Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AERIE PHARMACEUTICALS INC | d680132dex991.htm |
| 8-K - FORM 8-K - AERIE PHARMACEUTICALS INC | d680132d8k.htm |

AR-13324-CS205 Clinical Pilot Study Topline Results Exhibit 99.2
Important Information The information in this presentation is current only as of its date and may have changed or may change in the future. We undertake no obligation to update this information in light of new information, future events or otherwise. We are not making any representation or warranty that the information in this presentation is accurate or complete. Certain statements in this presentation are “forward-looking statements” within the meaning of the federal securities laws. Words such as “may,” “will,” “should,” “would,” “could,” “believe,” “expects,” “anticipates,” “plans,” “intends,” “estimates,” “targets,” “projects,” “potential” or similar expressions are intended to identify these forward-looking statements. These statements are based on the Company’s current plans and expectations. Known and unknown risks, uncertainties and other factors could cause actual results to differ materially from those contemplated by the statements. In evaluating these statements, you should specifically consider various factors that may cause our actual results to differ materially from any forward-looking statements. These risks and uncertainties are described more fully in the quarterly and annual reports that we file with the SEC, particularly in the sections titled “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations.” In particular, the topline AR-13324-CS205 data presented herein is preliminary and based solely on information available to us as of the date of this presentation and additional information about the results may be disclosed at any time. Such forward-looking statements only speak as of the date they are made. We undertake no obligation to publicly update or revise any forward-looking statements, whether because of new information, future events or otherwise, except as otherwise required by law. For Investor Use
Executive Summary Netarsudil 0.02% and 0.04% were safe and generally well tolerated in Japanese-American subjects Netarsudil 0.02% and 0.04% were efficacious in lowering IOP in Japanese-American subjects Both concentrations appear to have similar efficacy, but need larger sample size before drawing conclusions Netarsudil IOP lowering in Japanese-American subjects similar to non-Japanese data from Phase 1 (low baseline IOP) and Phase 3 studies Netarsudil efficacy in this study (mean baseline IOPs 18-20 mmHg) predicts strong efficacy in low tension glaucoma For Investor Use ++Data on File
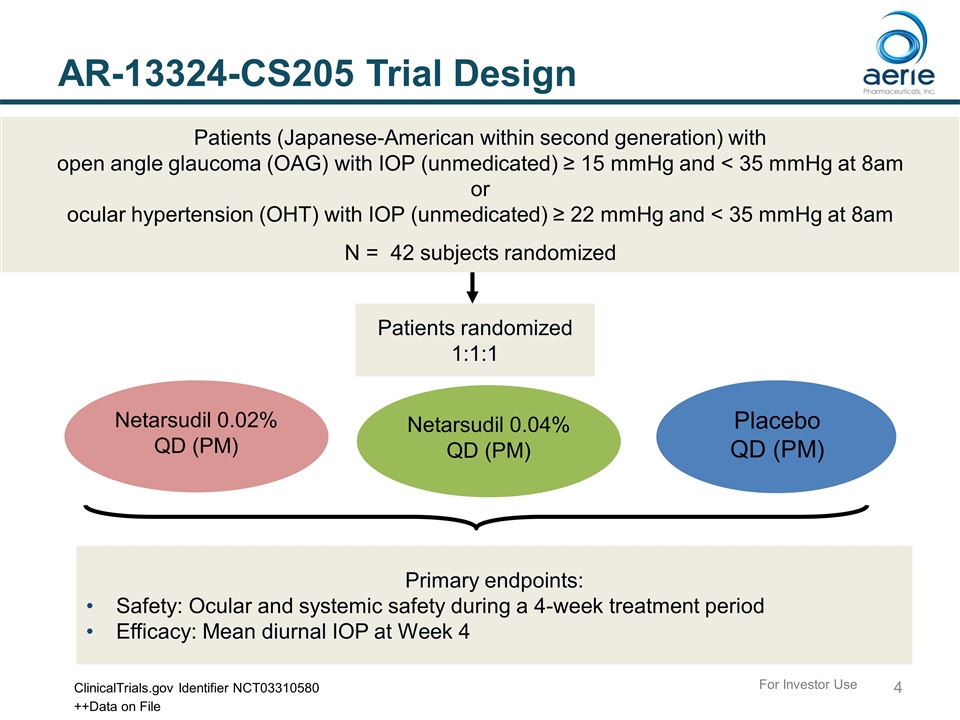
AR-13324-CS205 Trial Design Patients randomized 1:1:1 Primary endpoints: Safety: Ocular and systemic safety during a 4-week treatment period Efficacy: Mean diurnal IOP at Week 4 Patients (Japanese-American within second generation) with open angle glaucoma (OAG) with IOP (unmedicated) ≥ 15 mmHg and < 35 mmHg at 8am or ocular hypertension (OHT) with IOP (unmedicated) ≥ 22 mmHg and < 35 mmHg at 8am N = 42 subjects randomized Netarsudil 0.02% QD (PM) Placebo QD (PM) ClinicalTrials.gov Identifier NCT03310580 Netarsudil 0.04% QD (PM) For Investor Use ++Data on File
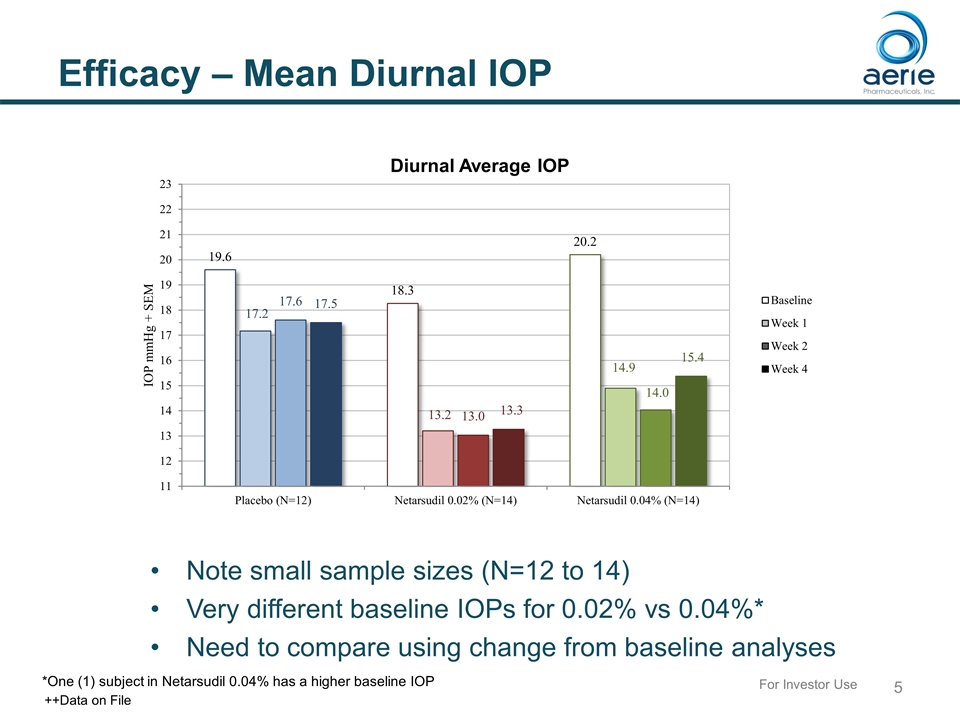
Efficacy – Mean Diurnal IOP Note small sample sizes (N=12 to 14) Very different baseline IOPs for 0.02% vs 0.04%* Need to compare using change from baseline analyses *One (1) subject in Netarsudil 0.04% has a higher baseline IOP For Investor Use ++Data on File
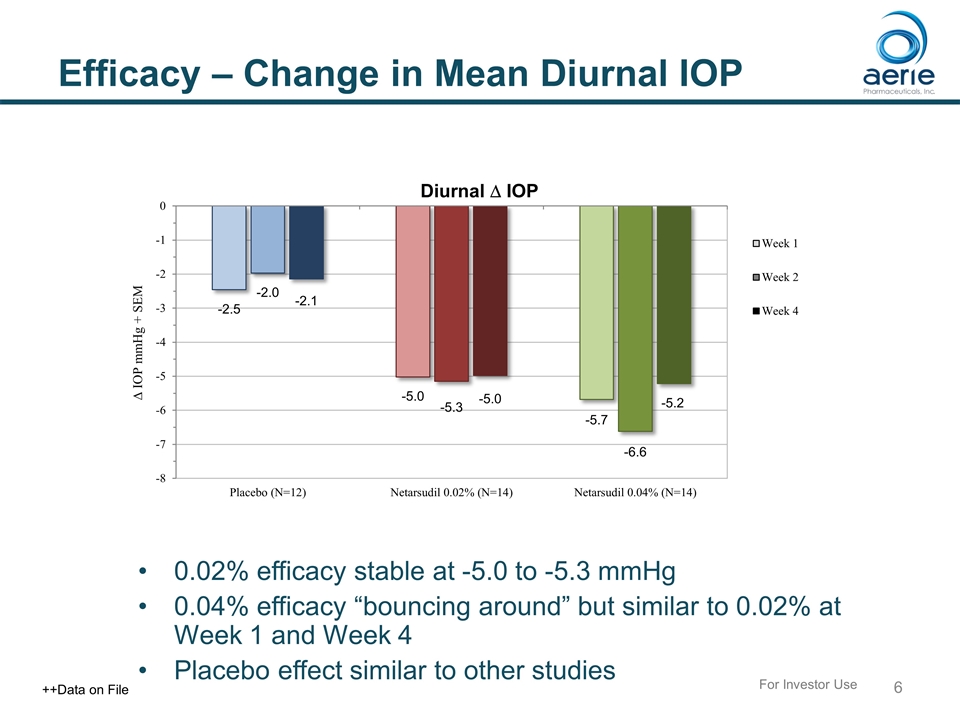
Efficacy – Change in Mean Diurnal IOP 0.02% efficacy stable at -5.0 to -5.3 mmHg 0.04% efficacy “bouncing around” but similar to 0.02% at Week 1 and Week 4 Placebo effect similar to other studies For Investor Use ++Data on File
Safety/Tolerability Overview of Netarsudil There were no serious adverse events (SAEs) The most common adverse event was conjunctival hyperemia and was scored as mild in the majority of the patients For Investor Use ++Data on File
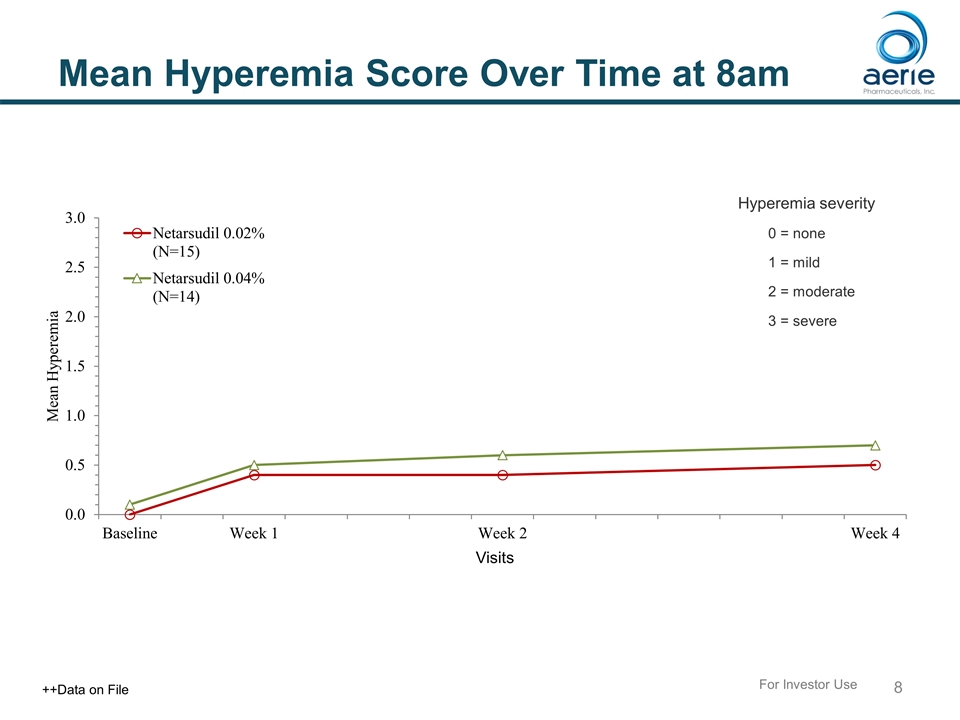
Mean Hyperemia Score Over Time at 8am For Investor Use ++Data on File
