Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Allena Pharmaceuticals, Inc. | d663422dex991.htm |
| EX-10.2 - EXHIBIT 10.2 - Allena Pharmaceuticals, Inc. | d663422dex102.htm |
| EX-10.1 - EXHIBIT 10.1 - Allena Pharmaceuticals, Inc. | d663422dex101.htm |
| 8-K - FORM 8-K - Allena Pharmaceuticals, Inc. | d663422d8k.htm |

Bringing First-in-Class Oral Enzyme Therapeutics to Patients with Rare and Severe Metabolic and Kidney Disorders January 2019 Exhibit 99.2

Allena Pharmaceuticals, Inc. These slides, and any accompanying presentation, contain forward-looking statements and information. The use of words such as “may,” “might,” “will,” “should,” “expect,” “plan,” “anticipate,” “believe,” “estimate,” “project,” “intend,” “future,” “potential,” or “continue,” and other similar expressions are intended to identify forward-looking statements. All forward-looking statements are based on estimates and assumptions by our management that, although we believe them to be reasonable, are inherently uncertain. All forward-looking statements are subject to risks and uncertainties that may cause actual results to differ materially from those that we expected, including those material risks and uncertainties that are described under the heading “Risk Factors” in our Quarterly Report on Form 10-Q for the quarter ended September 30, 2018 filed with the Securities and Exchange Commission on November 7, 2018, as well as discussions of potential risks, uncertainties and other important factors in our subsequent filings with the Securities and Exchange Commission. Any forward-looking statement speaks only as of the date on which it was made. We undertake no obligation to publicly update or revise any forward-looking statement, whether as a result of new information, future events or otherwise, except as required by law.
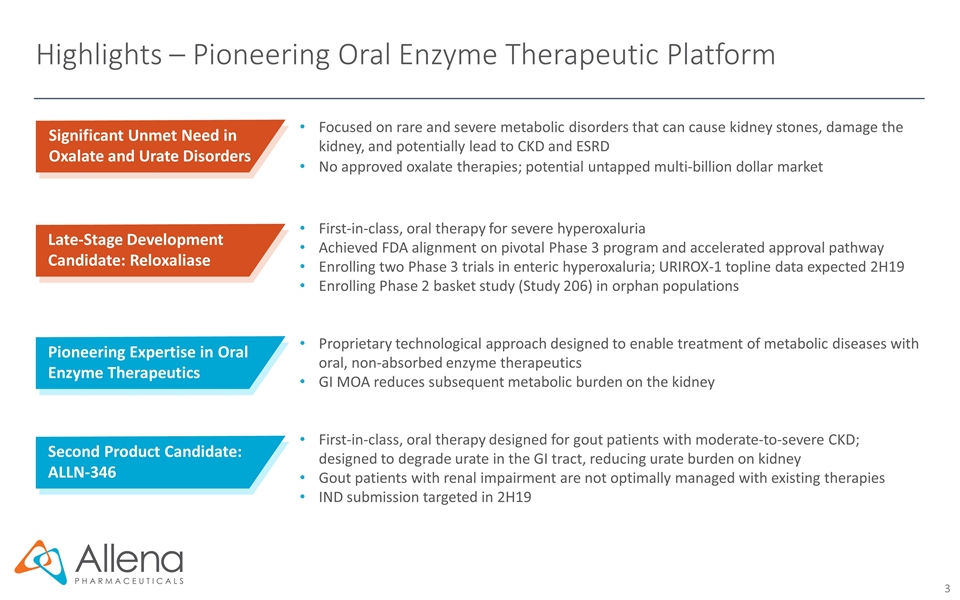
Highlights – Pioneering Oral Enzyme Therapeutic Platform Significant Unmet Need in Oxalate and Urate Disorders Focused on rare and severe metabolic disorders that can cause kidney stones, damage the kidney, and potentially lead to CKD and ESRD No approved oxalate therapies; potential untapped multi-billion dollar market First-in-class, oral therapy for severe hyperoxaluria Achieved FDA alignment on pivotal Phase 3 program and accelerated approval pathway Enrolling two Phase 3 trials in enteric hyperoxaluria; URIROX-1 topline data expected 2H19 Enrolling Phase 2 basket study (Study 206) in orphan populations Proprietary technological approach designed to enable treatment of metabolic diseases with oral, non-absorbed enzyme therapeutics GI MOA reduces subsequent metabolic burden on the kidney First-in-class, oral therapy designed for gout patients with moderate-to-severe CKD; designed to degrade urate in the GI tract, reducing urate burden on kidney Gout patients with renal impairment are not optimally managed with existing therapies IND submission targeted in 2H19 Late-Stage Development Candidate: Reloxaliase Pioneering Expertise in Oral Enzyme Therapeutics Second Product Candidate: ALLN-346

Post-Approval Clinical Benefit Phase In December 2018, Allena Achieved FDA Alignment on Accelerated Approval Pathway for Reloxaliase and Design of URIROX-2 1° efficacy endpoint: Percent change from baseline in mean UOx excretion during Weeks 1-4 2° efficacy endpoint: Proportion of patients with ≥20% reduction in mean UOx excretion during Weeks 1-4 and percent change from baseline in mean UOx excretion during Weeks 16-24 (URIROX-2) Long-term efficacy endpoints to confirm clinical benefit: Primary: Proportion of subjects with kidney stone disease progression Secondary: Change in eGFR from baseline and ER visits/hospitalizations/ procedures for management of kidney stones Expect to submit an accelerated approval BLA filing to the FDA after ~400 patients have been randomized and followed for six months. URIROX Phase 3 program – a landmark study for patients suffering from enteric hyperoxaluria: Urinary oxalate (UOx) biomarker as surrogate endpoint URIROX-2 has the same primary efficacy endpoint as URIROX-1 with topline data expected in 2H19 Kidney stone disease progression as long-term endpoint for clinical benefit Adaptive design elements to streamline clinical benefit phase of URIROX-2
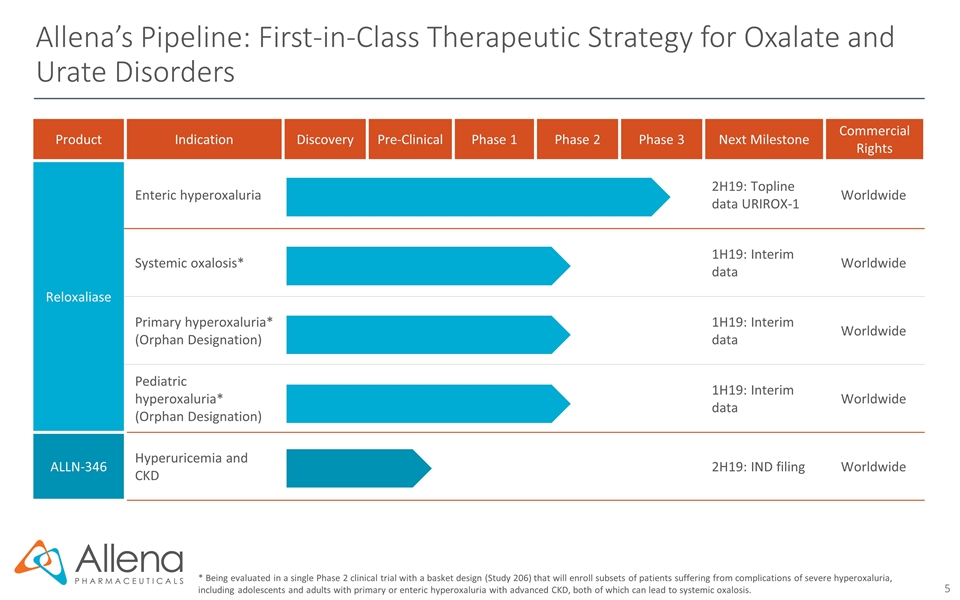
Product Indication Discovery Pre-Clinical Phase 1 Phase 2 Phase 3 Next Milestone Commercial Rights Reloxaliase Enteric hyperoxaluria 2H19: Topline data URIROX-1 Worldwide Systemic oxalosis* 1H19: Interim data Worldwide Primary hyperoxaluria* (Orphan Designation) 1H19: Interim data Worldwide Pediatric hyperoxaluria* (Orphan Designation) 1H19: Interim data Worldwide ALLN-346 Hyperuricemia and CKD 2H19: IND filing Worldwide Allena’s Pipeline: First-in-Class Therapeutic Strategy for Oxalate and Urate Disorders * Being evaluated in a single Phase 2 clinical trial with a basket design (Study 206) that will enroll subsets of patients suffering from complications of severe hyperoxaluria, including adolescents and adults with primary or enteric hyperoxaluria with advanced CKD, both of which can lead to systemic oxalosis.

First-in-Class Therapeutic Strategy Expertise and Proprietary Technological Approach in Enzyme Therapeutics Enables First-in-Class Therapeutic Strategy for Oxalate and Urate Disorders Pioneering Expertise in Oral Enzyme Therapeutics Design, formulation, and delivery of non-absorbed and stable enzymes orally for activity in GI tract First-in-Class Enzyme Therapeutics Oral enzymes designed to rapidly degrade a specific metabolite within the gut, reducing absorption in blood and urine, and in turn, diminishing disease burden on kidney Proprietary and Scalable Manufacturing Capabilities Proven ability to produce large quantities of oral enzymes COGS anticipated to be comparable to small molecule therapeutics
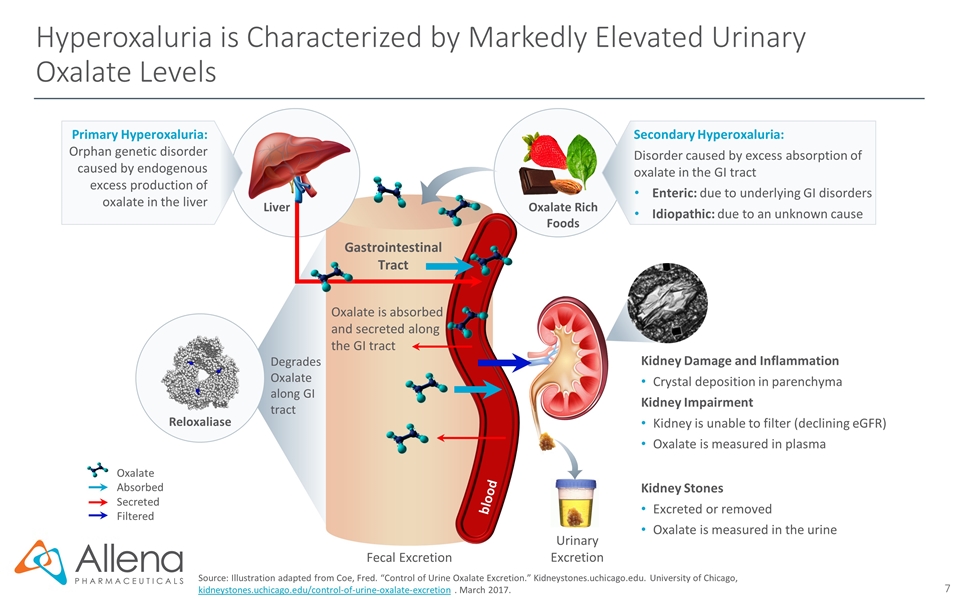
Hyperoxaluria is Characterized by Markedly Elevated Urinary Oxalate Levels Source: Illustration adapted from Coe, Fred. “Control of Urine Oxalate Excretion.” Kidneystones.uchicago.edu. University of Chicago, kidneystones.uchicago.edu/control-of-urine-oxalate-excretion . March 2017. Fecal Excretion Gastrointestinal Tract Oxalate is absorbed and secreted along the GI tract Urinary Excretion Kidney Damage and Inflammation Crystal deposition in parenchyma Kidney Impairment Kidney is unable to filter (declining eGFR) Oxalate is measured in plasma Kidney Stones Excreted or removed Oxalate is measured in the urine Oxalate Rich Foods Oxalate Absorbed Secreted Filtered Primary Hyperoxaluria: Orphan genetic disorder caused by endogenous excess production of oxalate in the liver Liver blood Secondary Hyperoxaluria: Disorder caused by excess absorption of oxalate in the GI tract Enteric: due to underlying GI disorders Idiopathic: due to an unknown cause Degrades Oxalate along GI tract Reloxaliase
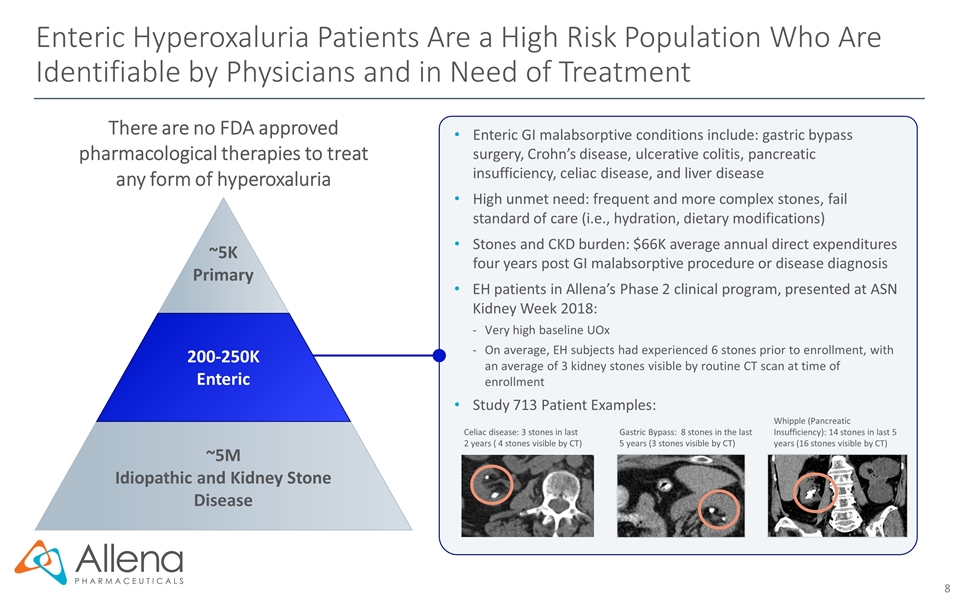
Enteric Hyperoxaluria Patients Are a High Risk Population Who Are Identifiable by Physicians and in Need of Treatment There are no FDA approved pharmacological therapies to treat any form of hyperoxaluria Enteric GI malabsorptive conditions include: gastric bypass surgery, Crohn’s disease, ulcerative colitis, pancreatic insufficiency, celiac disease, and liver disease High unmet need: frequent and more complex stones, fail standard of care (i.e., hydration, dietary modifications) Stones and CKD burden: $66K average annual direct expenditures four years post GI malabsorptive procedure or disease diagnosis EH patients in Allena’s Phase 2 clinical program, presented at ASN Kidney Week 2018: Very high baseline UOx On average, EH subjects had experienced 6 stones prior to enrollment, with an average of 3 kidney stones visible by routine CT scan at time of enrollment Study 713 Patient Examples: Whipple (Pancreatic Insufficiency): 14 stones in last 5 years (16 stones visible by CT) Celiac disease: 3 stones in last 2 years ( 4 stones visible by CT) Gastric Bypass: 8 stones in the last 5 years (3 stones visible by CT) ~5K Primary 200-250K Enteric ~5M Idiopathic and Kidney Stone Disease
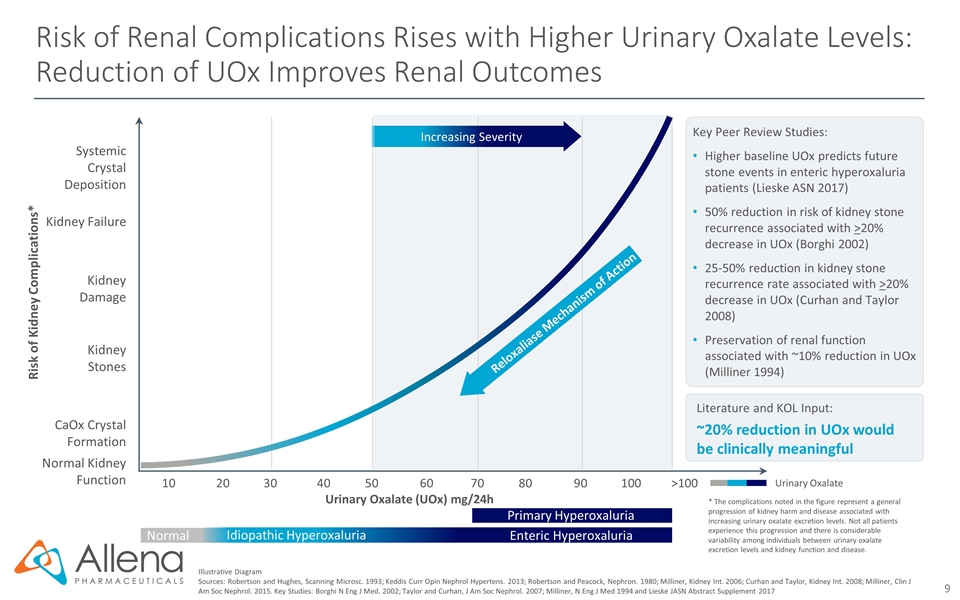
Risk of Renal Complications Rises with Higher Urinary Oxalate Levels: Reduction of UOx Improves Renal Outcomes Illustrative Diagram Sources: Robertson and Hughes, Scanning Microsc. 1993; Keddis Curr Opin Nephrol Hypertens. 2013; Robertson and Peacock, Nephron. 1980; Milliner, Kidney Int. 2006; Curhan and Taylor, Kidney Int. 2008; Milliner, Clin J Am Soc Nephrol. 2015. Key Studies: Borghi N Eng J Med. 2002; Taylor and Curhan, J Am Soc Nephrol. 2007; Milliner, N Eng J Med 1994 and Lieske JASN Abstract Supplement 2017 Urinary Oxalate Literature and KOL Input: ~20% reduction in UOx would be clinically meaningful Key Peer Review Studies: Higher baseline UOx predicts future stone events in enteric hyperoxaluria patients (Lieske ASN 2017) 50% reduction in risk of kidney stone recurrence associated with >20% decrease in UOx (Borghi 2002) 25-50% reduction in kidney stone recurrence rate associated with >20% decrease in UOx (Curhan and Taylor 2008) Preservation of renal function associated with ~10% reduction in UOx (Milliner 1994) Urinary Oxalate (UOx) mg/24h Kidney Damage 102030405060708090100>100 Kidney Failure Normal Kidney Function Kidney Stones CaOx Crystal Formation Systemic Crystal Deposition Risk of Kidney Complications* Increasing Severity Reloxaliase Mechanism of Action * The complications noted in the figure represent a general progression of kidney harm and disease associated with increasing urinary oxalate excretion levels. Not all patients experience this progression and there is considerable variability among individuals between urinary oxalate excretion levels and kidney function and disease. Idiopathic Hyperoxaluria Enteric Hyperoxaluria Primary Hyperoxaluria Normal
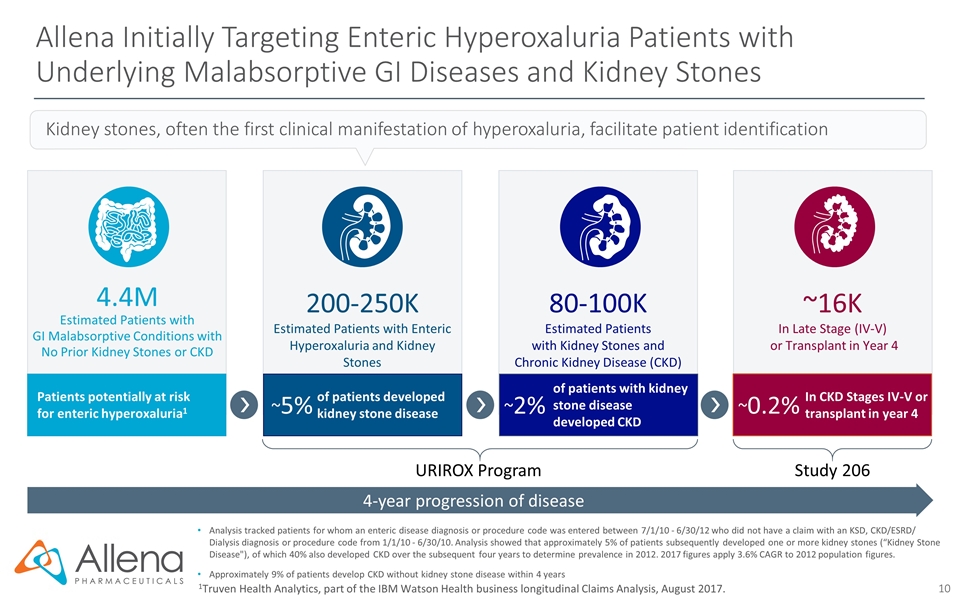
Allena Initially Targeting Enteric Hyperoxaluria Patients with Underlying Malabsorptive GI Diseases and Kidney Stones 1Truven Health Analytics, part of the IBM Watson Health business longitudinal Claims Analysis, August 2017. 4-year progression of disease 4.4M Estimated Patients with GI Malabsorptive Conditions with No Prior Kidney Stones or CKD Patients potentially at risk for enteric hyperoxaluria1 Kidney stones, often the first clinical manifestation of hyperoxaluria, facilitate patient identification of patients with kidney stone disease developed CKD 80-100K Estimated Patients with Kidney Stones and Chronic Kidney Disease (CKD) ~2% 200-250K Estimated Patients with Enteric Hyperoxaluria and Kidney Stones of patients developed kidney stone disease ~5% In CKD Stages IV-V or transplant in year 4 ~16K In Late Stage (IV-V) or Transplant in Year 4 ~0.2% Analysis tracked patients for whom an enteric disease diagnosis or procedure code was entered between 7/1/10 - 6/30/12 who did not have a claim with an KSD, CKD/ESRD/ Dialysis diagnosis or procedure code from 1/1/10 - 6/30/10. Analysis showed that approximately 5% of patients subsequently developed one or more kidney stones (“Kidney Stone Disease"), of which 40% also developed CKD over the subsequent four years to determine prevalence in 2012. 2017 figures apply 3.6% CAGR to 2012 population figures. Approximately 9% of patients develop CKD without kidney stone disease within 4 years URIROX Program Study 206
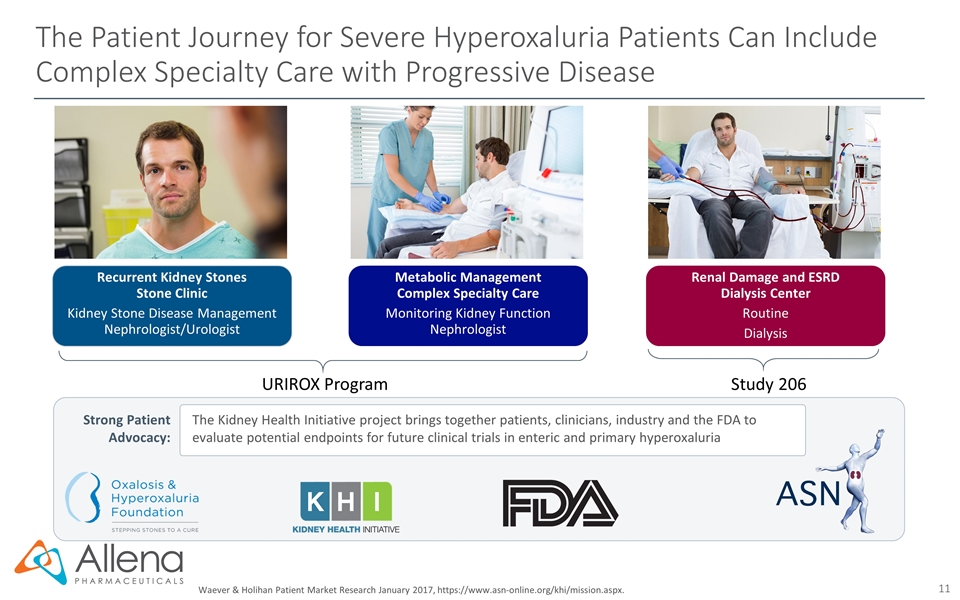
The Patient Journey for Severe Hyperoxaluria Patients Can Include Complex Specialty Care with Progressive Disease Strong Patient Advocacy: The Kidney Health Initiative project brings together patients, clinicians, industry and the FDA to evaluate potential endpoints for future clinical trials in enteric and primary hyperoxaluria Waever & Holihan Patient Market Research January 2017, https://www.asn-online.org/khi/mission.aspx. Recurrent Kidney Stones Stone Clinic Kidney Stone Disease Management Nephrologist/Urologist Metabolic Management Complex Specialty Care Monitoring Kidney Function Nephrologist Renal Damage and ESRD Dialysis Center Routine Dialysis URIROX Program Study 206

Reloxaliase for the Treatment of Hyperoxaluria
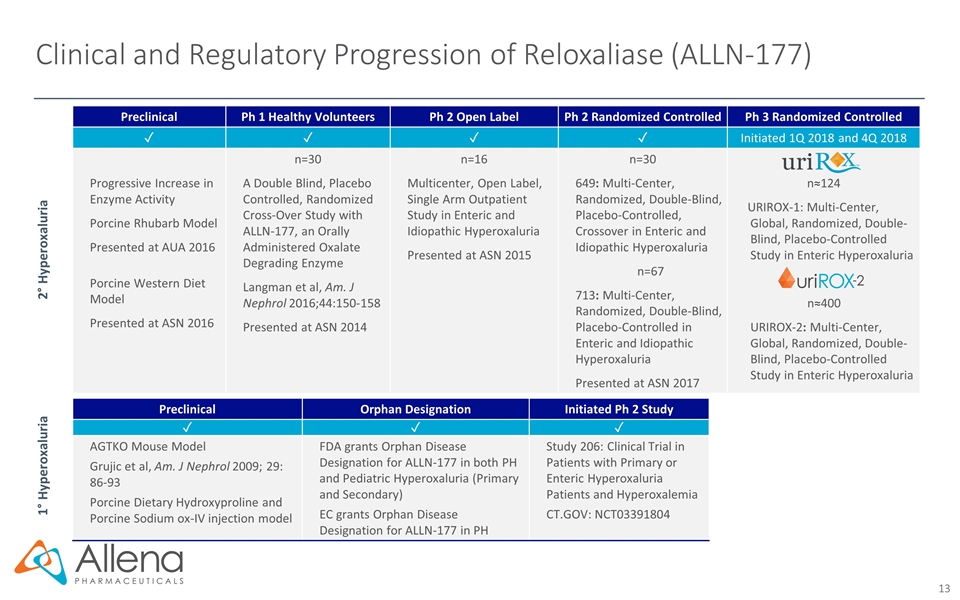
Clinical and Regulatory Progression of Reloxaliase (ALLN-177) Preclinical Ph 1 Healthy Volunteers Ph 2 Open Label Ph 2 Randomized Controlled Ph 3 Randomized Controlled ✓ ✓ ✓ ✓ Initiated 1Q 2018 and 4Q 2018 Progressive Increase in Enzyme Activity Porcine Rhubarb Model Presented at AUA 2016 Porcine Western Diet Model Presented at ASN 2016 n=30 A Double Blind, Placebo Controlled, Randomized Cross-Over Study with ALLN-177, an Orally Administered Oxalate Degrading Enzyme Langman et al, Am. J Nephrol 2016;44:150-158 Presented at ASN 2014 n=16 Multicenter, Open Label, Single Arm Outpatient Study in Enteric and Idiopathic Hyperoxaluria Presented at ASN 2015 n=30 649: Multi-Center, Randomized, Double-Blind, Placebo-Controlled, Crossover in Enteric and Idiopathic Hyperoxaluria n=67 713: Multi-Center, Randomized, Double-Blind, Placebo-Controlled in Enteric and Idiopathic Hyperoxaluria Presented at ASN 2017 n≈124 URIROX-1: Multi-Center, Global, Randomized, Double-Blind, Placebo-Controlled Study in Enteric Hyperoxaluria n≈400 URIROX-2: Multi-Center, Global, Randomized, Double-Blind, Placebo-Controlled Study in Enteric Hyperoxaluria 2° Hyperoxaluria 1° Hyperoxaluria Preclinical Orphan Designation Initiated Ph 2 Study ✓ ✓ ✓ AGTKO Mouse Model Grujic et al, Am. J Nephrol 2009; 29: 86-93 Porcine Dietary Hydroxyproline and Porcine Sodium ox-IV injection model FDA grants Orphan Disease Designation for ALLN-177 in both PH and Pediatric Hyperoxaluria (Primary and Secondary) EC grants Orphan Disease Designation for ALLN-177 in PH Study 206: Clinical Trial in Patients with Primary or Enteric Hyperoxaluria Patients and Hyperoxalemia CT.GOV: NCT03391804
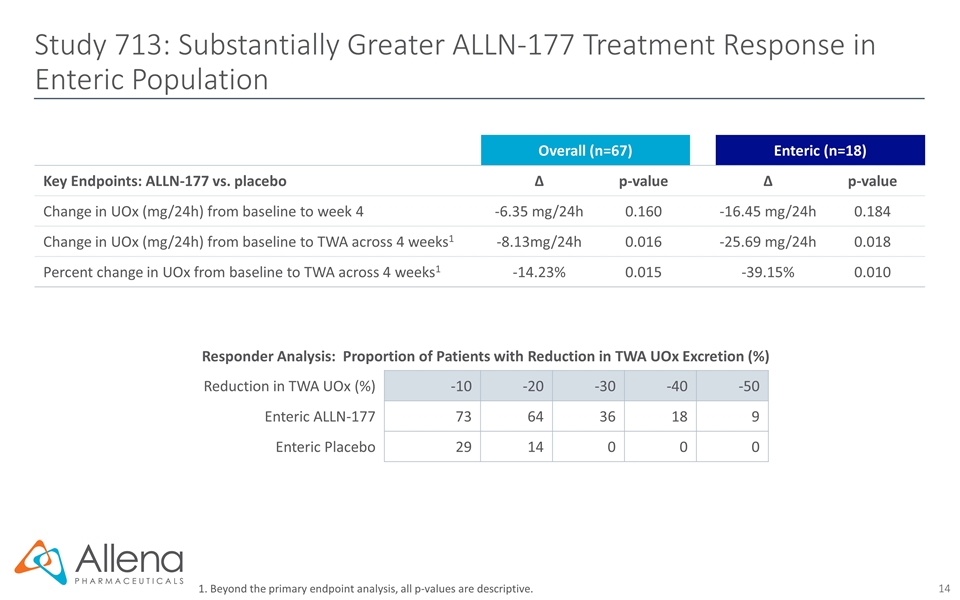
Study 713: Substantially Greater ALLN-177 Treatment Response in Enteric Population 1. Beyond the primary endpoint analysis, all p-values are descriptive. Overall (n=67) Enteric (n=18) Key Endpoints: ALLN-177 vs. placebo ∆ p-value ∆ p-value Change in UOx (mg/24h) from baseline to week 4 -6.35 mg/24h 0.160 -16.45 mg/24h 0.184 Change in UOx (mg/24h) from baseline to TWA across 4 weeks1 -8.13mg/24h 0.016 -25.69 mg/24h 0.018 Percent change in UOx from baseline to TWA across 4 weeks1 -14.23% 0.015 -39.15% 0.010 Responder Analysis: Proportion of Patients with Reduction in TWA UOx Excretion (%) Reduction in TWA UOx (%) -10 -20 -30 -40 -50 Enteric ALLN-177 73 64 36 18 9 Enteric Placebo 29 14 0 0 0
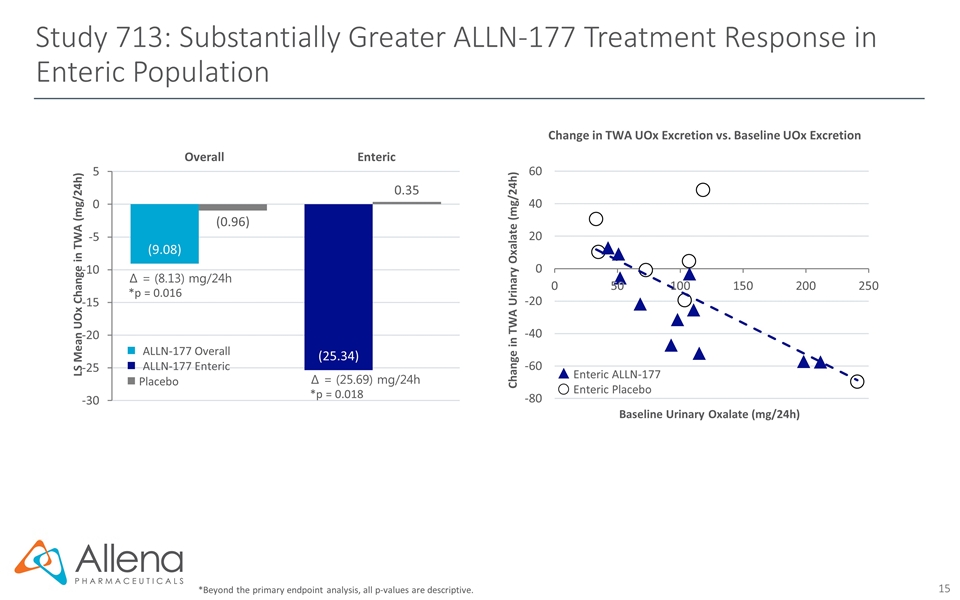
Study 713: Substantially Greater ALLN-177 Treatment Response in Enteric Population *Beyond the primary endpoint analysis, all p-values are descriptive. Enteric ALLN-177 Enteric Placebo Change in TWA Urinary Oxalate (mg/24h) Baseline Urinary Oxalate (mg/24h) Change in TWA UOx Excretion vs. Baseline UOx Excretion Create table ∆ = (8.13) mg/24h *p = 0.016 ∆ = (25.69) mg/24h *p = 0.018 LS Mean UOx Change in TWA (mg/24h) Overall Enteric ALLN-177 Overall ALLN-177 Enteric n Placebo
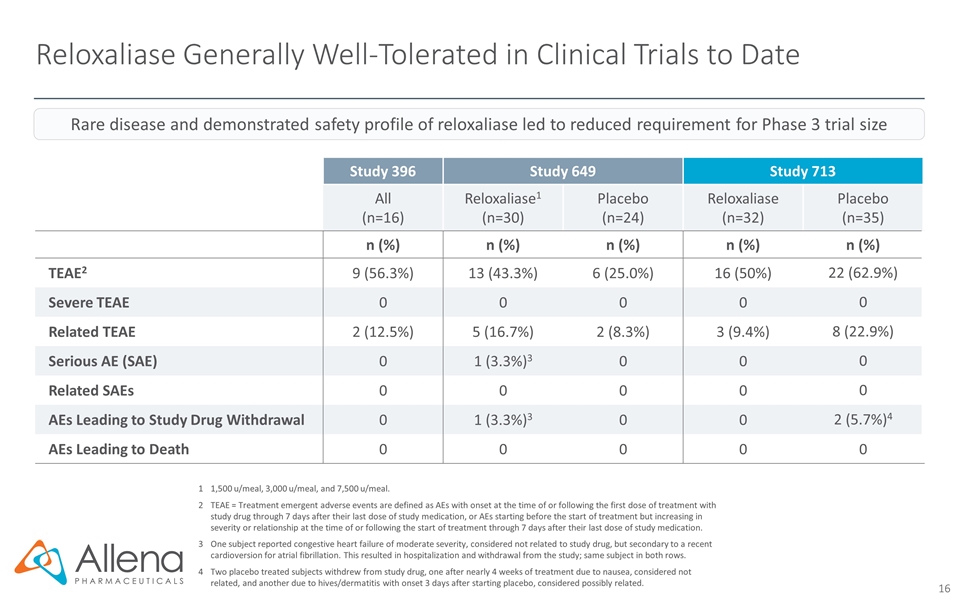
Reloxaliase Generally Well-Tolerated in Clinical Trials to Date 11,500 u/meal, 3,000 u/meal, and 7,500 u/meal. 2TEAE = Treatment emergent adverse events are defined as AEs with onset at the time of or following the first dose of treatment with study drug through 7 days after their last dose of study medication, or AEs starting before the start of treatment but increasing in severity or relationship at the time of or following the start of treatment through 7 days after their last dose of study medication. 3One subject reported congestive heart failure of moderate severity, considered not related to study drug, but secondary to a recent cardioversion for atrial fibrillation. This resulted in hospitalization and withdrawal from the study; same subject in both rows. 4Two placebo treated subjects withdrew from study drug, one after nearly 4 weeks of treatment due to nausea, considered not related, and another due to hives/dermatitis with onset 3 days after starting placebo, considered possibly related. Study 396 Study 649 Study 713 All (n=16) Reloxaliase1 (n=30) Placebo (n=24) Reloxaliase (n=32) Placebo (n=35) n (%) n (%) n (%) n (%) n (%) TEAE2 9 (56.3%) 13 (43.3%) 6 (25.0%) 16 (50%) 22 (62.9%) Severe TEAE 0 0 0 0 0 Related TEAE 2 (12.5%) 5 (16.7%) 2 (8.3%) 3 (9.4%) 8 (22.9%) Serious AE (SAE) 0 1 (3.3%)3 0 0 0 Related SAEs 0 0 0 0 0 AEs Leading to Study Drug Withdrawal 0 1 (3.3%)3 0 0 2 (5.7%)4 AEs Leading to Death 0 0 0 0 0 Rare disease and demonstrated safety profile of reloxaliase led to reduced requirement for Phase 3 trial size
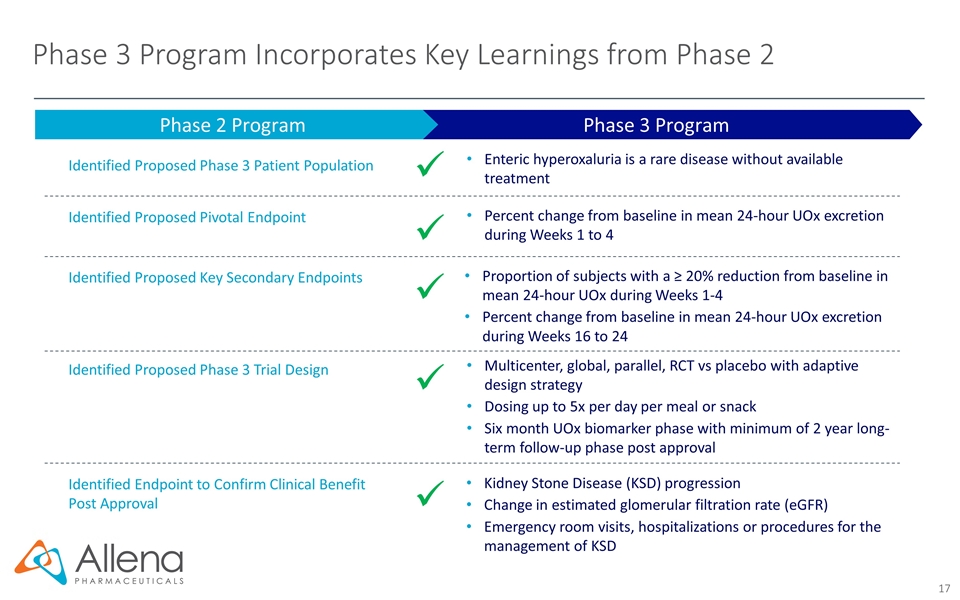
Phase 3 Program Incorporates Key Learnings from Phase 2 Phase 3 Program Phase 2 Program Identified Proposed Phase 3 Patient Population Identified Proposed Pivotal Endpoint Identified Proposed Phase 3 Trial Design ü Identified Proposed Key Secondary Endpoints Enteric hyperoxaluria is a rare disease without available treatment ü Percent change from baseline in mean 24-hour UOx excretion during Weeks 1 to 4 ü Proportion of subjects with a ≥ 20% reduction from baseline in mean 24-hour UOx during Weeks 1-4 Percent change from baseline in mean 24-hour UOx excretion during Weeks 16 to 24 ü Multicenter, global, parallel, RCT vs placebo with adaptive design strategy Dosing up to 5x per day per meal or snack Six month UOx biomarker phase with minimum of 2 year long-term follow-up phase post approval ü Identified Endpoint to Confirm Clinical Benefit Post Approval Kidney Stone Disease (KSD) progression Change in estimated glomerular filtration rate (eGFR) Emergency room visits, hospitalizations or procedures for the management of KSD
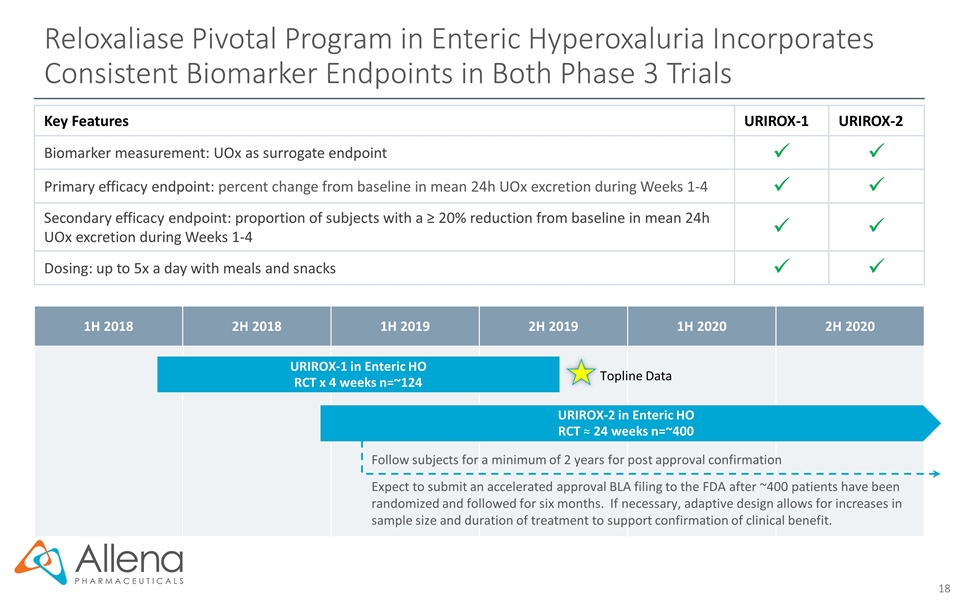
1H 2018 2H 2018 1H 2019 2H 2019 1H 2020 2H 2020 Reloxaliase Pivotal Program in Enteric Hyperoxaluria Incorporates Consistent Biomarker Endpoints in Both Phase 3 Trials Follow subjects for a minimum of 2 years for post approval confirmation URIROX-1 in Enteric HO RCT x 4 weeks n=~124 Topline Data URIROX-2 in Enteric HO RCT ≈ 24 weeks n=~400 Key Features URIROX-1 URIROX-2 Biomarker measurement: UOx as surrogate endpoint Primary efficacy endpoint: percent change from baseline in mean 24h UOx excretion during Weeks 1-4 Secondary efficacy endpoint: proportion of subjects with a ≥ 20% reduction from baseline in mean 24h UOx excretion during Weeks 1-4 Dosing: up to 5x a day with meals and snacks Expect to submit an accelerated approval BLA filing to the FDA after ~400 patients have been randomized and followed for six months. If necessary, adaptive design allows for increases in sample size and duration of treatment to support confirmation of clinical benefit.
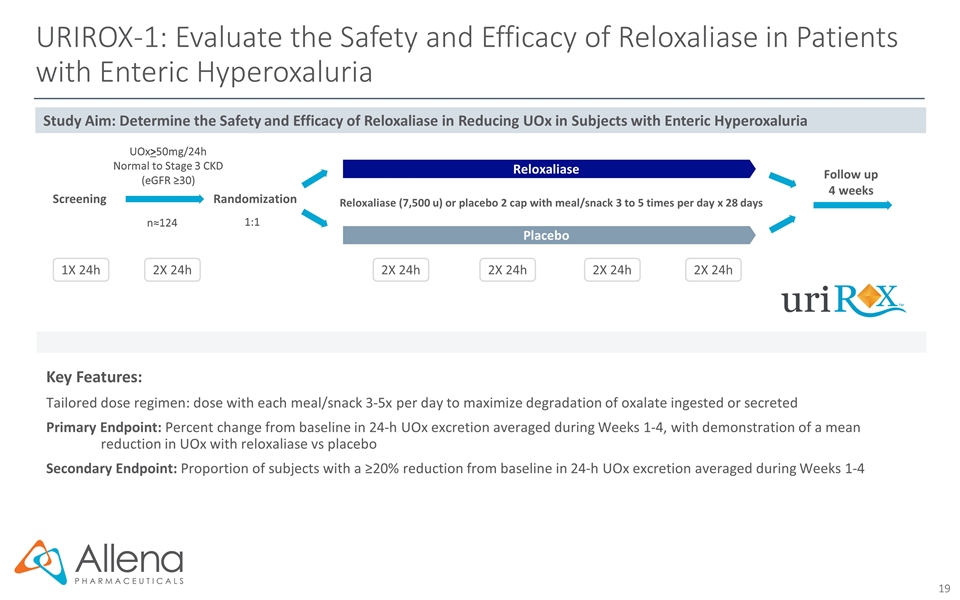
URIROX-1: Evaluate the Safety and Efficacy of Reloxaliase in Patients with Enteric Hyperoxaluria Key Features: Tailored dose regimen: dose with each meal/snack 3-5x per day to maximize degradation of oxalate ingested or secreted Primary Endpoint: Percent change from baseline in 24-h UOx excretion averaged during Weeks 1-4, with demonstration of a mean reduction in UOx with reloxaliase vs placebo Secondary Endpoint: Proportion of subjects with a ≥20% reduction from baseline in 24-h UOx excretion averaged during Weeks 1-4 Randomization Reloxaliase (7,500 u) or placebo 2 cap with meal/snack 3 to 5 times per day x 28 days UOx>50mg/24h Normal to Stage 3 CKD (eGFR ≥30) 1:1 n≈124 2X 24h 1X 24h 2X 24h 2X 24h 2X 24h 2X 24h Screening Study Aim: Determine the Safety and Efficacy of Reloxaliase in Reducing UOx in Subjects with Enteric Hyperoxaluria Follow up 4 weeks Reloxaliase Placebo
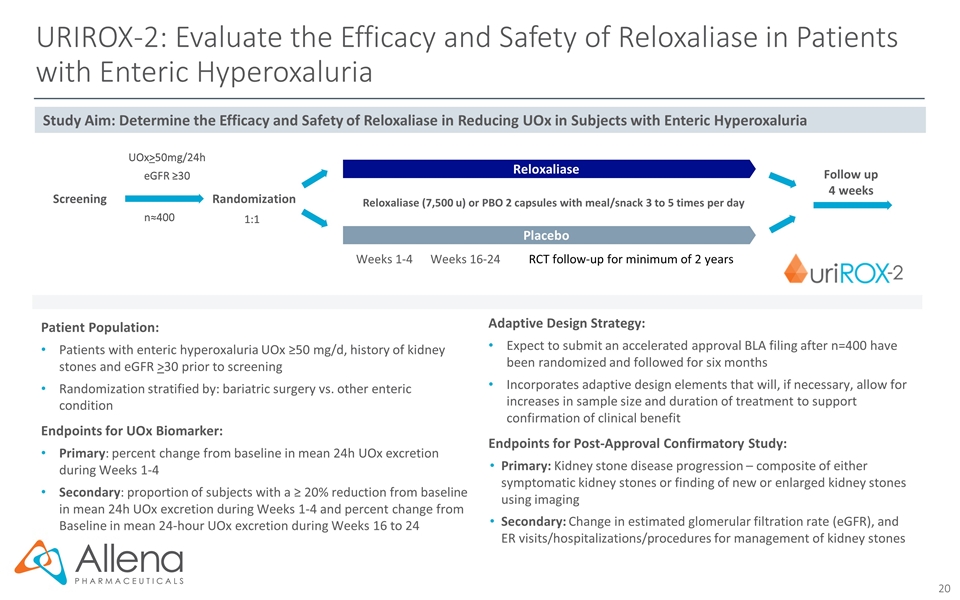
Randomization UOx>50mg/24h eGFR ≥30 1:1 n≈400 RCT follow-up for minimum of 2 years Patient Population: Patients with enteric hyperoxaluria UOx ≥50 mg/d, history of kidney stones and eGFR >30 prior to screening Randomization stratified by: bariatric surgery vs. other enteric condition Endpoints for UOx Biomarker: Primary: percent change from baseline in mean 24h UOx excretion during Weeks 1-4 Secondary: proportion of subjects with a ≥ 20% reduction from baseline in mean 24h UOx excretion during Weeks 1-4 and percent change from Baseline in mean 24-hour UOx excretion during Weeks 16 to 24 Adaptive Design Strategy: Expect to submit an accelerated approval BLA filing after n=400 have been randomized and followed for six months Incorporates adaptive design elements that will, if necessary, allow for increases in sample size and duration of treatment to support confirmation of clinical benefit Endpoints for Post-Approval Confirmatory Study: Primary: Kidney stone disease progression – composite of either symptomatic kidney stones or finding of new or enlarged kidney stones using imaging Secondary: Change in estimated glomerular filtration rate (eGFR), and ER visits/hospitalizations/procedures for management of kidney stones Reloxaliase Placebo Reloxaliase (7,500 u) or PBO 2 capsules with meal/snack 3 to 5 times per day Study Aim: Determine the Efficacy and Safety of Reloxaliase in Reducing UOx in Subjects with Enteric Hyperoxaluria Weeks 1-4 Weeks 16-24 URIROX-2: Evaluate the Efficacy and Safety of Reloxaliase in Patients with Enteric Hyperoxaluria Follow up 4 weeks Screening

Reloxaliase Additional Indications
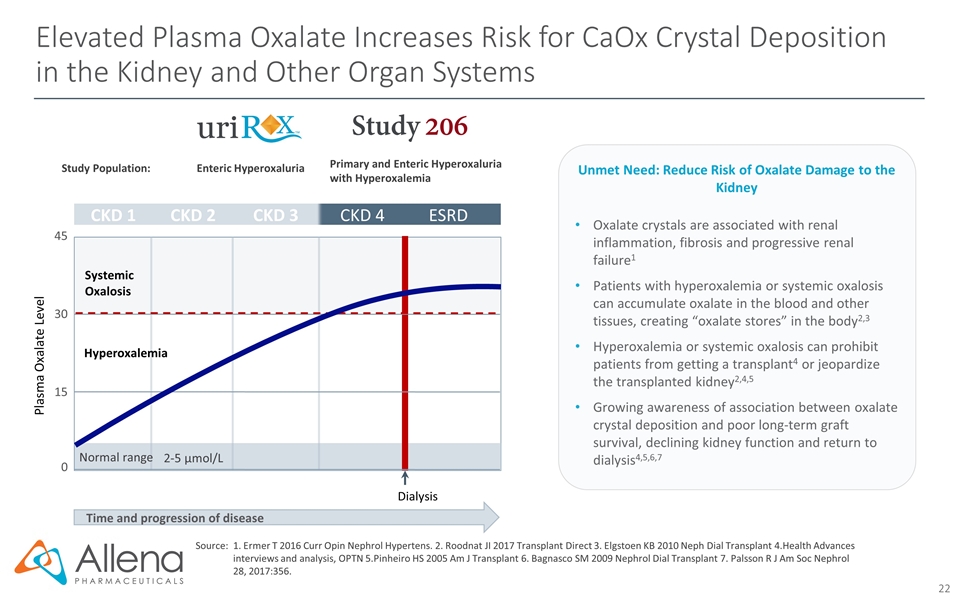
Elevated Plasma Oxalate Increases Risk for CaOx Crystal Deposition in the Kidney and Other Organ Systems Time and progression of disease Plasma Oxalate Level CKD 1 CKD 2 CKD 3 CKD 4 ESRD 0 15 30 45 2-5 µmol/L Normal range Systemic Oxalosis Dialysis Hyperoxalemia Study Population: Enteric Hyperoxaluria Primary and Enteric Hyperoxaluria with Hyperoxalemia Source:1. Ermer T 2016 Curr Opin Nephrol Hypertens. 2. Roodnat JI 2017 Transplant Direct 3. Elgstoen KB 2010 Neph Dial Transplant 4.Health Advances interviews and analysis, OPTN 5.Pinheiro HS 2005 Am J Transplant 6. Bagnasco SM 2009 Nephrol Dial Transplant 7. Palsson R J Am Soc Nephrol 28, 2017:356. Oxalate crystals are associated with renal inflammation, fibrosis and progressive renal failure1 Patients with hyperoxalemia or systemic oxalosis can accumulate oxalate in the blood and other tissues, creating “oxalate stores” in the body2,3 Hyperoxalemia or systemic oxalosis can prohibit patients from getting a transplant4 or jeopardize the transplanted kidney2,4,5 Growing awareness of association between oxalate crystal deposition and poor long-term graft survival, declining kidney function and return to dialysis4,5,6,7 Unmet Need: Reduce Risk of Oxalate Damage to the Kidney
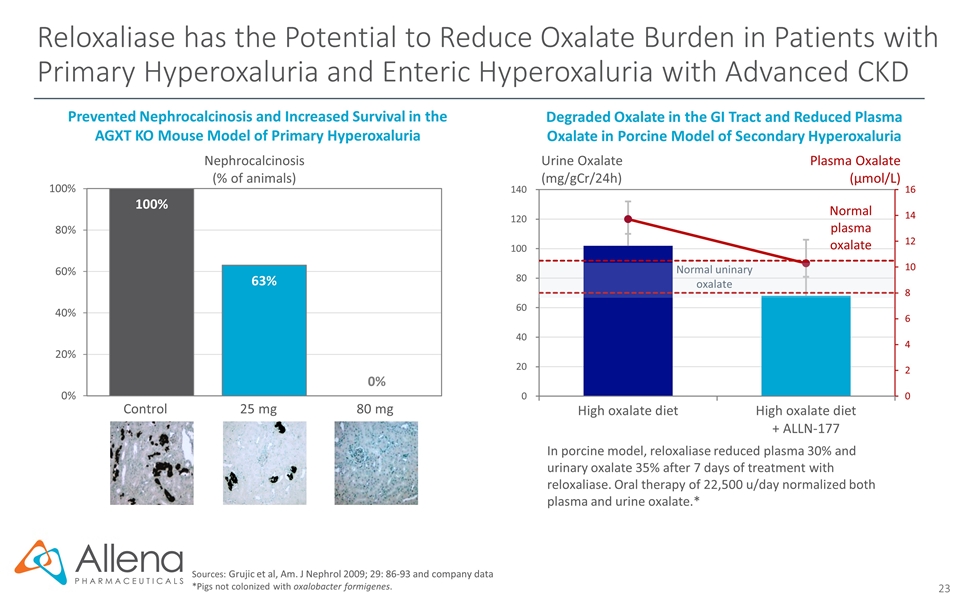
Reloxaliase has the Potential to Reduce Oxalate Burden in Patients with Primary Hyperoxaluria and Enteric Hyperoxaluria with Advanced CKD Sources: Grujic et al, Am. J Nephrol 2009; 29: 86-93 and company data *Pigs not colonized with oxalobacter formigenes. Degraded Oxalate in the GI Tract and Reduced Plasma Oxalate in Porcine Model of Secondary Hyperoxaluria In porcine model, reloxaliase reduced plasma 30% and urinary oxalate 35% after 7 days of treatment with reloxaliase. Oral therapy of 22,500 u/day normalized both plasma and urine oxalate.* Urine Oxalate (mg/gCr/24h) Plasma Oxalate (µmol/L) Nephrocalcinosis (% of animals) Prevented Nephrocalcinosis and Increased Survival in the AGXT KO Mouse Model of Primary Hyperoxaluria Control 25 mg 80 mg
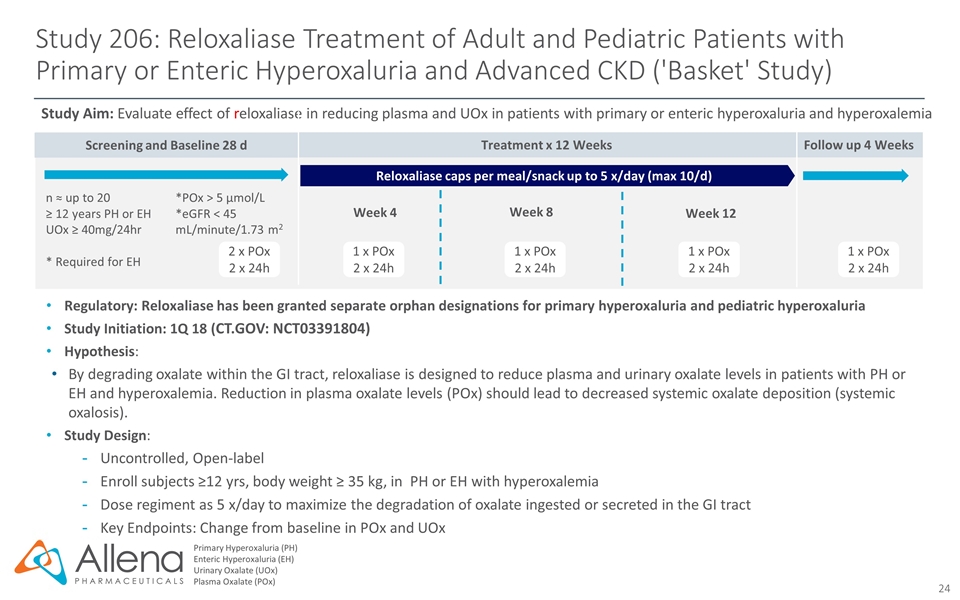
Screening and Baseline 28 d Follow up 4 Weeks Study 206: Reloxaliase Treatment of Adult and Pediatric Patients with Primary or Enteric Hyperoxaluria and Advanced CKD ('Basket' Study) Primary Hyperoxaluria (PH) Enteric Hyperoxaluria (EH) Urinary Oxalate (UOx) Plasma Oxalate (POx) Regulatory: Reloxaliase has been granted separate orphan designations for primary hyperoxaluria and pediatric hyperoxaluria Study Initiation: 1Q 18 (CT.GOV: NCT03391804) Hypothesis: By degrading oxalate within the GI tract, reloxaliase is designed to reduce plasma and urinary oxalate levels in patients with PH or EH and hyperoxalemia. Reduction in plasma oxalate levels (POx) should lead to decreased systemic oxalate deposition (systemic oxalosis). Study Design: Uncontrolled, Open-label Enroll subjects ≥12 yrs, body weight ≥ 35 kg, in PH or EH with hyperoxalemia Dose regiment as 5 x/day to maximize the degradation of oxalate ingested or secreted in the GI tract Key Endpoints: Change from baseline in POx and UOx Study Aim: Evaluate effect of reloxaliase in reducing plasma and UOx in patients with primary or enteric hyperoxaluria and hyperoxalemia 1 x POx 2 x 24h n ≈ up to 20 ≥ 12 years PH or EH UOx ≥ 40mg/24hr * Required for EH *POx > 5 µmol/L *eGFR < 45 mL/minute/1.73 m2 2 x POx 2 x 24h 1 x POx 2 x 24h 1 x POx 2 x 24h 1 x POx 2 x 24h Reloxaliase caps per meal/snack up to 5 x/day (max 10/d) Treatment x 12 Weeks Week 8 Week 12 Week 4
ALLN-346: Significant Opportunity in Gout Patients with Moderate-to-Severe CKD The Gout Market is Incompletely Served by Existing Therapies ~375,000 gout patients with moderate to severe CKD who have uncontrolled gout on urate lowering therapy (ULT)* Gout patients with renal impairment are not optimally managed due to limitations of existing therapies Gout patients with kidney and liver problems are contraindicated for allopurinol, Uloric, and Zurampic Current ULT’s may interact with other medications Co-morbidities (e.g. cardiovascular) may also limit ULT options Significant unmet need for safe and effective therapy that can be used in patients with renal impairment Sources: . *Lim JJ, Fu AC, and Reasner D. Prevalence of CKD and Uncontrolled Gout Among US Adults: Results from NHANES 2007-2012. Poster presented at: The National Kidney Foundation Spring Clinical Meetings; April 18-22, 2017; Orlando Florida. Fletcher Spaght Analysis July 2016; Image: Retailleau, P., Colloc'h, N., Vivares, D., Bonnete, F., Castro, B., El Hajji, M., Prange, T. (2005) Urate oxidase from Aspergillus flavus: new crystal-packing contacts in relation to the content of the active site. Acta Crystallogr.,Sect.D, 61, 218-229; D. Grujic Urol Res 2008, 193. ALLN-346 Therapeutic Strategy: Novel urate degrading enzyme optimized for stability in the GI tract MOA: orally administered, gut restricted enzyme therapeutic Animal POC: demonstrated a robust reduction in urine and plasma uric acid levels in a severe animal model of hyperuricemia with advanced CKD Data presented at American College of Rheumatology meeting October 22, 2018
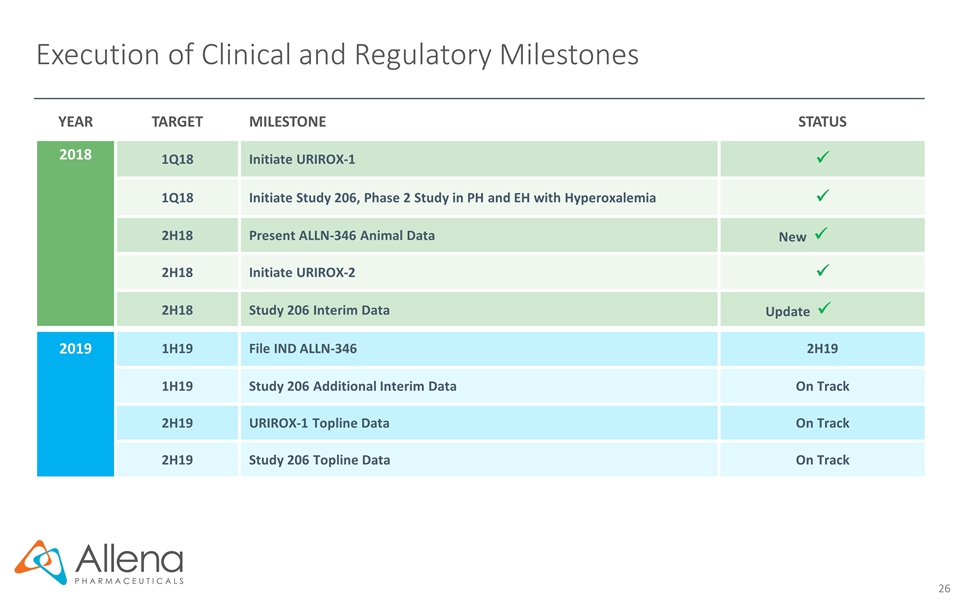
Execution of Clinical and Regulatory Milestones YEAR TARGET MILESTONE STATUS 2018 1Q18 Initiate URIROX-1 ü 1Q18 Initiate Study 206, Phase 2 Study in PH and EH with Hyperoxalemia ü 2H18 Present ALLN-346 Animal Data New ü 2H18 Initiate URIROX-2 ü 2H18 Study 206 Interim Data Update ü 2019 1H19 File IND ALLN-346 2H19 1H19 Study 206 Additional Interim Data On Track 2H19 URIROX-1 Topline Data On Track 2H19 Study 206 Topline Data On Track
Highlights – Pioneering Oral Enzyme Therapeutic Platform Significant Unmet Need in Oxalate and Urate Disorders Focused on rare and severe metabolic disorders that can cause kidney stones, damage the kidney, and potentially lead to CKD and ESRD No approved oxalate therapies; potential untapped multi-billion dollar market First-in-class, oral therapy for severe hyperoxaluria Achieved FDA alignment on pivotal Phase 3 program and accelerated approval pathway Enrolling two Phase 3 trials in enteric hyperoxaluria; URIROX-1 topline data expected 2H19 Enrolling Phase 2 basket study (Study 206) in orphan populations Proprietary technological approach designed to enable treatment of metabolic diseases with oral, non-absorbed enzyme therapeutics GI MOA reduces subsequent metabolic burden on the kidney First-in-class, oral therapy designed for gout patients with moderate-to-severe CKD; designed to degrade urate in the GI tract, reducing urate burden on kidney Gout patients with renal impairment are not optimally managed with existing therapies IND submission targeted in 2H19 Late-Stage Development Candidate: Reloxaliase Pioneering Expertise in Oral Enzyme Therapeutics Second Product Candidate: ALLN-346
