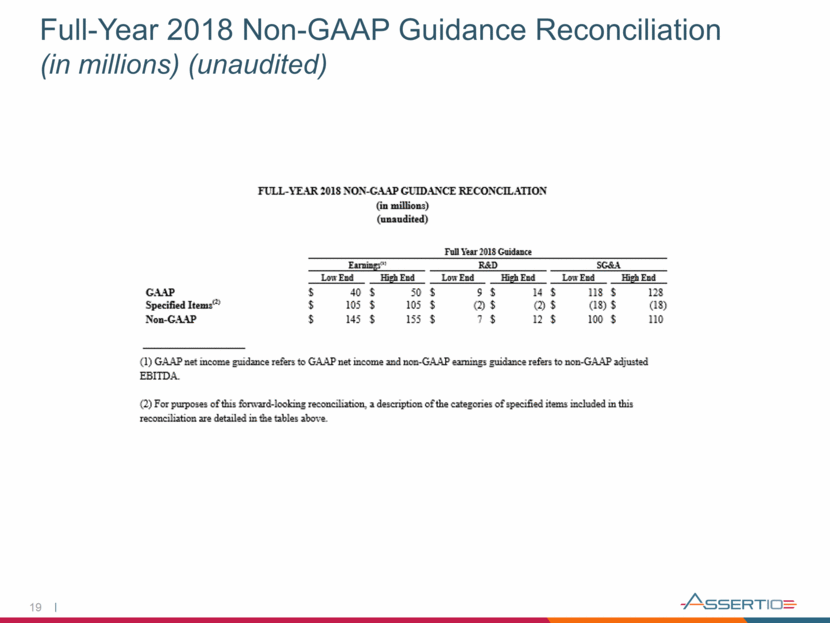
Full-Year 2018 Financial Guidance 14 Prior 2018 Guidance Current 2018 Guidance Neurology Franchise Net Sales $105 to $110 million $105 to $110 million GAAP SG&A Expense $118 to $128 million $118 to $128 million GAAP R&D Expense $9 to $14 million $9 to $14 million Non-GAAP SG&A Expense $100 to $110 million $100 to $110 million Non-GAAP R&D Expense $7 to $12 million $7 to $12 million GAAP Net Loss/Income ($8) to ($18) million $40 to $50 million* Non-GAAP Adjusted EBITDA $145 to $155 million $145 to $155 million Note: Guidance as of November 8, 2018. *Connotes modified 2018 guidance
Appendix

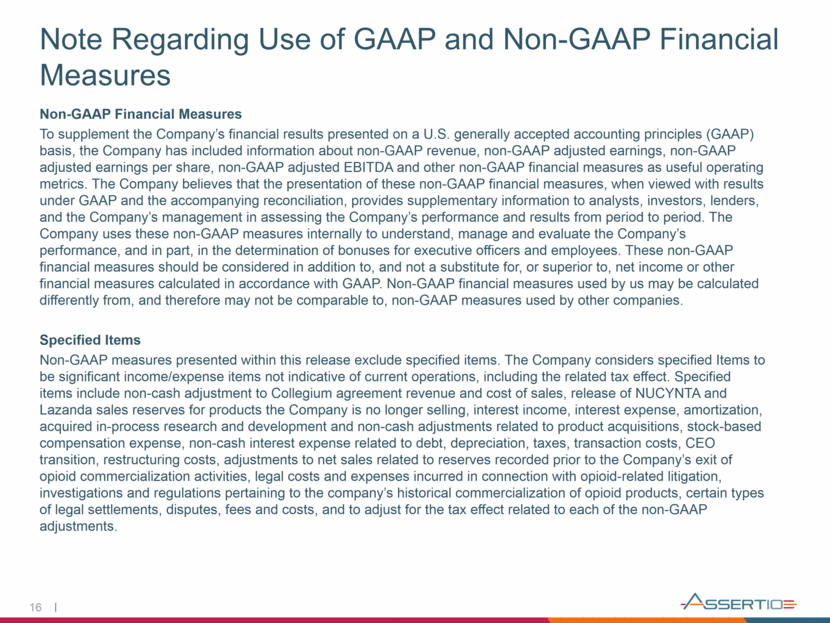
16 Note Regarding Use of GAAP and Non-GAAP Financial Measures Non-GAAP Financial Measures To supplement the Company’s financial results presented on a U.S. generally accepted accounting principles (GAAP) basis, the Company has included information about non-GAAP revenue, non-GAAP adjusted earnings, non-GAAP adjusted earnings per share, non-GAAP adjusted EBITDA and other non-GAAP financial measures as useful operating metrics. The Company believes that the presentation of these non-GAAP financial measures, when viewed with results under GAAP and the accompanying reconciliation, provides supplementary information to analysts, investors, lenders, and the Company’s management in assessing the Company’s performance and results from period to period. The Company uses these non-GAAP measures internally to understand, manage and evaluate the Company’s performance, and in part, in the determination of bonuses for executive officers and employees. These non-GAAP financial measures should be considered in addition to, and not a substitute for, or superior to, net income or other financial measures calculated in accordance with GAAP. Non-GAAP financial measures used by us may be calculated differently from, and therefore may not be comparable to, non-GAAP measures used by other companies. Specified Items Non-GAAP measures presented within this release exclude specified items. The Company considers specified Items to be significant income/expense items not indicative of current operations, including the related tax effect. Specified items include non-cash adjustment to Collegium agreement revenue and cost of sales, release of NUCYNTA and Lazanda sales reserves for products the Company is no longer selling, interest income, interest expense, amortization, acquired in-process research and development and non-cash adjustments related to product acquisitions, stock-based compensation expense, non-cash interest expense related to debt, depreciation, taxes, transaction costs, CEO transition, restructuring costs, adjustments to net sales related to reserves recorded prior to the Company’s exit of opioid commercialization activities, legal costs and expenses incurred in connection with opioid-related litigation, investigations and regulations pertaining to the company’s historical commercialization of opioid products, certain types of legal settlements, disputes, fees and costs, and to adjust for the tax effect related to each of the non-GAAP adjustments.
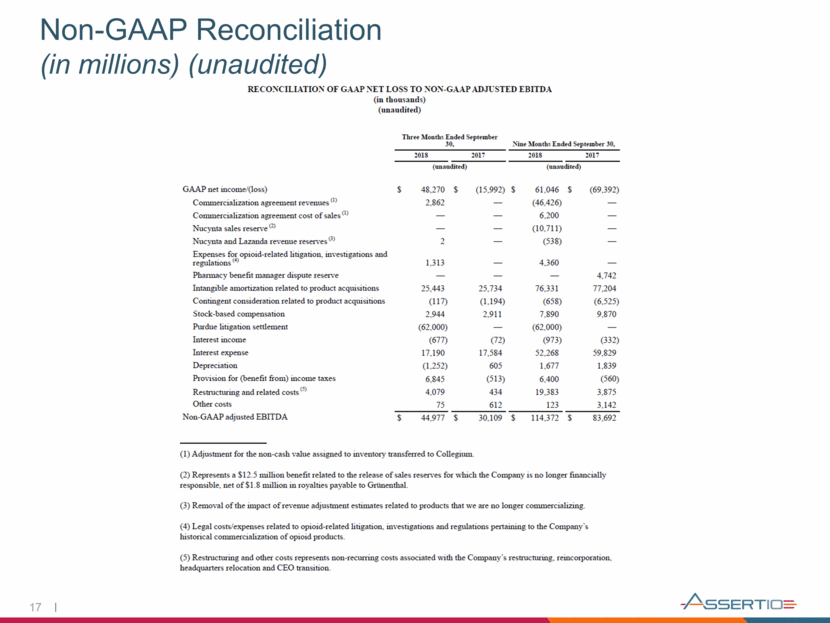
17 Non-GAAP Reconciliation (in millions) (unaudited)
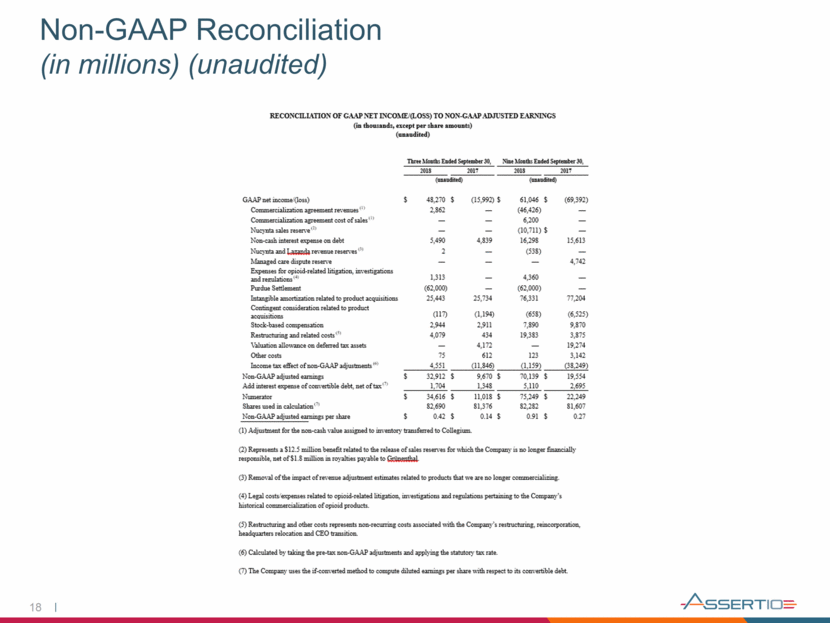
18 Non-GAAP Reconciliation (in millions) (unaudited)
19 Full-Year 2018 Non-GAAP Guidance Reconciliation (in millions) (unaudited)
January 2019

