Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - Sesen Bio, Inc. | sesenupdatedvisattrialda.htm |
| 8-K - 8-K - Sesen Bio, Inc. | sesn-form8xk1032018updated.htm |

Updated Phase 3 VISTA Trial Data in Patients with BCG-unresponsive Non-muscle Invasive Bladder Cancer January 3, 2019 NASDAQ SESN

FORWARD-LOOKING STATEMENTS This presentation contains forward-looking statements that involve substantial risks and our ability to successfully develop our product candidates and complete our planned uncertainties. All statements, other than statements of historical facts, contained in this clinical programs, our ability to obtain marketing approvals for our product candidates, presentation, including statements regarding our strategy, future operations, clinical expectations regarding our ongoing clinical trials, availability and timing of data from development of our protein therapies, future financial position, future revenues, clinical trials, the adequacy of any clinical models, expectations regarding regulatory projected costs, prospects, plans and objectives of management, are forward-looking approvals, our ability to obtain, maintain and protect our intellectual property for our statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” technology and products, other matters that could affect the financial performance of the “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and Company, other matters that could affect the availability or commercial potential of the similar expressions are intended to identify forward-looking statements within the Company’s product candidates and other factors discussed in the “Risk Factors” section meaning of the Private Securities Litigation Reform Act of 1995, although not all forward- of the Company’s Annual Report on Form 10-K, Quarterly Reports on Form 10-Q and looking statements contain these identifying words. other reports on file with the Securities and Exchange Commission (SEC). The forward- looking statements contained in this presentation are made as of the date hereof, and We may not actually achieve the plans, intentions or expectations disclosed in our Sesen Bio assumes no obligation to update any forward-looking statements whether as a forward-looking statements, and you should not place undue reliance on our forward- result of new information, future events, or otherwise except as required by applicable looking statements. Actual results or events could differ materially from the plans, law. intentions and expectations disclosed in the forward-looking statements we make as a result of various important factors, including: the uncertainties inherent in the initiation and conduct of clinical trials, the possibility that the available preliminary data of the Phase 3 VISTA Trial are not indicative of final data from all patients in the Phase 3 VISTA Trial and final data may not be positive with regard to the safety or efficacy of Vicinium®, 2

OUR MISSION IS TO SAVE AND RENEW THE LIVES OF PEOPLE WITH CANCER sesen: an ancient Egyptian word for the giant lotus flower that first rose out of the watery chaos at the beginning of time lotus: a symbol of purity, rebirth and renewal bio: from Greek bios meaning life 3

2018 PROGRESS SETS UP FOR TRANSFORMATIONAL 2019 Completed enrollment in VISTA Trial - Phase 3 registration trial of Vicinium for high-grade non-muscle invasive bladder cancer (NMIBC) Presented positive preliminary 3-month efficacy data at American Urological Association Annual Meeting Granted Fast Track Designation for Vicinium for treatment of high-grade NMIBC Initiated enrollment in NCI-sponsored combination study of Vicinium + durvalumab in NMIBC Established agreement with FujiFilm for Vicinium manufacturing Reported new preclinical data on early-stage deBouganin program supporting broad potential Enhanced leadership team Strengthened financial position1 1$57.9M in cash and cash equivalents at end of 3Q18; operating runway into 2020 4
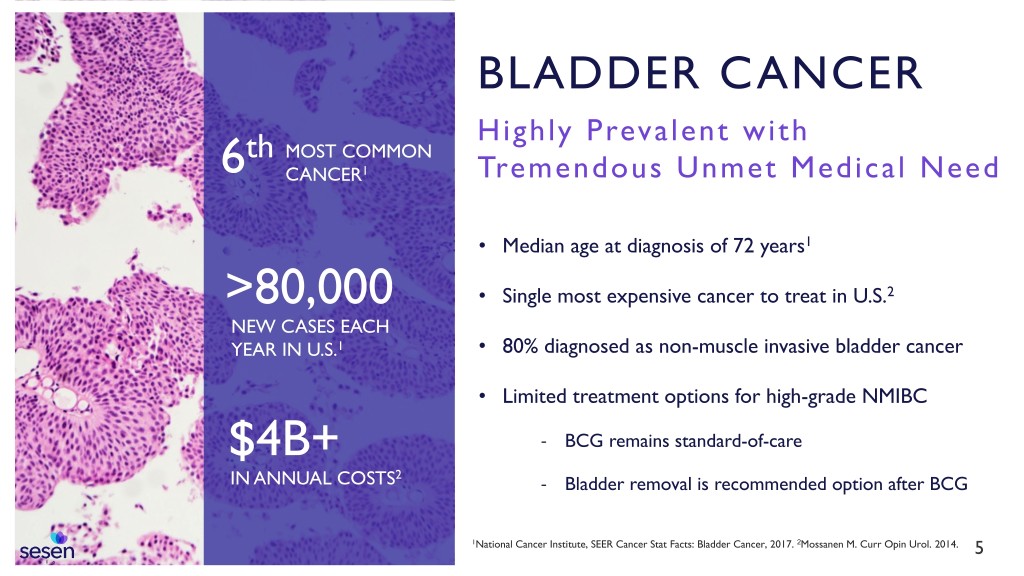
BLADDER CANCER Highly Prevalent with th MOST COMMON 6 CANCER1 Tremendous Unmet Medical Need • Median age at diagnosis of 72 years1 >80,000 • Single most expensive cancer to treat in U.S.2 NEW CASES EACH YEAR IN U.S.1 • 80% diagnosed as non-muscle invasive bladder cancer • Limited treatment options for high-grade NMIBC $4B+ - BCG remains standard-of-care 2 IN ANNUAL COSTS - Bladder removal is recommended option after BCG 1National Cancer Institute, SEER Cancer Stat Facts: Bladder Cancer, 2017. 2Mossanen M. Curr Opin Urol. 2014. 5

PROGRESSIVE NATURE OF BLADDER CANCER CIS • 60%-70% will recur1 • 20%-30% will progress to muscle invasive bladder cancer1 1Aldousari, S. Can Urol Assoc J. 2010 Feb. Image adapted from Knowles MA and CD Hurst, 2015. Nature Rev Cancer, 8 | Company Presentation 15: 25-41 6

BLOOD IN URINE See PCP; prescribed OUR PATH FORWARD STARTS misaligned antibiotics WITH THE PATIENT JOURNEY Still see urine in blood See blood in urine; try different antibiotic Confusion and concern on problem Begin tests Referred to urologist Shock and st CT Scan emotional Preparing for 1 TURBT struggle cancer treatment Cytology Bladder BCG BCG again Cystoscopy MRI Fear, anxiety Testing Fear cancer Testing is progressing Hope treatment DIAGNOSIS is working BCG TUMOR HAS RECURRED BCG again BCG has failed More testing What’s next? Urologist recommends Urologist visit 1Ronald de Wit, MD, PhD, group leader bladder removal of the Experimental Systematic Therapy of Urogenital Cancers program at Erasmus MC Cancer Institute, Rotterdam, The Netherlands, ESMO 2018 “Radical cystectomy is associated with significant morbidity and mortality and a negative impact on quality of life, and BLADDER LIFE WITH ? ? many patients actually refuse or are ineligible for cystectomy.”1 REMOVAL CANCER 7

VICINIUM HAS POTENTIAL TO PROVIDE VICINIUM CONTINUITY OF CARE FOR PATIENTS WITH NMIBC VICINIUM BCG TREATMENT TREATMENT 2-hour infusion • Antibody fragment tethered to a cytotoxic Administration through urinary catheter payload to form a single protein • Simple and cost-efficient manufacturing 2-hour “hold” for treatment • Inhibits protein synthesis Treated by urologist (same urologist) • Designed to be effective against multi-drug resistant tumors Medical support team throughout care (same team) • High potency relative to other available agents • Designed to kill both rapidly proliferating Response assessment every 3 months and slow-growing cells 8

VICINIUM Dual Mechanism of Action MECHANISM 2: 9

VISTA TRIAL: Phase 3 Trial to Support Approval of Vicinium for BCG-unresponsive NMIBC • Single-arm, open label, multi-center phase 3 trial of patients with BCG-unresponsive NMIBC - BCG-unresponsive defined as BCG-refractory or disease relapse after receiving adequate BCG (having completed 2+ courses (≥ 5 and ≥ 2 treatments resp.) of full dose BCG starting within 12 months of each other) • 3 cohorts by histology and time to recurrence after adequate BCG - Cohort 1: Carcinoma in situ with or without papillary tumors that recurred within 6 months of adequate BCG - Cohort 2: Carcinoma in situ with or without papillary tumors that recurred >6 months but ≤11 months of adequate BCG - Cohort 3: Papillary tumors (without Carcinoma in situ) that recurred within 6 months of adequate BCG • Dosing: intravesical instillation of 30 mg Vicinium in 50 ml buffered saline held for 2 hours - Induction: biweekly for 6 weeks → weekly for 6 weeks - Patients with complete response proceed to maintenance of every other week for 2 years total 10

VISTA TRIAL: Assessment of Response • PRIMARY ENDPOINT – Complete response rate and duration of response in patients with Carcinoma in situ enrolled in Cohort 1* • KEY SECONDARY ENDPOINTS – Event-free survival, time to disease recurrence, time to cystectomy, progression-free survival, overall survival,n=86 n=7 n=85 n=7 n=84 n=7 n=81 n=7 safety and tolerability Preliminary data as of December 3, 2018; Efficacy data from cohorts 1&2; Safety data from cohorts 1, 2 &3 *Complete response is defined as non-positive urinary cytology and either normal cystoscopy or abnormal cystoscopy with negative biopsy 11

VISTA Phase 3 Trial Design Aligns with FDA Final Guidance for NMIBC Treatment Development “In BCG- unresponsive NMIBC, a single- arm clinical trial with complete response rate and duration of response as the primary endpoint can provide primary evidence of effectiveness to support a marketing application.” “The goal of therapy in patients with BCG-unresponsive NMIBC is to avoid cystectomy.” BCG-unresponsive CIS defined as persistent or recurrent CIS with or without recurrent Ta/T1 disease within 12 months of completion of adequate BCG therapy BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment Guidance for Industry, February 2018 12

VISTA TRIAL: PATIENT DEMOGRAPHICS COHORT 1 & 2 COHORT 1 COHORT 2 COMBINED* CHARACTERISTICS CIS that recurred >6 months CIS that recurred within 6 CIS that recurred within but ≤11 months of adequate months of adequate BCG <11 months of adequate BCG BCG Total patients enrolled 86 7 93 Evaluable patients at 3-months 86 7 93 Evaluable patients at 6-months 85 7 92 Evaluable patients at 9-months 84 7 91 Evaluable patients at 12-months 81 7 88 Median age (years) 73 67 73 Males/Females 63/23 6/1 69/24 Median prior treatment for NMIBC BCG cycles 3 (range 1-14) Intravesical chemotherapy 0 (range 0-23) TURBT 3 (range 0-28) * FDA Guidance from February 2018 Preliminary data as of December 3, 2018; Efficacy data from cohorts 1&2; Safety data from cohorts 1, 2 &3 TURBT: transurethral resection of bladder tumor 13

Cohort 1 100% Cohort 2 90% VISTA TRIAL: 80% 70% Positive Complete 60% Response Rate in 50% 57% 57% Carcinoma in Situ 40% 43% Patients in 30% 37% Cohorts 1 and 2 20% 25% 18% 10% 14% 14% n=86 n=7 n=85 n=7 n=84 n=7 n=81 n=7 0% 3-Month CRR 6-Month CRR 9-Month CRR 12-Month CRR Preliminary data as of December 3, 2018; Efficacy data from cohorts 1&2; Safety data from cohorts 1, 2 &3 14

Complete Response Rate in Pooled Cohorts 1 & 2 60% 50% VISTA TRIAL: 40% Positive Efficacy Data 39% in BCG-unresponsive 30% Carcinoma in situ 20% 27% Patients within 12 20% months of Last BCG 10% 14% Treatment* 0% 3-Month CRR 6-Month CRR 9-Month CRR 12-Month CRR • Complete 12-month CRR to be between (n=93) (n=92) (n=91) (n=88) 13% - 18% once all patients are evaluable Preliminary data as of December 3, 2018; Efficacy data from cohorts 1&2; Safety data from cohorts 1, 2 &3 *FDA Guidance from February 2018 15

Complete Response Rate in Pooled Cohorts from 60% Phase 2 and Phase 3 Clinical Trials 50% Phase 2 Vicinium Trial (n = 45)1 VICINIUM Phase 3 VISTA Trial (n = 93)2 40% EFFICACY: 40% 39% CRR Data in 30% Phase 2 and 27% 27% 20% 20% Ongoing Phase 3 18% 16% Clinical Trials 10% 14% are Consistent 0% 3-Month CRR 6-Month CRR 9-Month CRR 12-Month CRR 1Kowalski M The Journal of Urology 2012; Phase 2 cohort 1 dose: 6 weekly induction doses, 6 weeks off, 3 maintenance doses every 3 months for 9 months; cohort 2 dose: 12 weekly induction doses, 3 maintenance doses every 3 months for 9 months 2Preliminary data as of December 3, 2018; Efficacy data from cohorts 1&2; Safety data from cohorts 1, 2 &3 16 VISTA Trial dosing: biweekly induction doses for 6 weeks followed by weekly dosing for 6 weeks; if a CR achieved, proceed to maintenance of every other week dosing for 2 years total

VISTA TRIAL: Extended Durations of Response Cohort 1 Cohort 2 Patients still on treatment Preliminary data as of December 3, 2018; Efficacy data from cohorts 1&2; Safety data from cohorts 1, 2 &3 17

Patients (n=133) with: Treatment-Related All TEAEs TEAEs Treatment-Emergent Adverse Event1 All Grades Grade ≥3 All Grades Grade ≥3 VISTA TRIAL: Any TEAE 116 (87%) 29 (22%) 64 (48%) 5 (4%) Vicinium Treatment Urinary tract infection 41 (31%) 5 (4%) 15 (11%) 2 (2%) Results in Low Rate Dysuria 33 (25%) 0 (0%) 17 (13%) 0 (0%) of Discontinuations Hematuria 31 (23%) 2 (2%) 16 (12%) 0 (0%) Pollakiuria (frequency of 21 (16%) 0 (0%) 14 (11%) 0 (0%) • Majority of adverse events (78%) considered urination) mild/moderate Fatigue 17 (13%) 0 (0%) 10 (8%) 0 (0%) • Only 4% (n=5) patients discontinued trial Diarrhea 16 (12%) 0 (0%) 3 (2%) 0 (0%) treatment due to adverse event Micturition urgency 16 (12%) 0 (0%) 12 (9%) 0 (0%) • Adverse events observed consistent with patient population and use of catheterization Nausea 13 (10%) 1 (<1%) 3 (2%) 0 (0%) Preliminary data as of December 3, 2018; Efficacy data from cohorts 1&2; Safety data from cohorts 1, 2 &3 1 Includes named TEAE occurring in more than 10% of patients regardless of treatment relationship 18

Treatment- Treatment- Patients (n=133) VISTA TRIAL: Emergent SAEs1 Related SAEs Vicinium Continues Any Serious AE 19 (14%) 3 (2%) Acute kidney injury to be Generally 4 (3%) 2 (2%) or renal failure Well-tolerated Small intestinal 3 (2%) 0 (0%) obstruction • 4 treatment-related serious adverse events reported in 3 patients Hematuria 2 (2%) 0 (0%) - 1 patient had grade 4 cholestatic hepatitis and Urinary Tract grade 5 renal failure 2 (2%) 0 (0%) infection - 1 patient had grade 3 acute kidney injury - 1 patient had grade 2 pyrexia Preliminary data as of December 3, 2018; Efficacy data from cohorts 1&2; Safety data from cohorts 1, 2 &3 1 SAEs reported in 2 or more patients 19
PRELIMINARY VISTA TRIAL DATA SUPPORT VICINIUM TREATMENT POTENTIAL FOR PATIENTS WITH CARCINOMA IN SITU • Encouraging preliminary efficacy data support planned Vicinium BLA submission • Prolonged duration of response observed in many patients • Favorable safety and tolerability with low rate of discontinuations due to AEs • Phase 3 preliminary data in-line with Phase 1 & 2 clinical trials • Convenient treatment administration, consistent with BCG • Opportunity to fulfill unmet medical need for BCG-unresponsive NMIBC patients; otherwise all patients recommended for bladder removal after BCG Preliminary data as of December 3, 2018; Efficacy data from cohorts 1&2; Safety data from cohorts 1, 2 &3 20

STRATEGIC PRIORITIES FOR A SUCCESSUL SESEN BIO FUTURE Bring Vicinium, a targeted fusion protein, to market for Carcinoma in situ patients with NMIBC Expand Vicinium utility as a combination treatment with immuno-oncology agents Explore partnerships to expand Vicinium into additional indications and geographies Leverage proprietary research capabilities to advance new fusion proteins for cancers 21

THANK YOU TO THE PATIENTS, FAMILIIES, CAREGIVERS, PHYSICIANS, NURSES AND MEDICAL STAFFS WHO SUPPORT AND HAVE PARTICIPATED IN VICINIUM CLINICAL TRIALS 22
