Attached files
| file | filename |
|---|---|
| 8-K - 8-K - CTI BIOPHARMA CORP | cti-item7018xk.htm |

Corporate Presentation January 7, 2019 © Copyright 2019 CTI BioPharma Corp. All rights reserved.

Forward Looking Statement This presentation includes forward-looking statements within the meaning of the Safe Harbor provisions of the Private Securities Litigation Reform Act of 1995. Such statements are subject to a number of risks and uncertainties, the outcome of which could materially and/or adversely affect actual future results and the trading price of CTI BioPharma's securities. Such statements include, but are not limited to, expectations with respect to results from PAC203, our ability to establish a dose for our randomized Phase 3 study of pacritinib, and our ability to commence said study, in 2019, our ability to interpret clinical trial data and results for PERSIST-2 despite not satisfying the pre-specified minimum evaluable patient goal, expectations with respect to the potential therapeutic utility of pacritinib, statements regarding CTI BioPharma’s expectations with respect to the potential of pacritinib to achieve treatment goals, the development of CTI BioPharma, including the advancement of pacritinib, CTI BioPharma’s ability to achieve its goals in 2019 and beyond, including achieving cost efficiency and year-on-year cost reduction goals. In particular, this presentation addresses top-line results regarding data from CTI BioPharma’s Phase 3 trial of pacritinib for the treatment of patients with myelofibrosis whose platelet counts are less than or equal to 100,000 per microliter. Meaningful interpretation of PERSIST-2 may not be possible because the pre-specified minimum evaluable patient goal was not met. Risks that contribute to the uncertain nature of the forward-looking statements include, among others, risks associated with the biopharmaceutical industry in general and with CTI BioPharma and its product and product candidate portfolio in particular including, among others, risks associated with the following: that CTI BioPharma cannot predict or guarantee the outcome of preclinical and clinical studies, the potential failure of pacritinib to prove safe and effective as determined by the FDA and/or the European Medicines Agency, changes to study protocol or design or sample size to address any patient safety, efficacy or other issues raised by the FDA or otherwise, that top-line results observed to date may differ from future results or that different conclusions or considerations may qualify such results once existing data has been more fully evaluated, that CTI BioPharma may not obtain favorable determinations by other regulatory, patent and administrative governmental authorities, that CTI BioPharma may experience delays in the commencement of preclinical and clinical studies, that the costs of developing pacritinib and CTI BioPharma’s other product candidates may rise; other risks, including, without limitation, competitive factors, technological developments, that CTI BioPharma may not be able to sustain its current cost controls or further reduce its operating expenses, that CTI BioPharma may not achieve previously announced goals, contractual milestones and objectives as or when projected, that CTI BioPharma’s average net operating burn rate may increase, that CTI BioPharma will continue to need to raise capital to fund its operating expenses, but may not be able to raise sufficient amounts to fund its continued operation as well as other risks listed or described from time to time in CTI BioPharma’s most recent filings with the Securities and Exchange Commission on Forms 10-K, 10-Q and 8-K. Except as required by law, CTI BioPharma does not intend to update any of the statements in this presentation upon further developments. 2

CTI BioPharma Focused on addressing the unmet needs of myelofibrosis patients • Development - Pacritinib − Development focused on myelofibrosis (MF) - Severe thrombocytopenia - Previous treatment with with ruxolitinib − Two Phase 3 MF trials completed − Phase 2 MF trial optimal dose study fully enrolled − Phase 3 MF study planned to start 3Q 2019 • Corporate - Major stockholders include BVF, NEA, Stonepine and OrbiMed* - Recent 50% reduction in workforce - Cash runway into 2020 ($81MM as of 9/30/18) 3 *As of 9/30/18

Pacritinib Development – Myelofibrosis Focus Program Indication Phase 1 Phase 2 Phase 3 Approved PAC203: Myelofibrosis Phase 2: Second-line Phase 3*: Severe thrombocytopenia (platelets <50,000/µL) Pacritinib PERSIST-2: Myelofibrosis (platelets ≤100,000/µL) PERSIST-1: Myelofibrosis (all platelet counts) *CTI intends to amend the PAC203 protocol to include the Phase 3 component and to commence the Phase 3 study in 3Q 2019. 4
Myelofibrosis and Unmet Medical Needs • Malignant bone marrow Debilitating Symptoms cancer with median survival 6 years after diagnosis • Only approved therapy is Jakafi/Jakavi (ruxolitinib) • Unmet medical needs in MF - Severe thrombocytopenia - Prior ruxolitinib therapy 5

Severe Thrombocytopenia Poor survival with platelet count <50,000/μL Overall survival is 15 months for patients with platelet count <50,000/μL1 6 1. Masarova et al., Eur J Haematolol. 2017

Ruxolitinib and Thrombocytopenia Significant and rapid decline in platelet counts with ruxolitinib at doses of 15-20mg BID1 ….associated with dose reductions ….which potentially reduces clinical benefit2 7 1. Verstovsek, et al., Haematologica, 2015 May; 100(4): 479–488; 2. Ruxolinitib NDA Clinical Pharmacology and Biopharmaceutical Review(s), FDA 2011.

Prior Ruxolitinib Therapy Poor survival following ruxolitinib discontinuation1,2 mOS = 7 months 50% of patients discontinue ruxolitinib by 3 years3,4 - Majority discontinue due to inability to tolerate an effective dose - Overall survival (OS) is 7-14 months1,2 - Shorter OS in thrombocytopenic patients (<100,000/µL)1 8 1. Newberry, Blood 2017; 2. Mehra, et al. ASH 2016 poster, Abstract 4769; 3. Cervantes, Blood 2013; 4. Verstovek, Haematologica 2013.

Pacritinib: Novel JAK2 inhibitor Pacritinib has demonstrated clinical activity in multiple trials • Oral JAK2 inhibitor • Over 1,200 patients treated in clinical trials • Orphan drug designation for myelofibrosis in US and EU • Patent protection until 2029/2030 (plus term extension) • Key completed Phase 3 studies: PERSIST-1: 2:1 randomization comparing pacritinib 400 mg QD to BAT, no prior ruxolitinib (n=327) PERSIST-2: Platelets <100,000 µL, 1:1:1 randomization, comparing pacritinib 200mg BID & 400 mg QD to BAT, prior ruxolitinib allowed (n=311) 9
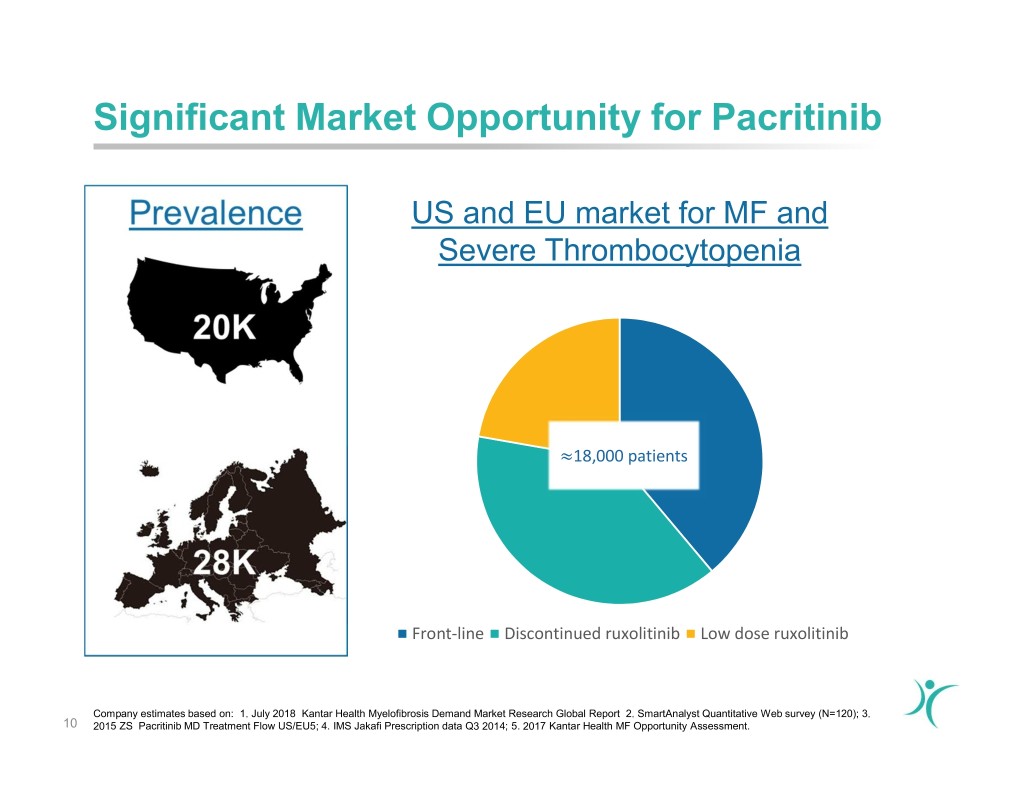
Significant Market Opportunity for Pacritinib US and EU market for MF and Severe Thrombocytopenia 18,000 patients ≈ Front-line Discontinued ruxolitinib Low dose ruxolitinib Company estimates based on: 1. July 2018 Kantar Health Myelofibrosis Demand Market Research Global Report 2. SmartAnalyst Quantitative Web survey (N=120); 3. 10 2015 ZS Pacritinib MD Treatment Flow US/EU5; 4. IMS Jakafi Prescription data Q3 2014; 5. 2017 Kantar Health MF Opportunity Assessment.

Completed Phase 3 Pacritinib Trials PERSIST-1 and PERSIST-2 PERSIST-1 1L therapy PERSIST-2 Thrombocytopenia 1L & 2L therapy SVR, spleen volume reduction; TSS, total symptom score; *n=211 completed 24 weeks on study; ** BAT may include ruxolitinib; Mesa RA, et al., Lancet Haematology 11 2017; Mascarenhas J, et al., JAMA Oncology 2018.

PERSIST-1: Efficacy Best Pacritinib Endpoint Available QD Dosing Therapy 19% 5% (N=220) (N=107) ≥35% SVR p=0.0003 - 19% 10% Reduction of (N=100) (N=48) ≥50% TSS* p=0.24 - 12 ..*Analysis using MPN-SAF TSS2.0 data only. SVR, spleen value reduction TSS, total symptom score; Mesa RA, et al., Lancet Haematology 2017.
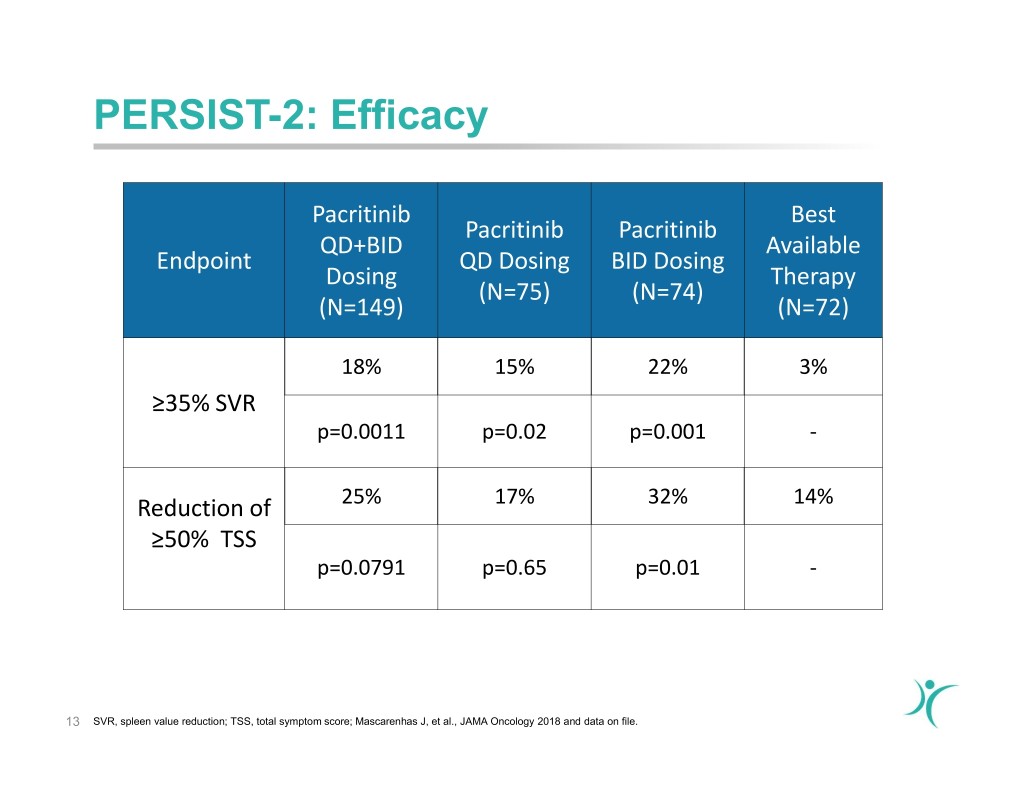
PERSIST-2: Efficacy Pacritinib Best Pacritinib Pacritinib QD+BID Available Endpoint QD Dosing BID Dosing Dosing Therapy (N=75) (N=74) (N=149) (N=72) 18% 15% 22% 3% ≥35% SVR p=0.0011 p=0.02 p=0.001 - Reduction of 25% 17% 32% 14% ≥50% TSS p=0.0791 p=0.65 p=0.01 - 13 SVR, spleen value reduction; TSS, total symptom score; Mascarenhas J, et al., JAMA Oncology 2018 and data on file.
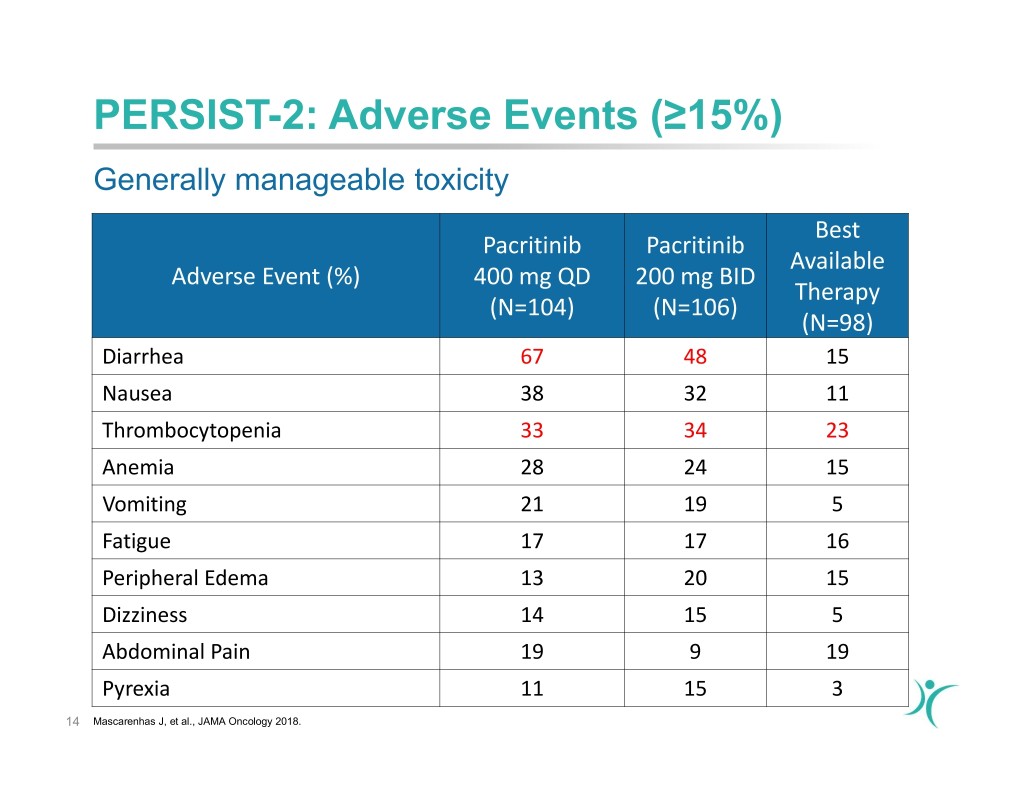
PERSIST-2: Adverse Events (≥15%) Generally manageable toxicity Best Pacritinib Pacritinib Available Adverse Event (%) 400 mg QD 200 mg BID Therapy (N=104) (N=106) (N=98) Diarrhea 67 48 15 Nausea 38 32 11 Thrombocytopenia 33 34 23 Anemia 28 24 15 Vomiting 21 19 5 Fatigue 17 17 16 Peripheral Edema 13 20 15 Dizziness 14 15 5 Abdominal Pain 19 9 19 Pyrexia 11 15 3 14 Mascarenhas J, et al., JAMA Oncology 2018.
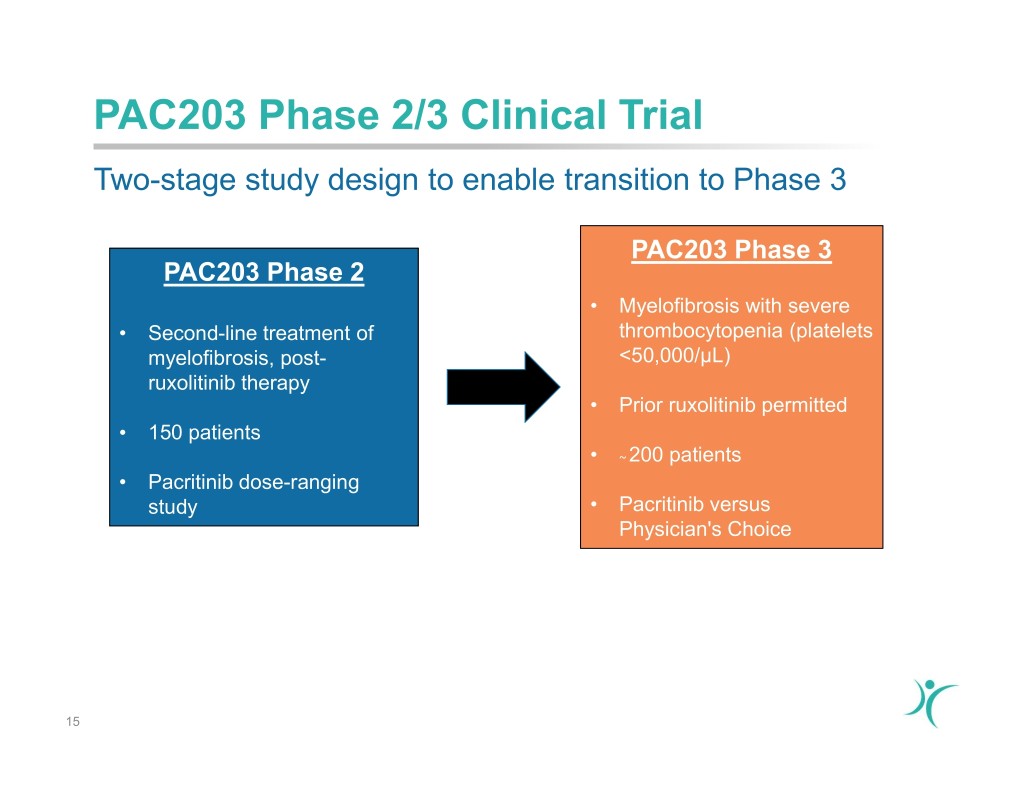
PAC203 Phase 2/3 Clinical Trial Two-stage study design to enable transition to Phase 3 PAC203 Phase 3 PAC203 Phase 2 • Myelofibrosis with severe • Second-line treatment of thrombocytopenia (platelets myelofibrosis, post- <50,000/µL) ruxolitinib therapy • Prior ruxolitinib permitted • 150 patients • ~ 200 patients • Pacritinib dose-ranging study • Pacritinib versus Physician's Choice 15

On-going PAC203 Phase 2 Clinical Trial Pacritinib Primary or 200 mg BID secondary 150 patients myelofibrosis Evaluate safety and Pacritinib SVR at 12 and 24 100 mg BID Prior ruxolitinib 1:1:1 weeks therapy randomization Pacritinib 100 mg QD • Second-line, post-ruxolitinib, pacritinib dose-ranging study • Fully enrolled 4Q 2018 • Optimal dose determination, following meeting with FDA, expected mid-2019 • Topline data expected 3Q 2019 16 SVR, ≥35% spleen volume reduction.

Pacritinib US Regulatory Strategy • Addressing unmet medical needs in MF • Severe thrombocytopenia (platelet <50,000/μL) • 1st and 2nd-line MF patients • Optimal dose determination expected mid-2019 • New Phase 3 expected to commence 3Q 2019 • Key elements of protocol discussed with FDA 17

Adaptation of PAC203 to a Phase 3 Clinical Trial 1º Endpoint: Primary or Pacritinib SVR at 24 weeks secondary ~200 patients myelofibrosis Severe 1:1 2º Endpoints: thrombocytopenia randomization Physician’s TSS at 24 weeks (platelets <50,000/µL) Choice Overall Survival • Targeting 80+ clinical sites worldwide • Prior ruxolitinib permitted • Cross-over not permitted • Enrollment expected to commence Q3 2019 • Top-line data expected mid-2021 18 SVR, ≥35% spleen volume reduction; TSS, ≥50% reduction in total symptom score.

Pacritinib and Severe Thrombocytopenia Clinical improvement observed with pacritinib therapy in myelofibrosis with severe thrombocytopenia Pacritinib Best available Endpoint BID/QD therapy Dosing 23% 2% (N=104) (N=48) ≥35% SVR p=0.0007 - 20% 11% ≥50% TSS (N=80) (N=37) reduction* p=0.294 - 19 Ad-hoc analysis of pooled data from PERSIST-1 and PERSIST-2, patients with platelet counts <50,000 per microliter. Data on file. *Analysis using MPN-SAF TSS2.0 data only.

European Marketing Authorization CHMP decision expected in 1Q 2019 • Responses to 2nd D180 List of Outstanding Issues submitted December 2018 • Oral Explanation to CHMP expected in 1Q 2019 • CHMP opinion expected in 1Q 2019 20

Regulatory Timeline 3Q 2018 4Q 2018 1Q 2019 2Q 2019 3Q 2019 PAC203 PAC203 PAC203 Interim Enrollment Optimal Dose Analysis Complete Type C Scientific Initiate Phase 3 Meeting Advice Potential EU CHMP Conditional Opinion Approval PAC203 Phase 2 milestones PAC203 Phase 3 milestones EU Regulatory milestones 21

Financial and Corporate

Financial Overview Ticker “CTIC” Exchange NASDAQ: CTIC Shares Outstanding as of 09/30/18 ~58 mm Cash as of 09/30/18 ~$81 mm Debt as of 09/30/18 ~$16 mm 23

Contact: Julia Balanova Solebury Trout jbalanova@troutgroup.com
