Attached files
Exhibit 99.2

Corporate Presentation January 2019 1

Cautionary Statement Regarding Forward - Looking Statements Certain statements contained in this presentation regarding matters that are not historical facts, are forward - looking statements within the meaning of Section 21 E of the Securities Exchange Act of 1934 , as amended, and the Private Securities Litigation Reform Act of 1995 , known as the PSLRA . These include statements regarding management’s intentions, plans, beliefs, expectations or forecasts for the future, and, therefore, you are cautioned not to place undue reliance on them . No forward - looking statement can be guaranteed, and actual results may differ materially from those projected . NeuBase Therapeutics Inc . (“ NeuBase ”) undertakes no obligation to publicly update any forward - looking statement, whether as a result of new information, future events or otherwise, except to the extent required by law . NeuBase uses words such as “anticipates,” “believes,” “plans,” “expects,” “projects,” “future,” “intends,” “may,” “will,” “should,” “could,” “estimates,” “predicts,” “potential,” “continue,” “guidance,” and similar expressions to identify these forward - looking statements that are intended to be covered by the safe - harbor provisions of the PSLRA . Such forward - looking statements are based on NeuBase’s expectations and involve risks and uncertainties ; consequently, actual results may differ materially from those expressed or implied in the statements due to a number of factors, including NeuBase’s plans to develop and commercialize its product candidates, including NT 0100 and NT 0200 ; the timing of initiation of NeuBase’s planned clinical trials ; the timing of the availability of data from NeuBase’s clinical trials ; the timing of any planned investigational new drug application or new drug application ; NeuBase’s plans to research, develop and commercialize its current and future product candidates ; the clinical utility, potential benefits and market acceptance of NeuBase’s product candidates ; NeuBase’s commercialization, marketing and manufacturing capabilities and strategy ; NeuBase’s ability to protect its intellectual property position ; and NeuBase’s estimates regarding future revenue, expenses, capital requirements and need for additional financing . New factors emerge from time to time and it is not possible for NeuBase to predict all such factors, nor can NeuBase assess the impact of each such factor on the business or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward - looking statements . Forward - looking statements included in this presentation are based on information available to NeuBase as of the date of this presentation . NeuBase disclaims any obligation to update such forward - looking statements to reflect events or circumstances after the date of this presentation, except as required by applicable law . This presentation does not constitute an offer to sell, or the solicitation of an offer to buy, any securities . 2
NeuBase Therapeutics • RNA therapeutics: pipeline of pre - clinical assets for gene silencing • DNA therapeutics: opportunity for genome editing, gene regulation and liquid biopsy • PATrOL ™ modular p eptide - nucleic acid (PNA) a n t isense ol igonucleotide platform • Advantages of small molecules with selectivity of antisense oligonucleotides (ASOs) • Strong IP composition of matter & field of use through 2037+ • Key advantages include rapid drug design, selectivity, blood brain barrier penetration, broad systemic distribution for multi - tissue disease and cell permeability • Central nervous system diseases, high unmet need, and orphan focus • Ongoing development for Huntington’s (NT0100) and Myotonic Dystrophy (NT0200), with billion dollar peak sales opportunity in each indication Advanced Peptide Nucleic Acids Addressing Genetic Disease Expansive market opportunity First - in - class technology Advantages over traditional antisense oligonucleotides Focus on initial indications 3

Team 4 COLLEEN CASSIDY , PhD BIOLOGY DIRECTOR SHIVAJI THADKE , PhD CHEMISTRY DIRECTOR ROBERT FRIEDLANDER , MD HUNTINGTON’S PRELCINICAL DEVELOPMENT TODD LORENZ , MD CHIEF MEDICAL OFFICER (ACTING) DIETRICH STEPHAN , PHD CHAIRMAN & CEO, FOUNDER SHAWN TITCOMB STRATEGIC ADVISOR

Merger with Ohr Pharmaceuticals • NeuBase Therapeutics (“ NeuBase ”) entered into a definitive merger agreement with Ohr Pharmaceutical, Inc. (“ Ohr ”) (Nasdaq: OHRP) on January 2 nd • The merger is expected to close in the first half of calendar 2019 and is subject to the approval of Ohr shareholders and other customary closing conditions • Upon closing, the combined company will be named NeuBase Therapeutics, Inc. • NeuBase will own ~80% of the combined company after the concurrent private placement and merger are finalized • The current executive team of NeuBase will serve as the executive team, and a new board of directors will be seated for the newly combined company 5
Significant Unmet Need • There are estimated to be 30 million people in the USA, 35 million in the EU, and 350 - 700 million people across the globe suffering from rare diseases, 95% of whom have no therapeutic option 1 • Many biopharmaceutical companies are working to address these disorders, but each rare disease is currently being tackled separately, with no cohesive approach • While gene silencing via antisense therapies and small molecules have the potential to tackle these genetic diseases, to date these technologies have been limited due to technical constraints 6 1 https://globalgenes.org/rare - diseases - facts - statistics/
Gene Silencing can Treat Dominant Genetic Disease • 80% of rare disease is genetic 1 • Recessive mutations generally cause loss of function of both copies of a gene • Dominant mutations occur on one copy of a gene and cause a gain or change - of - function of the resultant protein leading to disease • “Silencing” of the single mutant copy of the gene can treat dominant disease at its root 7 1 https://globalgenes.org/rare - diseases - facts - statistics/

How Antisense Technology Silences Disease - Causing Genes 8 Legend : There are proposed to be two mechanisms through which ASOs silence gene expression in the cytosol. ( A ) The first is binding of the ASO to its target mRNA is a sequence - specific manner and causing a steric hindrance which does not allow ribosomes to translate the RNA into a protein, thereby eliminating the propagation of the mutation into a protein and causing dysfunction. ( B ) A second mechanism is ASO binding followed by degradation of the transcript by RNAse H. https://www.sigmaaldrich.com/technical - documents/articles/biology/antisense - oligonucleotides.html
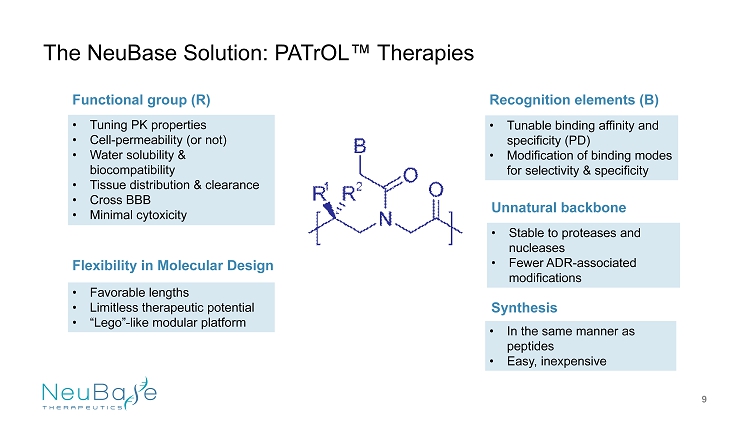
The NeuBase Solution: PATrOL ™ Therapies Recognition elements (B) • Tunable binding affinity and specificity (PD) • Modification of binding modes for selectivity & specificity Synthesis • In the same manner as peptides • Easy, inexpensive Unnatural backbone • Stable to proteases and nucleases • Fewer ADR - associated modifications • Favorable lengths • Limitless therapeutic potential • “Lego” - like modular platform Functional group (R) • Tuning PK properties • Cell - permeability (or not) • Water solubility & biocompatibility • Tissue distribution & clearance • Cross BBB • Minimal cytoxicity Flexibility in Molecular Design 9
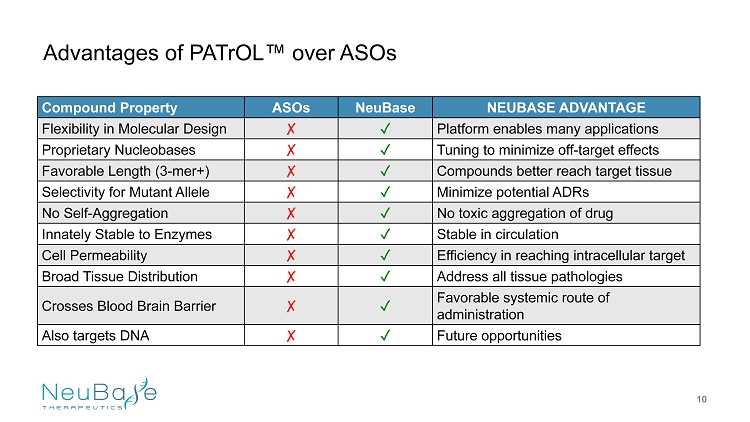
Compound Property ASOs NeuBase NEUBASE ADVANTAGE Flexibility in Molecular Design غ ض Platform enables many applications Proprietary Nucleobases غ ض Tuning to m inimize off - target effects Favorable Length (3 - mer+) غ ض Compounds better reach target tissue Selectivity for Mutant Allele غ ض Minimize potential ADRs No Self - Aggregation غ ض No toxic aggregation of drug Innately Stable to Enzymes غ ض Stable in circulation Cell Permeability غ ض Efficiency in reaching intracellular target Broad Tissue Distribution غ ض Address all tissue pathologies Crosses Blood Brain Barrier غ ض Favorable systemic route of administration Also t argets DNA غ ض Future opportunities Advantages of PATrOL ™ over ASOs 10
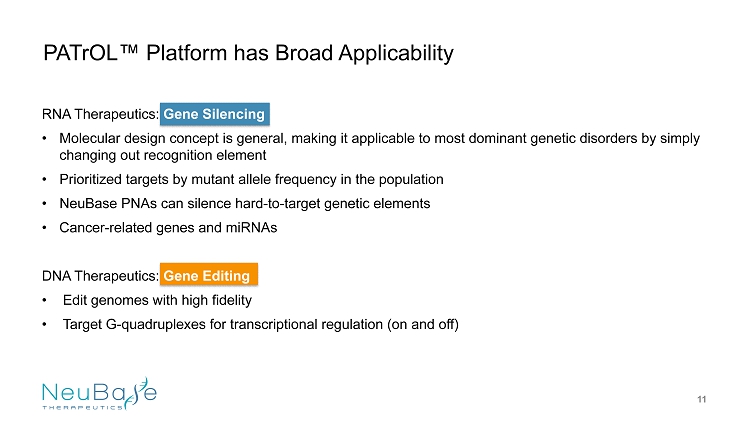
RNA Therapeutics: Gene Silencing • Molecular design concept is general, making it applicable to most dominant genetic disorders by simply changing out recognition element • Prioritized targets by mutant allele frequency in the population • NeuBase PNAs can silence hard - to - target genetic elements • Cancer - related genes and miRNAs DNA Therapeutics: Gene Editing • Edit genomes with high fidelity • Target G - quadruplexes for transcriptional regulation (on and off) PATrOL ™ Platform has Broad Applicability 11
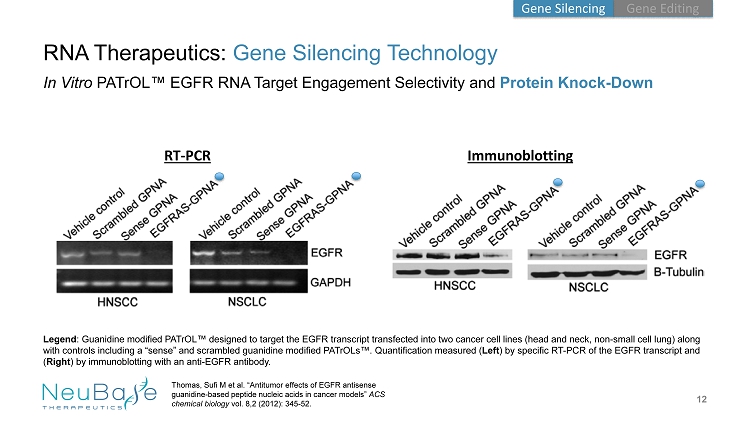
RNA Therapeutics: Gene Silencing Technology 12 Thomas, Sufi M et al. “Antitumor effects of EGFR antisense guanidine - based peptide nucleic acids in cancer models” ACS chemical biology vol. 8,2 (2012): 345 - 52. In Vitro PATrOL ™ EGFR RNA Target Engagement Selectivity and Protein Knock - Down RT - PCR Immunoblotting v v Legend : Guanidine modified PATrOL ™ designed to target the EGFR transcript transfected into two cancer cell lines (head and neck, non - small cell lung) along with controls including a “sense” and scrambled guanidine modified PATrOLs ™. Quantification measured ( Left ) by specific RT - PCR of the EGFR transcript and ( Right ) by immunoblotting with an anti - EGFR antibody. Gene Editing Gene Silencing

RNA Therapeutics: Gene Silencing Technology 13 In Vitro PATrOL ™ Dose Dependent RNA Target Engagement and Cell Senescence HNSCC NSCLC Thomas, Sufi M et al. “Antitumor effects of EGFR antisense guanidine - based peptide nucleic acids in cancer models” ACS chemical biology vol. 8,2 (2012): 345 - 52. Legend : Guanidine modified PATrOL ™ designed to target the EGFR transcript transfected into two cancer cell lines ( Left : head and neck, Right : non - small cell lung) along with controls including a scrambled guanidine modified PATrOLs ™ at increasing concentrations. Quantification measured ATP metabolism by luminescence. Gene Editing Gene Silencing
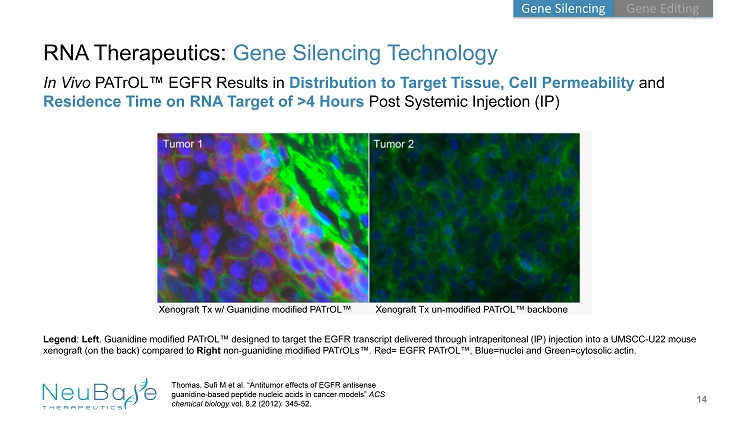
14 RNA Therapeutics: Gene Silencing Technology In Vivo PATrOL ™ EGFR Results in Distribution to Target Tissue, Cell Permeability and Residence Time on RNA Target of >4 Hours Post Systemic Injection (IP) Thomas, Sufi M et al. “Antitumor effects of EGFR antisense guanidine - based peptide nucleic acids in cancer models” ACS chemical biology vol. 8,2 (2012): 345 - 52. Xenograft Tx w/ Guanidine modified PATrOL ™ Xenograft Tx un - modified PATrOL ™ backbone Legend : Left . Guanidine modified PATrOL ™ designed to target the EGFR transcript delivered through intraperitoneal (IP) injection into a UMSCC - U22 mouse xenograft (on the back) compared to Right non - guanidine modified PATrOLs ™. Red= EGFR PATrOL ™, Blue=nuclei and Green=cytosolic actin. Gene Editing Gene Silencing
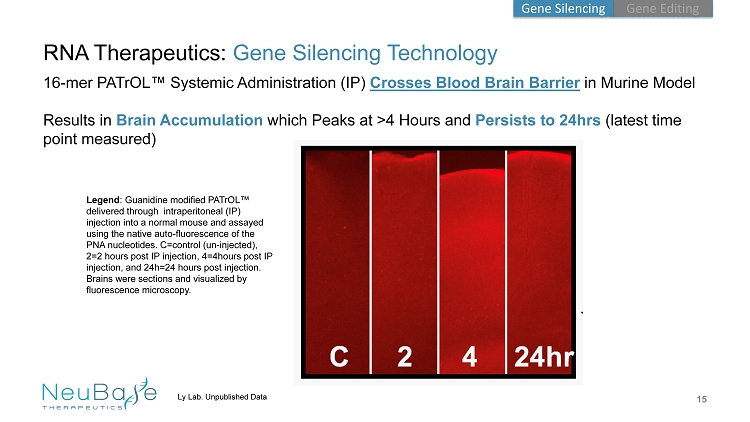
15 Ly Lab. Unpublished Data RNA Therapeutics: Gene Silencing Technology 16 - mer PATrOL ™ Systemic Administration (IP) Crosses Blood Brain Barrier in Murine Model Results in Brain Accumulation which Peaks at >4 Hours and Persists to 24hrs (latest time point measured) Legend : Guanidine modified PATrOL ™ delivered through intraperitoneal (IP) injection into a normal mouse and assayed using the native auto - fluorescence of the PNA nucleotides. C=control (un - injected), 2=2 hours post IP injection, 4=4hours post IP injection, and 24h=24 hours post injection. Brains were sections and visualized by fluorescence microscopy. Gene Editing Gene Silencing
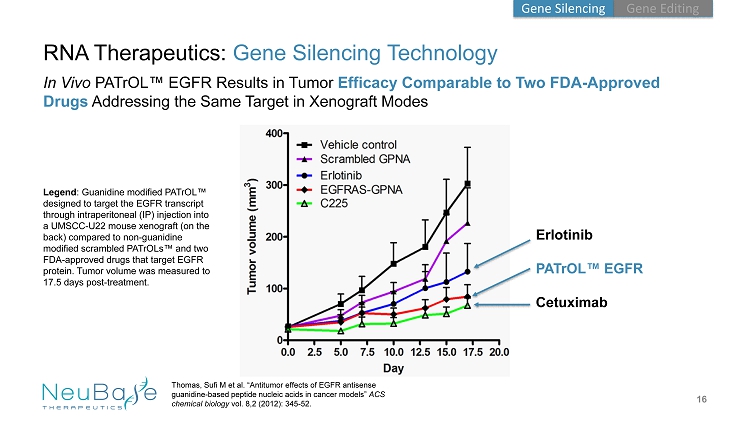
16 Erlotinib PATrOL ™ EGFR Cetuximab RNA Therapeutics: Gene Silencing Technology In Vivo PATrOL ™ EGFR Results in Tumor Efficacy Comparable to Two FDA - Approved Drugs Addressing the Same Target in Xenograft Modes Thomas, Sufi M et al. “Antitumor effects of EGFR antisense guanidine - based peptide nucleic acids in cancer models” ACS chemical biology vol. 8,2 (2012): 345 - 52. Legend : Guanidine modified PATrOL ™ designed to target the EGFR transcript through intraperitoneal (IP) injection into a UMSCC - U22 mouse xenograft (on the back) compared to non - guanidine modified scrambled PATrOLs ™ and two FDA - approved drugs that target EGFR protein. Tumor volume was measured to 17.5 days post - treatment. Gene Editing Gene Silencing
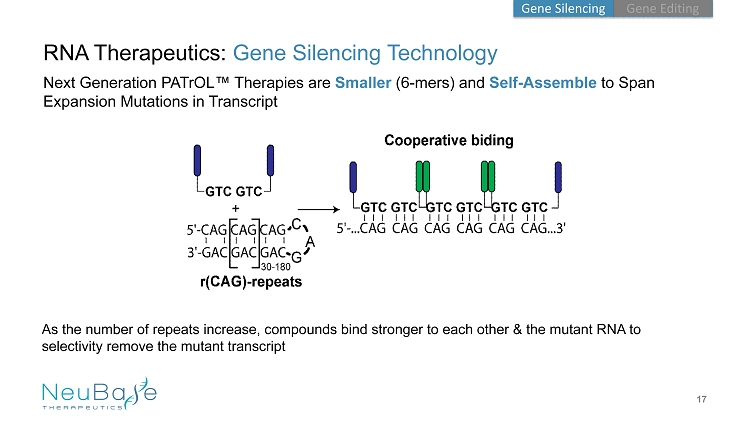
As the number of repeats increase, compounds bind stronger to each other & the mutant RNA to selectivity remove the mutant transcript 17 RNA Therapeutics: Gene Silencing Technology Next Generation PATrOL ™ Therapies are Smaller (6 - mers) and Self - Assemble to Span Expansion Mutations in Transcript Gene Editing Gene Silencing
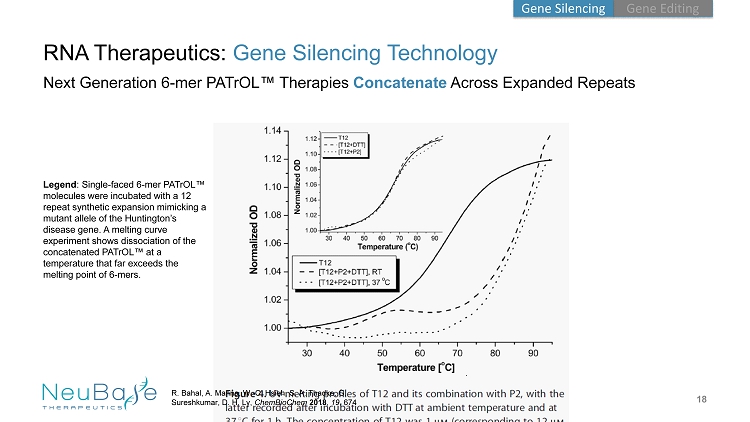
18 . RNA Therapeutics: Gene Silencing Technology Next Generation 6 - mer PATrOL ™ Therapies Concatenate Across Expanded Repeats R. Bahal , A. Manna, W. - C. Hsieh, S. A. Thadke, G. Sureshkumar , D. H. Ly, ChemBioChem 2018 , 19 , 674 Legend : Single - faced 6 - mer PATrOL ™ molecules were incubated with a 12 repeat synthetic expansion mimicking a mutant allele of the Huntington’s disease gene. A melting curve experiment shows dissociation of the concatenated PATrOL ™ at a temperature that far exceeds the melting point of 6 - mers. Gene Editing Gene Silencing

19 RNA Therapeutics: Gene Silencing Technology PATrOL ™ with proprietary Bifacial Janus Bases are 3 - mers have High Binding Energies and can Interpose Themselves in RNA hairpins under Normal Physiological Conditions Thadke, Shivaji A et al. “Design of Bivalent Nucleic Acid Ligands for Recognition of RNA - Repeated Expansion Associated with Huntington's Disease” Biochemistry vol. 57,14 (2018): 2094 - 2108. Legend : Left . Proprietary Bifacial Janus bases bind targets on both sides of the nuclear base and can intercalate into double - stranded RNA molecules (hairpins). Right . ( A ) is a 3 - mer Janus PNA with concatenation linkers on the 5’ and 3’ ends. ( B ) The molecular reaction that results in bifacial binding to the repeat expansion. These Janus PATrOLs ™ can also self - deactivate if they do not find their target. Gene Editing Gene Silencing
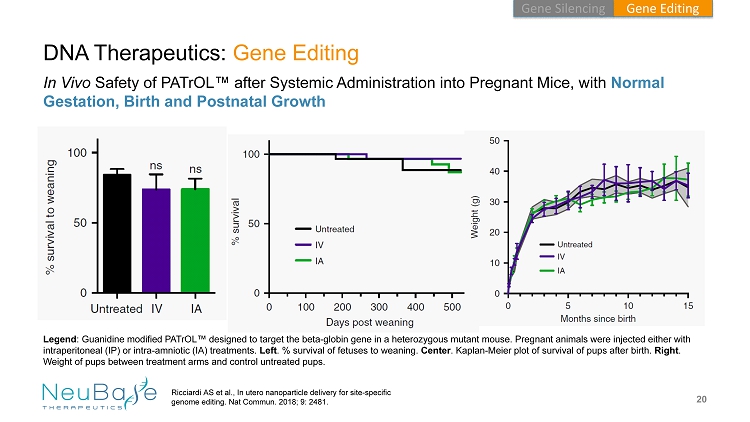
20 DNA Therapeutics: Gene Editing In Vivo Safety of PATrOL ™ after Systemic Administration into Pregnant Mice, with Normal Gestation, Birth and Postnatal Growth Ricciardi AS et al., In utero nanoparticle delivery for site - specific genome editing. Nat Commun . 2018; 9: 2481. Legend : Guanidine modified PATrOL ™ designed to target the beta - globin gene in a heterozygous mutant mouse. Pregnant animals were injected either with intraperitoneal (IP) or intra - amniotic (IA) treatments. Left . % survival of fetuses to weaning. Center . Kaplan - Meier plot of survival of pups after birth. Right . Weight of pups between treatment arms and control untreated pups. Gene Editing Gene Silencing

21 In Vivo Systemic Administration of PATrOL ™ Elicits No Immune Response Ricciardi AS et al., In utero nanoparticle delivery for site - specific genome editing. Nat Commun . 2018; 9: 2481. DNA Therapeutics: Gene Editing Legend : Guanidine modified PATrOL ™ designed to target the beta - globin gene in a heterozygous mutant mouse. Pregnant animals were injected intravenously (IV) with nano - particle encapsulated PNAs and controls. Gene Editing Gene Silencing
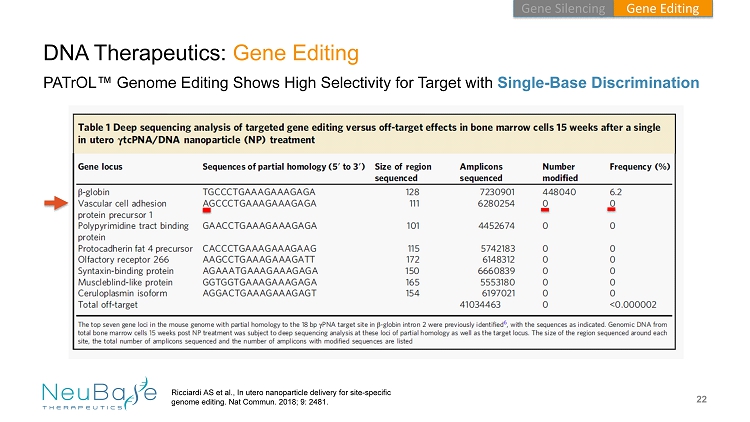
22 DNA Therapeutics: Gene Editing PATrOL ™ Genome Editing Shows High Selectivity for Target with Single - Base Discrimination Ricciardi AS et al., In utero nanoparticle delivery for site - specific genome editing. Nat Commun . 2018; 9: 2481. Gene Editing Gene Silencing

23 DNA Therapeutics: Gene Editing In Vivo PATrOL ™ Genome Editing Shows Functional Rescue of Thalassemia Model Ricciardi AS et al., In utero nanoparticle delivery for site - specific genome editing. Nat Commun . 2018; 9: 2481. P=0.0001 Legend : Guanidine modified PATrOL ™ designed to target the beta - globin gene in a heterozygous mutant mouse. Pregnant animals were injected IP with escalating doses of the gene editing PNA. Left . % reticulocytes edited back to wild - type increases with dose. Right . Gene corrected pups have 100% survival rates vs. non - corrected littermates. Gene Editing Gene Silencing
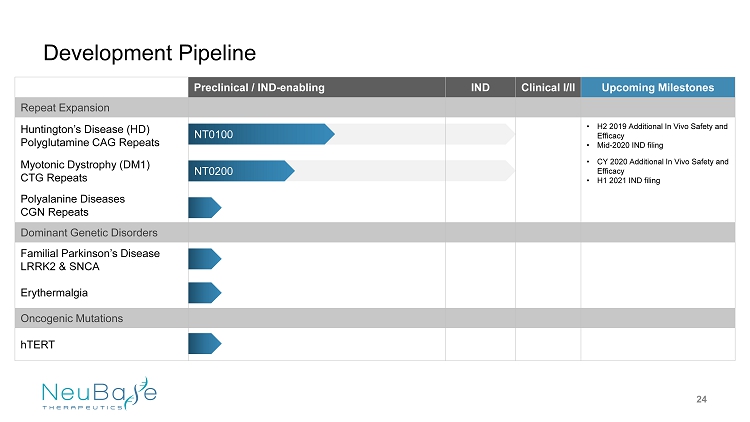
Preclinical / IND - enabling IND Clinical I/II Upcoming Milestones Repeat Expansion Huntington’s Disease (HD) Polyglutamine CAG Repeats • H2 2019 Additional In Vivo Safety and Efficacy • Mid - 2020 IND filing Myotonic Dystrophy (DM1) CTG Repeats • CY 2020 Additional In Vivo Safety and Efficacy • H1 2021 IND filing Polyalanine Diseases CGN Repeats Dominant Genetic Disorders Familial Parkinson’s Disease LRRK2 & SNCA Erythermalgia Oncogenic Mutations hTERT Development Pipeline 24 NT0100 NT0200
Lead Program NT0100: RNA Silencing in Huntington’s Disease 25 Huntington’s Disease (HD) affects 5.7 in 100,000 individuals in North America and Europe 1 and is characterized by mutant huntingtin protein aggregation and progressive loss of neurons in the striatum and cortex. Symptoms are mild at the beginning but worsen over time, ultimately leaving an individual fully dependent on others, with death usually occurring in the fifth decade of life. • Uncontrolled movements (chorea), slurred speech, difficulty swallowing • Cognitive impairments, forgetfulness and impaired judgement • Emotional disturbances, behavioral changes, personality changes, depression Pringsheim T, et al. The incidence and prevalence of Huntington's disease: a systematic review and meta - analysis. Mov Disord . 2012 Aug;27(9):1083 - 91.

Huntington Disease Development Program – NT0100 26 Prevailing Mechanisms of HD Pathology Caused by Formation of Hairpin Loops in the mHTT PATrOL ™ NT0100 Intercalates and Disrupts RNA Hairpins
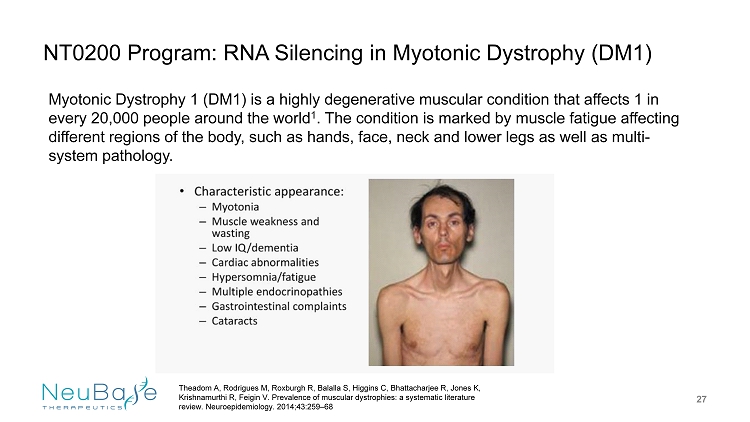
NT0200 Program: RNA Silencing in Myotonic Dystrophy (DM1) 27 Myotonic Dystrophy 1 (DM1) is a highly degenerative muscular condition that affects 1 in every 20,000 people around the world 1 . The condition is marked by muscle fatigue affecting different regions of the body, such as hands, face, neck and lower legs as well as multi - system pathology. Theadom A, Rodrigues M, Roxburgh R, Balalla S, Higgins C, Bhattacharjee R, Jones K, Krishnamurthi R, Feigin V. Prevalence of muscular dystrophies: a systematic literature review. Neuroepidemiology . 2014;43:259 – 68
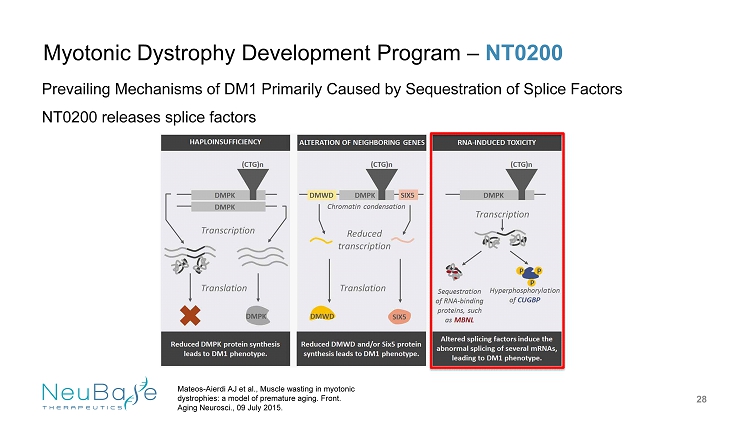
Myotonic Dystrophy Development Program – NT0200 28 Prevailing Mechanisms of DM1 Primarily Caused by Sequestration of Splice Factors NT0200 releases splice factors Mateos - Aierdi AJ et al., Muscle wasting in myotonic dystrophies: a model of premature aging. Front. Aging Neurosci ., 09 July 2015.

Mirkin . 2007. Nature Jun21;447(7147):932 - 40. PATrOL ™ Molecular Design Concept Applicable to Other Repeat Expansions by Changing out Recognition Elements HD is the first indication, and NT - 0100 could potentially be applicable in all 8 CAG (poly - glutamine) repeat diseases; DM1 drug NT0200 could potentially be applicable in two additional indications Potential Uses of NT0100 and NT0200 29

Spinraza (Biogen) is an antisense oligonucleotide for Spinal Muscular Atrophy that was approved in late 2016 • Set to be one of the best launches for a rare - disease drug ever • Developed by Ionis Pharmaceuticals • Limitations to the technology result in unfavorable intrathecal (spinal cord) route of administration NeuBase’s two initial indications have larger global prevalence numbers than SMA (HD=5.7/100,000 and DM1=5/100,000 vs. SMA=1 - 2/100,000) 2 . 1 http://www.loncarblog.com/spinraza - sales ; 2 Verhaart , Ingrid E C et al. “Prevalence, incidence and carrier frequency of 5q - linked spinal muscular atrophy - a literature review” Orphanet journal of rare diseases vol. 12,1 124. 4 Jul. 2017, doi:10.1186/s13023 - 017 - 0671 - 8 Antisense Drugs are Poised to be the next Pharmaceutical Blockbusters Spinraza Quarterly Sales 1 Example of Market Opportunity 30
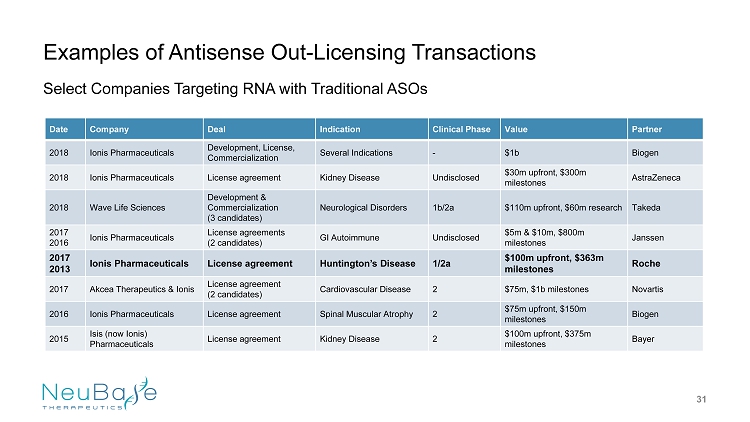
Date Company Deal Indication Clinical Phase Value Partner 2018 Ionis Pharmaceuticals Development , License, Commercialization Several Indications - $1b Biogen 2018 Ionis Pharmaceuticals License agreement Kidney Disease Undisclosed $30m upfront, $300m milestones AstraZeneca 2018 Wave Life Sciences Development & Commercialization (3 candidates) Neurological Disorders 1b/2a $110m upfront, $60m research Takeda 2017 2016 Ionis Pharmaceuticals License agreements (2 candidates) GI Autoimmune Undisclosed $5m & $10m, $800m milestones Janssen 2017 2013 Ionis Pharmaceuticals License agreement Huntington’s Disease 1/2a $100m upfront, $363m milestones Roche 2017 Akcea Therapeutics & Ionis License agreement (2 candidates) Cardiovascular Disease 2 $75m, $1b milestones Novartis 2016 Ionis Pharmaceuticals License agreement Spinal Muscular Atrophy 2 $75m upfront, $150m milestones Biogen 2015 Isis (now Ionis ) Pharmaceuticals License agreement Kidney Disease 2 $100m upfront, $375m milestones Bayer Examples of Antisense Out - Licensing Transactions 31 Select Companies Targeting RNA with Traditional ASOs

Strong IP Portfolio NeuBase Therapeutics holds an exclusive license to the PATrOL ™ technology, with 9 patents and applications covering matter and field of use of the platform Patents have 2037 expiration , not including “Hatch Waxman” extensions USPTO has Allowed Claims for Composition of Matter Developed at Carnegie Mellon University 32

Corporate Objectives NT - 0100 HD Objectives – H2 2019 Additional In Vivo Safety and Efficacy – Mid - 2020 IND filing 33 NT - 0200 DM1 Objectives – CY 2020 Additional In Vivo Safety and Efficacy – H1 2021 IND filing
NeuBase Therapeutics • RNA therapeutics: pipeline of pre - clinical assets for gene silencing • DNA therapeutics: opportunity for genome editing, gene regulation and liquid biopsy • PATrOL ™ modular p eptide - nucleic acid (PNA) a n t isense ol igonucleotide platform • Advantages of small molecules with selectivity of antisense oligonucleotides (ASOs) • Strong IP composition of matter & field of use through 2037+ • Key advantages include rapid drug design, selectivity, blood brain barrier penetration, broad systemic distribution for multi - tissue disease and cell permeability • Central nervous system diseases, high unmet need, and orphan focus • Ongoing development for Huntington’s (NT0100) and Myotonic Dystrophy (NT0200), with billion dollar peak sales opportunity in each indication Expansive market opportunity First - in - class technology Advantages over traditional antisense oligonucleotides Focus on initial indications 34 Expected to Soon to be a Publically Traded Company Developing Multiple Therapies for Genetic Disease
