Attached files
| file | filename |
|---|---|
| 8-K - 8-K - CHINOOK THERAPEUTICS, INC. | d670098d8k.htm |
Exhibit 99.1
Corporate Presentation January 2019 P I O N E E R I N G I M M U N O T H E R A P Y . T R A N S F O R M I N G L I V E S .

Note Regarding Forward-Looking Statements This presentation and the accompanying oral presentation include express and implied forward-looking statements regarding the current intentions, expectations, estimates, opinions and beliefs of Aduro Biotech, Inc. (“Aduro”) that are not historical facts. These forward-looking statements include statements regarding Aduro’s expectations for its product candidates (including their therapeutic and commercial potential, anticipated future development activities, anticipated timing of development activities, including initiation of clinical trials and presentations of clinical data and the indications Aduro and its collaborators plan to pursue), future results of operations andfinancial position, including current funds providing operating capital into 2021, business strategy, strategic collaborations, any royalty or milestone payments and Aduro’s ability to obtain and maintain intellectual property protection for its product candidates. Such forward-looking statements may be identified by words such as “believes”, “may”, “will”, “expects”, “endeavors”, “anticipates”, “intends”, “plans”, “estimates”, “projects”, “should”, “objective” and variations of such words and similar words. Forward-looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but not limited to, Aduro’s history of net operating losses and uncertainty regarding its ability to achieve profitability, Aduro’s ability to develop and commercialize its product candidates, Aduro’s ability to use and expand its technology to build a pipeline of product candidates, Aduro’s ability to obtain and maintain regulatory approval of its product candidates, Aduro’s ability to operate in a competitive industry and compete successfully against competitors that have greater resources than it does, Aduro’s reliance on third parties, and Aduro’s ability to obtain and adequately protect intellectual property rights for its product candidates. Aduro discusses many of these risks in greater detail under the heading “Risk Factors” in its most recent Quarterly or Annual Report on Form 10-Q or Form 10-K filed with the Securities and Exchange Commission. Any forward-looking statements that Aduro makes in this presentation and the accompanying oral presentation speak only as of the date of these presentations. Except as required by law, Aduro assumes no obligation to updateits forward-looking statements whether as a result of new information, future events or otherwise, after the date hereof. Nothing contained in this presentation is, or should be construed as, a recommendation, promise or representation by the presenter or Aduro or any director, employee, agent, or adviser of Aduro. This presentation does not purport to be all-inclusive or to contain all of the information you may desire. The content of this presentation is subject to copyright, which will be asserted by Aduro, and no part of this presentation may be reproduced, stored in a retrieval system, or transmitted in any form or by any means without prior permission in writing from Aduro. 2
Note Regarding Forward-Looking Statements intentions, This presentation expectations, and the estimates, accompanying opinions oral and presentation beliefs of include Aduro Biotech, express Inc. and (“Aduro”) implied forward-looking that are not historical statements facts. regarding These forward-looking the current potential, statements anticipated include statements future development regarding Aduro’s activities, expectations anticipated timing for its product of development candidates activities, (including including their therapeutic initiation of and clinical commercial trials and presentations position, including of clinical current data funds and providing the indications operating Aduro capital and through its collaborators 2021, business plan to pursue), strategy, future strategic results collaborations, of operations any and royalty financial or milestone payments statements and may Aduro’s be identified ability by to obtain words and such maintain as “believes”, intellectual “may”, property “will”, “expects”, protection “endeavors”, for its product “anticipates”, candidates. “intends”, Such forward-looking “plans”, “estimates”, performance “projects”, “should”, and are “objective” subject to and risks variations and uncertainties of such words that could and similar cause words. actual results Forward-looking and events statements to differ materially are not guarantees from those of anticipated, future including, to develop but and not commercialize limited to, Aduro’s its product history candidates, of net operating Aduro’s losses ability and to use uncertainty and expand regarding its technology its ability to to build achieve a pipeline profitability, of product Aduro’s ability candidates, industry and Aduro’s compete ability successfully to obtain against and maintain competitors regulatory that have approval greater of its resources product candidates, than it does, Aduro’s Aduro’s ability reliance to operate on third in parties, a competitive and greater Aduro’s detail ability under to obtain the heading and adequately “Risk Factors” protect in intellectual its most recent property Quarterly rights for or its Annual product Report candidates. on Form Aduro 10-Q discusses or Form 10-K many filed of with these the risks in Securities presentation and speak Exchange only as Commission. of the date of Any these forward-looking presentations. statements Except as that required Aduro by makes law, in Aduro this presentation assumes no obligation and the accompanying to update its forward- oral looking statements whether as a result of new information, future events or otherwise, after the date hereof. or Nothing any director, contained employee, in this presentation agent, or adviser is, or should of Aduro. be This construed presentation as, a recommendation, does not purport promise to be all-inclusive or representation or to contain by the all presenter of the information or Aduro you may desire. The stored content in a retrieval of this presentation system, or transmitted is subject to in copyright, any form or which by any will means be asserted without by prior Aduro, permission and no part in writing of this from presentation Aduro. may be reproduced, 2
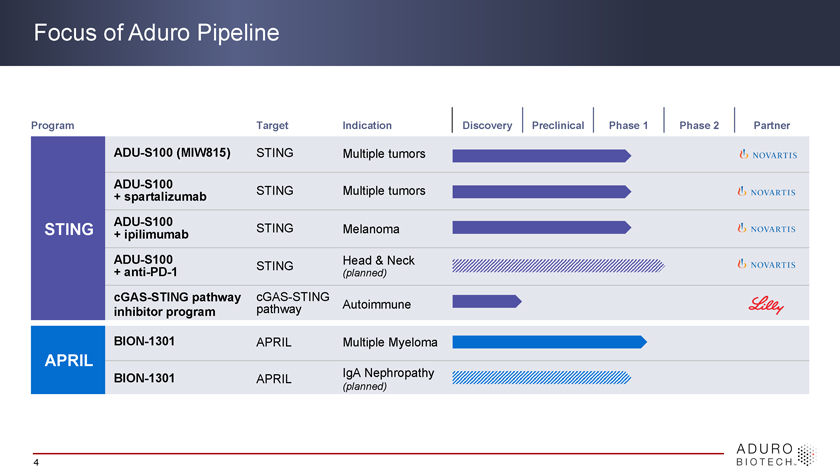
Focus of Aduro Pipeline Program Target Indication Discovery Preclinical Phase 1 Phase 2 Partner ADU-S100 (MIW815) STING Multiple tumors ADU-S100 + spartalizumab STING Multiple tumors ADU-S100 STING + ipilimumab STING Melanoma ADU-S100 Head & Neck + anti-PD-1 STING (planned) cGAS-STING pathway cGAS-STING pathway Autoimmune inhibitor program BION-1301 APRIL Multiple Myeloma APRIL BION-1301 APRIL IgA Nephropathy (planned) 4
ADU-S100 STING Agonist

STING Plays a Critical Role in Activation of Tumor Immunity • STimulator of INterferon Genes (STING) is a critical component of an innate immune pathway – STING protein function activated by cyclic dinucleotides – Immediate production of type I IFN and innate immunity – IFN-β is a signature cytokine of activated STING • STING activation is required for rejection of cancer in various mouse models 6
Key Attributes of ADU-S100 (MIW815) First-in-Class STING Agonist • Demonstrated preclinical anti-tumor activity – Induces local innate immune activation – cytokine production – in injected tumors – Activates tumor-specific CD8+ T cells and systemic tumor rejection, bridging innate to adaptive immunity – Effectively combined with checkpoint inhibitors to enhance efficacy and durable immunity • Well-tolerated and encouraging clinical signals in ADU-S100 (MIW815) ongoing early phase trials (X-ray bound crystal to STING structure) • Collaboration with Novartis provides $250M upfront, development cost share and profit share; Aduro leads U.S. commercialization 7 Aduro Biotech/Christian Lee, Genomics Institute of the Novartis Research Foundation
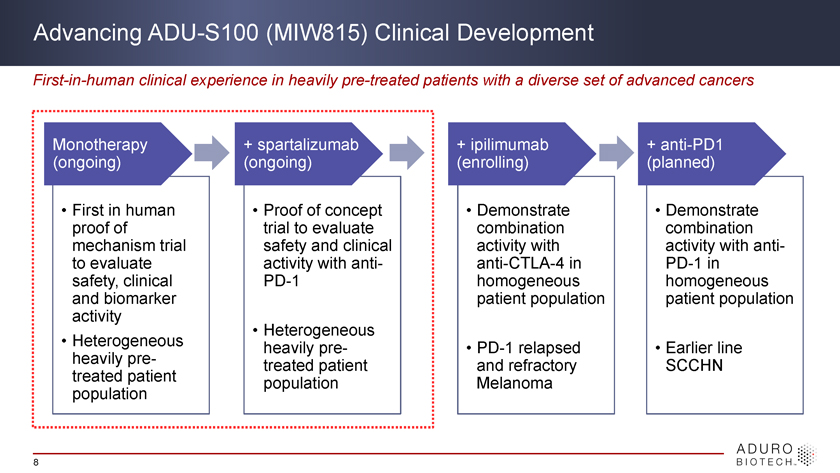
Advancing ADU-S100 (MIW815) Clinical Development First-in-human clinical experience in heavily pre-treated patients with a diverse set of advanced cancers Monotherapy + spartalizumab + ipilimumab + anti-PD1 (ongoing) (ongoing) (enrolling) (planned) • First in human • Proof of concept • Demonstrate • Demonstrate proof of trial to evaluate combination combination mechanism trial safety and clinical activity with activity with anti-to evaluate activity with anti- anti-CTLA-4 in PD-1 in safety, clinical PD-1 homogeneous homogeneous and biomarker patient population patient population activity • Heterogeneous • Heterogeneous heavily pre- • PD-1 relapsed • Earlier line heavily pre- treated patient and refractory SCCHN treated patient population Melanoma population 8
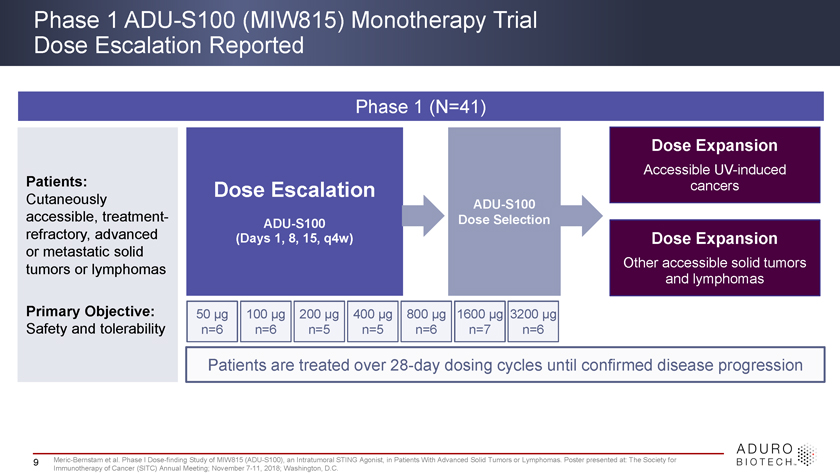
Phase 1 ADU-S100 (MIW815) Monotherapy Trial Dose Escalation Reported Phase 1 (N=41) Dose Expansion Accessible UV-induced Patients: Dose Escalation cancers Cutaneously ADU-S100 accessible, treatment- ADU-S100 Dose Selection refractory, advanced (Days 1, 8, 15, q4w) Dose Expansion or metastatic solid tumors or lymphomas Other accessible solid tumors and lymphomas Primary Objective: 50 ìg 100 ìg 200 ìg 400 ìg 800 ìg 1600 ìg 3200 ìg Safety and tolerability n=6 n=6 n=5 n=5 n=6 n=7 n=6 Patients are treated over 28-day dosing cycles until confirmed disease progression 9 Meric-Bernstam et al. Phase I Dose-finding Study of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients With Advanced Solid Tumors or Lymphomas. Poster presented at: The Society for Immunotherapy of Cancer (SITC) Annual Meeting; November 7-11, 2018; Washington, D.C.
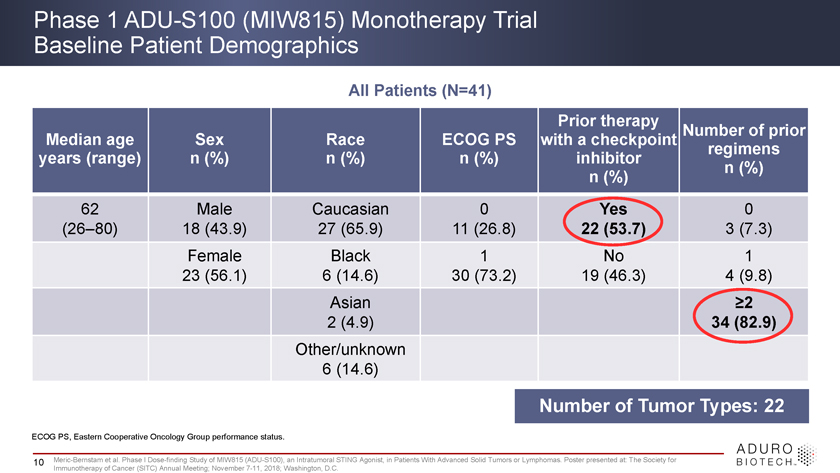
Phase 1 ADU-S100 (MIW815) Monotherapy Trial Baseline Patient Demographics All Patients (N=41) Prior therapy Number of prior Median age Sex Race ECOG PS with a checkpoint regimens years (range) n (%) n (%) n (%) inhibitor n (%) n (%) 62 Male Caucasian 0 Yes 0 (26–80) 18 (43.9) 27 (65.9) 11 (26.8) 22 (53.7) 3 (7.3) Female Black 1 No 1 23 (56.1) 6 (14.6) 30 (73.2) 19 (46.3) 4 (9.8) Asian ³2 2 (4.9) 34 (82.9) Other/unknown 6 (14.6) Number of Tumor Types: 22 ECOG PS, Eastern Cooperative Oncology Group performance status. 10 Meric-Bernstam et al. Phase I Dose-finding Study of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients With Advanced Solid Tumors or Lymphomas. Poster presented at: The Society for Immunotherapy of Cancer (SITC) Annual Meeting; November 7-11, 2018; Washington, D.C.
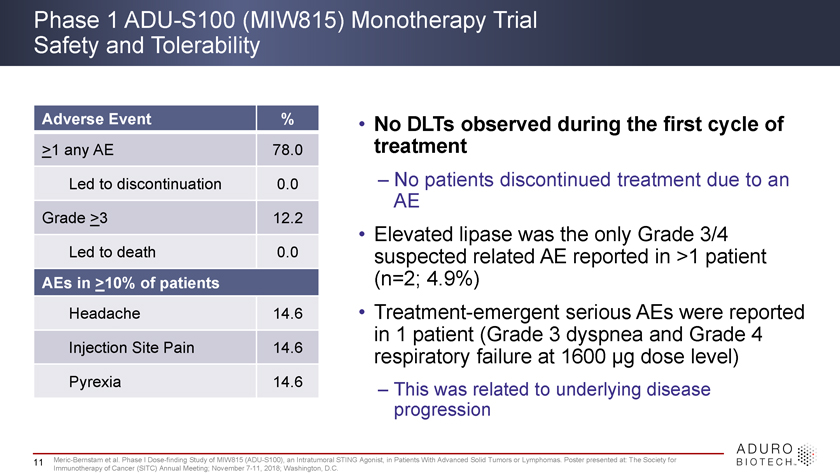
Phase 1 ADU-S100 (MIW815) Monotherapy Trial Safety and Tolerability Adverse Event % • No DLTs observed during the first cycle of >1 any AE 78.0 treatment Led to discontinuation 0.0 – No patients discontinued treatment due to an AE Grade >3 12.2 Led to death 0.0 • Elevated lipase was the only Grade 3/4 suspected related AE reported in >1 patient AEs in >10% of patients (n=2; 4.9%) Headache 14.6 • Treatment-emergent serious AEs were reported in 1 patient (Grade 3 dyspnea and Grade 4 Injection Site Pain 14.6 respiratory failure at 1600 ìg dose level) Pyrexia 14.6 – This was related to underlying disease progression 11 Meric-Bernstam et al. Phase I Dose-finding Study of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients With Advanced Solid Tumors or Lymphomas. Poster presented at: The Society for Immunotherapy of Cancer (SITC) Annual Meeting; November 7-11, 2018; Washington, D.C.
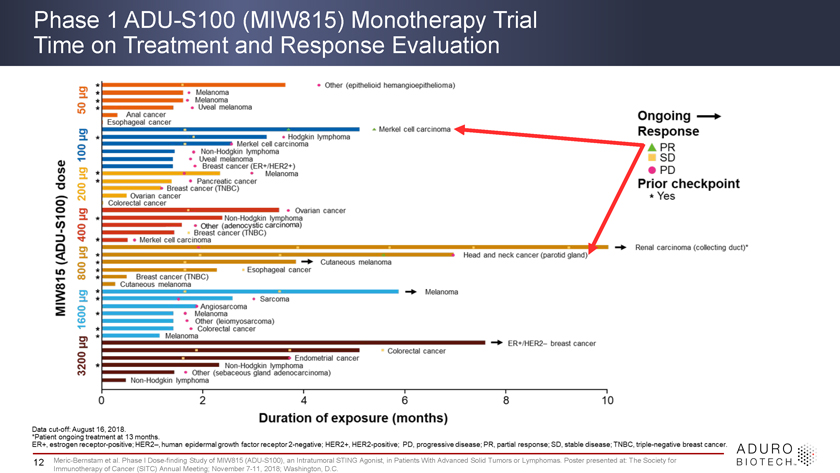
Phase 1 ADU-S100 (MIW815) Monotherapy Trial Time on Treatment and Response Evaluation 12 Meric-Bernstam et al. Phase I Dose-finding Study of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients With Advanced Solid Tumors or Lymphomas. Poster presented at: The Society for Immunotherapy of Cancer (SITC) Annual Meeting; November 7-11, 2018; Washington, D.C.
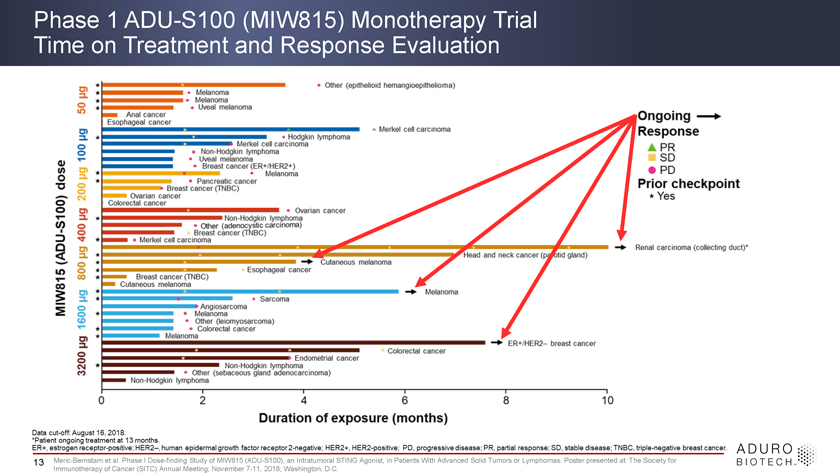
Phase 1 ADU-S100 (MIW815) Monotherapy Trial Time on Treatment and Response Evaluation 13 Meric-Bernstam et al. Phase I Dose-finding Study of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients With Advanced Solid Tumors or Lymphomas. Poster presented at: The Society for Immunotherapy of Cancer (SITC) Annual Meeting; November 7-11, 2018; Washington, D.C.
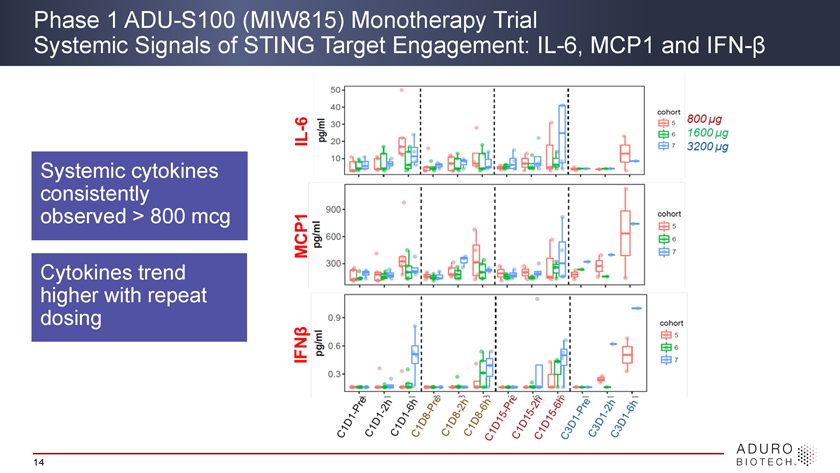
Phase 1 ADU-S100 (MIW815) Monotherapy Trial Systemic Signals of STING Target Engagement: IL-6, MCP1 and IFN-β Systemic cytokines consistently observed > 800 mcg Cytokines trend higher with repeat dosing 14
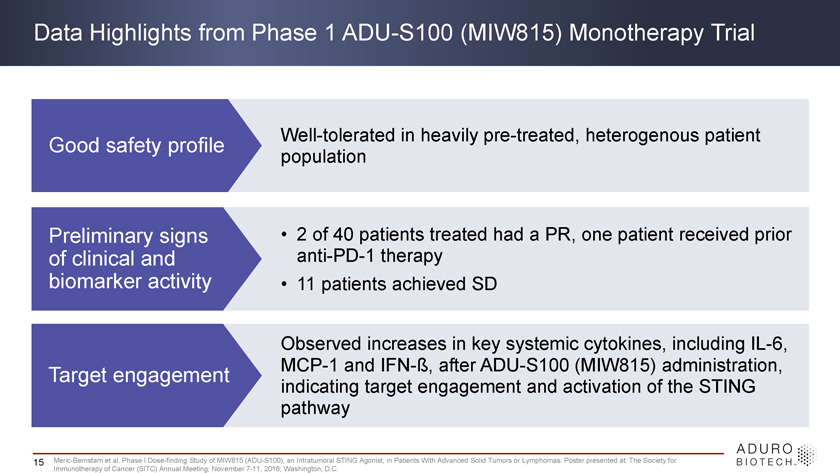
Data Highlights from Phase 1 ADU-S100 (MIW815) Monotherapy Trial Good safety profile Well-tolerated in heavily pre-treated, heterogenous patient population Preliminary signs • 2 of 40 patients treated had a PR, one patient received prior of clinical and anti-PD-1 therapy biomarker activity • 11 patients achieved SD Observed increases in key systemic cytokines, including IL-6, Target engagement MCP-1 and IFN-ß, after ADU-S100 (MIW815) administration, indicating target engagement and activation of the STING pathway 15 Meric-Bernstam et al. Phase I Dose-finding Study of MIW815 (ADU-S100), an Intratumoral STING Agonist, in Patients With Advanced Solid Tumors or Lymphomas. Poster presented at: The Society for Immunotherapy of Cancer (SITC) Annual Meeting; November 7-11, 2018; Washington, D.C.
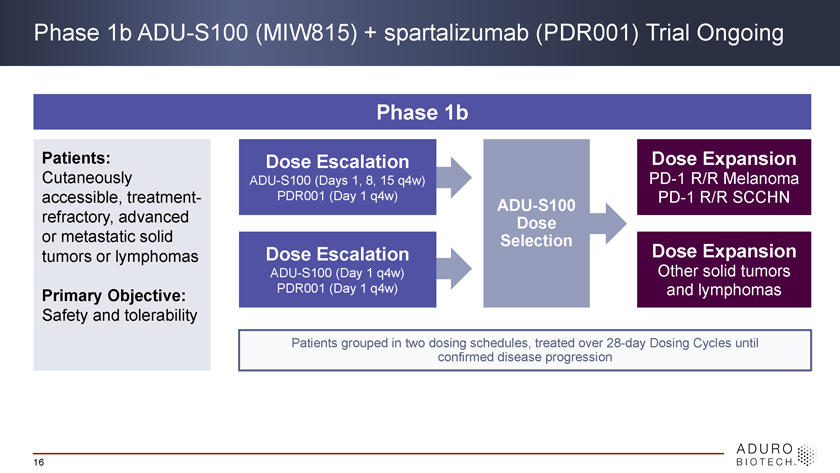
Phase 1b ADU-S100 (MIW815) + spartalizumab (PDR001) Trial Ongoing Phase 1b Patients: Dose Escalation Dose Expansion Cutaneously ADU-S100 (Days 1, 8, 15 q4w) PD-1 R/R Melanoma accessible, treatment- PDR001 (Day 1 q4w) PD-1 R/R SCCHN ADU-S100 refractory, advanced Dose or metastatic solid Selection tumors or lymphomas Dose Escalation Dose Expansion ADU-S100 (Day 1 q4w) Other solid tumors Primary Objective: PDR001 (Day 1 q4w) and lymphomas Safety and tolerability Patients grouped in two dosing confirmed schedules, disease treated progression over 28-day Dosing Cycles until 16
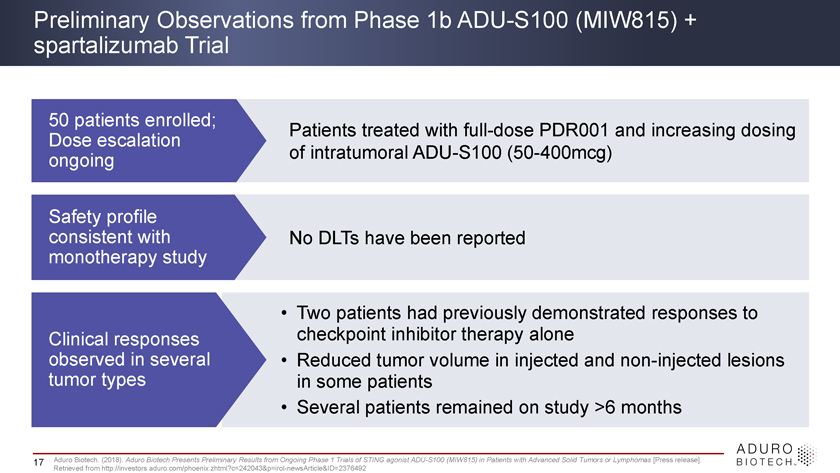
Preliminary Observations from Phase 1b ADU-S100 (MIW815) + spartalizumab Trial 50 patients enrolled; Patients treated with full-dose PDR001 and increasing dosing Dose escalation ongoing of intratumoral ADU-S100 (50-400mcg) Safety profile consistent with No DLTs have been reported monotherapy study • Two patients had previously demonstrated responses to Clinical responses checkpoint inhibitor therapy alone observed in several • Reduced tumor volume in injected and non-injected lesions tumor types in some patients • Several patients remained on study >6 months 17 Aduro Biotech. (2018). Aduro Biotech Presents Preliminary Results from Ongoing Phase 1 Trials of STING agonist ADU-S100 (MIW815) in Patients with Advanced Solid Tumors or Lymphomas [Press release]. Retrieved from http://investors.aduro.com/phoenix.zhtml?c=242043&p=irol-newsArticle&ID=2376492
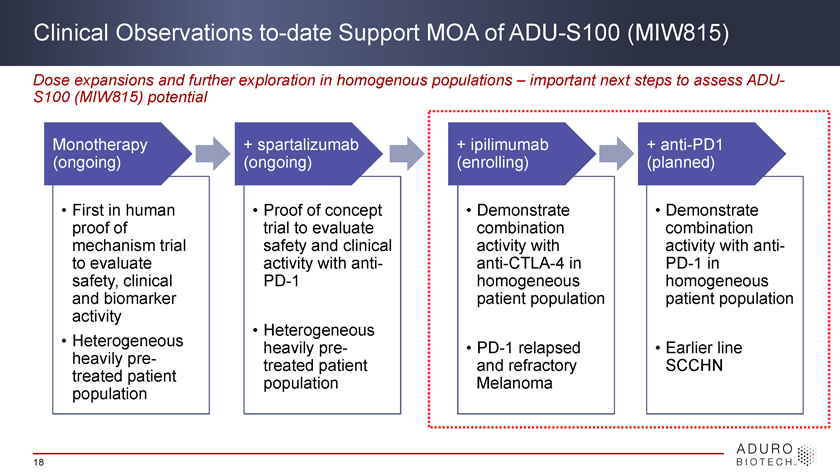
Clinical Observations to-date Support MOA of ADU-S100 (MIW815) Dose expansions and further exploration in homogenous populations – important next steps to assess ADU-S100 (MIW815) potential Monotherapy + spartalizumab + ipilimumab + anti-PD1 (ongoing) (ongoing) (enrolling) (planned) • First in human • Proof of concept • Demonstrate • Demonstrate proof of trial to evaluate combination combination mechanism trial safety and clinical activity with activity with anti-to evaluate activity with anti- anti-CTLA-4 in PD-1 in safety, clinical PD-1 homogeneous homogeneous and biomarker patient population patient population activity • Heterogeneous • Heterogeneous heavily pre- • PD-1 relapsed • Earlier line heavily pre- treated patient and refractory SCCHN treated patient population Melanoma population 18

BION-1301 APRIL Antibody
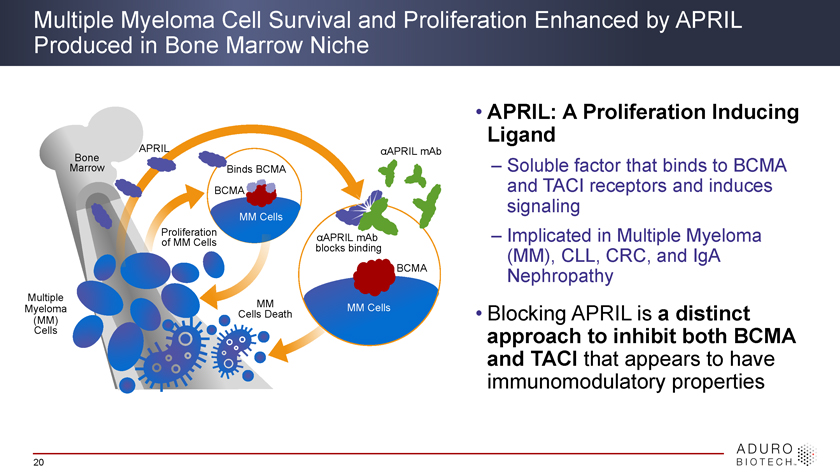
Multiple Myeloma Cell Survival and Proliferation Enhanced by APRIL Produced in Bone Marrow Niche • APRIL: Ligand A Proliferation Inducing APRIL áAPRIL mAb Bone Marrow Binds BCMA – Soluble factor that binds to BCMA BCMA and TACI receptors and induces signaling MM Cells Proliferation of MM Cells áAPRIL mAb – Implicated in Multiple Myeloma blocks binding (MM), CLL, CRC, and IgA BCMA Nephropathy Multiple Myeloma MM MM Cells Cells Death • Blocking APRIL is a distinct (MM) Cells approach to inhibit both BCMA and TACI that appears to have immunomodulatory properties 20
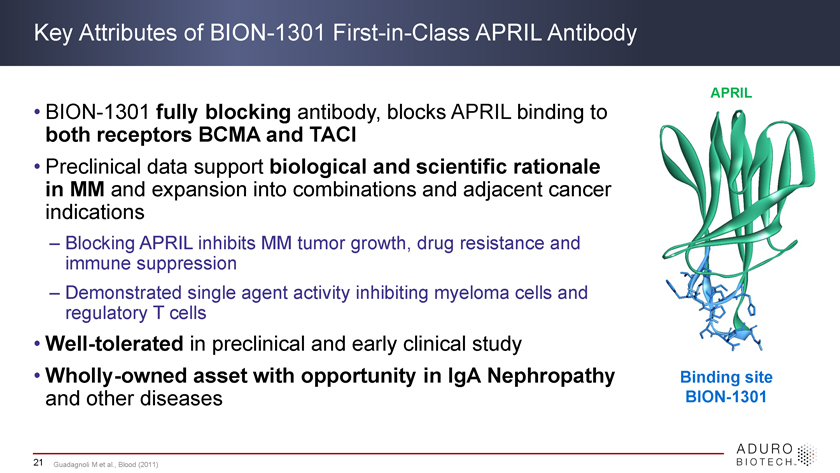
Key Attributes of BION-1301 First-in-Class APRIL Antibody APRIL • both BION-1301 receptors fully BCMA blocking and antibody, TACI blocks APRIL binding to • Preclinical MM data support biological and scientific rationale in and expansion into combinations and adjacent cancer indications – Blocking APRIL inhibits MM tumor growth, drug resistance and immune suppression – Demonstrated single agent activity inhibiting myeloma cells and regulatory T cells • Well-tolerated in preclinical and early clinical study • Wholly-owned asset with opportunity in IgA Nephropathy Binding site and other diseases BION-1301 21 Guadagnoli M et al., Blood (2011)
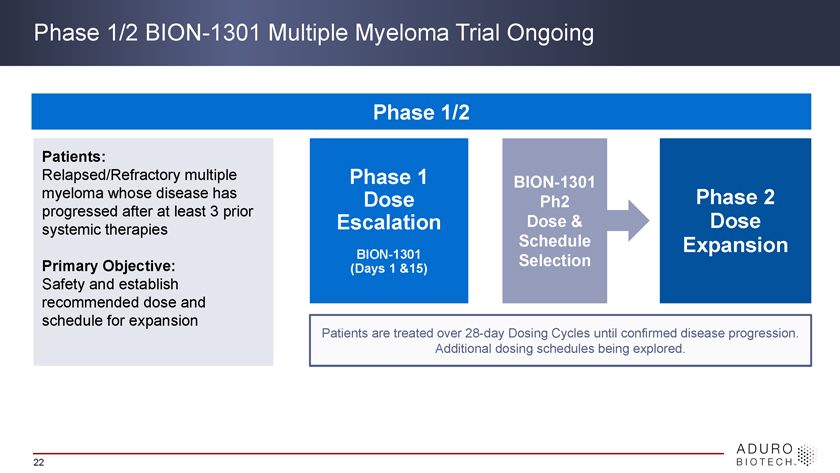
Phase 1/2 BION-1301 Multiple Myeloma Trial Ongoing Phase 1/2 Patients: Relapsed/Refractory multiple Phase 1 BION-1301 myeloma whose disease has Dose Ph2 Phase 2 progressed after at least 3 prior systemic therapies Escalation Dose & Dose Schedule Expansion BION-1301 Selection Primary Objective: (Days 1 &15) Safety and establish recommended dose and schedule for expansion Patients are treated over 28-day Dosing Cycles until confirmed disease progression. Additional dosing schedules being explored. 22
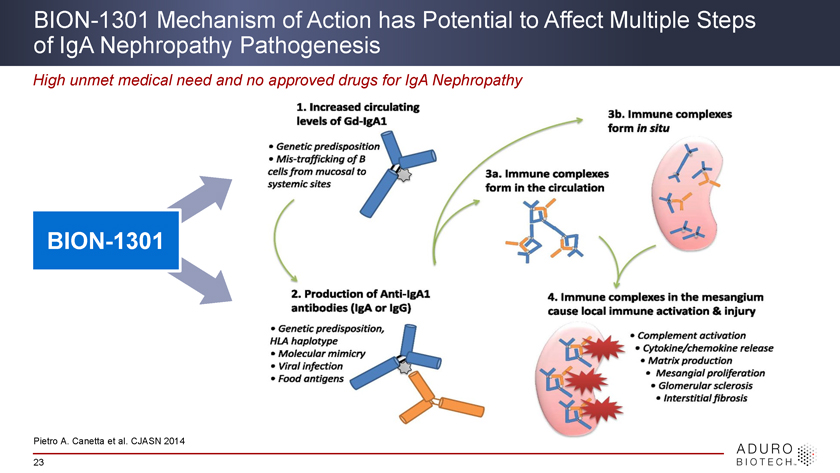
BION-1301 Mechanism of Action has Potential to Affect Multiple Steps of IgA Nephropathy Pathogenesis High unmet medical need and no approved drugs for IgA Nephropathy BION-1301 Pietro A. Canetta et al. CJASN 2014 23
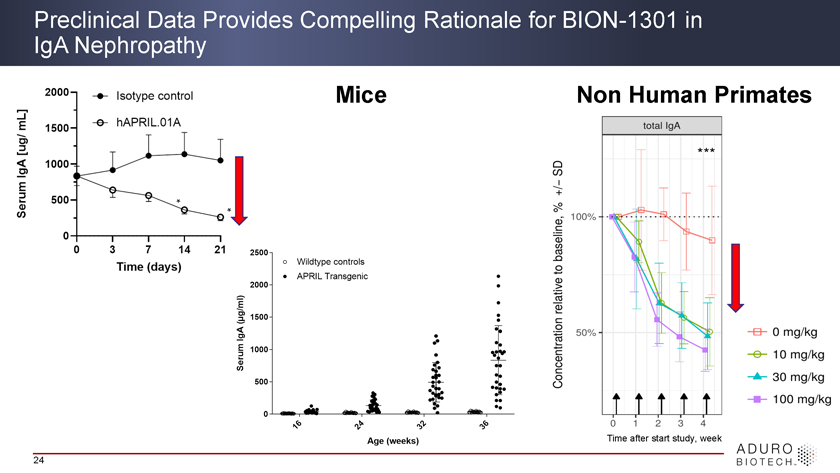
Preclinical Data Provides Compelling Rationale for BION-1301 in IgA Nephropathy Mice Non Human Primates Time after start study, week 24
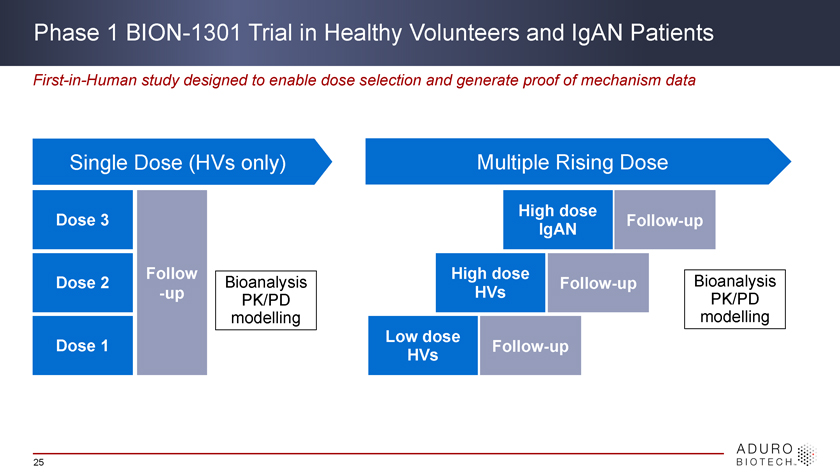
Phase 1 BION-1301 Trial in Healthy Volunteers and IgAN Patients First-in-Human study designed to enable dose selection and generate proof of mechanism data Single Dose (HVs only) Multiple Rising Dose High dose Dose 3 Follow-up IgAN Follow High dose Bioanalysis Dose 2 Bioanalysis Follow-up -up PK/PD HVs PK/PD modelling modelling Low dose Dose 1 Follow-up HVs 25
Business Overview
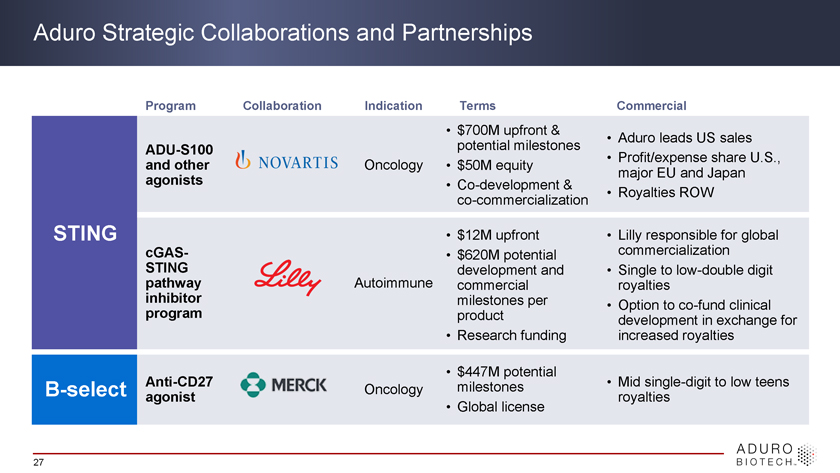
Aduro Strategic Collaborations and Partnerships Program Collaboration Indication Terms Commercial • $700M upfront & • Aduro leads US sales ADU-S100 potential milestones • Profit/expense share U.S., and other Oncology • $50M equity major EU and Japan agonists • Co-development & • Royalties ROW co-commercialization STING • $12M upfront • Lilly commercialization responsible for global cGAS- STING • $620M potential development and • Single to low-double digit inhibitor pathway Autoimmune commercial royalties milestones per • Option to co-fund clinical program product development in exchange for • Research funding increased royalties • $447M potential Anti-CD27 milestones • Mid single-digit to low teens B-select Oncology agonist royalties • Global license 27
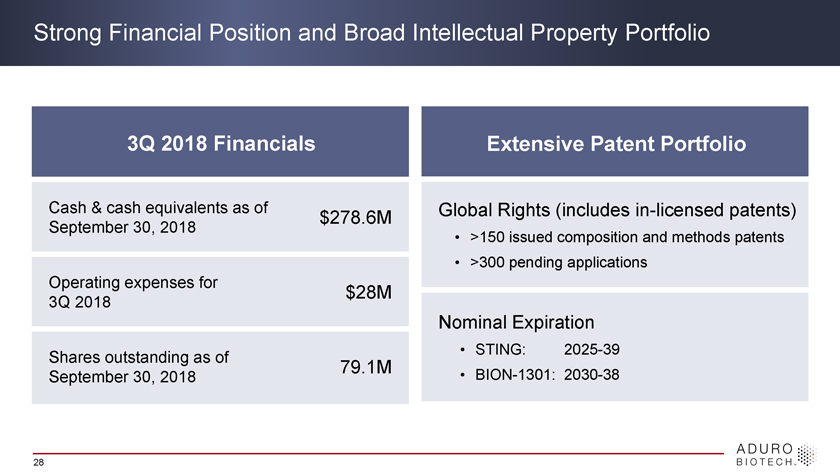
Strong Financial Position and Broad Intellectual Property Portfolio 3Q 2018 Financials Extensive Patent Portfolio Cash & cash equivalents as of $278.6M Global Rights (includes in-licensed patents) September 30, 2018 • >150 issued composition and methods patents • >300 pending applications Operating expenses for $28M 3Q 2018 Nominal Expiration Shares outstanding as of • STING: 2025-39 79.1M • BION-1301: 2030-38 September 30, 2018 28

Upcoming Anticipated Milestones H1 H2 2019 2019 ADU-S100 + ipilimumab Initiate Phase 1 dose escalation in PD-1 R/R melanoma ADU-S100 + spartalizumab Initiate Phase 1 dose expansion STING ADU-S100 + spartalizumab Report Phase 1 dose escalation results ADU-S100 + anti-PD-1 Initiate Phase 1b/2 dose escalation in earlier line head and neck cancer BION-1301 Initiate Phase 1 IgAN study in healthy volunteers APRIL BION-1301 Report Phase 1 MM dose escalation results BION-1301 Initiate Phase 2 MM dose expansion study 29

ADURO BIOTECH
