Attached files
Exhibit 10.15
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
DATED DECEMBER 18, 2018
(1) TCR2 THERAPEUTICS INC.
- and -
(2) CELL THERAPY CATAPULT LIMITED
COLLABORATION AGREEMENT

[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
THIS COLLABORATION AGREEMENT (the “Agreement) is dated the 18th of December 2018 (the “Effective Date”)
BETWEEN
| (1) | TCR2 Therapeutics Inc., a company incorporated in under the laws of the State of Delaware and located at 100 Binney Street, Suite 710, Cambridge, MA 02142, US (“COLLABORATOR”); and |
| (2) | Cell Therapy Catapult Limited, trading as Cell and Gene Therapy Catapult, a company incorporated and registered in England & Wales with company number 07964711 whose registered office is at 12th Floor Tower Wing, Guys Hospital, Great Maze Pond, London, SE1 9RT, United Kingdom (the “Catapult”). |
BACKGROUND
| (A) | Catapult’s purpose in commissioning the cell and gene therapy manufacturing centre is to further its broader aims within the UK to develop novel technologies, processes, supply chains, facilities, skills, and working practices for simultaneous and cost effective large-scale manufacture and distribution of multiple ATMP products. |
| (B) | COLLABORATOR is developing certain cell and gene therapy products. As part of this activity COLLABORATOR wishes to use the Centre in order to further develop and scale up manufacturing processes and capability for cell and gene therapy products. |
| (C) | COLLABORATOR and Catapult would each like to collaborate further with the other as further set forth in this Agreement (“Project” or “Collaboration”, as further described in the work streams set out at Schedule 1). |
| (D) | Other parties who collaborate with the Catapult and occupy the Centre in the same way will be referred to as a “Collaborator” or “Other Collaborator(s)” (and together with COLLABORATOR as “All Collaborators”). |
| (E) | This document aims to record the contributions of each party with respect to this Agreement, and the terms under which COLLABORATOR and Catapult will work together within the Centre. |
OPERATIVE PROVISIONS
| 1. | DEFINITIONS AND INTERPRETATION |
| 1.1 | In this Agreement, the following words will have the following meanings: |
| “Accompanied Access Areas” | means the areas of the Centre marked yellow on the Plans which are accessible by All Collaborators, but on condition such access is in the company of Catapult personnel. | |
| “Activity Related Inputs” | means the inputs provided by Catapult set out at Clause 9.2. | |
| “Activity Related Input Contributions” | means the financial contribution made by COLLABORATOR with respect to the provision of the Activity Related Inputs, and as described in Clause 8.4.3. | |
| “Additional Inputs” | means inputs COLLABORATOR requires Catapult to contribute to the Project, other than Activity Related Inputs and Integral Inputs, which will be arranged through the completion of an Additional Input Agreement in the form set out at Schedule 16 (“Additional Input Agreement”). | |
| “Affiliate” | in relation to a Party, means any person that Controls, is Controlled by, or is under common Control with that Party. | |
1
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| “Applicable Law” | means any:
(a) statute, statutory instrument, by-law, order, regulation, directive, treaty, decree, decision of the European Council or law;
(b) legally binding rule, policy, guidance or recommendation issued by any governmental, statutory or regulatory body with jurisdiction over this Agreement or the activities conducted hereunder; and/or
(c) legally binding industry code of conduct or guideline;
which relates to this Agreement and/or the Inputs and/or the activities which are comprised in the Project. | |
| “Background Intellectual Property” | (a) in relation to COLLABORATOR, means the Intellectual Property that is either (i) owned by or licensed to COLLABORATOR prior to the Effective Date, or (ii) that is developed or licensed by COLLABORATOR after the Effective Date and outside of the conduct of activities for the Project; and in the case of either (i) or (ii), that COLLABORATOR uses in the performance of the Project, other than Foreground Intellectual Property; and
(b) in relation to Catapult, means the Intellectual Property owned by or licensed to Catapult at the Effective Date, together with Intellectual Property that is developed by or licensed to Catapult after the Effective Date and outside of the conduct of activities for the Project; and in either case that Catapult uses in the performance of the Agreement, and that is not Foreground Intellectual Property. Catapult represents and warrants that to the best of Catapult’s knowledge and belief, as of the Effective Date, Catapult Background Intellectual Property, consists of the Intellectual Property as set forth in the attached Schedule 4, which Catapult will update from time to time as additional Catapult Background Intellectual Property is brought into the Project. | |
| “Business Rates” | means the portion of the business rates chargeable against the Centre paid for by COLLABORATOR in accordance with Clause 8.4.2 and in the amount set out at Schedule 3. | |
| “Centre” | means the Cell and Gene Therapy Catapult Manufacturing Centre located at Cell and Gene Therapy Catapult Manufacturing Centre, Gunnels Wood Road, Stevenage, Herts, SG1 2FX. The Centre outlines are edged in blue on the Plans. | |
| “CNC corridor” | means the controlled non-classified corridor forming part of the Common Access Areas. | |
| “Code of Conduct” | means the code of conduct set out at Schedule 5. | |
| “Collaborator Forums” | means the Quality Forum, the Health and Safety Forum, and the Operational Forum, each as more particularly referenced, and described in Clause 9.6 and Schedule 15. | |
| “COLLABORATOR Manufacturing Process” | means the process to be developed and operated by COLLABORATOR under the Agreement in order to enable the production of COLLABORATOR Product on a large scale as more particularly defined prior to signature of this contract in the Product Overview Document . It may be amended from time to time in accordance with Clause 7.2 and the QTA. | |
2
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| “COLLABORATOR Personnel” | means the employees or contractors of COLLABORATOR located at the Module from time to time. | |
| “COLLABORATOR Product” | means COLLABORATOR owned and developed product, or products, to be produced on a large scale through the creation and adoption of COLLABORATOR Manufacturing Process. | |
| “COLLABORATOR Responsibilities” | means the obligations on COLLABORATOR set out in Clause 10 (each one severally being a “COLLABORATOR Responsibility”). | |
| “Commissioning” | has the meaning given in Clause 6.1 | |
| “Common Access Areas” | means any part of the Centre shown edged green on the Plans which does not form part of the Module, the Restricted Access Areas, the Shared Restricted Access Areas or the Accompanied Access Areas, or that is designated by Catapult from time to time for common use by Catapult, and All Collaborators from time to time. | |
| “Compensations” | has the meaning given to it in Clause 18.1.1. | |
| “Conducting Media” | means any media for the transmission of Supplies. | |
| “Confidential Information” | means any information in any form or medium disclosed by one Party or such Party’s Affiliates, or their legal counsel, advisors, contractors (the “Disclosing Party”) to the other Party or its Affiliates, or their legal counsel, advisors, contractors (the “Receiving Party”) or to which either Party gains access as a result of COLLABORATOR’s occupancy of the Module and Centre or Catapult or Third Party’s use of the Centre at any time, or as a result of participation in any of the Collaborator Forums, concerning the business affairs, finances, technology, plans, strategy, products, manufacturing services, Know-how or services of (i) the Disclosing Party, (ii) any of its Affiliates, (iii) any other entity with which the Disclosing Party or any of its Affiliates is in business negotiations or has contracted or to which it owes a duty of confidence, or (iv) any other Collaborator ((iv) being an “Alternative Disclosing Party”), and all copies of the same. Confidential Information also includes any information disclosed by either Party under the terms of the confidentiality agreement between the Parties dated 24 August 2018 (“Pre-existing CDA”). | |
| “Contributions” | means the Activity Input Contributions, Integral Input Contributions, Establishment Input Contributions, the Additional Input Contributions and/or any other contributions as agreed in writing between the Parties and provided by Catapult to COLLABORATOR from time to time. | |
| “Control” | means (a) the direct or indirect ownership of fifty percent (50%) or more of the total voting power of securities or other evidences of ownership interest in a party or (b) the power to direct or cause the direction of the management and policies of such party, directly or indirectly, whether through ownership of voting securities, by contract or otherwise; and the terms “controlling” and “controlled” have meanings correlative to the foregoing, as the case may be. | |
| “Disclosing Party” | has the meaning given in the definition of “Confidential Information” . | |
| “Effective Date” | has the meaning given in the preamble of this Agreement. | |
| “Establishment Inputs” | means the inputs provided by Catapult set out at Clause 9.3. | |
| “Establishment Input Contributions” | means the financial contributions payable in accordance with cause 8.1.5, to be made by COLLABORATOR with respect to the provision of the Establishment Inputs by Catapult, which are detailed in the Establishment Input Statement. | |
3
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| “Establishment Input Statement” | means the Establishment Input Contributions Statement by and between Catapult and Collaborator dated December [***], 2018. | |
| “Expected Occupation Date” | means 1 March 2019, the contemplated date by which COLLABORATOR will occupy the Manufacturing Space, or such other date as mutually agreed by the parties in writing. | |
| “Facility Contribution” | means the contribution to be made by COLLABORATOR with respect to the provision of the Module and other capital aspects, as more particularly described at Clause 8.4.1 .1, and at Schedule 3. | |
| “Foreground Intellectual Property” | means the results, technical information, knowledge, inventions, improvements, experience, materials and data developed and arising directly from and as a direct result of the Project, together with any Intellectual Property in such items. | |
| “GMP” or “EU-GMP” | means good manufacturing practice, being the standard required under the Laws of the European Union. | |
| “GMP Requirements” | means the guidance for the interpretation of the principles and guidelines of good manufacturing practices for medicinal products for human and veterinary use laid down in the Commission 2003/94/EC as amended and set out in Volume 4 of Eudralex (the rules governing medicinal products in the European Union), and the MHRA Rules and Guidance for Pharmaceutical Manufacturers and Distributors (The Orange Guide). | |
| “Ground Level” | means the Level 0 Ground Level as shown on the Plans. | |
| “Health and Safety Forum” |
means the forum in which COLLABORATOR, Other Collaborators, and Catapult will convene to discuss health and safety matters as more particularly described in Clause 9.6 and Schedule 15. | |
| “HVAC” | heating, ventilation and air-conditioning. | |
| “Improvement” | means, with respect to any Intellectual Property or material: (a) all improvements, modifications and /or adaptions of such Intellectual Property or materials; and (b) all other Intellectual Property in, derived from, relating to, or otherwise within the scope of any claim, right, or interest which would impair or restrict the use of, application, or rights comprised in, such Intellectual Property or material. | |
| “Insured Risks” | means the risks covered by the policies of insurance under Clauses 19.1 and 19.2, in each case to the extent that cover is generally available on normal commercial terms in the UK insurance market at the time the insurance is taken out and any other risks against which Catapult reasonably insures from time to time, subject in all cases to any excesses, limitations and exclusions imposed by the insurers. | |
| “Integral Input Contributions” | means the financial contribution made by COLLABORATOR with respect to the provision of the Integral Inputs, as more particularly described in Clause 8.4.4, and at Schedule 3. | |
| “Integral Inputs” | the inputs provided by Catapult set out at Clause 9.1. | |
| “Intellectual Property” | means any and all issued patents and patent applications, inventions, utility models, registered and unregistered trademarks and service marks, registered designs, unregistered design rights, domain names, trade or business names, copyright, database rights, rights in respect of confidential information, rights under data exclusivity laws, rights under licences, rights under orphan drug laws, property rights in biological or chemical materials, topography rights, Know-how, extension of the terms of any such rights (including supplementary protection certificates), applications for and the right to apply any of the foregoing registered property and rights, and similar or analogous rights anywhere in the world. | |
4
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| “IT Infrastructure” | the information technology facilities in the Centre for use by COLLABORATOR and, where applicable, by Other Collaborators as more particularly described in Schedule 10. | |
| “Know-how” | means unpatented technical information (including without limitation information relating to inventions, discoveries, concepts, methodologies, models, research, development, and testing procedures; the results of experiments, tests, and trials; manufacturing processes, techniques, and specifications; and quality control data, analyses, reports, and submissions) that is not in the public domain. | |
| “Lease” | means a lease dated 1 October 2015 made between the (1) Stevenage Bioscience Catalyst and (2) Cell Therapy Catapult Limited. | |
| “Liability” | means liability arising out of this Agreement, whether in contract, tort, misrepresentation, restitution, under statute or otherwise, including any liability under an indemnity contained in this Agreement. | |
| “Letter Agreement” | Means the heads of terms entered into between the parties connected to COLLABORATOR’S occupation of the Centre dated 5 October 2018 | |
| “Licence Period” | means the Term. | |
| “MAL” | means material airlock. | |
| “Manufacturing Office” | means the manufacturing office space forming part of the Module, allocated for COLLABORATOR’s use in accordance with Clause 3, and more particularly described in Schedules 2 and 12. | |
| “Manufacturing Space” | means the manufacturing space forming part of the Module, allocated for COLLABORATOR’s use in accordance with Clause 3, more particularly described in Schedules 2 and 12. | |
| “Module” | means the specific Manufacturing Space, the Manufacturing Office, and Non-Manufacturing Office each allocated by Catapult under this Agreement for COLLABORATOR’s occupation and use at the Centre for carrying out the Project shown shaded in grey on the Plans, and which will include all fixtures and fittings and plant and machinery located within the Manufacturing Space, the Manufacturing Office and Non-Manufacturing Office, to be set out in the Schedule of Condition and Inventory of Module Fixtures and Fittings immediately prior to occupancy and appended as Schedule 7. | |
| “Necessary Consents” | means all planning permissions and all other consents, licences, permissions, certificates, authorisations and approvals whether of a public or private nature which will be required by any regulatory authority for the Permitted Use | |
| “Non-Manufacturing Office” | means the office space forming part of the Module allocated for COLLABORATOR’s use in Clause 3, the specifications for which are set out in Schedules 2 and 12. | |
| “Occupation Date” | means the date by which Catapult has materially completed its obligations contained in this Agreement and the Establishment Input Statement that are required to enable COLLABORATOR to physically occupy the Module. For the avoidance of doubt: (i) the Occupation Date shall not be triggered solely by the occupation of the Non-Manufacturing Office if such occupation occurs prior to the material completion of Catapult’s obligations under this Agreement and the Establishment Input Statement and (ii) COLLABORATOR will not have access to the Module until the later of: (a) the date that the Parties have agreed and entered into the QTA or (b) the Occupation Date. | |
5
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| “Office Hours | 9am – 5.30pm (UK time) Monday to Friday (inclusive) but excluding bank holidays and public holidays in England. | |
| “On-boarding” |
means part of the Establishment Inputs and a process completed by Catapult together with COLLABORATOR involving the risk assessment and regulatory oversight required to bring COLLABORATOR’s manufacturing processes and products into the Centre as referred to in Clause 9.3.1 (b), and more particularly set out at Schedule 6. | |
| “Operational Forum” | means the forum in which COLLABORATOR, Other Collaborators, and Catapult will convene to discuss operations matters connected with the Centre as more particularly described in Clause 9.6 and Schedule 15. | |
| PAL | means personnel airlock. | |
| “Parties” | COLLABORATOR and Catapult; “Party” shall mean either of them, and “Parties” shall mean both COLLABORATOR and Catapult. | |
| “Permitted Use” | means any activity strictly in connection with the performance of the Project. | |
| “Plans” | means the plans of the Module allocated to COLLABORATOR under this Agreement, and of the Centre generally, attached to this Agreement at Schedule 2, Part 4. | |
| “PrAL” | means product airlock. | |
| “Pre-Screen Questionnaire” | means a questionnaire described at Schedule 6 defining the COLLABORATOR Manufacturing Process and Product as part of the COLLAB0RATOR Selection phase. | |
| “Product Overview Document” or “POD” | means a quality document completed as part of the On-boarding Project defining the COLLABORATOR Manufacturing Process and COLLABORATOR Product. | |
| “Process Transfer” | means an Establishment Input, and the practical transfer of COLLABORATOR equipment and processes into the Centre under the control and responsibility of COLLABORATOR as referred to in Clause 9.3.1 (a) and more particularly set out at Schedule 6. | |
| “Project” | has the meaning given in Recital (C). | |
| “Quality Forum” | means the forum in which COLLABORATOR, Other Collaborators, and Catapult will convene to discuss quality matters connected with the Centre as more particularly described in Clause 9.4 and Schedule 15. | |
| “Quality Management System” or “QMS” | means a collection of business processes and governance structures focused on consistently meeting Regulatory Authority and GMP requirements. The Quality Management System is expressed as an organisational structure, policies, procedures, processes and resources needed to maintain compliance to Eudralex Vol 4, Chapter 1 that are set out in the Quality Technical Agreement, and encompass the contents of Schedule 8 (Warehouse and Procurement Management Provisions), Schedule 9 (Environmental Monitoring), Schedule 10 (IT Infrastructure), Schedule 12 (Module and Centre Specification). | |
6
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| “Quality Technical Agreement” or “QTA” | means the agreement between the Parties governing the quality aspects of the Centre that are comprised in the Quality Management System (as it may be amended from time to time) | |
| “Quarter” | means a period of three months commencing on 1 January, 1 April, 1 July, or 1 October; and “Quarterly” shall be construed accordingly. | |
| “Receiving Party” | has the meaning given in the definition of “Confidential Information”. | |
| “Registered Rights” | means patents, registrable design rights, trademarks, and all other registered Intellectual Property. | |
| “Regulatory Authority” | means the competent authority for each country or for any relevant grouping of countries legally responsible for authorising the sale or supply of human pharmaceutical products in that country or group of countries. | |
| “Restricted Access Area(s)” | means the parts of the Centre accessible only by Catapult personnel marked in pink on the Plan. | |
| “Shared Restricted Access Area” | means the areas shared between the Manufacturing Space and an adjacent manufacturing space belonging either to COLLABORATOR or to another Collaborator marked in turquoise on the Plans. | |
| “Supplies” | means water, gas, air, foul and surface water, drainage, electricity, oil, telephone, heating, telecommunications, internet, data communications and similar supplies or utilities. | |
| “Steering Committee” | the representatives of the COLLABORATOR and Catapult appointed as set out in Clause 9.5. | |
| “Technology Transfer” | means the transfer of COLLABORATOR’s existing production and/or manufacturing processes into the Module which is divided into On-boarding and Process Transfer. | |
| “Term” | means the period specified in Clause 17.1.17.1. | |
| “Termination Date” | means the date on which this Agreement expires or terminates for any reason. | |
| “Third Party” | means any person other than a Party or its Affiliates. | |
| “Warehouse and Procurement Management Provisions” | means the standards and obligations relating to the management of the warehouse set out at Schedule 8. | |
| “UPS” | means uninterrupted power supply. | |
| “Validation” | means documenting the way in which the equipment, facility or system used in a ‘process’ will result in an output meeting its specifications and predetermined quality attributes. | |
| “Year” | means the financial year ending 31 March. | |
| 1.2 | In this Agreement, unless otherwise specified: |
| 1.2.1 | references to Clauses and Schedules are to the clauses of, and schedules to, this Agreement; |
| 1.2.2 | headings are for convenience only and do not affect the interpretation of this Agreement; |
7
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 1.2.3 | references to a person includes a body corporate or unincorporated body, and references to a company includes any company, corporation or other body corporate, wherever and however incorporated or established; |
| 1.2.4 | unless the context otherwise requires, words in the singular will include the plural and vice versa; |
| 1.2.5 | references to approvals or notices being “in writing” or “written” will include email; |
| 1.2.6 | any reference to a statute or statutory provision is a reference to it as amended, extended, re-enacted and/or replaced from time to time; and |
| 1.2.7 | ‘including’ means ‘including but not limited to’ and ‘include’ and ‘includes’ will be construed accordingly. |
| 2. | CONDUCT OF THE PROJECT |
The Parties will undertake the Project in accordance with the provisions of this Agreement.
| 3. | OCCUPATION OF THE MODULE |
Catapult permits COLLABORATOR to occupy the Module on the terms set out in Schedule 2.
| 4. | MODULE SPECIFICATION |
| 4.1 | Catapult will ensure the Manufacturing Space will be in accordance with the specifications at Schedule 12 Part A. |
| 4.2 | Catapult will ensure the Manufacturing Office and Non-Manufacturing Office will be in accordance with the specifications at Schedule 12 Part B. |
| 5. | CENTRE SPECIFICATIONS |
The Centre is a UK-licensed EU-GMP-compliant facility developed in close relationship with the Medicines and Healthcare Products Regulatory Agency (the “MHRA”). comprising the facilities set out at Schedule 12, Part C. It is also planned to consult with the United States Food and Drug Agency in order to demonstrate FDA compliance
| 6. | COMMISSIONING AND QUALIFICATION OF THE CENTRE |
| 6.1 | In advance of COLLABORATOR being granted access to the Manufacturing Space and subject to Clause 7, Catapult will test equipment, facilities and/or plant which is Catapult owned or controlled and installed in order to verify it functions according to its design objectives or specifications (“Commissioning”). |
| 6.2 | Commissioning will not cover the formal qualification of manufacturing systems or manufacturing process equipment but will include the static and dynamic commissioning of the following: |
| 6.2.1 | the Building Management System (BMS); |
| 6.2.2 | the Quality Control Area HVAC |
| 6.2.3 | the electrical supply (single and three phase); |
| 6.2.4 | the boilers; |
| 6.2.5 | the chiller; |
| 6.2.6 | HVAC; |
| 6.2.7 | lighting – including emergency lighting; |
| 6.2.8 | back-up generator; |
8
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 6.2.9 | UPS systems; |
| 6.2.10 | door interlocks; |
| 6.2.11 | pharmaceutical grade gas supplies (air, oxygen, carbon dioxide and nitrogen); |
| 6.2.12 | CCTV; |
| 6.2.13 | fire alarm; |
| 6.2.14 | LN2 / Low level, temperature and oxygen monitors; |
| 6.2.15 | drainage; and |
| 6.2.16 | appropriate IT Infrastructure (including cable network, switches, and server rooms). |
| 6.3 | Where appropriate, Catapult will qualify the building, systems and equipment that form part of the Centre, and this will extend to installation qualification, operational qualification, and performance qualification of all GMP direct impacting systems and integral equipment (“Qualification”). The basis for this Qualification and results obtained as related to shared areas (including Shared Restricted Access Area) and the COLLABORATOR Module will be shared with quality representatives for COLLABORATOR on request. |
| 6.4 | Formal Qualification will be undertaken (which includes installation qualification and operational qualification) concurrent with leveraging the output of facility Commissioning. Performance qualification will only occur subsequent to the completion of Commissioning, installation qualification and operational qualification. Performance qualification will only be applied to those Inputs, systems and items of equipment that have been identified as having direct impact on product quality according to a formal system level impact assessment. These include, but are not necessarily limited to: |
| 6.4.1 | Manufacturing Space and all additional air locks HVAC |
| 6.4.2 | Warehouse HVAC |
| 6.4.3 | Environmental Monitoring System (EMS) |
| 6.4.4 | Grade C corridor and technical area HVAC |
| 6.4.5 | Carbon dioxide system |
| 6.4.6 | Nitrogen gas system |
| 6.4.7 | Liquid nitrogen system, storage tanks (PQ will be carried out in conjunction with a collaborator) and shared Controlled Rate Freezing equipment (COLLABORATOR will be responsible for their own cycle development and PQ) |
| 6.4.8 | Oxygen system; and |
| 6.4.9 | Cold room, fridges, freezers (PQ will be carried out in conjunction with COLLABORATOR) |
| 6.4.10 | the Environmental Monitoring System (EMS) |
Environmental monitoring equipment (e.g. viable air sampler, non-viable particulate monitors); PQ will be carried out in conjunction with a Collaborator
9
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 7. | PROCESS AND PRODUCT |
| 7.1 | VALIDATION |
| 7.1.1 | Process Validation is entirely the responsibility of COLLABORATOR. |
| 7.1.2 | Where COLLABORATOR has completed a Technology Transfer of COLLABORATOR’s Manufacturing Process to the Module, then COLLABORATOR’s Manufacturing Process validation will be the sole responsibility of COLLABORATOR. |
| 7.2 | PROCESS AND PRODUCT AMENDMENT |
| 7.2.1 | Catapult confirms that it approved the information contained in the initial POD provided as part of the On-boarding Project as describing the COLLABORATOR Process and COLLABORATOR Product that can and may be developed, implemented, and manufactured in the Module by COLLABORATOR. The initial COLLABORATOR Product provided as part of the On-boarding Project has been included in the QTA Product List, and, therefore, constitutes a COLLABORATOR Product. It further confirms that such approval of COLLABORATOR Product will remain valid during the Term on the condition that no amendments are made at a later stage. |
| 7.2.2 | The COLLABORATOR Product and Process will be again vetted and approved in advance of occupation as part of the On-boarding Process. In the event the On-boarding Process reveals any variations and/or additions to the information contained in the POD (“Product or Process Modifications”) these will be considered in accordance with the following procedure and in meeting the requirements of the QTA: |
| 7.2.2.1 | COLLABORATOR must notify Catapult in writing of its application for the intended changes (the “Notification”); |
| 7.2.2.2 | In response, Catapult will apply the principles under Clause 7.2.3. If the requested Product or Process Modifications meet the requirements of these principles, and are therefore capable of introduction, Catapult will always endeavour to approve the earliest date feasible for introduction, allowing for logistical constraints, and the competing interests of Other Collaborators in existence at the time of request (the “Introduction Date”); and |
| 7.2.2.3 | Catapult will confirm the outcome of the application of the criteria in Clause 7.2.3 and, if applicable, of the Introduction Date in writing to COLLABORATOR as soon as it is able to from the date of notification under Clause 7.2.2.1, but in any event within 30 calendar days of receipt of Notification by Catapult. Where Catapult indicates that the Modifications will not be permitted, it will identify the reasons why such Notification has been refused. |
| 7.2.3 | Catapult will permit (a) new COLLABORATOR Product(s), and/or COLLABORATOR Process(es) or COLLABORATOR modification(s) to such COLLABORATOR Product(s) or COLLABORATOR Process(es) if: |
| 7.2.3.1 | The new or modified COLLABORATOR Product(s), and/or COLLABORATOR Process(es) meet the requirements of the QTA; |
| 7.2.3.2 | The proposed product(s) is/are not a restricted product listed at Clause 7.3; |
| 7.2.3.3 | It does not affect Catapult’s ability to comply with GMP or GMP Requirements; |
10
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 7.2.3.4 | It does not compromise the safety of the Centre, or that of any Other Collaborator; |
| 7.2.3.5 | It does not place an additional, unreasonable demand on the resources of Catapult personnel and their ability to operate the Centre; |
| 7.2.3.6 | It does not interfere with the Catapult’s, COLLABORATOR’s, or any Other Collaborator’s compliance with their respective legal duties; and/or |
| 7.2.3.7 | It can be accommodated in the Centre, taking into account the overall capacity of the Centre. |
| 7.2.4 | In the event that COLLABORATOR does not agree with the outcome of Catapult’s application of the principles under Clause 7.2.3, the matter will be referred to the Steering Committee for resolution, and if no agreement is reached within a reasonable period, then the Parties will comply with the Expert Determination Procedure set out in Schedule 13. |
| 7.3 | RESTRICTED PRODUCTS |
COLLABORATOR will not be permitted, and Catapult undertakes that it will not allow any Other Collaborator to produce or utilise in their process the following products in the Centre (unless prior agreement is sought from all collaborators by Catapult):
| • | B Lactam Antibiotics |
| • | Other highly sensitising antibiotics |
| • | Pathogenic Organisms (Containment Level 3 or 4) |
| • | GMO 3 and above |
| • | Radiopharmaceuticals |
| • | Ectoparasiticides |
| • | Sources of ionising radiation (but excluding low energy laboratory scale X-irradiators which have been assessed and approved by Catapult) |
| 8. | CONTRIBUTIONS |
| 8.1 | A risk and capital contribution will be charged throughout the Term calculated at the rate of 10% of each Contribution, except for the Facility Contribution and Business Rates. The risk and capital contribution will remain fixed at the rate of 10% throughout the Term. |
| 8.2 | VAT, if applicable, will be added to all Contributions. The Parties acknowledge that on signature of the Letter Agreement COLLABORATOR made a reservation contribution to Catapult of £200,000. As a result, Catapult will reduce the aggregate of all Contributions due from COLLABORATOR on the Occupation Date by this amount. |
| 8.3 | Any changes to the Contributions (other than the Facility Contributions which are fixed for the Term) will be made once per year based on the new annual budget which will be discussed at the Operational Forum. |
| 8.4 | COLLABORATOR will make the following Contributions to the costs of the Collaboration: |
| 8.4.1 | subject to Clauses 8.1 to 8.3, from the Occupation Date, the Facility Contribution, payable quarterly in advance; |
| 8.4.2 | subject to Clauses 8.1 to 8.3, from the Occupation Date, for each Module occupied by COLLABORATOR, one-fifth of the total costs chargeable against Centre in the form of Business Rates, payable quarterly in advance; |
| 8.4.3 | the Activity Related Input Contributions as they are incurred, due individually from the COLLABORATOR; |
11
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 8.4.4 | subject to Clauses 8.1 to 8.3, from the Occupation Date, the Integral Input Contributions payable quarterly in advance and calculated in accordance with the following provisions of Clauses 8.4.4 (a), (b), and (c): |
| (a) | Catapult will estimate the aggregate Integral Input Contributions incurred for five (5) Modules on the Ground Level in concurrent occupation for the next year (the “Estimated Aggregate Integral Input Contributions”). COLLABORATOR will be responsible for a fixed amount, as set out at Schedule 3, such amount to be based on a 20% share of this Estimated Aggregate Integral Input Contributions. When five (5) Modules on the Ground Level are in concurrent occupation (“Full Occupation”), the contributions model in 8.4.4(b) will apply. In the event the share of the Estimated Aggregate Integral Input Contributions requested from the collaborators for the Year ending 31 March 2019 falls short of the actual amounts incurred by Catapult for that Year, Catapult will not be permitted to include any such shortfall in the COLLABORATOR’S share of the Estimated Integral Input Contributions for the Year beginning 1 April 2019. |
| (b) | From the date Full Occupation is achieved, COLLABORATOR will continue to pay a 20% share of the Estimated Aggregate Integral Input Contributions incurred. However, from and including the first anniversary date (the “First Anniversary Date”) that Full Occupation is achieved, a reconciliation will take place at the end of each Year and a refund or further contribution will be made to the COLLABORATOR with respect to its share of the Integral Input Contributions based on the difference between the Estimated Aggregate Integral Input Contributions, and the pro rata actual aggregate Integral Input Contributions incurred for that Year. |
| 8.4.5 | The Establishment Input Contributions will be payable directly to Catapult as detailed in the Establishment Input Statement. |
| 8.4.6 | Any further contributions payable by COLLABORATOR to Catapult pursuant to this Agreement will be payable within thirty (30) days of receipt of an accurate, complete and valid invoice by COLLABORATOR for such contributions. |
| 8.5 | Catapult will use reasonable endeavours to ensure the Occupation Date is not later than the Expected Occupation Date. In the event the Occupation Date is not achieved by the Expected Occupation Date, Facility Contributions, Business Rates and Integral Input Contributions will only accrue on a pro rata basis from the Occupation Date. |
| 8.6 | By being part of the Centre, COLLABORATOR has access to the wider Catapult supporting infrastructure which includes, but is not limited to, reimbursement support, clinical trial support, process development capability, and regulatory and market access consultancy expertise. The cost of such supporting inputs are to be agreed through separate negotiation and contractual agreement. |
| 8.7 | Catapult undertakes to keep full and proper books of account and records relating to the Integral Input Contributions and the Establishment Input Contributions. In addition COLLABORATOR will be provided with the opportunity to comment on such planned expenditure and consensus sought through participation in the Collaborator Forums (although for clarity, Catapult reserves its discretion in exercising its professional judgment in relation to making any final decisions with respect to the Integral Input Contributions, Activity Related Input Contributions and Establishment Input Contributions incurred, while being consistent with the objectives set out in the terms of reference for the Collaborator Forums, particularly with respect to maintaining a suitable level of services required for robust operation of a licensed facility suitable for late stage clinical and commercial manufacture of ATMPs in the most economical way). |
| 8.8 | At the beginning of each Year during the Term Catapult will provide to all Collaborators a budget setting out all anticipated contributions for the Year with respect to Integral and Activity Related Inputs to be provided in that Year. In addition to this, from the date Full Occupation is achieved, a quarterly statement will be provided to all Collaborators in the Centre comparing actuals to the budgeted amounts. |
| 8.9 | Catapult will procure an audit for each Year during the Term to be carried out by an independent auditor acceptable to all Collaborators. The Audit report will be made available to all Collaborators in the Centre. |
12
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 8.10 | [***]. |
| 8.11 | [***] |
In any event of conflict between this Clause 8.11 of this Agreement and the QTA, the QTA shall prevail
| 9. | CATAPULT INPUTS, FACILITIES AND SUPPORT |
The operation of the Centre is dependent on a range of critical inputs split between:
(i) Integral Inputs are inputs Catapult, using its reasonable judgment considers as fundamental to the operation of the Centre and that all collaborators will draw on in equal measure. Integral Inputs may be varied from time to time by Catapult, after prior discussion and consideration of concerns raised by COLLABORATOR and Other Collaborators in the Operational Forum, in order to cater for the common, but not necessarily universal requirements of the COLLABORATOR and Other Collaborators in the Centre.
(ii) Activity Inputs are inputs that are dependent on COLLABORATOR’s manufacturing processes and activity.
It is a condition of occupation that the Integral Inputs, Establishment Inputs and Activity Related Inputs will be procured through Catapult.
(iii) Additional Inputs are inputs COLLABORATOR requires Catapult to contribute to the Project. These will be arranged through the completion of an Additional Input Agreement in the form set out at Schedule 16 (“Additional Input Agreement”). Once signed by both Parties, the Additional Input Agreement will amend this agreement and an Additional Input will be deemed appended to the list of Additional Inputs at Clause 9.4 and any associated contributions from COLLABORATOR in consideration of the Additional Inputs will be deemed as having been inserted at Schedule 3.
(iii) Establishment Inputs which are activities to be performed by Catapult, in conjunction with COLLABORATOR, to support the On-boarding Project.
| 9.1 | Catapult will provide the following Integral Inputs: |
| 9.1.1 | The Quality Management System and supporting quality assurance function assuring all GMP services contributed by Catapult are maintained in compliance with GMP Requirements; |
| 9.1.2 | management and governance of the Quality Management System GMP compliance process; |
| 9.1.3 | GMP regulatory compliance of the Centre from start up, including handling of associated MHRA compliance governance activity such as routine audits inspections, subject to the following provisions: |
| (a) | From the Occupation Date, if any activity required to ensure GMP regulatory compliance of the Centre, including the handling of associated MHRA compliance governance activity such as inspections (“MHRA Compliance Activity”) impacts any COLLABORATOR Product and/or any COLLABORATOR Manufacturing Process, then the interactions with the MHRA related to such activity will be led by COLLABORATOR with assistance being provided by Catapult. COLLABORATOR will be entitled to nominate representatives to coordinate and accompany all inspections involved in such MHRA Compliance Activity on COLLABORATOR’s behalf; |
| (b) | If any MHRA Compliance Activity required as a result of COLLABORATOR activity in the Module or Centre impacts on any Catapult personnel, Other Collaborator personnel, Catapult activity within the Centre, Other Collaborator(s), or any part of the Centre other than the Module, then the interactions with the MHRA related to such activity will be led by Catapult and assisted by |
13
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| COLLABORATOR. During inspections, Catapult will be entitled to nominate representatives to coordinate and accompany all MHRA activity in this paragraph on Catapult’s behalf; and |
| (c) | To the extent any MHRA Compliance Activity impacts on COLLABORATOR use of the Module or performance of the COLLABORATOR process, Catapult will notify COLLABORATOR of such MHRA Compliance Activity and keep COLLABORATOR informed of the progress and communication relevant to such MHRA Compliance Activity. |
| 9.1.4 | insurance as described in Clause 19; |
| 9.1.5 | safety systems and equipment such as emergency light testing, fire extinguishers, health and safety equipment outside the Manufacturing Space, and associated safety audits; |
| 9.1.6 | a managed reception within Office Hours, and the provision of a mechanism for COLLABORATOR to access the Module at any time; |
| 9.1.7 | all utilities necessary for the operations of the Centre (but not the Manufacturing Space) as set out in Schedule 14; |
| 9.1.8 | support for IT Infrastructure; for the avoidance of doubt, this does not include applications support for COLLABORATOR; |
| 9.1.9 | hosting, maintenance and administration of all IT systems (BMS, EMS, eQMS, LIMS and WMS) plus all required access rights for IT systems required primarily for Centre functions and required even when no collaborators are in occupation (BMS, EMS); |
| 9.1.10 | receipt of incoming patient material and starting material into the Centre within Office Hours; |
| 9.1.11 | a system for booking in and managing COLLABORATOR owned inventory for raw materials, consumables, product contact materials and excipients within the warehouse; |
| 9.1.12 | short term final product and Drug Substance storage subject to terms to be agreed; |
| 9.1.13 | out of hours call out system for all facilities alarms (bar the Manufacturing Space alarms); |
| 9.1.14 | cleaning and disinfection of all areas outside of the Manufacturing Space and outside of any Quality Control laboratory space occupied by any collaborator; |
| 9.1.15 | (save with respect to the Manufacturing Space which is provided for in Clause 9.2.1 and for any Quality Control laboratory space occupied by any collaborator)) perform environmental monitoring in the form of viable and non-viable particulate monitoring required to demonstrate maintenance of the appropriate environmental classifications; |
| 9.1.16 | access to a controlled rate freezer and allocated short term storage capacity at the following temperatures: controlled room temperature, 2-8°C, -20°C, -80°C and gas phase of liquid nitrogen; |
| 9.1.17 | Toilet provision and services in relation to toilets available for COLLABORATOR use; |
| 9.1.18 | Security input as required for general security of Centre; |
| 9.1.19 | program and relationship management; |
14
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 9.1.20 | a kitchen area and vending machines for snacks, hot and cold drinks within the Centre; |
| 9.1.21 | a dedicated secure Manufacturing Office and a Non-Manufacturing Office per Module; and |
| 9.1.22 | any other Inputs which Catapult, using its reasonable judgment, considers as fundamental to the operation of the Centre and that the COLLABORATOR and all Other Collaborators will draw on in equal measure. |
As part of the Integral Inputs, Catapult will also ensure compliance with Applicable Laws and GMP Requirements in relation to the Restricted Access Areas, the Shared Restricted Access Areas or the Accompanied Access Areas by all collaborators. Should any default by any third-party Collaborator be identified by Catapult, Catapult will respond to such default as set out under the terms of the QTA.
| 9.2 | Catapult will provide the following Activity Related Inputs: |
| 9.2.1 | perform environmental monitoring in the form of viable monitoring and non-viable particulate monitoring required to demonstrate maintenance of the appropriate environmental classifications, including undertaking remote non-viable sampling in the Manufacturing Space, provided that the COLLABORATOR is responsible for performing viable sampling in the Manufacturing Space which will then be processed by Catapult; |
| 9.2.2 | measured electrical power to the Manufacturing Space; |
| 9.2.3 | upon request of COLLABORATOR, Manufacturing Space decontamination; |
| 9.2.4 | a measured supply of pharmaceutical grade oxygen, nitrogen, carbon dioxide, and air; |
| 9.2.5 | routine maintenance for the air handling system, including ULPA and HEPA filter changes |
| 9.2.6 | gowning for Catapult staff providing services to the collaborators, (COLLABORATOR gowning is ordered by COLLABORATOR from Catapult warehouse stock); |
| 9.2.7 | Leased telephone line with a data package from the supplier if required; |
| 9.2.8 | transfer of decontaminated clinical, biological and hazardous chemical liquid waste from the liquid waste staging area and arrange its removal from the Centre by appropriately licensed contractors; |
| 9.2.9 | transfer of decontaminated clinical and biological solid waste from the solid waste staging area and arrange its removal from the Centre by appropriately licensed contractors; ; |
| 9.2.10 | packing, and dispatch as described in Schedule 8; |
| 9.2.11 | warehouse movements over and above a fair usage level, such level to be defined prior to the Occupation Date |
| 9.2.12 | access rights to IT systems required for COLLABORATOR activity within the module: eQMS, LIMS, WMS; a stand-by facility and personnel to receive incoming patient material and starting material into the Centre outside of Office Hours; |
| 9.2.13 | provision of Quality Assurance inputs to support for COLLABORATOR operation within the Module; , in terms of handling non-process related deviations, Quality Events and planned changes, cleanroom environmental excursions, governance of Catapult generated GMP data provided to COLLABORATOR, providing GMP documentation to support QP certification of Drug Product; |
15
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 9.2.14 | engineering and maintenance support for COLLABORATOR operation within the Module. |
| 9.3 | Catapult will provide the following Establishment Inputs: |
| 9.3.1 | Catapult will work in cooperation with COLLABORATOR to define and implement an agreed strategy for Technology Transfer, made up of: |
| (a) | Process Transfer: defining, implementing and/or supporting conduct of Process Transfer, but such process under the control and responsibility of COLLABORATOR, and |
| (b) | On-boarding: the Parties agree the process is summarised in Schedule 6, but that the precise break down of responsibilities and the processes will be defined and agreed in the Establishment Input Statement. |
| 9.3.2 | Catapult will clean and decontaminate the Manufacturing Space and ensure the Manufacturing Space is operating at the specified cleanliness grade prior to COLLABORATOR occupation; |
| 9.3.3 | Catapult will decontaminate the Manufacturing Space at termination of the contract; and |
| 9.3.4 | Catapult will select and authorise all Collaborators and ensure their processes and procedures meet the minimum standards required by Catapult, such standard in each case sufficient to meet the requirement of this Agreement including GMP. |
| 9.4 | Additional Inputs |
Additional Inputs to be listed once the process is completed in accordance with Clause 9 (iii) above.
| 9.5 | Steering Committee |
The Parties will each nominate two representatives who will form the steering committee for the Project (“Steering Committee”). Where possible the representatives from both Parties will be the same as those serving on the steering committee for the On-boarding Project. The Steering Committee will meet (by telephone or in person) as required to discuss matters relating specifically to the COLLABORATOR and/or the Project (such as changes to Contributions and Inputs, staffing updates, collaboration performance and resolution of issues). The Steering Committee will also participate in dispute resolution as set out in Clause 33 as required.
| 9.6 | Collaborator Forums |
Catapult undertakes to COLLABORATOR it will ensure that the Collaborator Forums take place in accordance with the frequencies, the parameters, and all other terms set out in Schedule 15.
| 9.7 | Catapult Inputs: Failure or Prevention in Performance |
| 9.7.1 | If COLLABORATOR’s performance of its obligations under this Agreement is prevented or delayed by any act or omission of Catapult, its agents, sub-contractors or employees, COLLABORATOR will not be liable for any costs, charges or loss sustained or incurred by Catapult arising directly or indirectly from such prevention or delay. |
| 9.7.2 | If Catapult delays or does not perform its responsibilities under this Agreement that directly impact COLLABORATOR’S ability to develop the COLLABORATOR Process or to produce the COLLABORATOR Product, COLLABORATOR, directly or through a third-party, may perform such responsibilities in its place and Catapult will compensate COLLABORATOR for any costs it incurs in discharging such responsibilities on Catapult’s behalf. |
16
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 10. | COLLABORATOR RESPONSIBILITIES |
| 10.1 | COLLABORATOR will, and will ensure that COLLABORATOR Personnel will, comply with the following COLLABORATOR Responsibilities: |
| 10.1.1 | abide by the Code of Conduct and all other guidelines and protocols in force from time to time at the Centre; |
| 10.1.2 | perform health and safety and quality risk assessments for all materials and processes before such materials and processes enter or are carried out in (as applicable) the Manufacturing Space and provided always that such materials and processes have first been authorised by Catapult and provide copies of such risk assessments to Catapult on request; |
| 10.1.3 | handle all large volume liquid waste (such as culture media and buffers) within the cleanroom and securely and safely transfer it to the handling area in the Centre (as designated by Catapult from time to time); |
| 10.1.4 | collect all small volume liquid waste in a sealable container within the Manufacturing Space and decontaminate it in-situ before removing it from the cleanroom via the MAL out to a waste staging and disposal area; |
| 10.1.5 | remove all solid waste from the cleanroom via the MAL out to a staging area for removal by the Catapult; |
| 10.1.6 | maintain and implement in accordance with Catapult’s standard operating procedures cleaning regimes for the Manufacturing Space; |
| 10.1.7 | unless otherwise agreed with Catapult, define and implement Process Transfer; |
| 10.1.8 | perform as required by Catapult all appropriate environmental monitoring within the Manufacturing Space and make the plates available to Catapult for analysis; |
| 10.1.9 | obtain, and maintain insurance in accordance with Clause 19; and |
| 10.1.10 | comply with its obligations under the QTA. |
| 10.2 | If Catapult’s performance of its obligations under this Agreement is prevented or delayed by any act or omission of COLLABORATOR, its agents, sub-contractors or employees, Catapult will not be liable for any costs, charges or loss sustained or incurred by COLLABORATOR arising directly or indirectly from such prevention or delay. |
| 10.3 | If COLLABORATOR delays or does not perform its responsibilities under this Agreement, Catapult may perform such responsibilities in its place and COLLABORATOR will compensate Catapult for any costs it incurs in discharging such responsibilities on COLLABORATOR’s behalf. |
| 11. | BACKGROUND INTELLECTUAL PROPERTY |
| 11.1 | Subject to the provisions of this Agreement, COLLABORATOR hereby grants to Catapult a non-exclusive, fully paid-up, royalty-free, licence, under COLLABORATOR’s Background Intellectual Property solely for use in connection with the Project. |
| 11.2 | Subject to the provisions of this Agreement, Catapult hereby grants to COLLABORATOR a non-exclusive, fully paid-up, royalty-free, licence, under Catapult’s Background Intellectual Property to undertake the Project. |
| 11.3 | From the Termination Date, such license will extend to permit COLLABORATOR to replicate the Module, or in the alternative, to such extent as required to enable COLLABORATOR to otherwise replicate the COLLABORATOR Manufacturing Process, or to produce the COLLABORATOR Product, provided it is acknowledged that COLLABORATOR, at its own cost, will need to procure the consents required to use any Third Party Intellectual Property Rights forming any part of the following items that constitute the overall Catapult Background Intellectual Property for use outside the Centre: the Electronic Quality Management System, the Laboratory Information Management System, Warehouse |
17
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| Management System and Environmental Monitoring System. (it is acknowledged Catapult cannot procure the grant of such rights and that if COLLABORATOR does not procure such rights that Catapult accepts no liability whatsoever for claims resulting from breaches of any Third Party Intellectual Property Rights resulting from such inaction). |
| 11.4 | This Agreement does not affect the ownership of any Intellectual Property in any Background Intellectual Property, Know-how, or materials of a Party. Each Party will retain the sole and exclusive ownership rights in and to its Background Intellectual Property and except for the license granted to Catapult in Clause 11.1 and to COLLABORATOR in Clause 11.2, nothing in this Clause 11 will be construed as giving to either Party any rights to use any Background Intellectual Property of the other Party other than as expressly granted by this Agreement. Each Party will treat any other Party’s Background Intellectual Property as Confidential Information belonging to that other Party. |
| 12. | FOREGROUND INTELLECTUAL PROPERTY |
| 12.1 | All Foreground Intellectual Property excluding Catapult Foreground Intellectual Property (as defined in Clause 12.2 below), whether or not it is capable of being a Registered Right will be deemed to be the sole property of COLLABORATOR, regardless of which Party created such Foreground Intellectual Property. COLLABORATOR Foreground Intellectual Property will constitute Confidential Information belonging to COLLABORATOR. COLLABORATOR may take such steps as it may decide from time to time, and at its own expense, to register and maintain any protection for the Foreground Intellectual Property, including filing and prosecuting patent applications. Catapult will ensure that its employees involved in the creation of the Foreground Intellectual Property give COLLABORATOR such assistance as COLLABORATOR may reasonably request in connection with the registration and protection of the Foreground Intellectual Property, including filing and prosecuting patent applications, and taking any action in respect of any alleged or actual infringement of the Foreground Intellectual Property (for clarity, any costs connected with such assistance will be borne by COLLABORATOR). |
| 12.2 | All Foreground Intellectual Property that constitutes an Improvement to the Catapult Background Intellectual Property will be owned by Catapult (“Catapult Foreground Intellectual Property”). Subject to COLLABORATOR meeting the conditions under Clause 11.3, enabling Catapult to grant a license to the Catapult Background IP forming part of any Catapult Foreground Intellectual Property to be licensed under this clause, Catapult grants to COLLABORATOR a non-exclusive, fully paid-up, royalty-free, worldwide, sub-licensable licence under the Catapult Foreground Intellectual Property to undertake the Project. |
Subject to COLLABORATOR meeting the conditions under Clause 11.3, from the Termination Date, such license will extend to permit COLLABORATOR to replicate the Module, or in the alternative, to such extent as required to enable COLLABORATOR to otherwise replicate the COLLABORATOR Manufacturing Process, or to produce the COLLABORATOR Product.
Any costs associated with the licence of any Intellectual Property to COLLABORATOR under this Clause 12.2 will be borne by COLLABORATOR.
| 12.3 | To the extent that any Catapult Foreground Intellectual Property is capable of prospective assignment, COLLABORATOR now assigns the Catapult Foreground Intellectual Property to Catapult; and to the extent any Catapult Foreground Intellectual Property cannot prospectively be assigned, COLLABORATOR will assign such Catapult Foreground IP to Catapult as and when they are created, at the request of Catapult. |
| 12.4 | To the extent that any COLLABORATOR Foreground Intellectual Property is capable of prospective assignment, Catapult now assigns COLLABORATOR Foreground Intellectual Property to COLLABORATOR; and to the extent any COLLABORATOR Foreground Intellectual Property cannot prospectively be assigned, Catapult will assign such COLLABORATOR Foreground Intellectual Property to COLLABORATOR as and when they are created, at the request of COLLABORATOR. |
| 12.5 | In the event any COLLABORATOR Foreground Intellectual Property is required by any Other Collaborator or Catapult in order to operate within the Centre in accordance with GMP and the QTA, COLLABORATOR will grant a non-exclusive licence to such Other Collaborators or Catapult of such COLLABORATOR Foreground Intellectual Property to operate within the Centre. |
| 13. | CONFIDENTIAL INFORMATION |
| 13.1 | The Receiving Party undertakes: |
| 13.1.1 | to maintain as secret and confidential all Confidential Information of the Disclosing Party and its Affiliates; |
| 13.1.2 | where the Receiving Party is Catapult, to use such Confidential Information only for the purposes of making the Centre, Module and Inputs available to |
18
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| COLLABORATOR (and other Collaborators, solely with respect to their Confidential Information) in accordance with this Agreement and the QTA and for exercising its rights under this Agreement and the QTA, or where the Receiving Party is COLLABORATOR, for the purpose of exercising its rights, and complying with its obligations, under this Agreement and the QTA (in each case, a “Permitted Purpose”, and together, the “Permitted Purposes” as it applies to each Party); and |
| 13.1.3 | Subject to Clause 13.3, to disclose such Confidential Information only to those of its employees, officers, contractors, consultants, advisors, legal counsel, Affiliates and sub-licensees pursuant to this Agreement (if any) to whom and to the extent that such disclosure is reasonably necessary for the Permitted Purposes. For clarity, notwithstanding the foregoing, Catapult shall not disclose COLLABORATOR’s or its Affiliates’ Confidential Information (or the Confidential Information of any other entity with which COLLABORATOR or any of its Affiliates is in business negotiations or has contracted or to which it owes a duty of confidence) to any other Collaborator, without (i) COLLABORATOR’s express prior written permission, on a case by case basis, and (ii) Catapult complying with Clause 13.6and ensuring that such collaborator is made aware of the confidential nature of the Confidential Information. |
| 13.2 | The provisions of Clause 13.1 shall not apply to Confidential Information which the Receiving Party can demonstrate by reasonable, written evidence: |
| 13.2.1 | was, prior to its receipt by the Receiving Party from the Disclosing Party or Alternative Disclosing Party, in the possession of the Receiving Party and at its free disposal; |
| 13.2.2 | is subsequently disclosed to the Receiving Party without any obligations of confidence by a Third Party who has not derived it directly or indirectly from the Disclosing Party or Alternative Disclosing Party; |
| 13.2.3 | is or becomes generally available to the public through no act or default of the Receiving Party or its agents, officers, employees, Affiliates, advisors, contractors, consultants, legal counsel or sub-licensees; |
| 13.2.4 | is independently developed by the Receiving Party by individuals who have not had any direct or indirect access to the Disclosing Party’s or Alternative Disclosing Party’s Confidential Information; |
| 13.2.5 | the Receiving Party is required to disclose (and so discloses) to the courts of any competent jurisdiction, or to any government regulatory agency or financial authority, provided that the Receiving Party shall (i) inform the Disclosing Party or Alternative Disclosing Party as soon as is reasonably practicable, and (ii) at the Disclosing Party’s or Alternative Disclosing Party’s request seek to persuade the court, agency or authority to have the information treated in a confidential manner, where this is possible under the court, agency, or authority’s procedures, in which case, if the Confidential Information is treated in a confidential manner by the applicable court, agency or authority, such that it retains its confidential nature, Clause 13.1 shall continue to apply to such Confidential Information; or |
| 13.2.6 | was disclosed by COLLABORATOR pursuant to Clause 13.8. |
| 13.3 | The Receiving Party shall procure that all of its employees, officers, contractors, consultants, advisors, legal counsel, Affiliates and sub licensees pursuant to this Agreement (if any) who have access to any of the Disclosing Party’s or Alternative Disclosing Party’s information to which Clause 13.1 applies, shall be made aware of and subject to these obligations and shall be subject to undertakings of confidentiality at least as restrictive as Clause 13.1 and which apply to the Disclosing Party’s or Alternative Disclosing Party’s Confidential Information before being given access to the Disclosing Party’s or Alternative Disclosing Party’s Confidential Information, and the Receiving Party shall be liable to the Disclosing Party or Alternative Disclosing Party for any breach of its employees, officers, contractors, consultants, advisors, legal counsel, Affiliates or sub licensees of the terms of this Clause 13. |
19
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 13.4 | Upon any termination or expiry of this Agreement, the Receiving Party shall return to the Disclosing Party or Alternative Disclosing Party any documents or other materials that contain the Disclosing Party’s or Alternative Disclosing Party’s Confidential Information, including all copies made, and make no further use or disclosure thereof save that the Receiving Party shall not be obliged to purge or delete Confidential Information of the Disclosing Party or Alternative Disclosing Party from its IT systems that is stored by any automated back-up system, and shall be permitted to retain one (1) copy of all such Confidential information in its legal files solely for purposes of ensuring compliance with the terms of this Agreement, and shall not otherwise use or disclose such Confidential Information. |
| 13.5 | For the avoidance of doubt, and in light of Catapult’s objective to disseminate best practices and foster the development of the regenerative medicine sector in the UK, Catapult shall be entitled to publish or otherwise disclose any of its Confidential Information (but excluding the terms of this Agreement), including the Catapult Background Intellectual Property and Catapult Foreground Intellectual Property. |
| 13.6 | Catapult (a) will procure that each other Collaborator, and any other Third Party contractor or other business partner working under contract with Catapult or any other Collaborator that has access to the Centre, has agreed in writing to obligations of confidence equivalent to those set out in this Agreement with Catapult in relation to COLLABORATOR’s Confidential Information (and COLLABORATOR’s Affiliates’ Confidential Information (and the Confidential Information of any other entity with which COLLABORATOR or any of its Affiliates is in business negotiations or has contracted or to which it owes a duty of confidence)) and (b) will procure that (wherever possible with respect to such Third Parties other than Collaborators, but in all cases for Collaborators) COLLABORATOR will be given a third party right to enforce such confidentiality provisions. |
| 13.7 | For the purposes of this Agreement, Confidential Information shall also include any confidential information disclosed between COLLABORATOR and Catapult under (a) the Pre-existing CDA (defined in the definition of Confidential Information), and (b) the Letter Agreement relating to the Collaboration between the Parties dated 5 October 2018. In relation to Confidential Information disclosed under these prior agreements, the terms of this Agreement shall supersede the terms in those prior agreements. |
| 13.8 | COLLABORATOR shall refer to its intended occupation of the Centre in regulatory filings and COLLABORATOR shall be entitled to disclose the existence of this Agreement if required by applicable law, including any rules or regulations of the U.S. Securities and Exchange Commission (the “SEC”). COLLABORATOR shall provide Catapult with a reasonable opportunity to review and comment on any such proposed disclosure. In the event this Agreement must be included with any report, statement or other document to be filed with such regulatory or other government agency, COLLABORATOR will use good faith efforts to obtain confidential treatment from the SEC, or similar regulatory agency, of Catapult’s confidential financial information or other information of a competitive or confidential nature, and will include in such confidentiality request such provisions of this Agreement as may be reasonably requested by Catapult. |
| 14. | WARRANTIES |
| 14.1 | All Party Warranties |
Each Party warrants, represents and undertakes to the other that:
| 14.1.1 | it has full capacity and authority to enter into and to perform this Agreement; |
| 14.1.2 | there are no: |
| (a) | actions, suits or proceedings pending or, to its knowledge, threatened against or affecting it before any court or administrative body or arbitration tribunal; or |
| (b) | investigations by any Regulatory Authority pending or, to its knowledge, threatened against or affecting it; |
| 14.1.3 | once duly executed, this Agreement will constitute its legal, valid and binding obligations; and |
| 14.1.4 | it is not aware of any matters which might adversely affect its ability to perform its obligations pursuant to this Agreement. |
20
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 14.2 | Catapult Warranties |
Catapult warrants, represents and undertakes to COLLABORATOR that from the Effective Date until the Termination Date:
| 14.2.1 | it will use reasonable commercial endeavours to ensure it will at all times have the ability and all rights, titles and Necessary Consents to perform its obligations under this Agreement; |
| 14.2.2 | comply with its obligations under the QTA; |
| 14.2.3 | it will maintain the Lease and perform all of its obligations thereunder; |
| 14.2.4 | it will provide the Inputs with all due care and skill and in any event in accordance with Applicable Law; and |
| 14.2.5 | it will make available the Centre and the Module in accordance with the provisions of this Agreement and in any event in accordance with all Applicable Law. |
| 14.3 | COLLABORATOR Warranties |
COLLABORATOR warrants, represents and undertakes to Catapult that from the Effective Date until the Termination Date it will:
| 14.3.1 | use reasonable commercial endeavours to ensure it will at all times have the ability and all rights, titles and Necessary Consents to perform its obligations under this Agreement; |
| 14.3.2 | perform both its obligations under this Agreement and all activities in respect of the Project in accordance with all Applicable Law and the Code of Conduct; and |
| 14.3.3 | perform COLLABORATOR’s Responsibilities. |
| 14.4 | Intellectual Property Warranties |
Each Party represents, warrants, and undertakes to the other Party that:
| 14.4.1 | To its knowledge and belief, it has all right, title, and interest in and to its Background Intellectual Property (Subject to the Third Party Intellectual Property Rights in the categories of Catapult Background in Clause 11.3); and |
| 14.4.2 | it has not done, and will not do nor agree to do during the continuation of this Agreement, anything that would be inconsistent with the exercise by the other Party of the rights granted to it under this Agreement. |
| 14.5 | Except as expressly provided in Clause 11.3 and 14.4, neither Party makes any representation nor gives any warranty or undertaking: |
| 14.5.1 | as to the efficacy or usefulness of its Background Intellectual Property; |
| 14.5.2 | that the use of any of its Background Intellectual Property or the exercise of any of the rights granted under this Agreement will not infringe any other Intellectual Property or other rights of any other person; |
| 14.5.3 | that the use of any of its Background Intellectual Property under or in connection with this Agreement will produce Products of satisfactory or merchantable quality or fit for their intended purpose or that any Product will not have any latent or other defects, whether or not discoverable; or |
| 14.5.4 | as imposing any obligation on it to bring or prosecute actions or proceedings against third parties for infringement. |
21
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 15. | INDEMNITY |
| 15.1 | COLLABORATOR agrees to indemnify, and hold Catapult harmless from and against Liabilities that Catapult suffers or incurs arising out of or in connection with: |
| 15.1.1 | any claim or proceedings made, brought or threatened against Catapult by a Third Party in respect of the COLLABORATOR Product or Process, or when in respect of the Project, whenever a Third-Party claim or proceeding is made, brought or threatened against Catapult because of the negligence, omission or wilful misconduct of COLLABORATOR, its employees, agents, visitors or subcontractors; |
| 15.1.2 | any loss of or damage to the tangible property or equipment belonging to a Third Party caused by or resulting from the negligence, omission or wilful misconduct of COLLABORATOR, its visitors, employees, agents or subcontractors; |
| 15.1.3 | any costs relating to an investigation, action or proceeding by a Regulatory Authority which arises as a result of COLLABORATOR’s material breach of this Agreement; |
| 15.2 | Indemnification of Catapult under this Clause 15.1 is conditional upon: (a) the extent that the indemnified claim is caused by or resulting from the negligence, omission or wilful misconduct of Catapult, its employees, agents or subcontractors, or their failure to take any measures as are reasonable in the relevant circumstances to mitigate the loss or damage that has occurred or may occur (in which case any portion of the claim not caused by or resulting from the negligence, omission or wilful misconduct of Catapult, its employees, agents or subcontractors or failure to mitigate will remain valid for indemnification); (b) Catapult promptly, on becoming aware of such claim, notifying COLLABORATOR of the existence of the relevant claim ; (c) Catapult refraining from making any admissions in respect of the relevant claim; and (d) COLLABORATOR having sole control over the defence and/or settlement of the relevant claim. |
| 15.3 | Catapult agrees to indemnify, and hold COLLABORATOR harmless from and against Liabilities that COLLABORATOR suffers or incurs arising out of or in connection with: |
| 15.3.1 | any claim or proceedings made, brought or threatened against COLLABORATOR by a Third Party in respect of the fabric or the operation of the Centre, and/or in respect of any property within the Centre’s demise, whenever a Third-Party claim or proceeding is made, brought or threatened against COLLABORATOR because of the negligence, omission or wilful misconduct of Catapult, its employees, agents, visitors or subcontractors; |
| 15.3.2 | any loss of or damage to the tangible property or equipment belonging to a Third Party caused by or resulting from the negligence, omission or wilful misconduct of Catapult, its visitors, employees, agents or subcontractors; |
| 15.3.3 | any costs relating to an investigation, action or proceeding by a Regulatory Authority which arises as a result of Catapult’s material breach of this Agreement; |
| 15.4 | Indemnification of COLLABORATOR under this Clause 15.3 is conditional upon: (a) the extent that the indemnified claim is caused by or resulting from the negligence, omission or wilful misconduct of COLLABORATOR, its employees, agents or subcontractors, or their failure to take any measures as are reasonable in the relevant circumstances to mitigate the loss or damage that has occurred or may occur (in which case any portion of the claim not caused by or resulting from the negligence, omission or wilful misconduct of COLLABORATOR, its employees, agents or subcontractors or failure to mitigate will remain valid for indemnification); (b) COLLABORATOR promptly, on becoming aware of such claim, notifying Catapult of the existence of the relevant claim ; (c) COLLABORATOR refraining from making any admissions in respect of the relevant claim; and (d) Catapult having sole control over the defence and/or settlement of the relevant claim. |
| 16. | LIMITATION OF LIABILITY |
| 16.1 | Collaborating organisations occupying the Centre generally, and COLLABORATOR and Catapult in particular with respect to this Agreement, in choosing to employ the Centre as a base for GMP manufacturing activities accept and acknowledge a degree of risk inherent in any multi-mode, shared occupancy manufacturing facility and the nature of the biological processes undertaken within. Occasional unforeseen situations may arise associated with, for example utilities, equipment and associated processes that have the potential to disrupt or have a detrimental impact on processing, including on the COLLABORATOR Product(s), and/or the COLLABORATOR Process(es). |
22
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 16.1.1 | In light of this, Catapult will procure from each Collaborator, prior to their occupation of the Centre, contractual agreement not to commence or sustain legal proceedings against COLLABORATOR (or any other Collaborators or Catapult) for damages, or any other financial reimbursement (“Agreement Not to Sue”), as a consequence of any unexpected and unintended consequences as a result of such a situation at the Centre as described in this Clause 16.1 (“Unforeseen Risks”) unless it is a result of attributable gross negligence or wilful misconduct of COLLABORATOR (or any other Collaborators or Catapult, as applicable), breach of confidence, material breach of any obligation under the relevant Collaborator’s (or, COLLABORATOR’S, or Catapult’s) collaboration agreement or quality agreement for the Centre , or material breach of Catapult SOPs or breach of Applicable Laws, by COLLABORATOR (or any other Collaborators or Catapult, as applicable) and shall procure a direct right of enforcement of such Agreement Not to Sue by COLLABORATOR against each such Collaborator or Catapult pursuant to the Contracts (Rights of Third Parties) Act 1999. |
| 16.1.2 | With respect to each Collaborator that Catapult has obtained an enforceable Agreement Not to Sue in accordance with Clause 16.1.1, which COLLABORATOR has a direct right to enforce against such Collaborator pursuant to the Contracts (Rights of Third Parties) Act 1999, COLLABORATOR agrees that it shall not commence or sustain legal proceedings against such a Collaborator for damages, or any other financial reimbursement, as a consequence of any Unforeseen Risks unless it is a result of attributable gross negligence or wilful misconduct of, or a breach of confidence, material breach of any obligation under the relevant Collaborator’s collaboration agreement or quality agreement for the Centre, or material breach of Catapult SOPs or breach of Applicable Laws by, such a Collaborator. |
| 16.1.3 | Catapult and COLLABORATOR, each agree not to commence or sustain legal proceedings against the other Party for damages, or any other financial reimbursement, as a consequence of any Unforeseen Risks unless it is a result of attributable gross negligence or wilful misconduct of the other Party, or is a breach of confidence, material breach of any obligation under this Agreement or the QTA, or a material breach of Catapult SOPs or breach of Applicable Laws, by the other Party. |
| 16.1.4 | This Clause 16.1 is not intended to qualify, and is subject to and without prejudice to, each Party’s rights and obligations under Clause 15 (Indemnity). |
| 16.2 | All Parties are obliged to abide by the operating requirements of the Centre in order to comply with GMP (as defined in the Quality Technical Agreement) and exercise an appropriate duty of care such as to minimise the frequency and severity of events alluded to in Clause 16.1, thus offering each other mutual protection. In order to ensure no Party exposes themselves to unreasonable financial risk as a result of such a situation, Catapult and COLLABORATOR (and Catapult will procure this of each collaborating organisation in occupation of the Centre) will ensure the continued and uninterrupted maintenance of an appropriate level of public liability, business continuity and professional indemnity insurances. |
| 16.3 | Without prejudice to Clauses 15.1, 16.1, 16.5, 16.6 and 16.7, the maximum aggregate Liability of COLLABORATOR which arises from any single event which occurs in any Year will be limited to five million pounds sterling (£5,000,000) for any single event, with no limit on the number of claims made against it from Catapult. |
| 16.4 | Without prejudice to Clauses 15.3, 16.1, 16.5 and 16.6, the maximum aggregate Liability of Catapult which arises from any single event which occurs in any Year will be limited to five million pounds sterling (£5,000,000) for any single event, with no limit on the number of claims made against it from COLLABORATOR. |
23
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 16.5 | In no circumstance will any Party have any Liability for: |
| 16.5.1 | any indirect, special or consequential loss; or |
| 16.5.2 | any loss of profits, revenue, business opportunity, data, or goodwill (in each case whether such loss is direct or indirect). |
| 16.6 | Nothing in this Agreement limits or excludes any person’s liability to the extent that it may not be so limited or excluded by law, including any such liability for death or personal injury caused by that person’s negligence, or liability for fraud or fraudulent misrepresentation. |
| 16.7 | Without prejudice to Clause 16.6, nothing in this Agreement will operate to exclude or restrict COLLABORATOR’s Liability: |
| 16.7.1 | under the indemnity contained in Clause 15; or (in 16.7.2 below, other than when the specific conditions stipulated in the Agreement are met so as to justify otherwise); or |
| 16.7.2 | to pay the Contributions that are correctly incurred under this Agreement; or |
| 16.7.3 | to pay the Compensations. |
| 16.8 | The Parties agree that they have negotiated this Clause 16 and the allocation of risk in this Clause is a fair and equitable position. |
| 17. | DURATION AND TERMINATION |
| 17.1 | This Agreement, and the licences granted hereunder, will come into effect on the Effective Date and, unless terminated earlier in accordance with this Clause 17 or unless specified in the continuing obligations provisions of this Agreement as having continued effect, will continue in force until the date 3 years after the Occupation Date (the “Term”), and on such date this Agreement will terminate automatically by expiry. |
| 17.2 | On the conditions that (a) COLLABORATOR has consistently complied with its obligations in the Collaboration Agreement throughout the Term up to the date the option is exercised, and (b) the Option is exercised not less than 12 months before the end of the Term, then COLLABORATOR will have a six-month period during which it will be entitled to negotiate with Catapult the duration, terms and conditions of an extension to its occupation of a module .At the end of such 6 month period the option will lapse and Catapult will have no obligation to continue such negotiations with COLLABORATOR. |
| 17.3 |
| i. | [***]. |
| ii. | For a maximum period of 12 calendar months from the date that all the modules in the second release of modules at the Centre are available for reservation (the “Option Term”), Catapult grants COLLABORATOR the non-transferrable option to negotiate for one additional module in that second release (the “Option”). |
| iii. | [***]. |
| iv. | [***]. |
| v. | [***]. |
| 17.4 | The Parties may terminate this Agreement at any time by mutual written consent, signed by the authorised signatories of the Parties and the provisions of Clauses 18.1 and 18.2 will not apply. |
| 17.5 | Either Party may elect to terminate this Agreement at any time by notice in writing to the other Party, such notice to take effect as specified in the notice: |
| 17.5.1 | if the other Party is in material breach of this Agreement and, in the case of a breach capable of remedy within 90 days, the breach is not remedied within 90 days of the party receiving notice specifying the breach and requiring its remedy; or |
24
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 17.5.2 | if (A) the other Party becomes insolvent or unable to pay its debts as and when they become due; or (B) an order is made or a resolution is passed for the winding up of the other Party (other than voluntarily for the purpose of solvent amalgamation or reconstruction); or (C) a liquidator, administrator, administrative receiver, receiver, or trustee is appointed in respect of the whole or any part of the other party’s assets or business; or (D) the other Party makes any composition with its creditors; or (E) the other Party ceases to continue its business; or (F) as a result of debt and/or maladministration the other party takes or suffers any similar or analogous action in any jurisdiction. |
| 17.6 | COLLABORATOR may terminate this Agreement at any time after the Effective Date by notice in writing to the Catapult specifying the Termination Date of this Agreement, in which case the provisions of Clause 18.1.1 or 18.1.2 (as applicable) will apply, and this Agreement will end on the Termination Date. |
| 17.7 | COLLABORATOR may terminate this Agreement hereunder immediately upon written notice in the event that Catapult is in material breach of its obligations under Clause 20. Catapult will have no claim against COLLABORATOR for any loss of whatever nature by virtue of the termination of this Agreement in accordance with this Clause 17.7. To the extent that the laws of the territory provide for any such compensation to be paid to Catapult upon the termination of this Agreement, or under circumstances where breach is caused in any part by COLLABORATOR, Catapult hereby expressly agrees to waive (to the extent possible under the laws of the territory) any such compensation. |
| 17.8 | A Party’s right of termination under this Agreement, and the exercise of any such right, will be without prejudice to any other right or remedy (including any right to claim damages) that such Party may have in the event of a breach of contract or other default by the other Party. |
| 18. | CONSEQUENCES OF TERMINATION |
| 18.1 | COLLABORATOR recognises that considerable planning and advance preparation is required to ensure timely COLLABORATOR occupation of the Module. In recognition of the opportunity cost of reserving a Module for COLLABORATOR, if COLLABORATOR serves notice to terminate this Agreement in any circumstances other than as set out in Clause 17.4, or Clause 17.7, COLLABORATOR will compensate Catapult as follows: |
| 18.1.1 | where notice to terminate is served after the Effective Date but before the Expected Occupation Date, COLLABORATOR will pay to Catapult the sum equivalent to 1 year’s income and which will include: the Facility Contribution; the Business Rates; Integral Input Contributions, any Establishment Input Contributions and any other unavoidable costs resulting from the Module remaining unoccupied for up to a period of 1 year (the “Compensations”). Catapult will, to the extent that it is reasonably able to do so in the circumstances, release COLLABORATOR from its obligations where a suitable alternative collaborator is secured by Catapult.; |
| 18.1.2 | Thereafter, where notice to terminate is served from and including the Expected Occupation Date, COLLABORATOR will pay the Catapult Compensations until the earliest to occur of: (i) the expiration of one (1) year, (ii) through the end of the Term, or (iii) the date on which a suitable alternative collaborator (if any) is secured by the Catapult. In the event this Agreement is terminated in accordance with Clause 8.10.1, the Compensations due to Catapult following the Termination Date shall be determined pursuant to Clause 8.10.2. |
| 18.2 | The provisions of Clause 18.1 will not apply where this Agreement is terminated in accordance with Clause 20. |
| 18.3 | Upon termination of this Agreement for any reason (and unless otherwise agreed by the Parties in a subsequent, written agreement, including any agreement entered into in accordance with the provisions of Clause 26.1): |
| 18.3.1 | the provisions of 0, 11, 12, 13, 15 14, 18, and 19 will remain in force; and |
| 18.3.2 | the Collaboration will terminate, subject to any subsisting and continuing obligations. |
25
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 19. | INSURANCE |
| 19.1 | Catapult will take out with a reputable insurance company and maintain at all times during the Term of this Agreement professional indemnity, and public liability insurance including against all loss of and damage to the Module, the Centre, and injury to persons including death arising out of or in connection with this Agreement. Such insurances may be limited in respect of one claim provided that such limit must be at least £5 million (five million pounds). Product liability insurance will continue to be maintained for a further six years from the end of the term of this Agreement. |
| 19.2 | COLLABORATOR will take out with a reputable insurance company, and maintain at all times during the Term of this Agreement public and product liability insurance including against all loss of and damage to the Module and Centre, injury to persons including death arising out of or in connection with this Agreement, and against all loss of and damage to any COLLABORATOR owned equipment, or COLLABORATOR personnel personal effects within the Module or in the Centre generally. Such insurances may be limited in respect of one claim provided that such limit must be at least £5 million (five million pounds). Product liability insurance will continue to be maintained for a further six years from the end of the term of this Agreement. COLLABORATOR acknowledges that Catapult will have no responsibility for any COLLABORATOR owned equipment or any COLLABORATOR personnel personal effects located in the Module or any other part of the Centre. |
| 19.3 | If there is destruction or damage to the Centre that leaves the whole or substantially the whole of the Centre and / or the Module unfit for occupation and use or inaccessible, the Catapult may terminate this Agreement immediately by written notice to COLLABORATOR at any time within the period of 2 years after the date of such damage or destruction. |
| 19.4 | If there is destruction or damage to the Centre that leaves the whole or substantially the whole of the Centre and / or the Module unfit for occupation and use or inaccessible and the Module has not been made fit for occupation and use by COLLABORATOR within 2 years of the date of such destruction or damage then either Party may serve notice on the other to terminate this Agreement with immediate effect, but before such reinstatement is completed. |
| 19.5 | If there is destruction or damage to the Centre by any of the Insured Risks that leaves the whole or substantially the whole of the Centre and / or the Module unfit for occupation and use or inaccessible, then, save to the extent that the Catapult’s insurance has not been vitiated or policy moneys refused because of any act or default of COLLABORATOR then the Facility Charge, the Activity Related Input Contributions, the Integral Input Contributions, and the Business Rates or a fair proportion of them will not be payable from and including the date of such damage or destruction until the earlier of the date that the Module is once again fit for occupation and use and accessible and the date 2 years from and including the date of such damage or destruction. |
| 20. | ANTI-BRIBERY AND ANTI-CORRUPTION |
| 20.1 | Each Party agrees that, in connection with this Agreement and the Projects, they will each, (and will procure that their respective officers, employees, agents and any other persons who perform services for them or on their behalf in connection with this Agreement will): |
| 20.1.1 | not commit any act or omission which causes or could cause the other Party to breach, or commit an offence under, any laws relating to anti-bribery and/or anti-corruption; |
| 20.1.2 | keep accurate and up to date records showing all payments made and received and all other advantages given and received in connection with this Agreement and the steps taken to comply with this Clause 20, and permit the other Party to inspect those records as reasonably required; |
| 20.1.3 | promptly notify the other Party of: |
| (a) | any request or demand for any financial or other advantage received by it (or that person); and |
| (b) | any financial or other advantage it (or that person) give or intend to give |
whether directly or indirectly in connection with this Agreement; and
20.1.4 promptly notify the other Party of any breach of this Clause 20.
26
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 21. | PUBLICITY |
The Parties agree consent is required before use of the other’s name, or any adaptation of their name, or any of their logo(s), trademark(s), or other of their device(s) in any advertising, promotional, or sales materials (however, they also agree that such consent is not to be unreasonably withheld).
| 22. | STATE AID |
| 22.1 | The parties acknowledge that Catapult is a ‘Research Organisation’ as defined under European Union legislation and has an obligation to ensure, and is subject to audits to demonstrate, that all activities it undertakes are compliant with EU state aid rules, including its activities under this Agreement. The parties therefore agree that, notwithstanding any other provision of this Agreement: |
Catapult will be entitled to cooperate fully with any investigation by any grant funder of Catapult or by the European Commission or any court of law with respect to this Agreement regarding the grant/alleged grant of state aid and the provision of Inputs hereunder and COLLABORATOR will, if so requested by Catapult, promptly provide to Catapult all reasonable and necessary assistance in connection with any such investigation(s);
Catapult will keep COLLABORATOR informed of any active and specific investigation into this Agreement and, where possible, liaise with COLLABORATOR concerning any response to the European Commission; and
the parties will comply with any ruling of the European Commission or court of law in relation to the application of the EU state aid rules to this Agreement.
| 22.2 | The obligations set out in Clause 22.1 22.1 above will subsist for a period of 10 years from the date of this Agreement, notwithstanding any earlier termination of this Agreement. |
| 23. | NOTICES |
| 23.1 | Any notice required to be given under this Agreement will be given in writing and sent by prepaid airmail post or courier, delivered personal, or sent by email to the following addresses or such other address as may be notified by the relevant party from time to time in writing: |
| To Catapult: |
To COLLABORATOR: | |
| If sent by post to:
Cell Therapy Catapult 12th Floor Tower Wing Guy’s Hospital Great Maze Pond London SE1 9RT United Kingdom |
If sent by post to:
TCR2 Therapeutics Inc. | |
| For the attention of: | For the attention of: | |
| Matthew Durdy, CBO | Garry Menzel, CEO | |
| If sent by email, to:
matthew.durdy@ct.catapult.org.uk |
If sent by email, to:
garry@tcr2.com | |
| With a copy to:
Margaret Siegel, Corporate Counsel margaret.siegel@tcr2.com | ||
27
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 23.2 | Any notice so sent will be deemed to have been duly given: |
| 23.2.1 | if sent by personal delivery or courier, on delivery at the address of the relevant party; |
| 23.2.2 | if sent by prepaid airmail post, five days after the date of posting; and |
| 23.2.3 | if sent by email, only on acknowledgement of receipt, such acknowledgement not being an automated message. |
| 24. | FURTHER ASSURANCES |
Each party will, as and when requested by the other party and without charge, do all such acts and execute all such documents as may be reasonably necessary to give full effect to the provisions of this Agreement.
| 25. | ENTIRE AGREEMENT |
| 25.1 | This Agreement constitutes the entire agreement between the parties and supersedes and replaces any and all previous agreements, understandings or arrangements between the parties, whether oral or in writing, relating to its subject matter, including the Pre-Existing CDA. |
| 25.2 | The Parties acknowledge that in entering into this Agreement they do not rely on any statement, representation (including, without limitation, any negligent misrepresentation but excluding any fraudulent misrepresentation), warranty, course of dealing, custom, understanding or promise except for those expressly set out in this Agreement. |
| 25.3 | Except as expressly set forth in this Agreement, neither party grants to the other by implication, estoppel or otherwise, any right, title, licence or interest in any Intellectual Property Right. |
| 26. | VARIATION |
| 26.1 | Subject to Clause 26.2, no variation or amendment to this Agreement will be effective unless it is made in writing and signed by the duly authorised representatives of both parties. |
| 26.2 | The following principles will be adhered to in the event a change is proposed by Catapult to Schedule 8 (Warehouse and Procurement Management Provisions), Schedule 9 (Environmental Monitoring), Schedule 10 (IT Infrastructure), and Schedule 12 (Module and Centre Specifications), and Clause 10 only: |
| 26.2.1 | If the proposed change has no material impact on the COLLABORATOR Product(s) or Process(es), or COLLABORATOR’s compliance with GMP guidelines, Catapult may enact the change by a written notification (signed by a member of Catapult’s Quality team) to COLLABORATOR, such written notification forming an amendment to this Agreement; and |
| 26.2.2 | If the proposed change has a material impact on the COLLABORATOR Product(s) or Process(es), such change will require the mutual written consent of the Parties in the form of an amendment to this Agreement signed by authorised signatories of their respective Quality Teams. |
| 26.2.3 | If COLLABORATOR disagrees with Catapult’s assessment that a change under Clause 26.2.1 has no material impact, the matter will be resolved through the use of the Expert Determination Procedure under Schedule 13. |
| 27. | ASSIGNMENT AND SUB-CONTRACTING |
| 27.1 | Subject to Clause 27.2 and except as provided in Clause 27.3, neither Party will assign, sub-contract, mortgage, charge, or otherwise transfer any rights or obligations under this Agreement, without the prior written consent of the other Party. |
| 27.2 | Either Party may assign and transfer all its rights and obligations under this Agreement to an Affiliate or to any company to which it transfers all or substantially all of its assets or business to which this Agreement relates, provided that the assignee undertakes to the other Party to be bound by and perform the obligations of the assignor under this Agreement. However, COLLABORATOR’S right to assign will be subject to Catapult’s consent, such consent not to be unreasonably withheld or delayed, and a Party will not have such a right to assign this Agreement if it is insolvent or any other circumstance described in Clause 17.5.2 applies to it. |
28
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 27.3 | Catapult will be entitled to sub-contract any of its obligations under this Agreement, provided that it will ensure any relevant obligations are passed on to such sub-contractor and Catapult will be responsible for the performance of such sub-contractor. In the event Catapult changes its operating model from that in place on the Expected Occupation Date such that it sub-contracts any additional material obligation not set out under this Agreement, Catapult will provide notice of the same to COLLABORATOR. |
| 28. | WAIVER |
No failure or delay by a party to exercise any right or remedy provided under this Agreement or by law will constitute a waiver of that or any other right or remedy, nor will any single or partial exercise of any right or remedy preclude the further exercise of such right or remedy.
| 29. | SEVERABILITY |
If any provision (or part of any provision) of this Agreement is held to be invalid, void or otherwise unenforceable by a court of competent jurisdiction from whose decision no appeal is available, or from whose decision no appeal is made within the applicable time limit, then the provision (or relevant part of the provision) will be omitted and the remaining provisions of this Agreement (and parts of the relevant provision, as applicable) will continue in full force and effect.
| 30. | RELATIONSHIP OF THE PARTIES |
Nothing in this Agreement is intended to, or will be deemed to, establish or imply any agency, partnership or joint venture between the parties. Neither party will act or describe itself as the agent of the other party and neither party will have, or hold itself out as having any authority to make commitments for or on behalf of the other party.
| 31. | THIRD PARTY RIGHTS |
This Agreement does not create any right enforceable by any person who is not a Party to it save for (i) with respect to Clause 13 which the Parties agree may be directly enforceable (as applicable, as an Alternative Disclosing Party) by any other Collaborator that has occupied the Centre concurrently with COLLABORATOR, provided that COLLABORATOR is able to directly enforce provisions equivalent to Clause 13 with respect to COLLABORATOR’s Confidential Information in a collaboration agreement between such Collaborator and Catapult, and (ii) as provided in Clause 16.1.
| 32. | GOVERNING LAW AND JURISDICTION |
| 32.1 | This Agreement and any dispute or claim arising out of or in connection with it or its subject matter or formation (including non-contractual disputes or claims) will be governed by and construed in accordance with the laws of England and Wales. |
| 32.2 | The parties to this Agreement irrevocably agree that the courts of England will have exclusive jurisdiction to settle any dispute or claim that arises out of or in connection with this Agreement or its subject matter or formation (including non-contractual disputes or claims), except that a Party may seek an interim injunction in any court of competent jurisdiction |
| 33. | DISPUTE RESOLUTION PROCEDURE |
| 33.1 | If a dispute arises out of or in connection with this Agreement or the performance, validity or enforceability of it (Dispute), then, except as expressly provided in specific clauses of this Agreement, the Parties will follow the procedure set out in this clause: |
| 33.1.1 | either Party will give to the other written notice of the Dispute, setting out its nature and full particulars (Dispute Notice), together with relevant supporting documents. On service of the Dispute Notice, the Chief Business Officer of the Catapult, and the head of manufacturing of COLLABORATOR or another authorized representative of COLLABORATOR will attempt in good faith to resolve the Dispute; |
29
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 33.1.2 | if the Chief Business Officer of Catapult and the COLLABORATOR representative are for any reason unable to resolve the Dispute within 30 days of service of the Dispute Notice, the Parties agree to enter into mediation in good faith to settle the Dispute in accordance with the CEDR Model Mediation Procedure. To initiate the mediation, a Party must serve notice in writing (ADR notice) to the other Party to the Dispute, referring the dispute to mediation. A copy of the ADR notice should be sent to CEDR. Unless otherwise agreed between the Parties within 10 days of service of the ADR Notice, the mediator will be nominated by CEDR. Unless otherwise agreed between the Parties, the mediation will start not later than 15 days after the date of the ADR notice. |
| 33.2 | No Party may commence any court proceedings under Clause 32 (Governing Law and Jurisdiction) in relation to the whole or part of the Dispute until 40 days after service of the ADR notice, provided that the right to issue proceedings is not prejudiced by a delay. |
| 33.3 | If the Dispute is not resolved within 40 days after service of the ADR notice, or either Party fails to participate or ceases to participate in the mediation before the expiry of that 40-day period, or the mediation terminates before the expiry of that 40-day period, the Dispute will be finally resolved by the courts of England and Wales in accordance with Clause 32 (Governing Law and Jurisdiction) in this Agreement. |
| 34. | COUNTERPARTS |
This Agreement may be executed in any number of counterparts, each of which when executed and delivered will constitute a duplicate original of this agreement, but all the counterparts will together constitute the same agreement. If this Agreement is executed in counterparts, it will not be effective unless and until each party has executed and delivered a counterpart to the other party.
| 35. | FORCE MAJEURE |
Neither Party will have any liability or be deemed to be in breach of this Agreement for any delays or failures in performance of this Agreement that result from circumstances beyond the reasonable control of that Party, including without limitation labour disputes involving that Party. The Party affected by such circumstances will promptly notify the other Party in writing when such circumstances cause a delay or failure in performance and when they cease to do so.
| 36. | DATA PROTECTION |
| 36.1 | “Data Protection Legislation” means the the EU General Data Protection Regulation 2016/679) and all other applicable legislation relating to privacy or data protection in the United Kingdom. |
| 36.2 | In this Agreement the terms: |
| 36.2.1 | “Personal Data”, “Data Subject” and “Process” are as defined inthe EU General Data Protection Regulation 2016/679) (“Act”). Each Party shall comply with its respective obligations under the provisions of the Act and all applicable Data Protection Legislation; |
| 36.2.2 | “Data Processor” and “Data Controller” are as “Data Processor” and “Data Controller” are defined in the EU General Data Protection Regulation 2016/679). |
| 36.3 | COLLABORATOR shall act as the Data Controller in respect of any Personal Data Processed by Catapult on behalf of COLLABORATOR in connection with this Agreement, the Project and/or Inputs and in compliance with COLLABORATOR’s obligations as such under the Act. Nothing in this Clause 36 relieves Catapult of its own obligations as Data Processor under the Data Protection Legislation; some of the material Processing it will perform, including examples of the Personal Data and categories of data subject connected to this Processing, is broadly (but not exhaustively) set out in the Catapult Data Processing Summary in Schedule 11 to the rear of this Agreement. |
| 36.4 | Insofar as COLLABORATOR provides or otherwise makes available Personal Data to Catapult and such Personal Data is Processed by Catapult, or if Catapult is required to Process Personal Data on behalf of COLLABORATOR in connection with this Agreement, the Project and/or Inputs, COLLABORATOR shall ensure that it has all rights, consents and authority to permit Catapult to lawfully Process such Personal Data and will comply with applicable Data Protection Legislation generally including ensuring that all Personal Data provided or otherwise made available pursuant to this Agreement to Catapult is: |
(a) adequate, relevant and limited to what is necessary for Catapult to discharge its obligations, and to enjoy its rights under this Agreement; and
30
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
(b) accurate and, where necessary, kept up to date.
| 36.5 | In so far as Catapult Processes any Personal Data on behalf of COLLABORATOR, Catapult shall: |
| 36.5.1 | Process the Personal Data on behalf of COLLABORATOR only to the extent necessary for performing this Agreement and only in accordance with the specific written instructions of COLLABORATOR (save to the extent that Catapult considers that such instructions infringe the Data Protection Legislation, in which case Catapult shall notify COLLABORATOR) or as required by any regulator or Applicable Law; |
| 36.5.2 | not otherwise modify, amend or alter the contents of the Personal Data or disclose or permit the disclosure of any of the Personal Data to any third party unless: (i) specifically authorised in writing by COLLABORATOR or (ii) required in order for Catapult to perform its obligations under this Agreement or enjoy its rights under this Agreement (including where such obligations are sub-contracted to a third party); |
| 36.5.3 | implement appropriate technical and organisational methods to maintain the security of such Personal Data and preventing unauthorised or unlawful Processing and against accidental loss, destruction, damage, alteration or disclosure; |
| 36.5.4 | keep and procure that all of its employees, contractors and agents keep, the Personal Data confidential in accordance with Catapult’s confidentiality obligations under Clause 13; |
| 36.5.5 | maintain a record of its Processing activities and, at COLLABORATOR’s cost in respect of Catapult’s out of pocket expenses, provide all information to COLLABORATOR as is reasonably necessary for COLLABORATOR to demonstrate compliance with its obligations pursuant to Article 28 of EU General Data Protection Regulation 2016/679, or any provisions under equivalent legislation that is implemented in the UK after the date of this Agreement. |
| 36.5.6 | permit audits, including inspections, conducted by or on behalf of COLLABORATOR or its regulators including permitting audits to the extent that these are demanded by a Regulatory Authority overseeing compliance with the GDPR. To the extent the following do not contravene the conditions imposed by a Regulatory Authority COLLABORATOR will: not conduct more than one audit per calendar year, give Catapult reasonable (and at least 14 days’) prior written notice of each such audit and ensure that each audit is carried out during Catapult’s normal business hours, so as to cause the minimum disruption to Catapult’s business and without COLLABORATOR or its auditor having any access to any data belonging to a person other than COLLABORATOR; |
| 36.5.7 | notify COLLABORATOR in writing without undue delay and in any event within 48 hours of discovery of, and provide reasonable cooperation (at COLLABORATOR’s cost, except where the breach is caused by Catapult or its agents or subcontractors in which case such cooperation will be provided at Catapult’s cost) in the event of, any breach of security leading to the accidental or unlawful destruction, loss, alteration, unauthorised disclosure of, or access to, Personal Data in Catapult’s possession or control; |
| 36.5.8 | notify COLLABORATOR in writing without undue delay and in any event within ten days of receipt if it receives a request from a Data Subject to have access to that person’s Personal Data, a complaint or request relating to COLLABORATOR’s obligations under Data Protection Legislation or any other communication relating to the Processing of Personal Data, in each case where such request, complaint or communication relates directly to the Processing of Personal Data undertaken by Catapult on behalf of COLLABORATOR under or in connection with this Agreement; and |
31
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 36.5.9 | insofar as is possible, assist COLLABORATOR (at COLLABORATOR’s cost, except where the complaint or request arises from Catapult’s, or its agents or subcontractors processing of that Personal Data other than in accordance with Clause 36, in which case such assistance will be provided at Catapult’s cost) in relation to any complaint or request made by a regulator or data subject in respect of any Personal Data Processed by Catapult on behalf of COLLABORATOR under or in connection with this Agreement. |
| 36.5.10 | at the choice of COLLABORATOR, delete or return all Personal Data to COLLABORATOR promptly after termination or expiry of this Agreement, and delete all copies of the Personal Data (save to the extent that retention of copies is required by Applicable Law or that electronic copies are made on the Catapult IT systems as part of regular business archiving activity, but Catapult will ensure that any such archived electronic copies are kept confidential and in compliance with this Clause 36) providing confirmation of such deletion to COLLABORATOR; |
| 36.6 | COLLABORATOR authorises Catapult to transfer Personal Data outside of the European Economic Area on condition that Catapult shall: |
| 36.6.1 | provide an adequate level of protection to any Personal Data that is transferred outside of the EEA (which may include ensuring that the entity located outside the EEA to which Personal Data is transferred enters into the standard contractual clauses for the transfer of Personal Data from the EU to processors in third countries); and |
| 36.6.2 | comply with any other reasonable instructions notified to it by COLLABORATOR, |
in any event, Catapult confirms that its subcontractors with respect to LIMS and eQMS do not process such Personal Data outside of the European Economic Area.
| 36.7 | COLLABORATOR authorises Catapult to engage other Data Processors and sub-processors in respect of any Personal Data Processing that is undertaken in connection with this Agreement and in accordance with its terms. Where a sub-processor is duly engaged to carry out specific Processing activities on behalf of COLLABORATOR, Catapult shall ensure that it enters into a written contract with such sub-processor containing data protection obligations no less onerous than those set out in this Clause 36 which shall apply to the sub-processor. Catapult shall remain liable for the acts and omission of any such sub-processor. |
| 36.8 | Catapult shall without undue delay (and in any event within 48 hours of it becoming aware) notify COLLABORATOR in the event that it becomes aware of any breach of the Data Protection Legislation by Catapult or any of the subcontractors of Catapult in connection with this Agreement. |
| 36.9 | Subject to Clause 16.6, COLLABORATOR agrees that Catapult shall have no liability (whether in contract, tort, misrepresentation, restitution, under statute or otherwise, including under any indemnities, in each case howsoever caused including if caused by negligence) to COLLABORATOR in connection with any act and/or omission by Catapult to the extent that the liability arises from: |
(a) any breach of the Data Protection Legislation by COLLABORATOR; or
(b) any breach by COLLABORATOR of its obligations under this Clause 36; or
(c) Catapult complying with any written instructions from COLLABORATOR in accordance with Clause 36.5.1.
| 36.10 | Subject to Clause 16.6, Catapult agrees that COLLABORATOR shall have no liability (whether in contract, tort, misrepresentation, restitution, under statute or otherwise, including under any indemnities, in each case howsoever caused including if caused by negligence) to Catapult in connection with any act and/or omission by COLLABORATOR to the extent that the liability arises from: |
(a) any breach of the Data Protection Legislation by Catapult; or
32
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
(b) any material breach by Catapult of its obligations under this Clause 36
| 36.11 | As of the date of this Agreement the Parties acknowledge that no Personal Data has been provided, and that they do not intend to provide Personal Data from either of them to the other but that if this changes they agree they will enter into an amendment to record any change in Schedule 11. |
| 37. | JOINT AND SEVERAL OBLIGATIONS. |
COLLABORATOR covenants and agrees to incorporate and registered a wholly owned subsidiary in England & Whales (the “SUB”). COLLABORATOR will procure that the SUB shall be jointly and severally liable with respect to the obligations of COLLABORATOR under this Agreement by causing the SUB to execute a Joinder Agreement in the form attached in Schedule 17 to confirm its joint and several liability hereunder as soon as practicable following formation of the SUB.
| 38. | NON-SOLICITATION |
During the Term and for a period of twelve (12) months following any termination or expiration of this Agreement, each Party agrees, on behalf of itself and its Affiliates, not to solicit for employment, employ or otherwise retain any employee of the other Party or its Affiliates except with the prior written consent of the other Party; provided, however, that it will not be a violation of the non-solicitation obligation of this Section 38 (Non-Solicitation) if an employee of the other Party responds to an indirect solicitation (e.g., advertisements in media of general circulation).
[Signature Page Follows]
33
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
SCHEDULE 1
WORK STREAMS
| 1. | Development and operation of a COLLABORATOR-operated Manufacturing Space facility for GMP manufacture of COLLABORATOR product, including QMS and QC which will be governed under a separate agreement |
| 2. | Development and operation of aspects of a multi-product manufacturing centre and its associated quality management system that are connected with the production of the COLLABORATOR’s Product |
| 3. | Development and operation of a supply and distribution chain in connection with Work Stream 1 |
34
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
SCHEDULE 2
Part 1 - Occupation of Module
| 1. | Definitions |
In this Schedule 2:
| • | “Co-location Fee” means the aggregate of the Facility Contribution and Business Rates payable under clauses 8.1.1 and 8.1.2 of this Agreement. |
| • | “end of the Licence Period” means the expiry of the Term or its earlier termination pursuant to Clause 17 or 18 of this Agreement and “Licence Period” will have the same meaning as “Term”. |
| 2. | Occupation of the Module |
Catapult permits COLLABORATOR to occupy the Module for the Permitted Use from the later of: (a) the Occupation Date and (b) the date that the QTA is agreed, and entered into by the Parties, until the end of the Licence Period in common with Catapult and all others authorised by Catapult (so far as this is not inconsistent with the rights given to COLLABORATOR to use the Module for the Permitted Use) together with the rights mentioned in Part 2 of this Schedule and subject to the rights reserved to Catapult in Part 3 of this Schedule and subject further to payment of the Co-location Fee in accordance with Clause 8 of this Agreement.
Applicable only when a COLLABORATOR is party to Collaboration Agreements for 2 (two) adjoining modules which share a Restricted Access Area, will COLLABORATOR be provided with exclusive use of and access to the Shared Restricted Access Area between the two modules belonging to COLLABORATOR. For the avoidance of doubt, access to the PAL, MAL and PrAL of the Shared Restricted Access Area is restricted to COLLABORATOR, Catapult and their subcontractors via electronic access control. Access to the grade C corridor of the Shared Restricted Access Area is restricted to COLLABORATOR, Catapult and their subcontractors via Standard Operating Procedures. Catapult permits COLLABORATOR to employ the PrAL for their own purposes, in-line with Centre policies.
| 3. | COLLABORATOR’s Covenants and Acknowledgement |
| 3.1 | COLLABORATOR covenants with Catapult as follows: |
| 3.1.1 | to keep the Module clean, tidy and clear of rubbish; |
| 3.1.2 | not to use the Module other than for the Permitted Use; |
| 3.1.3 | not to make any alteration or addition to the Module or the Centre without the prior written consent of Catapult; |
| 3.1.4 | not to display any advertisement, signboards, nameplate, inscription, flag, banner, placard, poster, signs or notices at the Module or elsewhere in the Centre (that is not on agreed signage areas) without the prior written consent of Catapult, such consent not to be unreasonably withheld or delayed; |
| 3.1.5 | not to do or permit to be done in the Module anything which is illegal or which may be or become a disruption, nuisance (whether actionable or not), annoyance, inconvenience, or disturbance to Catapult, or to other occupiers of the Centre or to the owner or occupier of neighbouring property; |
| 3.1.6 | not to cause or permit to be caused any damage (other than general wear and tear as would be expected from general usage of the Module over time for the Permitted Use) to: |
| 3.1.6.1 | the Module, Centre or any neighbouring property; or |
| 3.1.6.2 | any property of the owners or occupiers of any neighbouring property; |
35
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 3.1.7 | not to obstruct the Common Access Areas, make them dirty or untidy or leave any rubbish on them and to otherwise keep the Common Access Areas free and clear of any equipment, materials or personal property of COLLABORATOR; |
| 3.1.8 | not to apply for any planning permission in respect of the Module unless agreed in advance in writing with Catapult; |
| 3.1.9 | not to do anything that will or might constitute a breach of any Applicable Law affecting the Centre or which will or might vitiate in whole or in part any insurance effected by Catapult in respect of the Centre from time to time and, in the case of the latter, to the extent that Catapult has made COLLABORATOR aware in writing of such requirements under its insurance; |
| 3.1.10 | (in as much as this applies to COLLABORATOR as the end user of any such supplies) to comply with all laws and with any recommendations of the relevant suppliers relating to the supply and removal of electricity, gas, water, sewerage, telecommunications and data and other services and utilities to or from the Module; |
| 3.1.11 | to observe any rules and regulations Catapult makes and notifies to COLLABORATOR from time to time in writing governing COLLABORATOR’s use of the Module and the Common Access Areas; and |
| 3.1.12 | not to do anything on or in relation to the Module and the Centre that would or might cause Catapult to be in breach of Catapult’s covenants and the conditions contained in the Lease, repeated, for reference, in Schedule 1 to this Agreement; and |
| 3.1.13 | to comply with Catapult’s reasonable requests for cooperation with respect to any further development of the Centre and the Module and not to raise any objection to any noise and disturbance resulting from such further development on condition Catapult uses reasonable endeavours to minimise any disruption to the COLLABORATOR’s activities within the Module and the Centre. |
| 3.2 | COLLABORATOR acknowledges that: |
| 3.2.1 | Catapult is entitled to exclusive control and possession of the Centre and the Module and nothing contained in this Agreement creates any relationship of landlord and tenant or any other relationship other than that of a licensor and licensee between Catapult and COLLABORATOR; and |
| 3.2.2 | this Agreement is personal to COLLABORATOR and is not assignable, and the rights set out in Schedule 2 may only be exercised by COLLABORATOR and its employees. |
| 4. | Relocation of Module |
| 5. | Catapult will be entitled, upon not less than 6 months’ written notice to Collaborator, from time to time, to relocate COLLABORATOR to a different location within the Centre provided that |
(a) relocation is not decided upon based on the requirements of any single other collaborator; (b) Catapult has first considered all reasonable alternatives to relocation, while discussing such alternatives with Collaborator; (c) any relocation does not affect Collaborator’s ability to continue manufacture, release and/or make shipment of Collaborator products in accordance with applicable law; (d) Catapult provides written notification to Collaborator of its intention to relocate Collaborator, (e) Collaborator is permitted to continue occupation of the Module for 3 months in tandem with that of the proposed replacement module for the latter 3 months of the 6 month notice period to enable a smooth handover, (f) the alternative module is in all material respects (including size, access and facilities) the same as the Module, (g) the costs and expenses incurred in relocating Collaborator shall be borne by Catapult.
36
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 6. | Termination |
| 6.1 | At the end of the Licence Period: |
| 6.1.1 | COLLABORATOR’s rights to occupy the Module will automatically terminate; |
| 6.1.2 | COLLABORATOR will leave the Module in the same state and condition (taking into account normal “wear and tear” usage), with all fixtures, fittings and equipment as were provided to it by Catapult as recorded in the Schedule of Condition and Inventory referred to at Schedule 7. |
| 6.2 | The termination of COLLABORATOR’s rights to occupy the Module will be without prejudice to any subsisting breach of COLLABORATOR’s obligations contained in this Schedule. |
Part 2 – Rights granted to COLLABORATOR
The following rights are granted to COLLABORATOR in common with Catapult, any person authorised by Catapult and all Other Collaborators and occupiers of the Centre but subject to Catapult’s rights:
| 7. | Running of services |
To connect to and use the existing service media at the Centre for the passage of supplies to and from and to the Module.
| 8. | Access and servicing |
| 8.1 | Access to and from the Module on foot only over the Common Access Areas from time to time designated by Catapult for COLLABORATOR’s use. |
| 8.2 | To use any service area from time to time designated by Catapult for COLLABORATOR’s use for loading and unloading and otherwise servicing the Module and the service roads with or without vehicles to come and go to and from that service area. |
| 9. | Refuse disposal |
To deposit rubbish in any receptacles or waste compactors within the Common Access Areas provided by Catapult for that purpose and designated by Catapult for the use of COLLABORATOR.
| 10. | Support and shelter |
Support and shelter for the Module from the Centre.
| 11. | Parking |
Use of parking spaces available for COLLABORATOR’S specific use, the location of which may be re-designated by Catapult from time to time,
| 12. | Signage |
To exhibit COLLABORATOR’s name in such form, shape and size as approved by Catapult and COLLABORATOR in writing.
| 13. | Toilet facilities |
To use any toilet facilities within the Common Access Areas designated by Catapult as facilities for the use of COLLABORATOR.
| 14. | Escape |
On foot only, in emergencies and for fire escape drills, to use all fire escape routes in the Centre designated by Catapult for the use of COLLABORATOR whether or not forming part of the Common Access Areas.
37
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 15. | QC Lab Space |
COLLABORATOR may at any time request access to, and use of any available of QC Lab Space; Catapult may, reserving its sole discretion, grant access to, and use of such QC Lab Space as may be available at the time of request.
Part 3 – Rights reserved to Catapult
The following rights are excepted and reserved to Catapult and all those authorised by Catapult:
| 16. | Support, shelter, light and air |
| 16.1 | Support and shelter for the remainder of the Centre from the Module. |
| 16.2 | All rights of light or air to the Module that now exist or that might (but for this reservation) be acquired over any other land. |
| 17. | Running of services |
The passage and running of Supplies from and to the remainder of the Centre through existing Conducting Media (if any) within the Module.
| 18. | Entry on to the Module |
| 18.1 | To enter the Module during regular business hours and on not less than 24 hours prior notice, but excluding any period in which COLLABORATOR Products are being manufactured in the Manufacturing Space (unless access is required in order for Catapult to fulfil any obligations under the QTA when no notice will be required) for any purpose including (without limitation) to: |
| 18.1.1 | estimate the current value or rebuilding cost of the Centre for insurance or any other purpose; |
| 18.1.2 | install, inspect, clean, maintain, replace and to take readings from metering equipment, heat cost allocators and thermostatic radiator valves within or relating to the Module and to prepare an energy performance certificate; and |
| 18.1.3 | do anything that Catapult is expressly entitled or required to do under this Agreement or the Lease or for any other reasonable purpose in connection with this Agreement including to inspect the state of repair and condition of the Module. |
| 18.1.4 | To enter the Module to carry out any works to the Module to improve their environmental performance. |
| 18.1.5 | If the relevant work cannot be reasonably carried out without entry onto the Module, to enter them to: |
| 18.1.5.1 | build on or into any boundary or party walls on or adjacent to the Module; |
| 18.1.5.2 | inspect, clean, maintain, repair, alter, decorate, rebuild or carry out works upon the Centre; |
| 18.1.5.3 | carry out any of the necessary inputs; or |
| 18.1.5.4 | for any other reasonable management purpose. |
| 19. | Common Access Areas and Conducting Media |
| 19.1 | In an emergency, or when works are being carried out to them, to close off or restrict access to the Common Access Areas, so long as (except an emergency) alternative facilities are provided that are not materially less convenient. |
| 19.2 | To change, end the use of or reduce the extent of any Common Access Areas or Conducting Media so long as alternative facilities are provided that are not materially less convenient or, if no alternative is provided, the use and enjoyment of the Module is not materially adversely affected. |
38
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 20. | Adjoining Property |
To carry out works of construction, demolition, alteration or redevelopment on Centre and any adjoining property (and to permit others to do so) as Catapult in its absolute discretion considers fit (whether or not these works interfere with the flow of light and air to the Module).
| 21. | Plant, equipment and scaffolding |
The right, where necessary, to bring plant and equipment onto the Module and to place scaffolding and ladders upon the exterior of or outside any buildings on the Centre (including the Module).
39
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Part 4: Plans of the Module and the Centre
[***]
40
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
SCHEDULE 3
Contributions – Initial Budget based on projected Full Occupancy Cost Base
| Contributions | ||||
| 1. | Facility Contributions |
[***] | ||
| 2. | Business Rates |
[***] | ||
| 3. | Integral Inputs Contribution |
[***] | ||
| 4. | Establishment Input Contribution |
[***] |
41
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
SCHEDULE 4
Catapult Background Intellectual Property
| • | Centre design (under licence from a third-party design firm) |
| • | QMS structure and content |
| • | Operating procedures (including, but not limited to: Warehouse; Common areas; Interactions with multiple collaborators; On-boarding process, Process transfer) |
| • | Cleaning and decontamination validation |
42
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
SCHEDULE 5
Code of Conduct
COLLABORATOR Agrees To:
| (a) | Operate in a manner consistent with EU GMP to maintain a compliant multiproduct environment; |
| (b) | Operate in the spirit of the Collaboration; |
| (c) | Respect the confidentiality, privacy and operations of Other Collaborators; |
| (d) | Wherever possible utilise the common infrastructure offered by the collection of cell and gene therapy related organisations in the local area, and nationally (the “Cluster”) in order to increase the benefits of the collaboration, and augment Cluster development, including the development of infrastructure connected with the Cluster ; |
| (e) | Maintain an environment within its Module in accordance with any procedures governing the Centre’s operation and the terms of occupation; |
| (f) | Adhere to facility quality policies and protocol; |
| (g) | Adhere to roles and responsibilities as detailed in the Quality Technical Agreement |
| (h) | Abide by incident reporting requirements communicated by Catapult; and |
| (i) | Operate in compliance with all appropriate environment, health and safety requirements (both national and local). |
43
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 6
New Business Introduction and On-boarding
Introduction
The Catapult is committed to providing excellence in new business introduction for our collaborators. The following objectives underpin the Catapult’s new business introduction philosophy:
| • | To guarantee that new business is introduced in accordance with our collaborators’ expectations; |
| • | To manage the new business introduction process to add value for our collaborators and for Catapult; |
| • | To ensure that new business introductions meet the requirements of the Catapult Quality and EHS policies; |
| • | To be open, honest and accurate in all of our new business introduction communications. |
To develop excellence in our employees through the development of world class project management skills.
The new business introduction process for the Centre is divided into (i) a selection process, (ii) a negotiation phase on a Heads of Terms basis and a subsequent Collaboration Agreement and (iii) the On-boarding phase.
Summary of the Selection process
The new business introduction process starts with the interest of a potential new collaborator in joining the Centre. After signing a CDA (Confidential Disclosure Agreement) Catapult will provide COLLABORATOR with a ‘Pre-Screen Questionnaire’ to complete. This document allows Catapult to assess whether the COLLABORATOR and the planned Collaborator Product(s) meet the requirements of the Centre’s standards. The process should ensure a smooth transition to the negotiation phase of the Collaboration Agreement from a quality and operational perspective.
The On-boarding process for introduction of COLLABORATOR Product and Process into the Module is detailed in the Establishment Input Statement
44
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 7
Schedule of Condition and Inventory of Module Fixtures and Fittings
To be provided under cover of a separate document, signed by both Parties immediately prior to, or contemporaneously with occupation, but incorporated into this Schedule 7 by reference.
45
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 8
Warehouse and Procurement Management Provisions
| 1. | General |
| 1.1 | Catapult is responsible for operating the warehouse area & processes in a GMP compliant manner. In summary this includes goods in, common consumables stock and COLLABORATOR owned stock, storage, picking, delivery and final product storage. |
| 1.2 | Catapult is responsible for the EHS within the warehouse and monitoring storage temperatures. |
| 1.3 | The Centre warehouse is an access controlled area limited to authorised Catapult personnel. COLLABORATOR personnel can only access the warehouse when accompanied by Catapult personnel. |
| 1.4 | Catapult will man the warehouse during Office Hours each week day (excluding bank holidays). |
| 1.5 | Catapult will provide a 24/7 call-out system for unexpected out of Office Hours’ deliveries, etc.as an Additional Input |
| 1.6 | Catapult may facilitate access to additional warehousing outside of the Centre if required by COLLABORATOR and subject to additional contributions. |
| 1.7 | All COLLABORATOR equipment, samples and materials must enter the Centre through the Centre’s goods in warehouse entrance. Samples and materials will be booked onto the Catapult Warehouse management system. |
| 1.8 | Transfer to the Manufacturing Space of all COLLABORATOR equipment, samples and materials must be formally authorised by Catapult personnel. |
| 1.9 | The Centre is considered a forward picking area and as such warehouse space is limited. |
| i. | Catapult will maintain a stock of common consumables. |
| (i) | Each COLLABORATOR will have allocated storage at ambient temperature (15°C to 25°C), 2-8oC, -20oC, -80oC and LN2 for their raw materials, product contact equipment & excipients. Catapult will be responsible for the qualification (IQ and OQ plus equipment-specific PQ with the units under load), monitoring, maintenance and functioning of the storage equipment and areas. Any COLLABORATOR-specific PQ will be an Additional Input. |
| ii. | Visibility of the COLLABORATOR’s inventory is through the warehouse management system (initially a paper based system).. Each collaborator will only have visibility of their inventory items. |
| 2. | Common consumables stock |
| 2.1 | CATAPULT will maintain a stock of an agreed list of commonly used consumables. |
| 2.2 | CATAPULT will be responsible for purchasing this stock or arranging a consignment stock with external vendors for direct purchase by COLLABORATOR, maintaining stock levels, undertaking the appropriate QC & putting the stock away. |
| 2.3 | CATAPULT will invoice the COLLABORATOR for the stock used and purchased by the COLLABORATOR |
| 2.4 | CATAPULT personnel will transfer the consumables to the COLLABORATOR’’s Grade C MAL staging area once a day or as agreed. |
| 2.5 | Common consumables stock will not be segregated between collaborators. |
| 3. | COLLABORATOR owned inventory |
| 3.1 | COLLABORATOR is responsible for the management of its own inventory supply chain including sourcing & auditing suppliers, price negotiation, purchasing and insurance. |
| 3.2 | Before purchasing any stock to be stored in the Centre, COLLABORATOR is responsible for providing a list of the inventory they will store & use within the Centre. Catapult reserves the right to reject any inventory item that is not in compliance with CATAPULT policies & procedures e.g. EHS |
| 3.3 | COLLABORATOR is responsible for the completion & submission of a Material Specification for each item which will include such information as product container size, weight, required stock level, minimum stock level, QC sampling and testing regime. |
| 3.4 | CATAPULT will be responsible for notifying COLLABORATOR when the stock has reached its minimum stock level. The stock will then be re-ordered by COLLABORATOR. |
| 3.5 | COLLABORATOR must give at least 48 hours’ notice of a delivery of their items, and must be delivered during the Office Hours unless by prior agreement. |
| 3.6 | CATAPULT personnel will book the goods into the Warehouse management system, attach appropriate labels, undertake the initial goods inspection, notify COLLABORATOR of the goods receipt (and promptly notify COLLABORATOR of any issues identified on initial goods inspection) and place the goods in a location. Catapult will store COLLABORATOR inventory in a separate location from that of other Collaborators |
| 3.7 | COLLABORATOR is responsible for the management of all retention samples. |
| 3.8 | COLLABORATOR will be responsible for the Quality Control (QC) of their inventory & Pass labelling. |
46
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| 3.9 | If the products fail QC then COLLABORATOR personnel will be responsible for attaching Reject labels. CATAPULT personnel will transfer these Reject products to the relevant Reject product storage area. CATAPULT will store these Reject products for up to 30 days during which time it is expected that the COLLABORATOR will arrange appropriate disposal. If this is not arranged CATAPULT will manage the appropriate disposal with additional costs being charged to the COLLABORATOR. |
| 4. | Picking & delivery of stock for the COLLABORATOR |
| 4.1 | For non-batch related common consumables stock such as clean room clothing CATAPULT manage the supply of these items. |
| 4.2 | For batch specific common consumables and for COLLABORATOR owned materials, COLLABORATOR will provide CATAPULT with a Bill of Materials (BoM) and a schedule identifying when the full or part BoMs are required. |
| 4.3 | A member of the COLLABORATOR staff will formally receive the BoM. If there are any queries or discrepancies with the BoM these will be highlighted & addressed at this time. |
| 4.4 | For Biological material or cold-stored items, through prior arrangement, the COLLABORATOR with a CATAPULT representative will pick and transfer these items to the Manufacturing Space. |
| 5. | Final Product storage |
| 5.1 | The Centre will provide final product and product intermediate storage at the following temperatures: |
| i. | -20oC |
| ii. | -80oC |
| iii. | LN2 (vapour phase) |
| 5.2 | The final product (or any intermediate thereof as requested by COLLABORATOR) will be stored in multi-collaborator storage equipment unless by previous agreement |
| 5.3 | COLLABORATOR can store final product in these storage areas for up to 44 days unless by agreement as an Additional Input for which there may be an Additional Contributions payable. |
| 5.4 | Access to the product storage areas will be strictly controlled and will only be possible when accompanied by an appropriately trained and authorised CATAPULT representative |
| 5.5 | Centre will be responsible for the qualification and maintenance of all equipment in this area including the temperature monitoring system and 24/7 emergency cover |
| 6. | Drug Substance (DS) or Drug Product (DP) packing area |
| 6.1 | CATAPULT will provide either a supervised GMP packing area or perform GMP packing. |
| 6.2 | CATAPULT will provide an area to charge dry shippers with liquid nitrogen. |
| 6.3 | CATAPULT will provide storage area for a reasonable supply of packing materials and boxes. |
| 7. | Drug Substance (DS) or Drug Product (DP) shipping |
| 7.1 | If required CATAPULT will provide access to cold chain GMP compliant courier service. |
| 8. | Examples of the warehouse & logistics Additional Inputs not included within Integral or Activity Related Inputs |
| 8.1 | Out of hours support. |
| 8.2 | Sampling of COLLABORATOR raw materials. |
| 8.3 | QC analysis of COLLABORATOR raw materials. |
| 8.4 | Auditing the COLLABORATOR supply chain. |
| 8.5 | Purchasing the COLLABORATOR raw materials. |
| 8.6 | Storing COLLABORATOR raw materials or starting materials for longer than the specified period. |
| 8.7 | Managing and storing retention samples. |
| 8.8 | GMP packing. |
| 8.9 | Arranging GMP shipping service through a GMP compliant logistics service provider. |
| 8.10 | Offsite additional storage space. |
47
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 9
Environmental Monitoring Schedule
Introduction
Catapult is committed to providing and maintaining manufacturing and manufacturing support environments that are fit for their intended purpose with regard to air quality. These environments will be appropriately controlled and monitored based on the room classification requirements defined in Eudralex Volume 4 Annex 1. This will be achieved by:
| • | The regular application of qualified cleaning procedures to all GMP environments within the Centre |
| • | The training and qualification of personnel to assure the consistent and appropriate execution of gowning and de-gowning procedures |
| • | The development of and adherence to an appropriate environmental monitoring program |
| • | Regular reporting and trending of data generated by the program |
| • | The creation and dissemination of procedures for the appropriate handling of starting materials, raw materials, consumables, samples, in-process and final product and waste within the manufacturing facility |
Summary of the environmental monitoring process
| • | The environmental monitoring (EM) program will be established by CATAPULT to comply with the requirements of Eudralex Volume 4 Annex 1 – Manufacture of sterile medicinal products. |
| • | CATAPULT Quality will establish and periodically reassess (based on historical data) action and alert limits for EM test result values or all types of monitoring. |
| • | CATAPULT will supply and perform routine calibration and servicing of the following calibrated EM sampling and measuring equipment per module for COLLABORATOR use, unless COLLABORATOR elects to employ their own monitoring equipment within isolators. |
| • | 3 portable active air samplers (for ‘in-operation’ viable air monitoring) |
| • | 3 portable non-viable particulate monitors |
| • | 5 fixed sampling points and associated non-viable particulate monitors |
| • | Catapult will maintain a stock in the Catapult warehouse of all the necessary consumables to facilitate COLLABORATORs to undertake viable ‘in-operation’ environmental monitoring (including sufficient TSA & SDA settle plates and contact plates to cover monitoring of the entire daily processing period). |
| • | Responsibility for the execution of the manufacturing facility environmental monitoring program will be shared between Catapult and the COLLABORATORs per the QTA: |
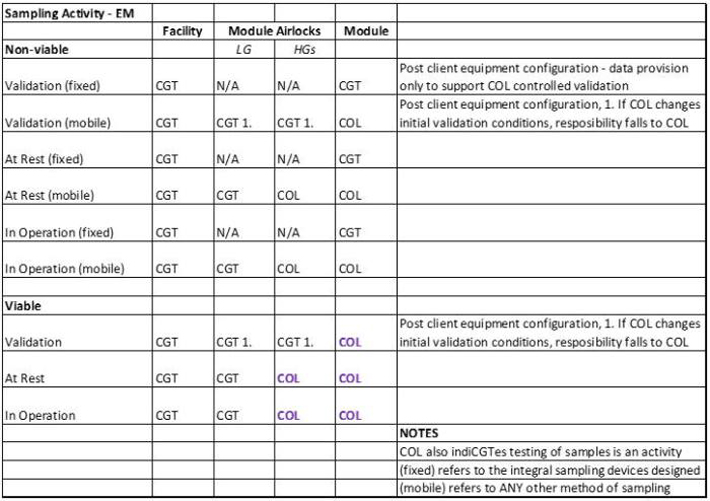
48
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
| • | When requested by COLLABORATOR staff, CATAPULT Technical Services staff are responsible for the delivery of EM consumables to the relevant Materials Air Lock of the COLLABORATOR’s Manufacturing Space |
| • | COLLABORATOR collected EM samples should be appropriately labelled and packaged immediately subsequent to exposure. |
| • | When requested by COLLABORATOR staff, CATAPULT Technical Services staff are responsible for the collection of exposed EM samples, their transportation to CATAPULT QC Microbiology, documentation of their receipt and transfer to QC staff for storage prior to testing. |
| • | CATAPULT QC Microbiology staff are responsible for the appropriate processing of EM samples (incubation, enumeration and speciation as required), documenting the results and providing trended data to COLLABORATOR. |
| • | All Manufacturing Space specific EM data and that collected from sampling of the common Centre areas will be made available to individual collaborators. |
| • | Data will be presented per specific EM session and as a trend graph on a mutually agreed frequency. Data will include the results of any speciation undertaken as a result of an action or alert limit breach. |
| • | Alert limit breach trends and any action limit breaches will result in CATAPULT QC staff raising a record in the Quality Management System to document the event, investigate root cause (with COLLABORATOR assistance if appropriate) and identify the appropriate preventative and corrective actions necessary to mitigate the risk of recurrence. |
49
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 10
IT infrastructure
The Centre will accommodate several collaborators consecutively, each of whom could potentially use the Centre in a different way.
The underlying IT infrastructure has been configured for each module to have its own self-contained secure network. This will allow independent network scenarios, the configuration of these requirements will be carried out, administered and monitored by CATAPULT IT staff.
COLLABORATOR will have its own dedicated secure virtual local area networks (VLANs). Catapult will provide COLLABORATOR all documentation related to the VLAN, including but not limited to, information regarding it’s: configuration, patching, and administration.
All collaborators will however be subject to the CATAPULT’s information security policy.
Internet provision is not provided as standard however we can provide the following:
| • | Synchronous fibre broadband provided by CATAPULT at current market rates. |
| • | COLLABORATOR supplies their own internet connectivity subject to wayleave |
| • | COLLABORATOR organises their own lease Line (PPTP) connectivity between their own sites and the Centre, subject to wayleave. |
Server infrastructure can also be provided by CATAPULT, the options that are available are as follows:
| • | Physical server on the premises – This will be located in one of our communications rooms with restricted access (all access will be accompanied by CATAPULT IT staff) |
| • | Virtual server on the premises – This will be located in the CATAPULT virtual environment; remote access will be provided to the COLLABORATOR – SSL VPNs will be provided for access from external sites. |
| • | Virtual server in private cloud – This will be located on CATAPULT `s own private cloud; remote access will be provided to COLLABORATOR – SSL VPNs will be provided for access from external sites. |
If COLLABORATOR does not wish to use the options above, CATAPULT may offer, subject to availability and feasibility, the following option:
| • | Physical / Virtual server on COLLABORATOR’s own site – An SSL site to site Tunnel will be provided with access controlled by dedicated VLAN`s |
50
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 11
Catapult Data Processing Summary
This Schedule 11 includes certain details of the Processing of Personal Data as required by Article 28(3) GDPR.
| 1 | Controller |
[***]
| 2 | Processor |
[***]
| 3 | Subject matter and duration of processing |
[***]
| 4 | Nature and purpose of processing |
[***]
| 5 | Types of Personal Data (including special categories of data, where applicable) |
The Personal Data Processed concern the following categories of data:
[***]
| 6 | Categories of data subjects |
The Personal Data Processed concern the following categories of Data Subjects:
[***]
| 7 | Obligations and rights of the Controller |
[***]
51
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 12
Module and Centre Specifications
Part A: Manufacturing Space Specification
It :
| • | Is part of a UK-licensed EU-GMP-compliant facility developed in close relationship with the Medicines and Healthcare Products Regulatory Agency |
| • | is of a design, construction, fit and finish in compliance with governing environment, health and safety legislation; |
| • | was designed, built, fitted and finished in compliance with Applicable Law including 2001/83/EC and 2001/20/EC; |
| • | has individual personnel access control |
| • | includes a positive pressure maintained cleanroom with production area of not less than 86m² and a culture room of not less than 15m2; |
| • | has appropriate pressure cascades with negative pressure sinks in all entry and exit routes to minimise the risk of ingress and/or egress of contamination; |
| • | has walk-on ceilings and a technical corridor; |
| • | has a high-efficiency particulate arrestance (“HEPA”) filtered HVAC supplying segregated air as single pass through, with heat recovery; |
| • | has a gas supply delivered through services plates (details of the service plates are set out in Schedule 7); |
| • | has single and three phase power supply, partly with UPS and emergency generator back-up, supplied through service plates (details of the service plates are set out in Schedule 7); and |
| • | has dedicated adjacent material air locks (“MALs”) and dedicated, adjacent personnel air locks (“PALs”) |
Part B: Manufacturing Office and Non-Manufacturing Office Specification
There is:
| • | a Manufacturing Office of not less than 15m2 as set out on the Plans with direct access from the controlled, non-classified corridor (CNC); and designed for occupation by 4 people a Non- Manufacturing Office of not less than 28m2 on the first or second floor of the Centre, as set out in the Plans, and designed for occupation for 2 people |
Both of these offices:
| • | are equipped for normal administrative functions only; |
| • | areequipped with lighting in line with British standards; |
| • | have lockable doors compliant with insurers requirements; |
| • | are furnished with desks, chairs and storage as agreed with the Centre staff, to accommodate the number of occupants they are designed to house |
| • | have small power outlets suitable for normal small office equipment use. The electricity usage will be measured and recharged |
| • | have service media outlets for IT and Telephones. Internet services can be provided on request and recharged as appropriate as an Activity Related Contribution; |
| • | have a telephone for communication with the Catapult. Outgoing calls will be charged at the service provider’s standard rate; and |
| • | are heated and cooled from the central Centre systems. Temperature control will be only via the Building Management System (BMS) under the control of the Centre team. |
52
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Part C: Centre Specifications
Facilities include:
| • | a 2-8°C cold room |
| • | a secure cryo storage area |
| • | a packing and dispatch area; operated in accordance with Schedule 8; |
| • | a solid waste staging area; |
| • | a liquid waste staging and disposal area; |
| • | male and female changing areas to access the controlled non-classified- (CNC) corridor; |
| • | PALs in and MALs in to access the grade C corridor; and |
| • | PrALs to access the CNC corridor |
| • | PALs out and MALs out to access the CNC corridor |
| • | a reception area |
53
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 13
Expert Determination
Definition
Expert: a person appointed in accordance with Clause 7.2.5 to resolve a disagreement as to the acceptability of a new product and/or process, and/or the introduction of any modification to an existing COLLABORATOR Product or Process into the Centre applying the criteria in Clause 7.2.4 OR in accordance with clause 26.2 to resolve a disagreement connected with a change to the schedules listed at that clause.
| 1. | EXPERT |
| 1 | An Expert is a person appointed in accordance with this clause to resolve a disagreement as to the acceptability of a new product or process, and/or the introduction of any modification to an existing COLLABORATOR Product or Process into the Centre. |
| 2 | The Parties shall agree on the appointment of an independent Expert and shall agree with the Expert the terms of their appointment. |
| 3 | If the Parties are unable to agree on an Expert or the terms of their appointment within seven days of either Party serving details of a suggested expert on the other, either Party shall then be entitled to request the Centre for Effective Dispute Resolution (CEDR) to appoint an Expert of professional repute and for the CEDR to agree with the Expert the terms of appointment. |
| 4 | The Expert is required to prepare a written decision including reasons and give notice (including a copy) of the decision to the parties within a maximum of three months of the matter being referred to the Expert. |
| 5 | If the Expert dies or becomes unwilling or incapable of acting, or does not deliver the decision within the time required by this Clause then: |
| (b) | either Party may apply to the London Court of International Arbitration to discharge the Expert and to appoint a replacement Expert with the required expertise; and |
| (c) | this Clause shall apply to the new Expert as if they were the first Expert appointed. |
| 6 | All matters under this clause must be conducted, and the Expert’s decision shall be written, in the English language. |
| 7 | The Parties are entitled to make submissions to the Expert including oral submissions and will provide (or procure that others provide) the Expert with such assistance and documents as the Expert reasonably requires for the purpose of reaching a decision. |
| 8 | Each Party shall with reasonable promptness supply each other with all information and give each other access to all documentation and personnel and/or things as the other Party may reasonably require to make a submission under this clause. |
| 9 | The Expert shall act as an expert and not as an arbitrator. The Expert shall determine the matter under the agreement. The Expert may award interest as part of their decision. The Expert’s written decision on the matters referred to them shall be final and binding on the parties in the absence of manifest error or fraud. |
| 10 | The Expert’s fees and any costs properly incurred by them in arriving at their determination (including any fees and costs of any advisers appointed by the Expert) shall be borne by the Parties equally or in such other proportions as the Expert shall direct. |
| 11 | All matters concerning the process and result of the determination by the Expert shall be kept confidential among the Parties and the Expert. |
| 12 | Each Party shall act reasonably and co-operate to give effect to the provisions of this clause and otherwise do nothing to hinder or prevent the Expert from reaching their determination. |
| 13 | The Expert and Nominating Body shall have no liability to the parties for any act or omission in relation to this appointment; save in the case of bad faith. |
54
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 14
List of Centre Utilities as referenced at Clause 9.1.7
| Utility |
| Natural Gas |
| Electricity |
| Water |
| Sewerage |
55
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 15
Collaborator Forums
The purposes of the collaborator forums (the “Forums”) are:
| • | to facilitate open and transparent exchange of information between Catapult and collaborators, and between collaborators, |
| • | to enable all parties to contribute to the safe, efficient, and successful operation of the Centre, and of the collaborators’ manufacturing activity, |
| • | to enable the standards of the centre to be maintained at an appropriate cost. |
The Forums will be supplemented by regular informal ad-hoc meetings and weekly/bi-weekly surgeries involving Catapult and any collaborator as is necessary.
Key objectives of the Forums include:
| 1. | Updating any requirements needed to continue to maintain a suitable level of services for robust operation of a licensed facility suitable for late stage clinical and commercial manufacture of ATMPs in the most economical way; |
| 2. | Considering COLLABORATOR input into the relevant aspects of the management and operation of the Centre; |
| 3. | Ensuring Catapult and COLLABORATOR compliance with all relevant Quality, Health & Safety and Legal requirements; |
| 4. | Discussing any modifications to any Module or the Centre with the potential to impact any collaborator (Prior to being raised in the relevant Forum, Catapult will consider all collaborator requests for facility modifications that require any Other Collaborator’s cleanroom to be non-operational for any period of time, or that affect the facility license and will discuss feasibility with the all Collaborators. For clarity, such modifications should remain part of the notification to collaborators of the agenda for any Collaborator Forum); |
| 5. | Having formal two-way communications between Catapult and collaborators to discuss common issues; |
| 6. | Raising awareness of issues and incidents with potential for impact on the Catapult and Other Collaborators; |
| 7. | Encouraging and facilitating the sharing of best practice between collaborators; and |
| 8. | Examining appropriate ways of managing costs. |
The Forums will be advisory in their nature and initially take place monthly, with their frequency being reviewed/varied as required. However, the frequency of Forums will be no less than quarterly.
The agenda, format, time and venue will be set and reasonable notice given in advance by Catapult, with the agenda being subject to change based on operational experience and input from collaborators. Relevant issues will be discussed and appropriate recommendations made during the Forums. Outputs of any key decisions that need to be made separately outside of the Forums will be communicated prior to the following meeting.
Key issues and follow-up actions will be summarised and circulated to all Collaborators by Catapult after each Forum.
Collaborators will be fully consulted prior to any key decisions being made. In recognition of the fact that the Catapult has overall responsibility for the operation of the centre Catapult reserves the right to make the final decision in the best interests of all Collaborators and the Catapult.
There will be 3 Forums covering 3 key areas, with the relevant Catapult chairs, Catapult leads, and Terms of Reference being summarised in the table below.
56
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
The Forums with be separated into 3 key areas, with the relevant Catapult chairs, Catapult leads or their representatives, and Terms of Reference being summarised in the table below.
| FORUM |
CATAPULT |
CATAPULT |
TERMS OF REFERENCE | |||
|
Quality Forum |
Director of Quality |
Head of QA |
• Environmental Monitoring Trends
• Collective discussion of recent deviations or changes with a shared impact
• Audit findings (internal and external)
• Audit findings of shared Vendors
• Regulatory Trends
• Best Practise Information
• Training Requirements
• Updates to Facility Management Procedures
• Updates to Foundation Documents
• Quality Agreement Compliance | |||
|
Health & Safety Forum |
Manufacturing Centre Director |
H&S Representative |
• Review of Catapult & collaborator accidents, incidents and near misses since last meeting
• Review of accident, incident and near miss trends
• Review of collaborators EHS concerns
• Catapult H&S update as it relates to:
• People
• Facilities, offices & equipment
• Facility modifications
• Biological & chemical
• Contractor management
• Catapult Environmental update
• New or updated EHS legislation
| |||
|
Operational Forum |
Manufacturing Centre Director |
Operations Lead(s) |
• Summary of current key discussions in the Quality and H&S forum, to ensure that any business-critical topics receive broad attention
• Catapult general operational updates
• Collaborator general operational updates
• Area specific issues/updates
• Welfare
• Process
• Equipment
• Materials & Product
• Waste Management
• IT
• Communications
• Proposed Facility modifications – requirements and costs
• Schedule for any planned shutdowns
• People & Training
• Budgetary and resource issues/updates
• Proposed capital expenditure
• Update on any expected changes in Integral, Activity Related Input Contributions and Additional Input Contributions
• Staff resources |
57
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 16
ADDITIONAL INPUT AGREEMENT NO. [INSERT]
This Additional Input Agreement is entered into between TCR2 Therapeutics Inc. (TCR2) and Cell Therapy Catapult Limited in accordance with, and in relation to, the Collaboration Agreement entered into between TCR2 and Catapult and dated [INSERT] (the “Collaboration Agreement”) [and a licence for alterations entered between TCR2 and Catapult on or about the date of this agreement (“Licence for Alterations”)].
For the avoidance of doubt this Additional Input Agreement makes no amendments or changes to the Collaboration Agreement other than, and solely with respect to, the Additional Inputs under this Additional Input Agreement.
The Collaboration Agreement is otherwise un-amended and unchanged and shall continue in full force and effect.
Collaborator TCR2 Therapeutics Inc. (“TCR2”) TCR2 Manager: [INSERT]
Catapult: Cell Therapy Catapult Limited (“Catapult”) Catapult statement author: [INSERT]
Date of this ADDITIONAL INPUT AGREEMENT:
1. Additional Inputs Required:
a.Title: [INSERT]
b.Project scope and required specification: [INSERT]
c. Sub-contracted Works: [INSERT]
[TCR2 permits the works inserted here to be sub-contracted by Catapult.]
2. Contributions associated with this Additional Input Agreement:
| Inputs |
Contributions | |
| 1. [INSERT] | £XXX.00 | |
| 2. [INSERT] | £XXX.00 | |
| 3. Sub contracted works as set out in section 1c above | £XXX.00 | |
| 4. Sub-contracted works as set out in section 1c above | £XXX.00 | |
| Total cost without 10% capital & risk charge | £XXX.00 | |
| 10% capital & risk charge | £XXX.00 | |
| TOTAL | £XXX.00 |
58
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
All prices are valid until [INSERT]
All costs are subject to vat at the prevailing rate
All costs are subject to a 10% capital and risk charge (itemised in the contributions table above)
Payment Schedule:
Payment will be due within 30 days of receipt of invoice
3. Additional terms required as a result of the changes to be made under this Additional Input Agreement:
[If any of the Works are required to take place outside of normal working hours due to any preference or actions of TCR2, the subcontractors will request an additional payment, which will be charged as a pass-through cost to TCR2.]
[If any of the Works are required to take place outside of normal working hours in order for Catapult to avoid interference with the normal business operation of either Catapult or other collaborators, the subcontractors will request an additional payment, which will be charged as a pass-through cost to TCR2
[Drafter’s Note: Amend as necessary]
4. Responsibility for the specification and/or design of any works performed as part of the Additional Inputs (“Works”):
[The Parties agree that they have worked together to prepare the correct specifications and/or designs for any Works inserted in this Additional Input Agreement under section 1b (the “Specifications”).
Catapult will be responsible only with respect to how the Specifications, and the Works themselves are compatible for use within the Module and/or the Manufacturing Centre. Other than this, Catapult undertakes no liability, and makes no representation or warranty that the Specifications and the Works will be fit for any particular purpose (including with respect to their suitability for TCR2’s Products or Processes, or any other use to which TCR2 will put the Works) or that they will conform with any other specification or quality standard that is not imposed by a regulatory body or authority governing those works.
TCR2 agrees it will have sole responsibility for ensuring the Specification and the Works will be suitable for the TCR2 Products or Processes, or any other use to which it wishes to put the Works to.
59
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
All other liability will be governed under the terms of the [Licence for Alterations and] the Collaboration Agreement (as applicable).] [Drafter’s Note: Amend as necessary]
5. Works Acceptance:
On completion of the Works in accordance with the Specifications set out in Section 1b of this Additional Input Agreement Catapult will issue the final invoice to TCR2.
6. Further Works:
The Parties agree that as part of the ongoing progress of the Works, further works may be required from time to time that are not included in this Additional Input Agreement.
When the need for such further works arise, Catapult will send an email to TCR2’s manager (set out in the header of this Additional Input Agreement) (the “TCR2 Manager”) requesting acknowledgment and formal approval for the further works and attributable costs that will be set out in such email.
TCR2 confirms [the COLLABORATOR’S manager] is legally authorised to bind it and it agrees that an email response from the COLLABORATOR’S manager] confirming acceptance of such further works, their scope and their cost will be a legally binding and valid communication from TCR2 confirming acceptance of such further works and their cost. Catapult confirms that the scope and other terms described within this email will constitute a formal extension of the scope of this original Additional Input Agreement and be subject to the terms of this original Additional Input Agreement.
This Additional Input Agreement is accepted:
| For and on behalf of: TCR2 Therapeutics Inc. |
For and on behalf of Cell Therapy Catapult Limited | |||||||
| Signed: |
|
Signed: |
| |||||
| Full Name: |
|
Full Name: |
| |||||
| Job Title: |
|
Job Title: |
| |||||
60
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Schedule 17
JOINDER AGREEMENT
This Joinder Agreement (“Joinder Agreement”) is executed on , 20 , by the undersigned (“NewCo”) pursuant to the Clause 37 of that certain Collaboration Agreement dated as of December , 2018 (the “Agreement”), by and among TCR2 Therapeutics Inc. (“COLLABORATOR”) and Cell Therapy Catapult Limited (“Catapult”). Capitalized terms used but not defined in this Joinder Agreement shall have the respective meanings ascribed to such terms in the Agreement.
By the execution of this Joinder Agreement, NewCo hereby accepts and becomes a party to the Agreement and shall be jointly and severally liable for all obligations of COLLABORATOR under the Agreement.
AGREED by the duly authorised representative on the date written at the start of this Joinder Agreement:
| For and on behalf of: [NEWCO] |
||||||||
| Signed: |
|
|||||||
| Full Name: |
|
|||||||
| Job Title: |
|
|||||||
ACCEPTED and AGREED by the parties through their duly authorised representatives on the date written at the start of this Joinder Agreement:
| For and on behalf of: TCR2 Therapeutics Inc. |
For and on behalf of Cell Therapy Catapult Limited | |||||||
| Signed: |
|
Signed: |
| |||||
| Full Name: |
|
Full Name: |
| |||||
| Job Title: |
|
Job Title: |
| |||||
61
[***] INDICATES MATERIAL THAT HAS BEEN OMITTED AND FOR WHICH CONFIDENTIAL TREATMENT HAS BEEN REQUESTED. ALL SUCH OMITTED MATERIAL HAS BEEN FILED WITH THE SECURITIES AND EXCHANGE COMMISSION PURSUANT TO RULE 406 PROMULGATED UNDER THE SECURITIES ACT OF 1933, AS AMENDED
Signature Page of Collaboration Agreement
AGREED by the parties through their duly authorised representatives on the date written at the start of this Agreement:
| For and on behalf of: TCR2 Therapeutics Inc. |
For and on behalf of Cell Therapy Catapult Limited | |||||||
| Signed: |
|
Signed: |
| |||||
| Full Name: | Garry E. Menzel | Full Name: |
| |||||
| Job Title: | President and Chief Executive Officer | Job Title: |
| |||||
END OF DOCUMENT
62
