Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - Mawson Infrastructure Group Inc. | f8k010419_wizepharma.htm |
Exhibit 99.1

Wize Pharma INC All Rights Reserved J.P. Morgan Healthcare Conference January 2019 Corporate Presentation

Forward - Looking Information This presentation contains forward - looking statements about our expectations, beliefs or intentions regarding, among other thing s, our product development efforts, business, financial condition, results of operations, strategies or prospects. In addition, from time to time, we or our repr ese ntatives have made or may make forward - looking statements, orally or in writing. Forward - looking statements can be identified by the use of forward - looking words such as “beli eve,” “expect,” “intend,” “plan,” “may,” “should” or “anticipate” or their negatives or other variations of these words or other comparable words or by the fact that the se statements do not relate strictly to historical or current matters. These forward - looking statements may be included in, but are not limited to, this presentation, various fili ngs made by us with the SEC, press releases or oral statements made by or with the approval of one of our authorized executive officers. Forward - looking statements relate to a nticipated or expected events, activities, trends or results as of the date they are made. Because forward - looking statements relate to matters that have not yet occurred, these statements are inherently subject to risks and uncertainties that could cause our actual results to differ materially from any future results expressed or implied by the forward - looking statements. Many factors could cause Wize’s actual activities or results to differ materially from the activities and results anticipated in forward - looking statements, including, but not limited to, the following: risks related to the substantial debt that we have incurred; our needs for additional financing an d c ertain limits on our abilities to raise funds; our dependence on a single compound, Lo 2 A and on the continuation of our license to commercialize LO 2 A; our inability to expand our rights under our license of LO 2 A; the initiation, timing, progress and results of our trials and product candidate development efforts; our ability to advance LO 2 A into clinical trials or to successfully complete our preclinical studies or clinical trials; our receipt of regulatory approvals for LO 2 A, and the timing of other regulatory filings and approvals; the clinical development, commercialization and market acceptance of LO 2 A; our ability to establish and maintain corporate collaborations; the implementation of our business model and strategic plans for our business and product candidates; the scope of protection we are able to establish and maintain for intellectual pr operty rights covering LO 2 A and our ability to operate our business without infringing the intellectual property rights of others; estimates of our expenses, future revenue s, and capital requirements; competitive companies, technologies and our industry; and statements as to the impact of the political and security situation in Israel o n o ur business. More detailed information about the risks and uncertainties affecting Wize is included in filings Wize has made and may make with the SEC in the future. These statements are only current predictions and are subject to known and unknown risks, uncertainties and other factors tha t m ay cause our or our industry’s actual results, levels of activity, performance or achievements to be materially different from those anticipated by the forward - looking stateme nts. Given these uncertainties, you should not rely upon forward - looking statements as predictions of future events. All forward - looking statements attributable to us or persons acting on our behalf included in, but not limited to, this presenta tion speak only as of the date hereof and are expressly qualified in their entirety by the foregoing. We undertake no obligations to update or revise forward - looking statemen ts to reflect events or circumstances that arise after the date made or to reflect the occurrence of unanticipated events. In evaluating forward - looking statements, you should c onsider these risks and uncertainties. All Rights Reserved

Welcome to Wize Pharma The Company

Corporate Overview Wize Pharma , Inc. (OTCQB: WIZP) is focused on the treatment of ophthalmic disorders, including dry eye syndrome, a $ 3.7 billion global market. Wize’s eye drop formula, LO 2 A, is proven safe and effective in certain countries in Europe for the treatment of dry eye syndrome. The Company is pursuing a regulatory approval for LO 2 A in additional indications, including Conjunctivochalasis (CCh) and Sjögren’s syndrome. All Rights Reserved

Wize Pharma: At - A - Glance Ticker symbol: OTCQB (WIZP) Main shareholders: • Ridge Valley Corp: ~ 16 % • Other large investors: ~ 29 % HQ: Israel Founded: 2015 Market Cap: ~$ 8 M as of 12 / 26 / 1 8 Manufacturing facilities: Pharma Stulln, Fareva All Rights Reserved

The Product: LO 2 A Eye Drops All Rights Reserved Wize has licensed certain rights to develop, purchase, market, sell and distribute LO 2 A for ophthalmic disorders in the United States, Israel, Ukraine and China. Wize has certain rights to add additional territories. Wize has the option to purchase licensor's agreements with its existing distributors in Switzerland, Germany and Holland .

The Product : LO 2 A Eye Drops Established sales history in Europe * Approved to treat DES in four European countries * Switzerland, Germany, Hungary and the Netherlands Anti - Irritant Anti - irritant properties Compatible Suitable for use with contact lenses Preservative - Free Removes potential for irritation and sensitization which can damage the ocular surface and cornea Multi - dose Multi - dose form under development All Rights Reserved *Sales made by the licensor. Based on information provided by licensor. Estimated 1.53 M packages sold in 2017 (*)

LO 2 A: The Opportunity Proven eye drops formulation Developed by the licensor, Prof. Dikstein, for treatment of dry eye syndrome (DES), sold exclusively in Europe (*) with lubricant and anti - irritant properties Completed and ongoing clinical trials, results for FDA pre - IND submission Finished Phase II study to evaluate safety and efficacy of LO 2 A for patients with moderate to severe CCh . Conducting ongoing Phase IV trial to evaluate the safety and efficacy of LO 2 A eye drops for symptomatic improvement of dry dye in Patients with Sjögren’s Syndrome. Studies involved a combined 100 + patients. Approved to treat DES in four European countries Estimated 1.53 M packages sold in 2017 * Significant business development opportunities North America, China, and other parts of the world. Potential strategic partnerships. Novel promising indications Treatment of patients suffering from DES and CCh as well as patients suffering from DES and Sjögern’s Syndrome Existing Manufacturing Facilities in France and Germany All Rights Reserved *Sales made by the licensor. Based on information provided by licensor.

Competitive Landscape The Market

European Sales Territory Manufactured in Distributor Packages sold in 2017 Hungary Pharma Stulln (Germany) PannonPharma 8,000 Germany Pharma Stulln (Germany) Pharma Stulln 265,000 The Netherlands Pharma Stulln (Germany) Tramedico 158,000 Switzerland Fareva (France) Thea Pharmaceuticals Ltd 1,100,000 Israel Pharma Stulln (Germany) Pharmidas Sales commence d in 2018 Ukraine Pharma Stulln (Germany) EkoTechProm Premium Ltd. Seeking local regulatory approval China Pharma Stulln (Germany) HPGC Seeking local regulatory approval Future Sales Reseller Model Existing Royalties Model (*) All Rights Reserved (*) Existing sales by the licensor. Based on information provided by the licensor.

+ + + Dry Eye Most common, instillation site irritation and decrease in visual acuity ( 5 - 25 % of patients). Blurred vision, eye irritation, conjunctival redness, pruritus (occur in 1 - 5 % of patients) 2 Most common, ocular burning ( 17 %). Conjunctival redness, tearing, blurred vision, eye pain, pruritus (occur in 1 - 5 % of patients) 1 Minor, transient ocular events expected with ophthalmic formulations, e.g., blurred vision Side effects $ 259 M (launched Aug 2016 ) 4 $ 1.5 B 3 ~$ 11 M (estimated) 7 Sales in 2017 + + + Preservative free - - + Use with contact lenses - - + 5 Conjunctivalchalasis (CCh) - - + 6 Sjögren’s Syndrome LO2A Vs. Competition Allergan Shire LO2A 1 https://www.allergan.com/assets/pdf/restasis_pi.pdf 2 https://www.shire.com/ - /media/shire/shireglobal/shirecom/pdffiles/media% 20 library/xiidra - clinical - development - program - infographic.pdf 3 Allergan 2017 annual report ( 10 K) 4 http://investors.shire.com/~/media/Files/S/Shire - IR/quarterly - reports/ 2018 /full - year - 2017 - results.pdf 5 Approved in Hungary 6 Approved in the Netherlands 7 Estimate of wholesale sales of LO 2 A carried out by the inventor distributors All Rights Reserved

Multiple Indications: Sjögren’s Syndrome Affects about 0.2 % - 4 % of the adult population 9,10 Wide range, in part, reflects the lack of uniform diagnostic criteria Male/female ratio of 1:9 9 https://emedicine.medscape.com/article/332125 - overview#a6 10 https://www.ncbi.nlm.nih.gov/pubmed/20012976 A chronic autoimmune disorder characterized by exocrine gland dysfunction, affecting the salivary & lacrimal glands All Rights Reserved

Multiple Indications: CCh Refers to redundant folds of loose conjunctiva Increases dramatically with age 12 Current treatment for severe cases is surgical procedure 11 http://journals.plos.org/plosone/article?id= 10.1371 /journal.pone. 0132656 12 https://www.ncbi.nlm.nih.gov/pubmed/ 23036571 Present in up to a third of dry eye patients 11 (approx. 7 million people in the US) All Rights Reserved

Multiple Indications: Dry Eye Syndrome More than 16 M adults in the US have DES 7 Approx. 19 M prescriptions were filled in the US in 2013 for anti - inflammatory drugs administered by eye drops for ocular diseases and conditions, resulting in sales of approximately $ 2.2 billion (Source: IMS Health) Economic burden to US economy is $ 55.4 B 8 7 http://www.sciencedirect.com/science/article/pii/S 0002939417302908 8 https://www.ncbi.nlm.nih.gov/pubmed/ 21045640 / All Rights Reserved

Clinical Trials & Intellectual Property

Dry Eye Syndrome • Several clinical trials in Europe involving more than 200 patients with Dry Eye • Documented beneficial effect of LO 2 A eye drops by both the investigators and the patient DES & Sjögren’s Syndrome See Appendix • Investigator - initiated study • Performed in Hungary • 21 patients applied LO 2 A eye drops to both eyes in a single arm, open label trial for 3 months • The study demonstrated improvement in all parameters examined (with the exception of tear production) DES & CCh See Appendix • Investigator - initiated study • Performed in Hungary • 20 patients with Grade 2 or 3 CCh, who had previously failed treatment on a variety of artificial tear preparations, applied LO 2 A in a single arm, open label study for 3 months • This study demonstrated CCh severity decreased significantly after 3 months Prior Clinical Trials * All Rights Reserved (*) The previous clinical trials were conducted by the inventor of LO 2 A .

DES and CCh Phase II: Overview DES & CCh Phase II Trial • Multi - center, double - masked, placebo - controlled study in Israel • Completed patient enrollment and treatment of all 62 patients • Evaluate safety and efficacy of LO 2 A for patients with moderate to severe CCh • Randomized using a 1:1 active (LO 2 A) to placebo (saline) ratio • Trial treatment with LO 2 A/Saline for 3 months • Designed according to US standards by Ora Clinic – a leading US full - service ophthalmic CRO and product development firm Primary Endpoint: • Change from baseline in Lissamine Green Conjunctival Staining (LGCS) score at 3 months Secondary Endpoints: • Change in LIPCOF 1 grade score at 1 month and 3 months • Change in LGCS score at 1 month compared to baseline • Change in TFBUT 2 at 1 month and 3 months compared to baseline • Change in OSDI 3 questionnaire score measured at 1 month and 3 months compared to baseline (Visit 2 ) All Rights Reserved ( 1 ) LIPCOF = LId Parallel Conjunctival Folds (grading system for CCh severity) ( 2 ) TFBUT = Tear film break - up time ( 3 ) OSDI = Ocular Surface Disease Index (Patient questionnaire measuring subjective symptom score)

DES and CCh Phase II: Initial Results DES & CCh Phase II Trial • Phase II trial successful met primary objective of lowering lissamine green conjunctival staining (LGCS) score from baseline at 3 months • Based on analysis of 49 fully evaluable patients, out of the 62 originally enrolled, using a mixed model with repeated measures (MMRM), utilizing all post baseline observations ( 1 - month and 3 - month follow - ups), demonstrated a statistical significance between the LO 2 A group and the placebo group (P= 0.0079 ) • An analysis of the average reduction in LGCS score from baseline at three months demonstrated a strong trend towards significance (P= 0.0713 ) with average reduction in LGCS score between baseline and 3 months of - 3.5 and - 1.6 in the LO 2 A and placebo groups, respectively All Rights Reserved

Ongoing Clinical Trial: DES & Sjögren’s Syndrome Phase IV Confirmatory Trial • Multi - center, double - masked study design in up to 60 patients in Israel • Currently enrolling patients • Evaluate safety and efficacy of LO 2 A for symptomatic improvement of dry eye in patients with Sjögren’s Syndrome • Randomized using a 1:1 active (LO 2 A) to placebo (Systane Ultra UD) ratio • Trial treatment with LO 2 A/Systane Ultra UD for 3 months Primary Endpoints: • Change in corneal and conjunctival staining scores at 3 months compared to baseline using the National Eye Institute (NEI)/Industry Grading System Secondary Endpoints: • Change in OSDI questionnaire score at 1 and 3 months compared to baseline • Change in corneal and conjunctival staining scores at 3 months compared to baseline using the NEI/Industry Grading System • Change in eye dryness score (VAS) at 1 and 3 months compared to baseline All Rights Reserved

Anticipated Timeline All Rights Reserved Q 1 / 2020 Q 4 / 2019 Q 3 / 2019 Q 2 / 2019 Q 1 / 2019 Q 4 / 2018 మ Phase II Trial CCh - results మ Phase IV Trial DES Sjögren’s Syndrome – complete recruitment మ Phase IV Trial DES Sjögren’s Syndrome – results మ FDA – pre IND

Intellectual Property Territory Manufactured in Distributor International Patent Application Publ. No. WO 2012150583 filed on April 5 , 2012 , assigned to Resdevco, Corresponding members include US Patent No. 8,912,166 , Israeli Patent No. 212725 , Japanese Patent No. 5957517 and pending European Pat. Appl. 2704747 Granted patents and patents to issue of this patent family will expire, without extension, in 2032 The use of LO 2 A for treating or alleviating CCh is protected by the patent family titled EYE DROPS FOR TREATMENT OF CONJUNCTIVOCHALASIS. Provisional Patent Application No. 62 / 467139 , filed on March 5 , 2017 , assigned to Resdevco Patents to issue based on this provisional application will expire, without extension, in 2038 . The use of LO2A for treating or alleviating irritation of the eye caused by non - infectious diseases, such as Sjögren’s, is covered by the patent application titled EYE DROPS FOR TREATMENT OF IRRITATION NOT DUE TO INFECTION. US Patent No. 5,106,615 Expired The LO2A composition and use thereof for treating or alleviating DES All Rights Reserved

Summa r y
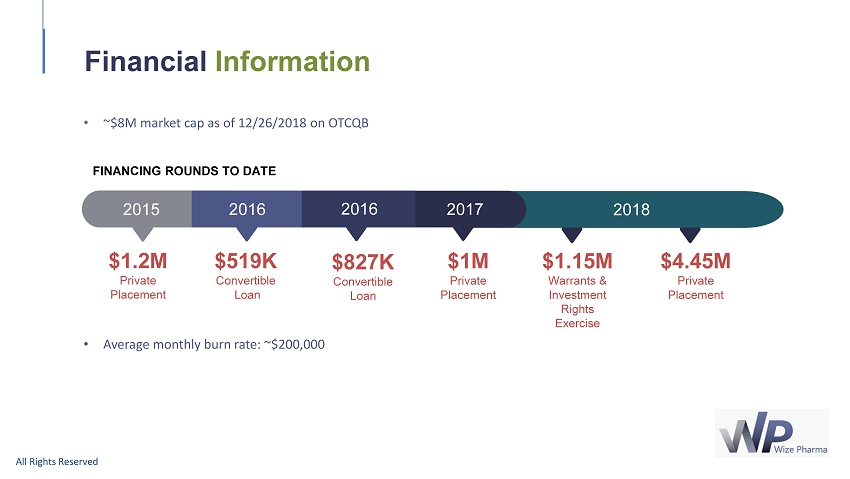
• ~$ 8 M market cap as of 12 / 26 / 2018 on OTCQB • Average monthly burn rate: ~$ 200,000 Financial Information All Rights Reserved FINANCING ROUNDS TO DATE $ 1.2 M Private Placement $519K Convertible Loan $827K Convertible Loan $1M Private Placement 2017 2015 2016 2016 $ 1.15 M Warrants & Investment Rights Exercise $4.45M Private Placement 2018

Capital Structure All Rights Reserved 2018 As of 11 / 13 / 2018 Weighted Average Exercise Price (WAEP) Common Stock 8,462,550 Convertible Loans 1,366,803 WAEP: $ 1.054 (Conversion Price) Investment Rights 890,138 WAEP: $ 1.321 Options 259,896 WAEP: $ 3.68 Warrants 10,015,871 WAEP: $ 1.119 Preferred Stock, Series A 1,350,000 1,350 preferred stock convertible into 1,350,000 shares of common stock Total – Fully Diluted Basis 22,345,258

Introducing Management Team Or Eisenberg CFO, ACTING CEO Joined March 2015 Formerly controller of Katzir Fund Group Formerly external controller/CFO of multiple TASE companies Formerly accountant at Ernst & Young BA in Economics & Accounting (from Haifa University) CPA in Israel OE Noam Danenberg Chairman Strategic advisor to Wize since April 2015, COO from December 2017 to November 2018 Served as a founder, director, investment advisor to a number of pharmaceutical and medical companies MBA from Boston University ND Dr. Adam Foley - Coper MEDICAL DIRECTOR Joined July 2015 Experience in development, regulatory strategy, clinical trial planning & execution, with leading international pharmaceutical companies MB Chb , University of Manchester Fellow of the Royal College of Surgeons M.Sc. from University College, London AF All Rights Reserved

Introducing Scientific Advisory Board Prof. Janos Nemeth Ophthalmologic surgeon and Board Member of the Drug Discovery and Safety Center at Semmelweis University in Budapest, Hungary Performed clinical trial with LO2A in the treatment of CCh JN All Rights Reserved Dr. Penny Asbell KOL in the treatment of DES PI in clinical studies that led to development of pharmaceuticals for pivotal treatment of ocular conditions including DES Prof. of Ophthalmology at Icahn School of Medicine, Mount Sinai Established Mount Sinai's Lowenstein Foundation Sjogren's Center & Founded Ocular Inflammatory Biomarker Laboratory Authored 100 s of articles, 25 book chapters and presented over 200 lectures and courses PA Dr. Joseph Tauber One of the world’s leading experts in the field of ocular surface diseases, including DES and meibomitis management PI in dozens of clinical programs including two FDA approved medications for DES Wrote 5 book chapters and over 60 articles in ophthalmology medical journals Received doctorate from Harvard Medical School Founder of Tauber Eye Center in Kansas City, Missouri JT

LO 2 A: The Opportunity Proven eye drops formulation Developed by the licensor, Prof. Disktein, for treatment of DES, sold exclusively in Europe (*) with lubricant and anti - irritant properties Completed and ongoing clinical trials, results for FDA pre - IND submission Finished Phase II study to evaluate safety and efficacy of LO 2 A for patients with moderate to severe CCh . Conducting ongoing Phase IV trial to evaluate the safety and efficacy of LO 2 A Eye Drops for symptomatic improvement of dry dye in patients with Sjögren’s Syndrome. Studies involved a combined 100 + patients. Approved to treat DES in four European countries Estimated 1.53M packages sold in 2017 * Significant business development opportunities North America, China, and other parts of the world. Potential strategic partnerships. Novel promising indications Treatment of patients suffering from DES and CCh as well as patients suffering from DES and Sjögern’s Syndrome Existing Manufacturing Facilities in France and Germany All Rights Reserved *Sales made by the licensor. Based on information provided by licensor.

24 Hanagar St. Hod Hasharon, Israel Tel: + 972 72 2600536 Cell: + 972 54 4318380 Fax: + 972 72 2600537 Thank You Visit wizepharma.com or email info@wizepharma.com

Appendix

Clinical Results: Hungarian CCh Study * Month 0 Month 1 Month 3 Comparison Month 3 vs Month 0 Comparison Month 3 vs Month 1 Mean ± SD Mean ± SD Mean ± SD Shift parameter and 95% Cl P value Shift parameter and 95% Cl P value Right Left Right Left Right Left Right Left Right Left Right Left Right Left LIPCOF Degree 2.9 ± 0.4 2.9 ± 0.4 1.8 ± 0.9 1.6 ± 0.8 1.4 ± 0.6 1.4 ± 0.7 - 2.0 ( - 2.0 to - 1.0) - 1.0 ( - 2.0 to - 1.0) <0.001 <0.001 0.0 ( - 1.0 to 0.0) 0.0 (0.0 to 0.0) 0.035 0.312 TFBUT value 4.8 ± 1.9 4.8 ± 1.9 5.4 ± 1.6 5.7 ± 2.1 5.9 ± 2.3 5.7 ± 1.8 1.1 (0.2 to 2.0) 0.9 (0.3 to 1.5) 0.020 0.004 0.5 ( - 0.3 to 1.3) - 0.0 ( - 0.7 to 0.6) 0.191 0.953 LGCS 1.3 ± 0.6 1.4 ± 0.6 0.6 ± 0.6 0.6 ± 0.5 0.3 ± 0.4 0.2 ± 0.4 - 1.0 ( - 1.0 to - 1.0) - 1.0 ( - 1.0 to - 1.0) <0.001 <0.001 0.0( - 1.0 to 0.0) 0.0 ( - 1.0 to 0.0) 0.016 0.039 OSDI Score 36.2 ± 25.3 22.6 ± 21.9 15.6 ± 16.7 - 13.1 ( - 25.0 to - 8.3) <0.001 - 2.4 ( - 5.7 to 0.0) 0.012 LIPCOF = LId Parallel Conjunctival Folds (grading system for CCh severity) TFBUT = Tear film break - up time LGCS = Lissamin Green Corneal Staining (according to the Oxford scale) OSDI = Ocular Surface Disease Index (Patient questionnaire measuring subjective symptom score) All Rights Reserved N= 20 patients (*) The previous clinical trials were conducted by the inventor of LO 2 A .

Clinical Results: Hungarian CCh Study * 1 2 3 0 1 2 3 Conjunctivochalasis (LIPCOF degree) Time (months) Right eyes 0 1 2 3 0 1 2 3 Conjunctivochalasis (LIPCOF degree) Time (months) Left eyes 0 The graph demonstrates a statistically significant improvement in treating patients with CCh according to LIPCOF (LId Parallel COnjunctival Folds) Grade Degree of the conjunctivochalasis in terms of LIPCOF degrees after 1 and 3 months of artificial tear treatment. Artificial te ar treatment and measurement of LIPCOF degree on 20 patients were performed as described in Methods. The artificial tear investigated caused a significant decrease of LIPCOF deg ree on both eyes after one month of use that advanced further on the right eye (filled diamonds, solid line) significantly, and showed the same tendency on the left eye (open rect ang les, dashed line) after three months of use. Means and their standard errors of the LIPCOF degree are shown. Statistical evaluation was performed using the Wilcoxon Signed Rank Tes t. All Rights Reserved (*) The previous clinical trials were conducted by the inventor of LO 2 A .

Clinical Results: Hungarian CCh Study * 0 2 2 0 15 22 6 17 15 34 6 1 BASELINE 1 MONTH 3 MONTH Number of Eyes Study Visit LIPCOF Grade following 1 and 3 month treatment with Conheal Grade 0 Grade 1 Grade 2 Grade 3 Bar chart demonstrating the improvement in CCh per eye, measured by LIPCOF Grade, after one and three months treatment with Conheal ® All Rights Reserved (*) The previous clinical trials were conducted by the inventor of LO 2 A .

Clinical Results: Hungarian Sjögren ’ s Study * Figure 1 : The artificial tears with high glycerine content ceased the corneal staining almost completely in the patients with Sjögren’s Sy ndrome by the end of the three - month treatment. In the patients the initial ocular sin face staining was developed in spite of the earlier lasting use of ar tif icial tears. All Rights Reserved Lissamin green staining (according to the Oxford scale) Lissamin green staining (according to the Oxford scale) Right eyes Duration (months) Duration (months) Left eyes N = 21 patients (*) The previous clinical trials were conducted by the inventor of LO 2 A .
