Attached files
| file | filename |
|---|---|
| EX-23.1 - CONSENT OF PARITZ & COMPANY, P.A. - NEXEON MEDSYSTEMS INC | fs12018a9ex23-1_nexeonmedsys.htm |
‘As filed with the Securities and Exchange Commission on December 17, 2018
Registration No. 333-224715
UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM S-1/A
(AMENDMENT NO. 9)
REGISTRATION STATEMENT UNDER THE SECURITIES ACT OF 1933
NEXEON MEDSYSTEMS INC
(Exact name of registrant as specified in its charter)
| Nevada | 3845 | 81-0756622 | ||
| (State or other jurisdiction of | (Primary Standard Industrial | (I.R.S. Employer | ||
| incorporation or organization) | Classification Code Number) | Identification Number) |
1910 Pacific Avenue
Suite 20000
Dallas, Texas 75201
Telephone: (844) 919-9990
(Address, including zip code, and telephone number, including area code, of registrant’s principal executive offices)
William Rosellini
Chief Executive Officer
Nexeon MedSystems Inc
1910 Pacific Avenue
Suite 20000
Dallas, Texas 75201
Telephone: (844) 919-9990
(Name, address, including zip code, and telephone number, including area code, of agent for service)
With copies to:
Gregory Sichenzia Esq. Tara Guarneri-Ferrara, Esq. |
Jeffrey J. Fessler, Esq. Sheppard, Mullin, Richter & Hampton LLP |
| Sichenzia Ross Ference LLP | 30 Rockefeller Plaza, 39th Floor |
| 1185 Avenue of the Americas, 37th Floor | New York, New York 10112 |
| New York, New York 10036 | (212) 653-8700 |
| (212) 930-9700 |
Approximate date of commencement of proposed sale to the public:
As soon as practicable after this Registration Statement is declared effective.
If any of the securities being registered on this Form are to be offered on a delayed or continuous basis pursuant to Rule 415 under the Securities Act of 1933, check the following box: ☒
If this Form is filed to register additional securities for an offering pursuant to Rule 462(b) under the Securities Act, please check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(c) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
If this Form is a post-effective amendment filed pursuant to Rule 462(d) under the Securities Act, check the following box and list the Securities Act registration statement number of the earlier effective registration statement for the same offering. ☐
Indicate by check mark whether the registrant is a large accelerated filer, an accelerated filer, a non-accelerated filer, or a smaller reporting company. See the definitions of “large accelerated filer,” “accelerated filer,” and “smaller reporting company” in Rule 12b-2 of the Exchange Act.
| Large accelerated filer ☐ | Accelerated filer ☐ |
| Non-accelerated filer ☒ | Smaller reporting company ☒ |
| Emerging growth company ☒ |
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ☒
The Registrant hereby amends this Registration Statement on such date or dates as may be necessary to delay its effective date until the Registrant shall file a further amendment that specifically states that this Registration Statement shall thereafter become effective in accordance with Section 8(a) of the Securities Act of 1933, as amended, or until the Registration Statement shall become effective on such date as the Securities and Exchange Commission, acting pursuant to Section 8(a), may determine.
The information in this prospectus is not complete and may be changed. These securities may not be sold until the Registration Statement filed with the Securities and Exchange Commission is effective. This prospectus is not an offer to sell, and is not soliciting an offer to buy these securities in any jurisdiction where the offer or sale is not permitted.
| PRELIMINARY PROSPECTUS | SUBJECT TO COMPETION | DATED DECEMBER 17, 2018 |
2,474,227 Units

Nexeon MedSystems Inc
We are offering 2,474,227 Units, with each Unit consisting of one share of common stock, $0.001 par value per share, and two warrants to purchase shares of common stock, each warrant exercisable for one share of common stock, of Nexeon MedSystems Inc (referred to herein as “we,” “us,” “our,” “Nexeon,” “Registrant,” or the “Company”).
The shares of common stock and warrants comprising the Units are immediately separable upon issuance and will be issued separately in this offering. Each warrant will have an initial exercise price of $ per share ( % of the public offering price of one unit), and will expire five years from the closing of this offering. The offering also includes the shares of common stock issuable from time to time upon exercise of the warrants.
Our common stock is presently quoted on the OTCQB tier of the OTC Markets Group, Inc. (“OTCQB”) under the symbol “NXNN”. In connection with this offering we have applied to have our common stock listed on the Nasdaq Capital Market under the symbol “NXMD” and intend to apply to have the warrants listed on the Nasdaq Capital Market under the symbol “NXMDW.” No assurance can be given that such listing will be approved or that a trading market will develop for the warrants.
On December 14, 2018, the last reported sale price for our common stock on the OTCQB was $4.95 per share. Quotes on the OTCQB may not be indicative of the market price of our common stock on a national securities exchange, including the Nasdaq Capital Market. We effected a 1-for-14 reverse stock split of our outstanding common stock, or, the “Reverse Stock Split”, on June 25, 2018 and, unless otherwise indicated, all per share amounts set forth herein have been retroactively restated to reflect the Reverse Stock Split.
The final public offering price per Unit will be determined through negotiation between us and the underwriter in this offering and will take into account the recent market price of our common stock, the general condition of the securities market at the time of this offering, the history of, and the prospects for, the industry in which we compete, and our past and present operations and our prospects for future revenues. The recent market price used throughout this prospectus may not be indicative of the public offering price per Unit.
We are an “emerging growth company” as the term is used in the Jumpstart Our Business Startups Act of 2012, and, as such, have elected to comply with certain reduced public company reporting requirements for future filings.
Our business and an investment in our securities involve a high degree of risk. See “Risk Factors” beginning on page 8 of this prospectus for a discussion of information that you should consider before investing in our securities.
Neither the Securities and Exchange Commission nor any state securities commission has approved or disapproved of these securities or determined if this prospectus is truthful or complete. Any representation to the contrary is a criminal offense.
| Per Unit | Total | |||||||
| Public offering price | $ | $ | ||||||
| Underwriting discounts and commissions(1) | $ | $ | ||||||
| Proceeds, before expenses, to us(2) | $ | $ | ||||||
| (1) | Does not include a non-accountable expense allowance equal to 1% of the gross proceeds (excluding any proceeds from exercise of the over-allotment option) of this offering payable to ThinkEquity, a division of Fordham Financial Management, Inc. (“ThinkEquity”), the representative of the underwriters. Please refer to “Underwriting” beginning on page 80 of this prospectus for additional information regarding underwriting compensation. |
| (2) | We estimate the total expenses of this offering payable by us, excluding the underwriting discount, will be approximately $475,000. |
We have granted the underwriters a 45-day option to purchase up to an aggregate of 371,134 additional shares of common stock and/or warrants to purchase up to 742,268 additional shares of common stock (equal to 15% of the common stock and warrants included within the units sold in the offering) in any combination thereof, solely to cover over-allotments, if any. We have also agreed to issue to the representative of the underwriters warrants to purchase a number of shares of our common stock equal to an aggregate of 6% of the common stock sold in this offering, not including any shares of our common stock sold in connection with the exercise by the underwriters of the over-allotment option.
The underwriters expect to deliver the shares of common stock and warrants against payment therefor on or about , 2018.
Sole Book-Running Manager
ThinkEquity
a division of Fordham Financial Management, Inc.
Co-Manager
Dougherty & Company
The date of this prospectus is ______, 2018
You should rely only on the information contained in this prospectus or in any free writing prospectus that we may specifically authorize to be delivered or made available to you. We have not authorized anyone to provide you with any information other than that contained in this prospectus or in any free writing prospectus we may authorize to be delivered or made available to you. We take no responsibility for, and can provide no assurance as to the reliability of, any other information that others may give you. This prospectus may only be used where it is legal to offer and sell our securities. The information in this prospectus is accurate only as of the date of this prospectus, regardless of the time of delivery of this prospectus or any sale of our securities. Our business, financial condition, results of operations, and prospects may have changed since that date. We are not making an offer of these securities in any jurisdiction where the offer is not permitted.
For investors outside the United States: We have not and the underwriter has not taken any action that would permit this offering or possession or distribution of this prospectus in any jurisdiction where action for that purpose is required, other than in the United States. Persons outside the United States who come into possession of this prospectus must inform themselves about, and observe any restrictions relating to, the offering of the securities covered hereby the distribution of this prospectus outside the United States.
This prospectus includes statistical and other industry and market data that we obtained from industry publications and research, surveys, and studies conducted by third parties. Industry publications and third-party research, surveys, and studies generally indicate that their information has been obtained from sources believed to be reliable, although they do not guarantee the accuracy or completeness of such information. We believe that the data obtained from these industry publications and third-party research, surveys, and studies are reliable. We are ultimately responsible for all disclosure included in this prospectus.
We further note that the representations, warranties, and covenants made by us in any agreement that is filed as an exhibit to the Registration Statement of which this prospectus is a part were made solely for the benefit of the parties to such agreement, including, in some cases, for allocating risk among the parties to such agreements, and should not be deemed to be a representation, warranty, or covenant to you. Moreover, such representations, warranties, or covenants were accurate only as of the date when made. Accordingly, such representations, warranties, and covenants should not be relied on as accurately representing the current state of our affairs.
This summary highlights information contained elsewhere in this prospectus, and does not contain all the information that you should consider in making your investment decision. Before investing in our common stock, you should carefully read this entire prospectus, including our financial statements and the related notes and information set forth under the headings “Risk Factors” and “Management’s Discussion and Analysis of Financial Condition and Results of Operations” in each case included elsewhere in this prospectus.
Unless the context otherwise requires, references to “we,” “our,” “us,” “Nexeon,” or the “Company” in this prospectus mean Nexeon MedSystems Inc, a Nevada corporation, on a consolidated basis with its wholly owned subsidiaries, as applicable. Unless otherwise indicated, except for our financial statements and the notes thereto, all share amounts and per share amounts in this prospectus have been presented to reflect the 1-for-14 reverse stock split of our outstanding shares of common stock that occurred on June 25, 2018 (the “Reverse Stock Split”).
Business Overview
We are a medical device company focused on the development, manufacturing, and commercialization of neurostimulation technology for the treatment of various neurological disorders through electrical stimulation of neural tissues. We believe our neurostimulation technology platform has the potential to provide treatment to patients in several established neurostimulator markets, including deep brain stimulation (DBS), peripheral electrical nerve stimulation (PENS), sacral nerve stimulation (SNS), spinal cord stimulation (SCS), vagus nerve stimulation (VNS), and other emerging neurostimulator markets.
We expect that our first commercial application of our platform will be the Viant™ Deep Brain Stimulation System (or the Viant™ System). We plan to pursue regulatory approval of the Viant™ System for Parkinson’s disease, essential tremor, and dystonia in Europe in 2019, and for Parkinson’s disease in the United States in the second half of 2019. The Viant™ DBS device is designed to deliver best-in-class stimulation with the capability to collect local field potential (“LFP”) recordings. Using LFP surveys, neurologists will be able to quickly and confidently determine where to stimulate to take full advantage of directional leads. Moreover, our devices are designed to be non-invasively upgradable, enabling both physicians and patients to benefit from the latest technology as it is developed, without the need for implantable pulse generator (“IPG”) replacement surgery to take advantage of new features.
The Viant™ System continues to meet critical milestones in its development program. Previous generations of the device received a certification mark that indicates conformity with health, safety, and environmental protection standards for products sold within the European Economic Area (“CE Mark”) and were manufactured and sold to GlaxoSmithKline plc, a British pharmaceutical company. We completed the ISO 13485 certification process, which is a pivotal hurdle, prior to regulatory submissions to the CE Mark authorities. Design verification, process validation, and testing requirements are nearly complete, and management expects we will complete the technical file in the first half of 2019. We expect to receive a CE Mark in early 2020. As related to the United States, we completed the pre-submission meetings with the United States Food and Drug Administration (“FDA”) in early 2018 to determine scope of requirements for approval of the Viant™ System. As a result of that meeting, we intend to submit an FDA Premarket Approval (“PMA”) application without a clinical study. Although the FDA is allowing us to submit an application for a PMA that leverages the clinical data from a previously approved product in accordance with Section 216 of the Food and Drug Administration Modernization Act of 1997, no assurance can be given that our application which will be submitted without a clinical study will be sufficient for approval by the FDA.
We have additional opportunities to license the neurostimulator platform to companies focused on enhancements to a comprehensive system offering for closed-loop, chronic disease therapeutics, including advanced computational biology, deep learning utilizing Internet of Medical Things technology, imaging solutions, e-health programs, and big data management and optimization, among others. Once we complete the development of the platform for our DBS application, we anticipate being able to potentially license the platform to capitalize on hundreds of diseases of the nervous system that might be therapeutically addressed with neurostimulation.
1
We also operate our wholly owned subsidiary, Medi-Line, a Belgium company, which currently serves over 30 medical device customers in 16 countries, including multi-year contracts with Fortune 500 healthcare companies. The Belgian manufacturer owns state-of-the-art facilities, which feature two validated clean rooms (one assembly cleanroom Class ISO 7 or C, and one extrusion/injection molding cleanroom Class ISO 8 or D) and 600m2 of production space. Their capabilities will enable us to de-risk our commercial launch and speed the development of our neurostimulation products.
Our operations to date include research and development activities.
Our consolidated operations include operations of the following wholly owned subsidiaries: Nexeon MedSystems Europe, SARL (“Nexeon Europe”), Nexeon MedSystems Puerto Rico Operating Company Corporation (“NXPROC”), NMB (which owns and operates Medi-Line), SA, and Pulsus Medical, LLC (“Pulsus”). Nexeon Europe is the holding company for NXPROC and NMB. NXPROC is focused on advanced computational biology and deep learning utilization associated with the Internet of Medical Things technology. Pulsus conducts research and development related to cardiovascular disease technology.
Corporate History
We were incorporated in the State of Nevada on December 7, 2015. Our principal corporate office is located at 1910 Pacific Avenue, Suite 20000, Dallas, Texas 75201, and our phone number is (844) 919-9990. Our Internet address is www.nexeonmed.com.
Risks:
We are a development-stage company and have generated minimal revenues to date. Since our inception, we have incurred substantial losses. Our business and our ability to execute our business strategy are subject to a number of risks of which you should be aware before you decide to buy our common stock. In particular, you should carefully consider the risks which are in “Risk Factors” beginning on page 8 of this prospectus.
2
Implications of Being an Emerging Growth Company
We qualify as an “emerging growth company” as defined in the Jumpstart Our Business Startups Act of 2012. As an emerging growth company, we may take advantage of specified reduced disclosure and other requirements that are otherwise applicable generally to public companies. These provisions include:
| ● | Being permitted to provide only two years of audited financial statements in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; |
| ● | Reduced disclosure obligations regarding executive compensation arrangements; |
| ● | Not being required to hold a non-binding advisory vote on executive compensation or golden parachute arrangements; and |
| ● | Exemption from the auditor attestation requirement in the assessment of our internal control over financial reporting. |
We have elected to use the extended transition period for complying with new or revised accounting standards under Section 102(b)(1) of the JOBS Act. This election allows us to delay the adoption of new or revised accounting standards that have different effective dates for public and private companies until those standards apply to private companies. As a result of this election, our financial statements may not be comparable to companies that comply with public company effective dates.
We will remain an emerging growth company until the earlier of (i) the last day of the fiscal year (a) following the fifth anniversary of the date we completed our initial public offering, (b) in which we have total annual gross revenue of at least $1.07 billion, or (c) in which we are deemed to be a large accelerated filer, which means the market value of our common stock that is held by non-affiliates exceeded $700.0 million as of the prior June 30th, or (ii) the date on which we have issued more than $1.0 billion in non-convertible debt during the prior three-year period. We may choose to take advantage of some but not all of these exemptions. We have taken advantage of reduced reporting requirements in this prospectus. Accordingly, the information contained herein may be different than the information you receive from other public companies in which you hold stock.
Notwithstanding the above, we are also currently a “smaller reporting company,” meaning that we are not an investment company, an asset-backed issuer, or a majority-owned subsidiary of a parent company that is not a smaller reporting company and has a public float of less than $75 million and annual revenues of less than $50 million during the most recently completed fiscal year. In the event that we are still considered a smaller reporting company at such time as we cease to be an emerging growth company, the disclosure we will be required to provide in our filings with the SEC will increase, but will still be less than it would be if we were not considered either an emerging growth company or a smaller reporting company. Specifically, similar to emerging growth companies, smaller reporting companies are able to provide simplified executive compensation disclosures in their filings; are exempt from the provisions of Section 404(b) of the Sarbanes-Oxley Act of 2002 (the “Sarbanes-Oxley Act”), requiring that independent registered public accounting firms provide an attestation report on the effectiveness of their internal control over financial reporting; and have certain other decreased disclosure obligations in their SEC filings, including, among other things, only being required to provide two years of audited financial statements in their annual reports.
3
THE OFFERING
The following summary contains basic information about our securities and the offering, and is not intended to be complete. It does not contain all the information that may be important to you. For a more complete understanding of our securities, you should read the section entitled “Description of Capital Stock” in this prospectus. All per share amounts, unless otherwise indicated, reflect the effectuation of the Reverse Stock Split.
| Securities offered by us | 2,474,227 Units, each Unit consisting of one (1) share of common stock and two (2) warrants to purchase shares of common stock, each warrant exercisable for one share of common stock. The shares of common stock and warrants comprising the Units are immediately separable upon issuance and will be issued separately in this offering. Each warrant will have an initial exercise price of $ per share ( % of the public offering price of one Unit), and will expire five years from the closing of this offering. The offering also includes the shares of common stock issuable from time to time upon exercise of the warrants. | |
| Assumed public offering price | $4.85 per Unit (based on the closing price of the common stock on the OTCQB on December 4, 2018) | |
| Common stock to be outstanding after this offering | 4,439,873 shares of common stock (not including shares of common stock issuable upon exercise of the warrants) (4,811,007 shares if the underwriter’s over-allotment option is exercised in full). | |
| Overallotment option | We have granted the underwriters a 45-day option to purchase up to an aggregate of 371,134 additional shares of common stock and/or warrants to purchase up to 742,268 additional shares of common stock (equal to 15% of the common stock and warrants included within the units sold in the offering) in any combination thereof, solely to cover over-allotments, if any. | |
| Use of proceeds | We intend to use the net proceeds received from this offering for seeking approval for the CE mark and PMA for our DBS device (approximately $5.2 million), filing a de novo request for our non-invasive auricular vagus nerve stimulator (aVNS) withdrawal product (approximately $1.2 million), European and United States sales launch for our DBS device (approximately $2.1 million), pre-payment of the outstanding senior secured convertible promissory note to Leonite Capital, LLC (approximately $1.3 million, including $1.1 million of principal and $145,600 in pre-payment penalties), working capital and general corporate purposes. See “Use of Proceeds.” | |
| Risk factors | See “Risk Factors” beginning on page 8 of this prospectus and the other information included in this prospectus for a discussion of factors you should carefully consider before investing in our securities. | |
| OTCQB trading symbol | NXNN. | |
| Proposed Nasdaq trading symbol and listing | We have applied to have our common stock listed on the Nasdaq Capital Market under the symbol “NXMD.” We intend to apply to have the warrants listed on the Nasdaq Capital Market under the symbol “NXMDW.” No assurance can be given that such listing will be approved or that a trading market will develop for the warrants. |
Unless we indicate otherwise, all information in this prospectus assumes no exercise by the underwriters of the over-allotment option or of the underwriter warrants, gives pro forma effect to the Reverse Stock Split and the corresponding adjustment of all common stock price per share and stock option and warrants exercise price data, except for the financial statements and the notes thereto, and is based on 1,965,646 shares of common stock issued and outstanding as of December 4, 2018, and excludes:
| ● | 261,089 shares of our common stock issuable upon exercise of outstanding vested options at a weighted average exercise price of $14.09 per share as of December 4, 2018, and 84,647 shares of our common stock issuable upon exercise of outstanding unvested options at a weighted average exercise price of $12.95 per share as of December 4, 2018 and 104,264 shares of common stock that remain available for issuance under the Company’s 2016 Omnibus Incentive Plan (the “2016 Plan”); | |
| ● | 82,926 shares of our common stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $19.39 per share as of December 4, 2018; and | |
| ● | 123,429 shares of our common stock issuable upon the conversion of the Leonite Capital, LLC senior secured convertible promissory note with an outstanding principal balance in the amount of $1,080,000 at a conversion price of $8.75 per share as of December 4, 2018. |
4
SUMMARY CONSOLIDATED FINANCIAL DATA
The following table summarizes our consolidated financial data. We have derived the summary consolidated balance sheet data as of September 30, 2018 and consolidated statements of operations data for the years ended December 31, 2017 and 2016 and the nine months ended September 30, 2018 and September 30, 2017 from our audited and unaudited consolidated financial statements include elsewhere in this prospectus. Our historical results are not necessarily indicative of our results in any future period. You should read the following summary consolidated financial data together with “Management’s Discussion and Analysis of Financial Condition and Results of Operations,” as well as our consolidated financial statements and the related notes included elsewhere in this prospectus. The summary consolidated financial data in this section are not intended to replace our consolidated financial statements and the related notes, and are qualified in their entirety by the consolidated financial statements and related notes included elsewhere in this prospectus.
| For the Nine Months Ended | For the Years Ended | |||||||||||||||
| September 30, | December 31, | |||||||||||||||
| Consolidated Statement of Operations Data: | 2018 | 2017 | 2017 | 2016 | ||||||||||||
| Revenues | $ | 7,666,827 | $ | 1,147,288 | $ | 3,302,775 | $ | 1,494,881 | ||||||||
| Cost of revenue | 5,533,173 | 737,664 | 2,321,756 | 39,129 | ||||||||||||
| Gross profit | 2,133,654 | 409,624 | 981,019 | 1,455,752 | ||||||||||||
| Selling, general and administrative expenses | 2,724,960 | 1,817,218 | 2,831,069 | 693,603 | ||||||||||||
| Research and development expenses – other | 1,881,198 | 1,659,560 | 2,942,981 | 751,434 | ||||||||||||
| Research and development expenses – related party | — | — | — | 8,068 | ||||||||||||
| Depreciation and amortization | 1,095,075 | 924,790 | 1,297,710 | 636,921 | ||||||||||||
| Income (loss) from operations | (3,567,579 | ) | (3,991,944 | ) | (6,090,741 | ) | (634,274 | ) | ||||||||
| Other Income (expense) | ||||||||||||||||
| Interest income | — | 3,216 | ||||||||||||||
| Interest income – related party | — | 2,009 | 2,036 | 19,049 | ||||||||||||
| Gain on bargain purchase | — | 624,211 | 4,311,554 | — | ||||||||||||
| Interest expense | (235,869 | ) | (31,762 | ) | (113,967 | ) | (13,738 | ) | ||||||||
| Loss on stock exchange | — | (37,788 | ) | (37,788 | ) | — | ||||||||||
| Write-off of related party loan | — | (171,946 | ) | (174,252 | ) | — | ||||||||||
| Loss on impairment of asset | — | — | (74,483 | ) | (173,500 | ) | ||||||||||
| Gain on disposition of patent | 160,000 | — | — | — | ||||||||||||
| Loss before provision (benefit) for taxes | (3,643,448 | ) | (3,604,004 | ) | (2,177,641 | ) | (802,463 | ) | ||||||||
| Provision (benefit) for taxes | (576,224 | ) | (13,203 | ) | — | — | ||||||||||
| Net (loss) | $ | (3,067,224 | ) | $ | (3,590,801 | ) | $ | (2,177,641 | ) | $ | (802,463 | ) | ||||
| Other comprehensive income | ||||||||||||||||
| Foreign currency translation adjustment | 22,147 | 5,002 | (15,719 | ) | (23,411 | ) | ||||||||||
| Comprehensive (loss) | (3,045,077 | ) | (3,585,799 | ) | (2,193,360 | ) | (825,874 | ) | ||||||||
| BASIC AND DILUTED PER SHARE DATA: | ||||||||||||||||
| Net (loss) per common share, basic and diluted | $ | (1.56 | ) | $ | (2.08 | ) | $ | (1.23 | ) | $ | (0.59 | ) | ||||
| Weighted average common shares outstanding, basic and diluted | 1,969,719 | 1,726,426 | 1,775,803 | 1,360,344 | ||||||||||||
| Net income (loss) per common share, basic and diluted and Weighted average common shares outstanding, basic and diluted restated giving effect to the Reverse Stock Split. |
5
| September 30, 2018 | ||||||||
| Consolidated Balance Sheet Data: | Actual | As
Adjusted(1) (2) | ||||||
| Cash and cash equivalents | $ | 125,573 | $ | 10,810,573 | ||||
| Working capital | (1,928,566 | ) | 8,756,434 | |||||
| Total assets | 18,577,891 | 29,262,891 | ||||||
| Total liabilities | 9,438,937 | 9,438,937 | ||||||
| Total stockholder’s equity | $ | 9,138,954 | $ | 19,823,954 | ||||
| (1) | Gives effect to the issuance and sale of the Units in this offering assuming a public offering price of $4.85 per Unit, and after deducting the estimated underwriting discount and estimated offering expenses payable by us. |
| (2) | A $1.00 increase (decrease) in the assumed public offering price of $4.85 per Unit would increase (decrease) each of cash and cash equivalents, working capital, total assets, and total stockholder’s equity by approximately $2,274,000, assuming that the number of Units offered by us, as set forth above, remains the same, and after deducting the estimated underwriting discount and estimated offering expenses payable by us. |
6
Investing in our securities involves a high degree of risk. Prospective investors should carefully consider the risks described below and other information contained in this prospectus, including our financial statements and related notes, before purchasing our securities. There are numerous and varied risks, known and unknown, that may prevent us from achieving our goals. If any of these risks actually occurs, our business, financial condition, or results of operations may be materially adversely affected. In that case, the trading price of our securities could decline, and investors in our common stock could lose all or part of their investment.
RISKS RELATED TO OUR BUSINESS AND INDUSTRY
We have only a limited history upon which an evaluation of our prospects and future performance can be made, and have no history of profitable operations. Since we have a limited operating history, it may be difficult to predict our future operating results.
We were incorporated in the State of Nevada on December 7, 2015, and as a result, we have only a limited history upon which an evaluation of our prospects and future performance can be made, and have no history of profitable operations on a consolidated basis. Due to our lack of operating history, our operations are subject to all business risks associated with new enterprises. The likelihood of our success must be considered in light of the problems, expenses, difficulties, complications, and delays frequently encountered in connection with the startup of a business, and operation in a competitive and regulated industry. We may sustain losses in the future as we implement our business plan. There can be no assurance that we will ever generate revenues to operate profitably. Investors should evaluate an investment in us in light of the uncertainties encountered by developing companies in a competitive environment. Our business is dependent upon the implementation of our business plan. We may not be successful in implementing such plan, and cannot guarantee that, if implemented, we will ultimately be able to attain profitability.
We may need to obtain additional financing to fund our operations.
We may need additional capital in the future to continue to execute our business plan. In that case, we would be dependent upon additional capital in the form of either debt or equity to continue our operations and commercialize our products. We may not be able to arrange enough investment within the time the investment is required, or, if it is arranged, that it will be on favorable terms.
Failure to raise the necessary capital could restrict our growth, limit our development of new products and services, and hinder our ability to compete.
We need to raise funds in order to achieve our business objectives. Failure to raise these funds may:
| ● | Restrict our growth; |
| ● | Limit our development of new products and services; and |
| ● | Hinder our ability to compete. |
Any of these aforementioned consequences would have a materially adverse effect on our business, operations, and financial position. If we cannot obtain the needed capital, we may not be able to become profitable, and may have to curtail or cease our operations.
Additionally, Leonite Capital LLC (“Leonite”), a holder of our outstanding convertible promissory note and warrants to purchase our common stock, received certain rights in connection with its August 2017 investment that may hinder our ability to raise funds in the future. Pursuant to the terms of its investment, Leonite has the right to participate in our future financings in an amount of up to 50% of the aggregate offering amount. Additionally, if we issue any securities with more favorable terms than those received by Leonite or if we issue securities at a price lower than the conversion and/or exercise price of Leonite’s note and warrant, respectively, Leonite is entitled to receive the benefit of such favorable terms and to have the conversion and/or exercise price adjusted to such lower priced issuance, as applicable. Furthermore, Leonite is entitled to “piggyback registration rights” with respect to the shares of common stock underlying its note and warrants. In the event we are required to obtain a waiver from Leonite with respect to the aforementioned rights, we may be required to provide additional consideration, including, but not limited to, consideration in the form of cash and/or additional shares of our capital stock and/or securities convertible into or exercisable for shares of our capital stock, in order to obtain such waiver. If we are unable to obtain any required waivers from Leonite when necessary for future offerings, we may be unable to raise additional funds. An inability to raise additional funds could have a material adverse effect on our financial condition, results of operations, ability to conduct our business and on the price of our common stock.
7
We have a history of losses, and we anticipate that we will continue to incur losses in the future. Our auditors have included in their audit report an explanatory paragraph as to substantial doubt as to our ability to continue as a going concern.
We have experienced net losses since our inception. For the year ended December 31, 2017, our net loss was $2,177,641, compared to a net loss of $802,463 for the year ended December 31, 2016. For the nine months ended September 30, 2018 and 2017, our net loss was $3,067,224 and $3,590,801, respectively. As of December 31, 2017, we had an accumulated deficit of $3,743,438. As of September 30, 2018, we had an accumulated deficit of $6,810,662. Our auditors have included in their audit report a “going concern” explanatory paragraph that there is substantial doubt as to our ability to continue as a going concern, which assumes the realization of our assets and the satisfaction of our liabilities and commitments in the normal course of business. We anticipate continuing to incur substantial additional losses over at least the next several years due to, among other factors, expenses related to the following: anticipated research and development activities, investor and public relations, Securities and Exchange Commission (SEC) compliance efforts, and the general and administrative expenses associated with each of these activities. We may never achieve profitability, and, even if we do, we may not be able to sustain being profitable.
Restrictions contained in our debt agreements may limit our ability to incur additional indebtedness.
Our existing debt facilities contain restrictive covenants, including restrictions on our ability to incur indebtedness. These restrictions could limit our ability to effectuate future acquisitions, limit our ability to pay dividends, limit our ability to make capital expenditures, or restrict our financial flexibility. Our ability to meet the financial covenants or requirements in our debt facilities may be affected by events beyond our control, and we may not be able to satisfy such covenants and requirements. A breach of these covenants or other restrictions contained in a debt facility could result in an event of default under one or more of our other debt facilities. Upon the occurrence of an event of default under a debt facility, and the expiration of any grace periods, the lenders could elect to declare all amounts outstanding under one or more of our other debt facilities, together with accrued interest, to be immediately due and payable. If this were to occur, our assets may not be sufficient to fully repay the amounts due under our debt facilities or our other indebtedness.
Consolidation in the health care industry could lead to demands for price concessions, or limit or eliminate our ability to sell to certain of our potential significant market segments.
The cost of health care has risen significantly over the past decade, and numerous initiatives and reforms initiated by legislators, regulators, and third-party payers to curb these costs have resulted in a consolidation trend in the medical device industry, as well as among our potential customers, including health care providers. This, in turn, has resulted in greater pricing pressures, which could limit our ability to sell to important market segments, group purchasing organizations, independent delivery networks, and large single accounts, such as the Veterans Administration in the United States, which continue to consolidate purchasing decisions for some of our potential health care provider customers. We expect that market demand, government regulation, third-party reimbursement policies, and societal pressures will continue to change the worldwide health care industry, resulting in further business consolidations and alliances that may exert further downward pressure on our potential product prices, which would adversely impact our business and financial condition, and the results of operations.
We are subject to stringent domestic and foreign medical device regulation, and any adverse regulatory action may materially adversely affect our financial condition and business operations.
We are subject to rigorous regulation by the FDA and numerous other federal, state, and foreign governmental authorities. To varying degrees, each of these authorities monitors and enforces our compliance with laws and regulations governing the development, testing, clinical study, manufacturing, labeling, packaging, marketing, and distribution of our medical devices. These laws and regulations are subject to change, and to evolving interpretations that could increase costs, prevent or delay future device clearance or approvals, or otherwise adversely affect our ability to market currently cleared or approved devices. The process of obtaining marketing approval or clearance from the FDA and comparable foreign bodies for new products, or for enhancements or modifications to existing products, could:
| ● | Take a significant amount of time; | |
| ● | Require the expenditure of substantial resources; | |
| ● | Involve rigorous pre-clinical and clinical testing, as well as increased post-market surveillance; | |
| ● | Involve modifications, repairs, or replacements of our products; and | |
| ● | Result in limitations on the indicated uses of our products. |
8
We cannot be certain that new medical devices or new uses for existing medical devices will be cleared or approved by the FDA or foreign regulatory agencies in a timely or cost-effective manner, if at all. In addition, the FDA may require post-market testing and surveillance, and may, depending on the results, prevent or limit further marketing of products. The failure to receive approval or clearance for significant new products or modifications to existing products, or the receipt of an approval of limited or reduced scope could have a materially adverse effect on our financial condition and results of operations.
Both before and after a product is commercially released, we have ongoing responsibilities under the United States Federal Food, Drug, and Cosmetic Act (FDCA) and FDA regulations, which govern virtually all aspects of a medical device’s design, development, testing, manufacturing, labeling, storage, record keeping, adverse event reporting, sale, promotion, distribution, and shipping. Compliance with applicable statutory and regulatory requirements is subject to continual review, and is monitored rigorously through periodic inspections by the FDA, which may result in observations on Form 483, and (in some cases) warning letters that require corrective action. If the FDA were to conclude that we are not in compliance with applicable laws or regulations, or that any of our medical devices are ineffective or pose an unreasonable health risk, the FDA could:
| ● | Require us to notify health professionals and others that the devices present unreasonable risk of substantial harm to public health; | |
| ● | Order us to recall, repair, replace, or refund the cost of any medical device that we manufactured or distributed; | |
| ● | Detain, seize, or ban adulterated or misbranded medical devices; | |
| ● | Refuse to provide us with documents necessary to export our products; | |
| ● | Refuse requests for 510(k) clearance or PMA of new products or new intended uses; | |
| ● | Withdraw 510(k) clearances or PMAs that are already granted; | |
| ● | Impose operating restrictions, including requiring a partial or total shutdown of production; | |
| ● | Enjoin or restrain conduct resulting in violations of applicable law pertaining to medical devices; and/or | |
| ● | Assess criminal or civil penalties against us or our officers and employees. |
Any adverse regulatory action, depending on its magnitude, may restrict us from effectively manufacturing, marketing, and selling our products. In addition, negative publicity and product liability claims resulting from any adverse regulatory action could have a materially adverse effect on our financial condition and results of operations.
In addition, the FDCA permits device manufacturers to promote products solely for the uses and indications set forth in the approved product labeling. The U.S. Department of Justice has initiated a number of enforcement actions against manufacturers that promote products for “off-label” uses, alleging, among other things, that “off-label” promotion caused the submission of false and fraudulent claims for reimbursement to federal health care programs in violation of the Federal False Claims Act. Government enforcement action can result in substantial fines, penalties, and/or administrative remedies, including exclusion from government reimbursement programs and entry into Corporate Integrity Agreements (CIAs) with governmental agencies, entailing significant additional obligations and costs.
Foreign governmental regulations have become increasingly stringent and more common, and we may become subject to even more rigorous regulation by foreign governmental authorities in the future. Changes in clearance, approvals, or standards that must be complied with prior to commercial marketing, or the enactment of additional laws or regulations may cause delays in or prevent the marketing of a product. Penalties for a company’s noncompliance with foreign governmental regulation could be severe, including revocation or suspension of a company’s business license, and criminal sanctions. Any domestic or foreign governmental medical device law or regulation imposed in the future may have a materially adverse effect on our financial condition and business operations.
9
The regulatory approval processes of the FDA and comparable foreign authorities are lengthy, time-consuming, and inherently unpredictable, and if we are ultimately unable to obtain regulatory approval for our product candidate, our business will be substantially harmed.
The time required to obtain approval by the FDA and comparable foreign authorities is unpredictable, but typically takes many years following the commencement of clinical trials, and depends upon numerous factors, including the substantial discretion of the regulatory authorities. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development, and may vary among jurisdictions. If we are unable to obtain timely approval of our product candidate, our business would be materially harmed.
Instability in international markets, or foreign currency fluctuations could adversely affect our results of operations.
We generate a significant amount of our revenue from outside the United States. As a result, we face currency and other risks associated with our international sales. We are exposed to foreign currency exchange rate fluctuations due to transactions denominated primarily in euros, which may potentially reduce the U.S. dollars we receive for sales denominated in any of these foreign currencies, and/or increase the U.S. dollars we report as expenses in these currencies, thereby affecting our consolidated results of operations. Fluctuations between the currencies in which we do business have caused and will continue to cause foreign currency transaction gains and losses. We cannot predict the effects of currency exchange rate fluctuations upon our future operating results because of the number of currencies involved, the variability of currency exposures, and the volatility of currency exchange rates.
In addition to foreign currency exchange rate fluctuations, there are a number of additional risks associated with our international operations, including those related to:
| ● | The imposition of or increase in import or export duties, surtaxes, tariffs, or customs duties; | |
| ● | The imposition of import or export quotas or other trade restrictions; | |
| ● | Foreign tax laws and potential increased costs associated with overlapping tax structures; | |
| ● | Compliance with various U.S. and foreign laws, including the Foreign Corrupt Practices Act, the UK Anti-Bribery Act, and import/export laws; |
| ● | Longer accounts receivable cycles in certain foreign countries, whether due to cultural, economic, or other factors; | |
| ● | Changes in medical reimbursement programs and regulatory requirements in international markets in which we operate; and |
| ● | Economic and political instability in international markets, including concerns over excessive levels of sovereign debt and budget deficits in countries where we market our products that could result in an inability to pay or timely pay outstanding payables. |
10
We are subject to anti-bribery, anti-corruption, and anti-money laundering laws, including the U.S. Foreign Corrupt Practices Act, as well as export control laws, customs laws, sanctions laws, and other laws governing our operations. If we fail to comply with these laws, we could be subject to civil or criminal penalties, other remedial measures, and legal expenses, which could adversely affect our business, results of operations, and financial condition.
As we grow our international presence and global operations, we will be increasingly exposed to trade and economic sanctions and other restrictions imposed by the United States, the European Union, and other governments and organizations. The U.S. Departments of Justice, Commerce, State, and Treasury, and other federal agencies and authorities have a broad range of civil and criminal penalties they may seek to impose against corporations and individuals for violations of economic sanctions laws, export control laws, the U.S. Foreign Corrupt Practices Act (FCPA), and other federal statutes and regulations, including those established by the Office of Foreign Assets Control (OFAC). Under these laws and regulations, as well as other anti-corruption laws, anti-money laundering laws, export control laws, customs laws, sanctions laws, and other laws governing our operations, various government agencies may require export licenses, may seek to impose modifications to business practices (including cessation of business activities in sanctioned countries or with sanctioned persons or entities, and modifications to compliance programs) that may increase compliance costs, and may subject us to fines, penalties, and other sanctions. A violation of these laws or regulations would negatively affect our business, financial condition, and results of operations.
We are in the process of implementing policies and procedures designed to ensure compliance by us and our directors, officers, employees, representatives, consultants, and agents with the FCPA, OFAC restrictions, and other export control, anti-corruption, anti-money-laundering, and anti-terrorism laws and regulations. We cannot assure you, however, that our policies and procedures are or will be sufficient, or that directors, officers, employees, representatives, consultants, and agents have not engaged and will not engage in conduct for which we may be held responsible; nor can we assure you that our business partners have not engaged and will not engage in conduct that could materially affect their ability to perform their contractual obligations to us, or even result in our being held liable for such conduct. Violations of the FCPA, OFAC restrictions, or other export control, anti-corruption, anti-money laundering, and anti-terrorism laws or regulations may result in severe criminal or civil sanctions, and we may be subject to other liabilities, which could have a materially adverse effect on our business, financial condition, and results of operations.
We may experience delays or unforeseen issues during the requisite studies and trials required prior to regulatory approval of our medical devices.
Although there are no foreseeable risks with respect to obtaining an Investigational Device Exemption (IDE), following biocompatibility and active animal safety testing, even minor issues with the testing and application process can delay the IDE, which would in turn delay the clinical studies and increase the costs to complete the testing. For our PMA application, we plan to hire a regulatory consultant to facilitate all interactions with the FDA and ensure that we make the most time- and capital-efficient steps towards regulatory approval.
We may not be able to compete effectively with larger companies in the medical device space with greater resources and market recognition.
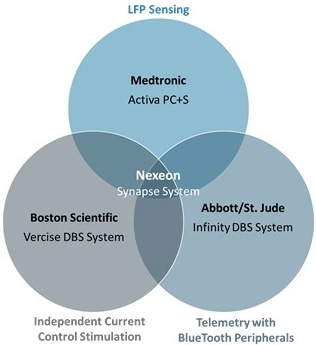
Our primary competitor, Medtronic, a provider of medical devices, has been involved in the manufacturing and sale of deep brain stimulation devices for several years. In addition, Boston Scientific and Abbott (formerly St. Jude Medical) have a CE mark and PMA to market and sell their neurostimulation implant devices in Europe and the U.S. These companies have substantially greater financial, research and development, manufacturing, marketing, and sales experience, and resources than us. As a result, our competitors may be more successful than us in developing their products, obtaining regulatory approvals, and marketing their products to users. We cannot assure investors that we will be able to compete effectively against current and future competitors.
We are dependent on a few significant customers for our businesses, and the loss of these customers could have an adverse effect on our business, results of operations, and financial condition.
In our original equipment manufacturer (OEM) solutions business, we sell to over 30 customers. For the year ended December 31, 2017, sales to our two largest customers represented 79.4% of our sales revenue. The loss or reduction in services to these significant customers, or other discontinuation of their relationship with us for any reason—or, if either of these significant customers reduces or postpones purchases that we expect to receive—could have an adverse impact on our business, results of operations, and financial condition.
11
Laws and regulations that could affect the industry in which we operate may be enacted, which could result in a delay or cessation of our research and development activities, or the imposition of additional costs that could hinder our ability to achieve and maintain profitable operations.
Current laws and regulations with respect to our industry, and additional laws and regulations that may be enacted in the future could impose new and/or unexpected operational considerations or constraints upon us. Complying with existing laws or regulations may require significant time and resource allocation for medical device manufacturers, including us. Additionally, changing or new legislation may force us to redesign one or more of our products. In such an event, our proprietary neurostimulation device may have to be altered or modified to ensure that it is in compliance with all applicable laws and regulations. Such alterations or modifications would cause us to incur substantial research and development costs. Moreover, if we cannot modify or alter our neurostimulation device’s design or functionality, then our device could be rendered obsolete, which would substantially reduce our future profitability and harm our business. Additionally, since we intend to operate both domestically and in the EU, we must remain cognizant of the legislative and regulatory landscape in both regions. Compliance with these regulations, when applicable, increases the research and development and production costs, and could make our proposed products and services less attractive to potential customers.
International operations conducted by us subject us to a number of risks, including unfavorable political, regulatory, labor, and tax conditions.
We sell medical devices to customers located outside the United States. International operations are subject to the legal, political, regulatory, and social requirements and economic conditions in the jurisdictions in which they are conducted. Risks inherent to international operations and sales include, but are not limited to, the following:
| ● | Exposure to violations of the Foreign Corrupt Practices Act of 1977, as amended; |
| ● | Difficulty in enforcing agreements, judgments, and arbitration awards in foreign legal systems; | |
| ● | Impediments to the flow of foreign exchange capital payments and receipts due to exchange controls instituted by certain foreign governments and the fact that the local currencies of these countries are not freely convertible; | |
| ● | Inability to obtain maintain or enforce our intellectual property rights; | |
| ● | Changes in general economic and political conditions in foreign countries; | |
| ● | Changes in foreign government regulations and technical standards, including additional regulation of medical devices, which may reduce or eliminate our ability to sell or license in certain markets; | |
| ● | Requirements or preferences of foreign nations for domestic technologies, which could reduce demand for our technologies; | |
| ● | Trade barriers such as export requirements, tariffs, taxes, and other restrictions and expenses, which could increase the prices of our technologies and make us less competitive; and | |
| ● | Longer payment cycles typically associated with international sales, and potential difficulties in collecting accounts receivable, which may reduce the future profitability of foreign sales or licensing. |
Conducting business in foreign jurisdictions requires us to respond to rapid changes in market conditions in these countries. We believe that our overall success as a global business depends on our ability to succeed in different legal, regulatory, economic, social, and political situations and conditions. We may not be able to develop and implement effective policies and strategies in each foreign jurisdiction where we may do business in the future.
12
We depend on our key management personnel for our future success.
Our success depends largely on the skills of our key management and technical personnel. The loss of one or more of our key management and technical personnel may materially and adversely affect business and results of operations. We do not maintain key person insurance for any of our employees. We cannot guarantee that we will be able to replace any of our key management personnel in the event that their services become unavailable.
Our chief executive officer beneficially owns a significant percentage of our outstanding capital stock, and will have the ability to significantly influence our affairs.
Our chief executive officer, William Rosellini, beneficially owns approximately 34.1% of our issued and outstanding capital stock, primarily through the holdings of Rosellini Scientific Holdings, LLC (“RSH”), of which he is the sole member and manager. By virtue of his holdings, he may significantly influence the election of the members of our board of directors, our management, and our affairs, and other corporate transactions (such as mergers, consolidations, or the sale of all or substantially all of our assets) that are submitted to shareholders for approval, and that may not be favorable from our standpoint or that of our other shareholders.
We only have a limited number of employees to manage and operate our neurostimulation business segment.
As of the date of this prospectus, our neurostimulation business segment employed a total of 7 full-time employees and 1 consultant. We cannot assure you that we will be able to retain adequate staffing levels to run our operations and/or to accomplish all of the objectives that we otherwise would seek to accomplish for the neurostimulation business segment.
Anti-takeover provisions may impede the acquisition of our Company.
Certain provisions of the Nevada Revised Statutes have anti-takeover effects and may inhibit a non-negotiated merger or other business combination. These provisions are intended to encourage any person interested in acquiring us to negotiate with, and to obtain the approval of, our board of directors in connection with such a transaction. But certain of these provisions may discourage a future acquisition of us, including an acquisition in which the stockholders might otherwise receive a premium for their shares. As a result, stockholders who might desire to participate in such a transaction may not have the opportunity to do so, which could cause our stock price to decline.
Our products may be subject to recalls, even after receiving FDA clearance or approval, which would harm our reputation, business, and financial results.
We will be subject to the medical device reporting regulations, which will require us to report to the FDA if our products may have caused or contributed to a death or serious injury, or have malfunctioned in a way that would likely cause or contribute to a death or serious injury if the malfunction were to occur. We will also be subject to the correction and removal reporting regulations, which will require us to report to the FDA any field corrections and device recalls or removals that we undertake to reduce a risk to health posed by the device, or to remedy a violation of the Federal Food, Drug and Cosmetic Act (FDCA) caused by the device that may present a risk to health. In addition, the FDA and similar governmental agencies in other countries have the authority to require the recall of our products if there is a reasonable probability that the products would cause serious adverse health consequences or death. A government-mandated or voluntary recall by us could occur as a result of manufacturing defects, labeling deficiencies, packaging defects, or other failures to comply with applicable regulations. Any recall would divert management attention and financial resources and harm our reputation with customers, and could have a materially adverse effect on our financial condition and results of operations.
We may be subject to product liability claims, and may not have sufficient product liability insurance to cover any such claims, which may expose us to substantial liabilities.
We may be exposed to product liability claims from users of our products. We currently do not have product liability insurance. It is possible that any product liability insurance coverage we obtain will be insufficient to protect us from future claims. Further, we may not be able to obtain or maintain insurance on acceptable terms, or guarantee that such insurance would be sufficient to cover any potential product liability claim or recall. Failure to obtain or maintain sufficient insurance coverage could have a materially adverse effect on our business, prospects, and results of operations if claims are made that exceed our coverage.
13
Our revenues will depend upon adequate reimbursement from public and private insurers and health systems.
Our success will depend on the extent to which reimbursement for the costs of our devices will be available from third-party payers, such as public and private insurers and health systems. There can be no guarantee that our neurostimulation device will be approved for reimbursement, which could adversely affect the adoption of our device. Government and other third-party payers attempt to contain health care costs by limiting both coverage and the level of reimbursement of new treatments. Even if our device and treatment are successful and approved for use, reimbursement may not be forthcoming, which could lead to slower-than-anticipated adoption of our technology. Therefore, significant uncertainty usually exists as to the reimbursement status of new health care treatments. If we are not successful in obtaining adequate reimbursement for our treatment from these third-party payers, the market’s acceptance of our treatment could be adversely affected. Inadequate reimbursement levels also likely would create downward price pressure on our treatment. Even if we succeed in obtaining widespread reimbursement for our treatment, future changes in reimbursement policies could have a negative impact on our business, financial condition, and results of operations.
We are subject to numerous federal and state health care laws and regulations, and failure to comply with such laws and regulations could have an adverse effect on our business and our ability to compete in the marketplace.
There are numerous laws and regulations that govern the means by which companies in the health care industry may market their treatments to health care professionals, and may compete by discounting the prices of their treatments, including, for example, the federal Anti-Kickback Statute, the federal False Claims Act (“FCA”), the federal Health Insurance Portability and Accountability Act of 1996 (“HIPAA”), and state law equivalents to these federal laws that are meant to protect against fraud and abuse (as well as analogous laws in foreign countries). Violations of these laws are punishable by criminal and civil sanctions, including but not limited to (in some instances) civil and criminal penalties, damages, fines, and exclusion from participation in federal and state health care programs, including Medicare and Medicaid. In addition, federal and state laws are also sometimes open to interpretation. Accordingly, we could potentially face legal risks if our interpretation differs from those of enforcement authorities. Further, from time to time we may find ourselves at a competitive disadvantage if our interpretation differs from those of our competitors.
Specifically, anti-kickback laws and regulations prohibit any knowing and willful offer, payment, solicitation, or receipt of any form of remuneration (direct or indirect, in case or in kind) in return for the referral, use, ordering, or recommending of the use of a product or service for which payment may be made by Medicare, Medicaid, or other government-sponsored health care programs. We have entered into consulting agreements, research agreements, and product development agreements with physicians, including some who may order our products or make decisions to use them. In addition, some of these physicians own our stock, which they purchased in arm’s-length transactions on terms identical to those offered to non-physicians, or received as stock awards from us as consideration for services performed by them. While these transactions were structured with the intention of complying with all applicable laws, including state anti-referral laws and other applicable anti-kickback laws, it is possible that regulatory or enforcement agencies or courts may in the future view these transactions as prohibited arrangements that must be restructured, or for which we would be subject to other significant civil or criminal penalties. There can be no assurance that regulatory or enforcement authorities will view these arrangements as being in compliance with applicable laws, or that one or more of our employees or agents will not disregard the rules we have established. Because our strategy relies on the involvement of physicians who consult with us on the design of our potential products, perform clinical research on our behalf, or educate the market about the efficacy and uses of our potential products, we could be materially impacted if regulatory or enforcement agencies or courts interpret our financial relationships with physicians who refer or order our potential products to be in violation of applicable laws, and determine that we would be unable to achieve compliance with such applicable laws. This could harm our reputation and the reputations of the physicians we engage to provide services on our behalf. In addition, the cost of noncompliance with these laws could be substantial since we could be subject to monetary fines and civil or criminal penalties. We could also be excluded from federally funded health care programs, including Medicare and Medicaid, for noncompliance. Further, even the costs of defending investigations of noncompliance could be substantial.
14
Also, the FCA imposes civil liability on any person or entity that submits, or causes the submission of, a false or fraudulent claim to the federal government. Damages under the FCA can be significant and consist of the imposition of fines and penalties. The FCA also allows a private individual or entity (i.e., a whistleblower) with knowledge of past or present fraud against the federal government to sue on behalf of the government, and to be paid a portion of the government’s recovery, which can include both civil penalties and up to three times the amount of the government’s damages (usually the amount reimbursed by federal health care programs). The U.S. Department of Justice (“DOJ”) on behalf of the government takes the position that the marketing and promotional practices of life sciences product manufacturers, including the off-label promotion of products, the provision of inaccurate or misleading reimbursement guidance, or the payment of prohibited kickbacks to doctors or other referral sources may cause the submission of improper claims to federal and state health care entitlement programs such as Medicare and Medicaid by health care providers that use the manufacturer’s products, which results in a violation of the FCA. In certain cases, manufacturers have entered into criminal and civil settlements with the federal government under which they entered into plea agreements, paid substantial monetary amounts, and entered into corporate integrity agreements that require, among other things, substantial reporting and remedial actions going forward.
In addition, there has been a recent trend of increased federal and state regulation of payments made to physicians and other health care providers. In addition to federal laws, some states, such as California, Massachusetts, and Vermont, mandate implementation of commercial compliance programs, along with the tracking and reporting of gifts, compensation, and other remuneration to physicians. The shifting commercial compliance environment and the need to build and maintain robust and expandable systems to comply with different compliance and/or reporting requirements in multiple jurisdictions increase the possibility that a health care company may run afoul of one or more of the requirements.
The scope and enforcement of all of these laws is uncertain, and subject to rapid change, especially in light of the lack of applicable precedent and regulations. There can be no assurance that federal or state regulatory or enforcement authorities will not investigate or challenge our current or future activities under these laws. Any investigation or challenge could have a materially adverse effect on our business, financial condition, and results of operations. Any state or federal regulatory or enforcement review of us, regardless of the outcome, would be costly and time consuming. Additionally, we cannot predict the impact of any changes in these laws, whether these changes are retroactive or will have effect on a going-forward basis only.
Our use of sensitive patient information is subject to complex regulations at multiple levels, and we would be adversely affected if we failed to adequately protect this sensitive information.
We process, maintain, and utilize personal health and other confidential and sensitive data. In particular, we have developed a web and mobile application through which our customers can communicate with physicians and others, which may involve sharing patient identifiable health information. The use and disclosure of such information is regulated at the federal, state, and international levels, and these laws, rules, and regulations are subject to change and increased enforcement activity, such as the audit program implemented by the U.S. Department of Health and Human Services under HIPAA. International laws, rules, and regulations governing the use and disclosure of such information are generally more stringent than in the United States, and they vary from jurisdiction to jurisdiction. Noncompliance with any privacy or security laws or regulations, or any security breach, cyber-attack, or cybersecurity breach, as well as any incident involving the theft, misappropriation, loss, or other unauthorized disclosure of, or access to, sensitive or confidential information, whether by us or by another third party, could require us to expend significant resources to remediate any damage, interrupt our operations, and damage our brand and reputation, and could also result in investigations, regulatory enforcement actions, material fines and penalties, loss of customers, litigation, or other actions that could have a materially adverse effect on our business, brand, reputation, cash flows, and operating results.
15
Our business depends on provider and patient willingness to entrust us with health-related and other sensitive personal information. Events that negatively affect that trust, including inadequate disclosure of our uses of their information, failure to keep our information technology systems and sensitive information secure from significant attack, theft, damage, loss, or unauthorized disclosure or access, whether as a result of our action or inaction, or that of third parties, could adversely affect our brand, reputation, and revenues, and also expose us to mandatory disclosure to the media, litigation (including class action litigation) and other enforcement proceedings, material fines, penalties and/or remediation costs, and compensatory, special, punitive, and statutory damages, as well as consent orders and/or injunctive relief, any of which could adversely affect our business, cash flows, operating results, or financial position. There can be no assurance that any such failure will not occur—or, if any does occur, that we will detect it, or that it can be sufficiently remediated.
To be commercially successful, we must convince physicians that our devices are safe and effective alternatives to existing medical devices, and that our devices should be used.
We believe physicians will only adopt our devices if they determine, based on experience, clinical data, and published, peer-reviewed journal articles, that the use of our devices is a favorable alternative to conventional devices/methods. Physicians may be slow to change their practices for the following reasons, among others:
| ● | Lack of evidence supporting additional patient benefits and the advantages of our devices over conventional devices/methods; | |
| ● | Perceived liability risks generally associated with the use of new devices; and | |
| ● | Limited availability of reimbursement from third-party payers. |
In addition, we believe that recommendations for and support of our devices by influential physicians are essential for market acceptance and adoption. If we do not receive this support or are unable to demonstrate favorable, long-term clinical data, physicians and hospitals may not use our devices, which would significantly reduce our ability to achieve revenue, and would prevent us from sustaining profitability.
The training required for physicians to use our Viant™ System could reduce the market acceptance of our products.
As with any new method or technique, physicians must undergo a thorough training program before they are qualified to perform the surgery to implant our Viant™ System. Physicians could experience difficulty with the technique necessary to successfully insert the device, and may not achieve the technical competency necessary to complete the training program. Even after successfully completing the training program, physicians could still experience difficulty implanting our Viant™ System, and, as a result, limit its use significantly in their practices, or cease utilizing it altogether.
In addition, we may experience difficulty growing the number of physicians who complete our training program if patient demand is low, if the length of time necessary to train each physician is longer than expected, if the capacity of our sales representatives to train physicians is less than expected, or if we are unable to sufficiently grow our sales organization. All of these events would lead to fewer trained physicians qualified to implant our Viant™ System, which could negatively affect our business, financial condition, and results of operations, and impair our ability to grow our business.
We are an “emerging growth company,” and the reduced disclosure requirements applicable to emerging growth companies may make our common stock less attractive to investors.
We are an “emerging growth company,” as defined in the Jumpstart Our Business Startups (“JOBS”) Act, and may remain an emerging growth company for up to five years. For so long as we remain an emerging growth company, we are permitted and intend to rely on exemptions from certain disclosure requirements that are applicable to other public companies that are not emerging growth companies. These exemptions include:
| ● | Being permitted to provide only two years of audited financial statements, in addition to any required unaudited interim financial statements, with correspondingly reduced “Management’s Discussion and Analysis of Financial Condition and Results of Operations” disclosure; | |
| ● | Not being required to comply with the auditor attestation requirements in the assessment of our internal control over financial reporting; |
16
| ● | Not being required to comply with any requirement that may be adopted by the Public Company Accounting Oversight Board regarding mandatory audit firm rotation, or a supplement to the auditor’s report providing additional information about the audit and the financial statements; | |
| ● | Reduced disclosure obligations regarding executive compensation; and | |
| ● | Exemptions from the requirements of holding a non-binding advisory vote on executive compensation and shareholder approval of any golden parachute payments not previously approved. |
We cannot predict whether investors will find our common stock less attractive if we rely on these exemptions. If some investors find our common stock less attractive as a result, there may be a less-active trading market for our common stock, and our stock price may be more volatile.
If we fail to maintain proper and effective internal control over financial reporting in the future, our ability to produce accurate and timely financial statements could be impaired, which could harm our operating results.
Pursuant to Section 404 of the Sarbanes-Oxley Act, our management is required to report upon the effectiveness of our internal control over financial reporting. When and if we become a “large accelerated filer” or an “accelerated filer” and are no longer a “smaller reporting company,” each as defined in the Securities Exchange Act of 1934, as amended (the “Exchange Act”), our independent registered public accounting firm will be required to attest to the effectiveness of our internal control over financial reporting. For so long as we remain a smaller reporting company, however, we intend to take advantage of certain exemptions from various reporting requirements that are applicable to smaller reporting companies, including, but not limited to, not being required to comply with the auditor attestation requirements of Section 404. Once we are no longer a smaller reporting company, or if, prior to such date, we opt to no longer take advantage of the applicable exemption, we will be required to include an opinion from our independent registered public accounting firm on the effectiveness of our internal controls over financial reporting. The rules governing the standards that must be met for management to assess our internal control over financial reporting are complex and require significant documentation, testing, and possible remediation. To comply with the requirements of being a reporting company under the Exchange Act, we will need to upgrade our systems, including information technology; implement additional financial and management controls, reporting systems, and procedures; and ensure we have hired sufficient accounting and finance staff.
Due to the recent acquisitions of foreign subsidiaries and integration of those entities, management is in the process of evaluating internal controls over financial reporting. As such, our chief executive officer and chief financial officer concluded that, as of September 30, 2018, our internal controls over financial reporting were not effective due to the lack of implementation of internal controls over financial reporting across all operating entities. If we fail to establish and maintain an effective system of internal control over financial reporting, we may not be able to accurately report our financial results, file our periodic reports on a timely basis, maintain our reporting status, or prevent fraud. At times, we have not had sufficient accounting and supervisory personnel with the appropriate level of technical accounting experience and training necessary, or adequate formally documented accounting policies and procedures to support effective internal controls. As we grow, we plan to hire additional personnel and engage in external temporary resources, and may implement, document, and modify policies and procedures to maintain effective internal controls. We may identify deficiencies and weaknesses, or fail to remediate previously identified deficiencies in our internal controls. If material weaknesses or deficiencies in our internal controls exist and go undetected or un-remediated, our financial statements could contain material misstatements that, when discovered in the future, could result in our operating results being materially impacted, and we could fail to meet our future reporting obligations.
17
If we discover material weaknesses or other deficiencies in our internal control and accounting procedures, our stock price could decline significantly, and raising capital could be more difficult.
If we fail to comply with the rules under the Sarbanes-Oxley Act of 2002 related to disclosure controls and procedures, or if we fail to remediate the material weaknesses or other deficiencies in our internal control and accounting procedures in a timely fashion, our stock price could decline significantly, and raising capital could be more difficult. Moreover, effective internal controls are necessary for us to produce reliable financial reports, and are important to helping prevent financial fraud. If we cannot provide reliable financial reports or prevent fraud, our business and operating results could be harmed, investors could lose confidence in our reported financial information, and the trading price of our common stock could drop significantly. In addition, we cannot be certain that material weaknesses or significant deficiencies in our internal controls will not be discovered in the future.
RISKS RELATED TO COMMERCIALIZATION
If we are unable to obtain required regulatory approvals, we will be unable to market and sell our product candidates.
Our product candidates are subject to extensive governmental regulations relating to development, clinical trials, manufacturing, oversight of clinical investigators, record keeping, and commercialization. Rigorous pre-clinical testing and clinical trials, and an extensive regulatory review and approval process are required to be successfully completed in the United States, and in each foreign jurisdiction in which we offer our products before a new drug or other product can be sold in such jurisdictions. Satisfaction of these and other regulatory requirements is costly, time-consuming, uncertain, and subject to unanticipated delays. The time required to obtain approval by the FDA or the regulatory authority in other jurisdictions is unpredictable and often exceeds five years following the commencement of clinical trials, depending upon the complexity of the product candidate and the requirements of the applicable regulatory agency.
In connection with the clinical development of our product candidates, we face risks that:
| ● | The product candidate may not prove to be safe and efficacious; |
| ● | Patients may die or suffer serious adverse effects for reasons that may or may not be related to the product candidate being tested; |
| ● | We may fail to maintain adequate records of observations and data from our clinical trials, to establish and maintain sufficient procedures to oversee, collect data from, and manage clinical trials, or to monitor clinical trial sites and investigators to the satisfaction of the FDA or other regulatory agencies; |
| ● | The results of later-phase clinical trials may not confirm the results of earlier clinical trials; and |
| ● | The results from clinical trials may not meet the level of statistical significance or clinical benefit-to-risk ratio required by the FDA or other regulatory agencies for marketing approval. |
Only a small percentage of product candidates for which clinical trials are initiated receive approval for commercialization. Furthermore, even if we do receive regulatory approval to market a product candidate, any such approval may be subject to limitations such as those on the indicated uses for which we may market a particular product candidate.
Specifically, design verification, process validation, and testing requirements for our Viant™ System are nearly complete, and management expects we will complete the technical file in the first half of 2019. We expect to receive a CE Mark in early 2020, but to date this has not been granted, and we cannot be certain that this will be granted.
Our product candidates have not completed sufficient clinical trials to obtain regulatory approval, and may never demonstrate sufficient safety and efficacy in order to do so.
Our product candidates are in the clinical and pre-clinical stages of development. In order to achieve profitable operations, we alone (or in collaboration with others) must successfully license, develop, manufacture, introduce, and market our products. The time frame necessary to achieve market success for any individual product—whether or not we develop it—is long and uncertain. The products we are currently developing will require significant additional research, development, and pre-clinical and clinical testing prior to application for commercial use or sale. A number of companies in the biotechnology and pharmaceutical industries have suffered significant setbacks in clinical trials, even after showing promising results in early or later-stage studies or clinical trials. Additionally, we may encounter problems in our clinical trials that may cause us to delay, suspend, or terminate those clinical trials.
18
If clinical trials or regulatory approval processes for our product candidates are prolonged, delayed, or suspended, we may be unable to commercialize our product candidates on a timely basis, which would require us to incur additional costs and delay our receipt of any revenue from potential product sales.
If our product candidates are licensed, we cannot predict whether we will encounter problems with any planned clinical trials that will cause us or any regulatory authority to delay or suspend those clinical trials, or delay the analysis of data derived from them. A number of events, including any of the following, could delay the completion of our ongoing and planned clinical trials, and negatively impact our ability to obtain regulatory approval for, and to market and sell, a particular product candidate:
| ● | Conditions imposed on us by the FDA or another foreign regulatory authority regarding the scope or design of our clinical trials; | |
| ● | Delays in obtaining, or our inability to obtain, required approvals from institutional review boards or other reviewing entities at clinical sites selected for participation in our clinical trials; | |
| ● | Insufficient supply of our product candidates or other materials necessary to conduct and complete our clinical trials; | |
| ● | Slow enrollment and retention rate of subjects in our clinical trials; | |
| ● | Serious and unexpected drug-related side effects related to the product candidate being tested; and | |
| ● | Delays in meeting manufacturing and testing standards required for production of clinical trial supplies. |
Commercialization of our product candidates may be delayed by the imposition of additional conditions on our clinical trials by the FDA or any other applicable foreign regulatory authority, or the requirement of additional supportive studies by the FDA or any foreign regulatory authority. In addition, clinical trials require sufficient patient enrollment, which is a function of many factors, including the size of the patient population, the nature of the trial protocol, the proximity of patients to clinical sites, the availability of effective treatments for the relevant disease, the conduct of other clinical trials that compete for the same patients as our clinical trials, and the eligibility criteria for our clinical trials. Our failure to enroll patients in our clinical trials could delay the completion of the clinical trials beyond our expectations. In addition, the FDA could require us to conduct clinical trials with a larger number of subjects than we may have projected for any of our product candidates. We may not be able to enroll a sufficient number of patients in a timely or cost-effective manner. Furthermore, enrolled patients may drop out of our clinical trials, which could impair the validity or statistical significance of the clinical trials.
We do not know whether our clinical trials will begin as planned, will need to be restructured, or will be completed on schedule, if at all. Delays in our clinical trials will result in increased development costs for our product candidates, and our financial resources may be insufficient to fund any incremental costs. In addition, if our clinical trials are delayed, our competitors may be able to bring products to market before we do, and the commercial viability of our product candidates could be limited.
Our product candidates will remain subject to ongoing regulatory review even if they receive marketing approval, and if we lose these approvals, the sale of any of our approved commercial products could be suspended.
Even if we receive regulatory approval to market a particular product candidate, the manufacturing, labeling, packaging, adverse event reporting, storage, advertising, promotion, and record keeping related to the product will remain subject to extensive regulatory requirements. If we fail to comply with the regulatory requirements of the FDA and other applicable domestic and foreign regulatory authorities, or discover any previously unknown problems with any approved product, manufacturer, or manufacturing process, we could be subject to administrative or judicially imposed sanctions, including:
| ● | Restrictions on the products, manufacturers, or manufacturing processes; | |
| ● | Warning letters; | |
| ● | Civil or criminal penalties; |
19
| ● | Fines; | |
| ● | Injunctions; | |
| ● | Product seizures or detentions; | |
| ● | Pressure to initiate voluntary product recalls; | |
| ● | Suspension or withdrawal of regulatory approvals; and | |
| ● | Refusal to approve pending applications for marketing approval of new products, or supplements to approved applications. |
Our industry is highly competitive, and our product candidates may become obsolete.
We are engaged in a rapidly evolving field. Competition from other pharmaceutical companies, biotechnology companies, and research and academic institutions is intense and likely to increase. Many of those companies and institutions have substantially greater financial, technical, and human resources than we do. Those companies and institutions also have substantially greater experience in developing products, conducting clinical trials, obtaining regulatory approval, and manufacturing and marketing pharmaceutical products. Many of our competitors have already succeeded in obtaining regulatory approval for their products, and may continue to do so more rapidly than we do. Competitors have developed or are in the process of developing technologies that are, or in the future may be, the basis for competitive products. Our competitors may succeed in developing products that are more effective and/or cost competitive than those we are developing, or that would render our product candidates less competitive or even obsolete. In addition, one or more of our competitors may achieve product commercialization or patent protection earlier than we do, which could materially adversely affect our business.
If physicians and patients do not accept our future products, or if the market for indications for which any product candidate is approved is smaller than expected, we may be unable to generate significant revenue, if any.
Even if any of our product candidates obtain regulatory approval, they may not gain market acceptance among physicians, patients, and third-party payers. Physicians may decide not to recommend our products for a variety of reasons, including:
| ● | Timing of market introduction of competitive products; | |
| ● | Demonstration of clinical safety and efficacy compared to other products; |
| ● | Cost-effectiveness; | |
| ● | Limited or no coverage by third-party payers; | |
| ● | Convenience and ease of administration; and | |
| ● | Ineffective marketing and distribution support of its products. |
If any of our product candidates are approved but fail to achieve market acceptance, or such market is smaller than anticipated, we may not be able to generate significant revenue, and our business would suffer.
20
Our internal computer systems, or those of our third-party service providers, licensees, licensors, collaborators, or other contractors or consultants, may fail or suffer security breaches, which could result in a material disruption in our business and operations.
Despite the implementation of security measures, our internal computer systems and those of our current and future service providers, licensees, licensors, collaborators, and other contractors and consultants are vulnerable to damage from computer viruses, unauthorized access, natural disasters, terrorism, war, and telecommunication and electrical failures. While we are not aware of any such material system failure, accident, or security breach to date, if such an event were to occur and cause interruptions in our operations, it could result in a material disruption of our development programs and business operations. For example, the loss of clinical trial data from completed, on-going, or future clinical trials could result in delays in our regulatory approval efforts and significant costs to recover or reproduce the data. Likewise, we rely on third parties to manufacture our drug candidates and conduct clinical trials, and similar events relating to their computer systems could also have a materially adverse effect on our business. To the extent that any disruption or security breach were to result in a loss of, or damage to, our data or applications, or inappropriate disclosure of confidential or proprietary information, we could incur liabilities, and the further development and commercialization of our product candidates could be delayed.
Due in part to our limited financial resources, we may fail to select or capitalize on the most scientifically, clinically, or commercially promising or profitable indications or therapeutic areas for our product candidates, or those that are in-licensed, and/or we may be unable to pursue the clinical trials that we would like to pursue.
We have limited technical, managerial, and financial resources to determine the indications on which we should focus the development efforts related to our product candidates. Due to our limited available financial resources, we may have curtailed clinical development programs and activities that might otherwise have led to more rapid progress of our product candidates through the regulatory and development processes.
We may make incorrect determinations with regard to the indications and clinical trials on which to focus the available resources that we do have. Furthermore, we cannot assure you that we will be able to retain adequate staffing levels to run our operations, and/or to accomplish all of the objectives that we otherwise would seek to accomplish. Our decisions to allocate our research, management, and financial resources toward particular indications or therapeutic areas for our product candidates may not lead to the development of viable commercial products, and may divert resources from better opportunities. Similarly, our decisions to delay or terminate drug development programs may also cause us to miss valuable opportunities.
If the third parties on which we rely for the conduct of our clinical trials and results do not perform our clinical trial activities in accordance with good clinical practices and related regulatory requirements, we may be unable to obtain regulatory approval for or commercialize our product candidates.
We use independent clinical investigators and other third-party service providers to conduct and/or oversee the clinical trials of our product candidates, and expect to continue to do so for the foreseeable future. We rely heavily on these parties for successful execution of our clinical trials. Nonetheless, we are responsible for confirming that each of our clinical trials is conducted in accordance with the FDA’s requirements and our general investigational plan and protocol.
The FDA requires us and our clinical investigators to comply with regulations and standards, commonly referred to as good clinical practices, for conducting clinical trials and recording and reporting their results to assure that data and reported results are credible and accurate, and that the trial participants are adequately protected. Our reliance on third parties that we do not control does not relieve us of these responsibilities and requirements. Third parties may not complete activities on schedule, or may not conduct our clinical trials in accordance with regulatory requirements or the respective trial plans and protocols. The failure of these third parties to carry out their obligations could delay or prevent the development, approval, and commercialization of our product candidates, or result in enforcement action against us.
21
We have limited manufacturing capacity, and have relied on—and expect to continue to rely on—third-party manufacturers to produce our product candidates.
We do not own or operate manufacturing facilities for the production of clinical or commercial quantities of our neurostimulation product candidates, and we lack the resources and the capabilities to do so. As a result, we currently rely on (and expect to do so for the foreseeable future) third-party manufacturers to supply our product candidates. Reliance on third-party manufacturers entails risks to which we would not be subject if we manufactured our product candidates or products ourselves, including:
| ● | Reliance on third-parties for manufacturing process development, regulatory compliance, and quality assurance; | |
| ● | Limitations on supply availability resulting from capacity and scheduling constraints of third-parties; | |
| ● | The possible breach of manufacturing agreements by third-parties because of factors beyond our control; and | |
| ● | The possible termination or non-renewal of the manufacturing agreements by the third party, at a time that is costly or inconvenient to us. |
If we do not maintain our key manufacturing relationships, we may fail to find replacement manufacturers or develop our own manufacturing capabilities, which could delay or impair our ability to obtain regulatory approval for our products, and substantially increase our costs or deplete profit margins, if any. If we do find replacement manufacturers, we may not be able to enter into agreements with them on terms and conditions favorable to us, and there could be a substantial delay before new facilities could be qualified and registered with the FDA and other foreign regulatory authorities.
The FDA and other foreign regulatory authorities require manufacturers to register manufacturing facilities. The FDA and corresponding foreign regulators also inspect these facilities to confirm compliance with current standards. Contract manufacturers may face manufacturing or quality control problems causing drug substance production and shipment delays, or a situation where the contractor may not be able to maintain compliance with the applicable GMP requirements. Any failure to comply with such requirements, or other FDA and comparable foreign regulatory requirements, could adversely affect our clinical research activities, and our ability to develop our product candidates and market our products following approval.
Our current and anticipated future dependence upon others for the manufacture of our product candidates may adversely affect our future profit margins, and our ability to develop our product candidates and commercialize any products that receive regulatory approval on a timely basis.
If product liability lawsuits are successfully brought against us, we may incur substantial liabilities, and may be required to limit commercialization of our product candidates and any products that we may develop.
The testing and marketing of medical products entail an inherent risk of product liability. Although we are not aware of any historical or anticipated product liability claims or specific causes for concern, if we cannot successfully defend ourselves against product liability claims, we may incur substantial liabilities, or be required to limit commercialization of our product candidates and any products that we may develop. In addition, product liability claims may also result in withdrawal of clinical trial volunteers, injury to our reputation, and decreased demand for any products that we may commercialize. If we are unable to obtain sufficient product liability insurance at an acceptable cost, potential product liability claims could prevent or inhibit the commercialization of any products that we may develop, alone or with corporate partners.
The medical device industry is highly competitive and subject to rapid technological change.
If our competitors are better able to develop and market products that are safer, more effective, less costly, or otherwise more attractive than any products that we may develop, our commercial opportunity will be reduced or eliminated.
22
We face competition from established pharmaceutical and biotechnology companies, as well as from academic institutions, government agencies, and private and public research institutions in the United States and abroad. Most of the companies developing or marketing competing products are publicly traded, or divisions of publicly traded companies, and these companies enjoy several competitive advantages, including:
| ● | Greater financial and human resources for product development, sales and marketing, and patent litigation; | |
| ● | Significantly greater name recognition; | |
| ● | Established relationships with health care professionals, customers, and third-party payers; | |
| ● | Additional lines of products, and the ability to offer rebates or bundle products to offer higher discounts or incentives to gain a competitive advantage; | |
| ● | Established distribution networks; and | |
| ● | Greater experience in conducting research and development, manufacturing, clinical trials, obtaining regulatory approval for products, and marketing approved products. |
For example, Boston Scientific, Abbott Laboratories, and Medtronic—three companies with far greater financial and marketing resources than we possess—have each developed and are actively marketing neurostimulation devices that have been approved by the FDA. We may be unable to demonstrate that our systems offer any advantages over theirs. Smaller or early-stage companies may also prove to be significant competitors, particularly through collaborative arrangements with, mergers with, or acquisitions by large and established companies, or through the development of novel products and technologies.
The industry in which we operate has undergone, and is expected to continue to undergo, rapid and significant technological change, and we expect competition to intensify as technical advances are made. Our competitors may develop and patent processes or products earlier than us, obtain regulatory approvals for competing products more rapidly than us, and develop more-effective or less-expensive products or technologies that render our technology or products obsolete or non-competitive. We also compete with our competitors in recruiting and retaining qualified scientific and management personnel, establishing clinical trial sites, and patient registration for clinical trials, as well as in acquiring technologies and technology licenses complementary to our programs or advantageous to our business. If our competitors are more successful than us in these matters, our business may be harmed.
If we are unable to establish sales and marketing capabilities, or enter and maintain arrangements with third parties to sell and market our products, our business may be harmed.
We do not have a sales organization, and have no experience as a company in the sales, marketing, and distribution of medical devices. To be successful in commercializing our products, we must either develop a sales and marketing infrastructure or enter into distribution arrangements with others to market and sell our products. We have not yet hired any European sales people or entered into any third-party distribution agreements.
We currently plan to establish our own direct U.S. sales force. If we develop our own marketing and sales capabilities, our sales force will be competing with the experienced and well-funded marketing and sales operations of our more established competitors. Developing a sales force is expensive and time consuming, and could delay or limit the success of any product launch. We may not be able to develop this capacity on a timely basis, or at all. If we are unable to establish sales and marketing capabilities, we will need to contract with third parties to market and sell our products in the United States. To the extent that we enter arrangements with third parties to perform sales, marketing, and distribution services in the United States or internationally, our product revenue could be lower than if we directly marketed and sold our products or related devices that we may develop.
Furthermore, to the extent that we enter co-promotion or other marketing and sales arrangements with other companies, any revenue received will depend on the skills and efforts of others, and we do not know whether these efforts will be successful. Some of our future distributors may market their own products or other companies’ products that compete with ours, and they may have an incentive not to devote sufficient efforts to marketing our products. If we are unable to establish and maintain adequate sales, marketing, and distribution capabilities, independently or with others, we may not be able to generate product revenue, and may not become profitable.
23
The manufacturing facilities of our special process suppliers (e.g., neurostimulation device manufacture, extension manufacture, lead manufacture, sterilization, and calibration) must also comply with strictly enforced regulatory requirements. If we fail to achieve regulatory approval for our own manufacturing facilities or those of our suppliers, our business and our results of operations would be harmed.
Completion of our clinical trials and commercialization of our products require access to, or the development of, manufacturing facilities that comply with the FDA’s Quality Systems Regulation and Good Manufacturing Practice requirements. The FDA must approve facilities that manufacture our products for domestic commercial purposes, as well as the manufacturing processes and specifications for the product.
We depend on single-source suppliers for some of the components in our neurostimulation system. The loss of these suppliers could delay our clinical trials, or prevent or delay commercialization of our products.
Although we have identified several vendors for the components of our products, some of our components are currently provided by only one vendor, or a single-source supplier. In addition, we do not have long-term contracts with our third-party suppliers of some of the equipment and components that are used in our manufacturing process, and we do not carry a significant inventory of most components used in our products. Establishing additional or replacement suppliers for these components, and obtaining any additional regulatory approvals that may result from adding or replacing suppliers will take a substantial amount of time. We may also have difficulty obtaining similar components from other suppliers that are acceptable to the FDA or foreign regulatory authorities. Furthermore, since some of these suppliers are located outside of the United States, we are subject to foreign export laws and U.S. import and customs regulations, which complicate and could delay shipments to us.
If we must switch to replacement suppliers, we will face additional regulatory delays, and the manufacture and delivery of our products would be interrupted for an extended period, which would delay completion of our clinical trials or commercialization of our products. In addition, we will be required to obtain prior regulatory approval from the FDA or foreign regulatory authorities to use different suppliers or components that may not be as safe or as effective. As a result, regulatory approval of our products may not be received on a timely basis, or at all.
If we do not achieve our projected development goals in the time frames we announce and expect, the commercialization of our products may be delayed and, as a result, the value of our stock may decline.
From time to time, we may estimate and publicly announce the anticipated timing of the accomplishment of various clinical, regulatory, and other product development goals, which we sometimes refer to as milestones. These milestones could include obtaining CE Mark approval in the European Union, the submission of PMA documentation to the FDA, initiation of our pivotal U.S. clinical trials, the enrollment of patients in our clinical trials, the release of data from our clinical trials, and other clinical and regulatory events. The actual timing of these milestones could vary dramatically compared to our estimates, in some cases for reasons beyond our control. We cannot assure you that we will meet our projected milestones. If we do not meet these milestones as publicly announced, the commercialization of our products may be delayed and, as a result, the value of our stock may decline.
We depend on our officers, and if we are not able to retain them or recruit additional qualified personnel, our business will suffer.
Due to the specialized knowledge each of our officers possesses with respect to interventional medicine and our operations, the loss of service of any of our officers could delay or prevent the successful completion of our neurostimulation device development and the commercialization of our products. Each of our officers may terminate their employment without notice and without cause or good reason.
24
Upon receiving regulatory approval for our products, we expect to expand our operations and grow our research and development, product development, and administrative operations. Our growth will require hiring a significant number of qualified clinical, scientific, manufacturing, commercial, and administrative personnel. Accordingly, recruiting and retaining such personnel in the future will be critical to our success. There is intense competition from other companies and research and academic institutions for qualified personnel in the areas of our activities. If we fail to identify, attract, retain, and motivate these highly skilled personnel, we may be unable to continue our development and commercialization activities.
If we are unable to manage our expected growth, we may not be able to commercialize our products, including our neurostimulation system.
If we obtain CE Mark and FDA approvals for our products, we intend to continue to expand our operations, grow our research and development, product development, and administrative operations, and invest substantially in our manufacturing facilities. This expansion has and is expected to continue to place a significant strain on our management and operational and financial resources. To manage any expected growth, and to commercialize our products, we will be required to improve existing and implement new operational and financial systems, procedures, and controls, and expand, train, and manage our growing employee base. Our current and planned personnel, systems, procedures, and controls may not be adequate to support our anticipated growth. If we are unable to manage our growth effectively, our business could be harmed.
RISKS RELATED TO OUR INTELLECTUAL PROPERTY
We may be unable to complete or integrate intellectual property acquisitions effectively, which may adversely affect our growth, profitability, and results of operations.
We expect future acquisitions of intellectual property to play a significant role in our product development growth. As of the date hereof, we have made four such acquisitions; we cannot, however, be certain that we will be able to continue to identify attractive acquisition opportunities, obtain financing for acquisitions on satisfactory terms if needed, or successfully acquire identified targets. Additionally, we may not be successful in integrating acquired intellectual property into our existing operations and realizing anticipated synergies. Competition for acquisition opportunities in the industry in which we operate may increase our costs of acquisitions or cause us to refrain from engaging in acquisitions. These and other factors relating to our acquisition of intellectual property could negatively and adversely impact our growth, profitability, and results of operations.
Variability in intellectual property laws may adversely affect our intellectual property position.
Intellectual property laws—and patent laws and regulations in particular—have been subject to significant variability, either through administrative or legislative changes to such laws or regulations, or changes or differences in judicial interpretation. It is expected that such variability will continue to occur. Additionally, intellectual property laws and regulations differ between countries. Variations in the patent laws and regulations or in interpretations of patent laws and regulations in the United States and other countries may diminish the value of our intellectual property, and may change the impact of third-party intellectual property on us. Accordingly, we cannot predict the scope of patents that may be granted to us, the extent to which we will be able to enforce our patents against third parties, or the extent to which third parties may be able to enforce their patents against us.
We may need to negotiate modifications or extensions of existing intellectual property agreements.
We license intellectual property from third parties. In the future, we may need to extend, modify, or otherwise negotiate changes to such licenses. There can be no assurance that the third parties with whom we have (or in the future may have) agreements will agree to such modifications, or that, if they do, they will do so on terms favorable to us.
We or the third parties whom we license intellectual property from may become involved in legal proceedings to protect or enforce our intellectual property rights, which could be expensive and time-consuming.
Competitors or others may infringe upon our intellectual property rights. To counteract infringement or unauthorized use, we or third parties whom we license intellectual property from may be required to file patent infringement claims, which can be expensive and time-consuming. In addition, in an infringement proceeding, a court may decide that certain of our intellectual property is not valid or is unenforceable, or the court may refuse to stop the other party from using the technology at issue on the grounds that our intellectual property rights do not cover such technology.
25
An adverse determination of any litigation or defense proceedings could put our intellectual property at risk of being invalidated or interpreted narrowly, and could put outstanding intellectual property applications at risk of not being granted.
Interference proceedings brought by the United States Patent and Trademark Office may be necessary to determine the priority of inventions with respect to our intellectual property applications. Litigation or interference proceedings may fail, and, even if successful, may result in substantial costs, diversion of resources, and distraction of our management. We or our licensors may not be able to prevent misappropriation of our proprietary rights, particularly in countries where the laws may not protect such rights as comprehensively as in the United States.
Furthermore, due to the substantial amount of discovery associated with intellectual property litigation, there is a risk that some of our (or our licensors’) confidential information could be compromised by disclosure. In addition, during the course of this litigation, there could be public announcement of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, then it could have a substantial adverse effect on the price of our common stock. We or our licensors may not prevail in any litigation or interference proceeding in which we may be involved in the future. Even if we or our licensors do prevail, such legal proceedings would likely be expensive and time-consuming.
If we are unable to protect the confidentiality of our proprietary information and know-how related to any of our product candidates, our competitive position would be impaired and our business, financial condition, and results of operations could be adversely affected.
Some of our technology, including our knowledge regarding the processing of our product candidates, is unpatented, and is maintained by us as trade secrets. In an effort to protect these trade secrets, the information is restricted to our employees, consultants, collaborators, and advisors on a need-to-know basis only. In addition, we require our employees, consultants, collaborators, and advisors to execute confidentiality agreements upon the commencement of their relationships with us. These agreements require that all confidential information developed by the individual or made known to the individual by us during the course of the individual’s relationship with us be kept confidential and not disclosed to third parties. These agreements, however, do not ensure protection against improper use or disclosure of confidential information, and these agreements may be breached. A breach of confidentiality could affect our competitive position. In addition, in some situations, these agreements and other obligations of our employees to assign intellectual property to us may conflict with or be subject to the rights of third parties with whom our employees, consultants, collaborators, or advisors have had previous employment or consulting relationships. Also, others may independently develop substantially equivalent proprietary information and techniques, or otherwise gain access to our trade secrets.
Adequate remedies may not exist in the event of unauthorized use or disclosure of our confidential information. The disclosure of our trade secrets would impair our competitive position, and could have a materially adverse effect on our business, financial condition, and results of operations.
We may become subject to claims of infringement of the intellectual property rights of others, which could prohibit us from developing our treatment, require us to obtain licenses from third parties or to develop non-infringing alternatives, and subject us to substantial monetary damages. We have not obtained and do not intend to obtain any legal opinion with regard to our freedom to practice our technology.
Third parties could assert that our processes, product candidates, or technology infringe upon their patents or other intellectual property rights. Whether a process, product, or technology infringes upon a patent or other intellectual property involves complex legal and factual issues, the determination of which is often uncertain. We cannot be certain that we will not be found to have infringed upon the intellectual property rights of others. Because patent applications may remain unpublished for certain periods of time and may take years to be issued as patents, there may be applications now pending of which we are unaware, and/or that do not currently contain claims of concern but may later result in issued patents that our product candidates, procedures, or processes will infringe upon. There may be existing patents that our product candidates, procedures, or processes infringe upon, of which infringement we are not aware. Third parties could also assert ownership over our intellectual property. Such an ownership claim could cause us to incur significant costs to litigate the ownership issues. If an ownership claim by a third party were upheld as valid, we may be unable to obtain a license from the third party on acceptable terms, to continue to make, use, or sell technology free from claims by that third party of infringement upon the third party’s intellectual property. We have not obtained and do not intend to obtain any legal opinion with regard to our freedom to practice our technology at this time.
26
If we are unsuccessful in actions we bring against the patents of other parties, and it is determined that we infringe upon the patents of third parties, we may be subject to injunctions, or otherwise prevented from commercializing potential products and/or services in the relevant jurisdiction; or may be required to obtain licenses to those patents, or develop or obtain alternative technologies, any of which could harm our business. Furthermore, if such challenges to our patent rights are not resolved in our favor, we could be delayed or prevented from entering into new collaborations, or from commercializing certain product candidates and/or services, which could adversely affect our business and results of operations.
If we are successful in obtaining patent protection, we may not be able to enforce those patent rights against third parties.
Successful challenge of any future patents, such as through opposition, reexamination, inter partes review, interference, or derivation proceedings could result in a loss of patent rights in the relevant jurisdiction. Furthermore, because of the substantial amount of discovery required in connection with intellectual property litigation, there is a risk that some of our confidential or sensitive information could be compromised by disclosure in the event of litigation. In addition, during the course of litigation, there could be public announcements of the results of hearings, motions, or other interim proceedings or developments. If securities analysts or investors perceive these results to be negative, it could have a substantial adverse effect on the price of our common stock.
We may not be able to protect our intellectual property in countries outside of the United States.
Intellectual property law outside the United States is uncertain, and, in many countries, is currently undergoing review and revisions. The laws of some countries do not protect patent and other intellectual property rights to the same extent as United States laws. Third parties may challenge our patents in foreign countries by initiating proceedings, including pre- and post-grant oppositions and invalidation proceedings. Developments during opposition or invalidation proceedings in one country may directly or indirectly affect a corresponding patent or patent application in another country in an adverse manner. It may be necessary or useful for us to participate in proceedings to determine the validity of our patents or our competitors’ patents that have been issued in countries other than the United States. This could result in substantial costs, divert our efforts and attention from other aspects of our business, and have a materially adverse effect on our results of operations and financial condition.
RISKS RELATED TO OUR COMMON STOCK
Our shares may be thinly traded with wide share price fluctuations, low share process, and minimal liquidity.
Our shares of common stock began trading on the OTCQB exchange on September 27, 2017, and have experienced limited trading activity. The share price may be volatile, with wide fluctuations in response to several factors, including:
| ● | Potential investors’ anticipated feelings regarding our results of operations; |
| ● | Increased competition; | |
| ● | Our ability or inability to generate future revenues; and | |
| ● | Market perception of the future of development of the products and services we offer. |
27
In addition, if our shares are quoted on another trading platform or exchange for which we qualify, our share price may be affected by factors that are unrelated or disproportionate to our operating performance. Our share price might be affected by general economic, political, and market conditions such as recessions, interest rates, or international currency fluctuations. In addition, stocks traded over the OTC Markets quotation system are usually thinly traded, highly volatile, and not followed by analysts. These factors, which are not under our control, may have a material effect on our share price, and cause the share price to drop below the price you pay in this offering.
Because we can issue additional shares of common stock, purchasers of our common stock may suffer immediate dilution, and may experience further dilution in the future.
We are authorized to issue up to 75,000,000 shares of common stock. As of December 4, 2018, 1,965,646 shares of common stock are issued and outstanding. Our board of directors has the authority to mandate the issuance of additional shares of common stock without the consent of any of our shareholders. Consequently, our shareholders may experience further dilution of their ownership in the Company in the future, which could have an adverse effect on the trading market for our common stock.
We have not paid cash dividends in the past, and do not expect to pay dividends in the future. Any return on investment may be limited to the value of our common stock.
We have never paid cash dividends on our common stock, and do not anticipate doing so in the foreseeable future. The payment of dividends on our common stock will depend on earnings, financial condition, and other business and economic factors affecting us at such time as our board of directors may consider relevant. If we do not pay dividends, our common stock may be less valuable because a return on your investment will only occur if our stock price appreciates.
If we are not successful in listing our common stock on the Nasdaq Capital Market or other national securities exchanges, our common stock is deemed a “penny stock,” which would make it more difficult for our investors to sell their shares.
Our common stock is subject to the “penny stock” rules adopted under Section 15(g) of the Securities Exchange Act. The penny stock rules generally apply to companies whose common stock is not listed on the Nasdaq Capital Market or other national securities exchanges, and trades at less than $4.00 per share (other than companies that have had average revenue of at least $6,000,000 for the last three years, or that have tangible net worth of at least $5,000,000 ($2,000,000 if the company has been operating for three or more years). These rules require, among other things, that brokers who trade penny stocks to persons other than “established customers” complete certain documentation, make suitability inquiries of investors, and provide investors with certain information concerning trading in the security, including a risk disclosure document and quote information under certain circumstances. Many brokers have decided not to trade penny stocks because of the requirements of the penny stock rules, and, as a result, the number of broker-dealers willing to act as market makers in such securities is limited. If we remain subject to the penny stock rules for any significant period, it could have an adverse effect on the market, if any, for our securities. If our securities are subject to the penny stock rules, investors will find it more difficult to dispose of our securities. We have applied to have our common stock listed on the Nasdaq Capital Market under the symbol “NXMD.” Although we believe we will satisfy the Nasdaq Capital Market listing requirements, no assurance can be given that such listing will be achieved in a timely manner, or at all.
Our stock price may be volatile; you may not be able to resell your shares at or above your purchase price.
The market prices for our securities and the securities of companies similar to ours have been highly volatile, with price and volume fluctuations, and may continue to be highly volatile in the future. The following factors, in addition to other risk factors described in this section, may have a significant impact on the market price of our common stock, some of which are beyond our control:
| ● | Announcements of technological innovations or new commercial products by our competitors or us; | |
| ● | Our issuance of equity or debt securities, or disclosure or announcements relating thereto; |
28
| ● | Developments concerning proprietary rights, including patents; | |
| ● | Regulatory developments in the United States and foreign countries; | |
| ● | Litigation; | |
| ● | Economic and other external factors, or other disasters or crises; or | |
| ● | Period-to-period fluctuations in our financial results. |
Risks Related To Our Reverse Stock Split
We effected a 1-for-14 Reverse Stock Split of our outstanding common stock on June 25, 2018. However, the price of our common stock may decline below the minimum bid price requirement and we may not be able to list our common stock thereon.
The Reverse Stock Split increased the market price of our common stock. However, there is no guarantee that the price will not decline and we will be able to meet the minimum bid price requirement of the Listing Rules of the Nasdaq Capital Market. If we are unable meet the minimum bid price requirement, we may be unable to list our shares on the Nasdaq Capital Market.
We cannot assure you that we will be able to continue to comply with the minimum bid price requirement of the Nasdaq Capital Market.
We cannot assure you that the market price of our common stock following the Reverse Stock Split will remain at the level required for continuing compliance with the minimum bid price requirements of the Nasdaq Capital Market. It is not uncommon for the market price of a company’s common stock to decline in the period following a reverse stock split. If the market price of our common stock declines following the effectuation of a reverse stock split, the percentage decline may be greater than would occur in the absence of a reverse stock split. In any event, other factors unrelated to the number of shares of our common stock outstanding, such as negative financial or operational results, could adversely affect the market price of our common stock and jeopardize our ability to meet or maintain the Nasdaq Capital Market’s minimum bid price requirement. In addition to specific listing and maintenance standards, the Nasdaq Capital Market has broad discretionary authority over the initial and continued listing of securities, which it could exercise with respect to the listing of our common stock.
There can be no assurance that we will be able to comply with other continued listing standards of the Nasdaq Capital Market.
We cannot assure you that we will be able to comply with the standards that we are required to meet in order to maintain a listing of our common stock on the Nasdaq Capital Market. Our failure to meet these requirements may result in our common stock being delisted from the Nasdaq Capital Market, irrespective of our compliance with the minimum bid price requirement.
RISKS RELATED TO THIS OFFERING
Investors in this offering will suffer immediate and substantial dilution.
Because the price per share of our common stock being offered is substantially higher than the net tangible book value per share of our common stock, you will suffer substantial dilution in the net tangible book value of the common stock you purchase in this offering. If you purchase shares of common stock and warrants to purchase shares of common stock in this offering, you will suffer immediate and substantial dilution of $2.60 per share in the net tangible book value of the common stock. See the section entitled “Dilution” below for a more detailed discussion of the dilution you will incur if you purchase common stock in this offering.
29
Management will have broad discretion as to the use of the net proceeds from this offering, and we may not use these proceeds effectively.
Our management will have broad discretion in the application of the net proceeds from this offering, and could spend the proceeds in ways that do not improve our results of operations or enhance the value of our common stock. Accordingly, you will be relying on the judgment of our management with regard to the use of these net proceeds, and you will not have the opportunity, as part of your investment decision, to assess whether the proceeds are being used appropriately. Our failure to apply these funds effectively could have a materially adverse effect on our business, delay the development of our product candidates, and cause the price of our common stock to decline.
Future sales or issuances of our common stock may cause the market price of our common stock to decline.
The sale of substantial amounts of our common stock, whether directly by us or in the secondary market by existing security holders (including holders of our outstanding warrants and convertible debt), as well as the perception that such sales could occur, or the availability for future sale of shares of our common stock or securities convertible into (or exchangeable or exercisable for) our common stock could materially and adversely affect the market price of our common stock, and our ability to raise capital through future offerings of equity or equity-related securities. Any such sales may result in significant dilution to our existing shareholders, including you. We cannot assure you that we will be able to sell shares or other securities in any other offering at a price per share that is equal to or greater than the price per share paid by investors in this offering. In addition, investors purchasing shares or other securities in the future could have rights superior to existing stockholders, which will result in additional dilution to you.
There is no guarantee that we will be accepted for listing on the Nasdaq Capital Market. If we are accepted, but are not at any point able to comply with the applicable continued listing requirements or standards of the Nasdaq Capital Market, the Nasdaq Capital Market could delist our common stock.
We have applied to have our common stock listed on the Nasdaq Capital Market under the symbol “NXMD”. Although we believe we will satisfy the Nasdaq Capital Market listing requirements, no assurance can be given that such listing will be achieved in a timely manner, or at all. In the event we successfully list our common stock on the Nasdaq Capital Market, in order to maintain that listing, we must satisfy minimum financial and other continued listing requirements and standards, including those regarding director independence and independent committee requirements, minimum stockholders’ equity, minimum share price, and certain corporate governance requirements. There can be no assurances that we will be able to comply with the applicable listing standards.
In the event that our common stock is delisted from the Nasdaq Capital Market and is not eligible to be listed on another national securities exchanges, trading of our common stock could be conducted in the over-the-counter market, or on an electronic bulletin board established for unlisted securities such as the OTC Pink or OTCQB. In such event, it could become more difficult to dispose of or obtain accurate price quotations for our common stock, and there would likely also be a reduction in our coverage by securities analysts and the news media, which could cause the price of our common stock to decline further. Also, it may be difficult for us to raise additional capital if we are not listed on a major exchange.
If our common stock is not listed on a national securities exchange, compliance with applicable state securities laws may be required for subsequent offers, transfers. and sales of the shares of common stock offered hereby.
The securities offered hereby are being offered pursuant to one or more exemptions from registration and qualification under applicable state securities laws. We have applied to have our common stock listed on the Nasdaq Capital Market under the symbol “NXMD”. Although we believe we will satisfy the Nasdaq Capital Market listing requirements, no assurance can be given that such listing will be achieved in a timely manner, or at all. No assurance can be given that our application will be approved, and as such we are not required to register or qualify in any state the subsequent offer, transfer, or sale of the common stock, until such time as is necessary. If our common stock is delisted from the Nasdaq Capital Market and is not eligible to be listed on another national securities exchange, subsequent transfers of the shares of our common stock offered hereby by U.S. holders may not be exempt from state securities laws. In such event, it will be the responsibility of the holder of shares to register or qualify the shares for any subsequent offer, transfer, or sale in the United States, or to determine that any such offer, transfer, or sale is exempt under applicable state securities laws.
30
The warrants are speculative in nature.
The warrants to be issued to investors in this offering do not confer any rights of common stock ownership on their holders, such as voting rights or the right to receive dividends, but rather merely represent the right to purchase shares of common stock at a fixed price for a limited period of time. There can be no assurance that the market price of the common stock will ever equal or exceed the exercise price of the warrants, and, consequently, whether it will ever be profitable for holders of the warrants to exercise the warrants.
If you purchase Units in this offering, as a holder of warrants, you will have no rights as a common stockholder with respect to the shares of common stock underlying the warrants until you acquire our common stock.
If you purchase Units in this offering, until you acquire our common stock upon exercise of your warrants, you will have no rights with respect to the common stock underlying the warrants. Upon exercise of your warrants, you will be entitled to exercise the rights of a common stockholder only as to matters for which the record date for actions to be taken by our common stockholders occurs after the date you exercise your warrants.
An active trading market for the warrants may not develop.
Although we intend to apply to have the warrants listed on the Nasdaq Capital Market, no assurance can be given that such listing will be approved or that a trading market will develop for the warrants. Without an active market, the liquidity of the warrants will be limited.
CAUTIONARY NOTE REGARDING FORWARD-LOOKING STATEMENTS
This prospectus contains forward-looking statements. Such forward-looking statements include those that express plans, anticipation, intent, contingency, goals, targets, future development, and/or otherwise that are not statements of historical fact. These forward-looking statements are based on our current expectations and projections about future events, and are subject to risks and uncertainties known and unknown that could cause actual results and developments to differ materially from those expressed or implied in such statements.
In some cases, you can identify forward-looking statements by terminology, such as “expects,” “anticipates,” “intends,” “estimates,” “plans,” “potential,” “possible,” “probable,” “believes,” “seeks,” “may,” “should,” “could,” or the negative of such terms or other similar expressions. Accordingly, these statements involve estimates, assumptions, and uncertainties that could cause actual results to differ materially from those expressed in them. Any forward-looking statements are qualified in their entirety by reference to the factors discussed throughout this prospectus.
You should read this prospectus and the documents that we reference herein and therein and have filed as exhibits to the registration statement (of which this prospectus is part) completely and with the understanding that our actual future results may be materially different from what we expect. You should assume that the information appearing in this prospectus is accurate as of the date on the front cover of this prospectus only. Because the risk factors referred to above could cause actual results or outcomes to differ materially from those expressed in any forward-looking statements made by us or on our behalf, you should not place undue reliance on any forward-looking statements. These risks and uncertainties, along with others, are described above under the heading “Risk Factors” beginning on page 8 of this prospectus. Further, any forward-looking statement speaks only as of the date on which it is made, and we undertake no obligation to update any forward-looking statement to reflect events or circumstances after the date on which the statement is made, or to reflect the occurrence of unanticipated events. New factors emerge from time to time, and it is not possible for us to predict which factors will arise. In addition, we cannot assess the impact of each factor on our business, or the extent to which any factor, or combination of factors, may cause actual results to differ materially from those contained in any forward-looking statements. We qualify all of the information presented in this prospectus, and particularly our forward-looking statements, by these cautionary statements.
31
We estimate that the net proceeds of this offering from the issuance and sale of 2,474,227 Units in this offering will be approximately $10,685,000, or approximately $12,341,000 if the underwriters exercise the over-allotment option in full, after deducting the estimated underwriting discount and estimated offering expenses payable by us.
A $1.00 increase (decrease) in the assumed public offering price of $4.85 per Unit would increase (decrease) the expected net proceeds of this offering by approximately $2,274,000, assuming the number of Units offered by us remains the same, and after deducting the estimated underwriting discount and estimated offering expenses payable by us. A 250,000 increase (decrease) in the assumed number of Units sold in this offering would increase (decrease) the expected net proceeds of this offering by approximately $1,114,000, assuming the assumed public offering price per Unit remains the same.
We intend to use the net proceeds received from this offering for seeking approval for the CE mark and PMA for our DBS device (approximately $5.2 million), filing a de novo request for our non-invasive auricular vagus nerve stimulator (aVNS) withdrawal product (approximately $1.2 million), European and United States sales launch for our DBS device (approximately $2.1 million), pre-payment of the outstanding senior secured convertible promissory note to Leonite Capital, LLC (approximately $1.3 million, including $1.1 million of principal and $145,600 in pre-payment penalties), working capital and general corporate purposes. The Leonite Capital, LLC debt bears a 12% annual interest rate and matures December 2019.
We believe that the net proceeds from this offering and our existing cash and cash equivalents, together with interest thereon, and grant funding awards will be sufficient to fund our operations for at least the next 12 months after the date of this prospectus.
We have not yet determined the amount of net proceeds to be used specifically for any of the foregoing purposes. Accordingly, we will retain broad discretion over the use of these proceeds.
PRICE RANGE OF COMMON STOCK AND RELATED MATTERS
There is a limited public market for our common shares. Our common stock has traded on the OTCQB under the ticker symbol “NXNN” since September 27, 2017. Prior to September 27, 2017 there was no public market for our common stock.
Trading in stocks quoted on the OTCQB platform is often thin, and is characterized by wide fluctuations in trading prices due to many factors that may be unrelated to a company’s operations or business prospects. We cannot assure you that there will be a market for our common stock in the future.
In connection with the offering made hereby, we have applied for listing of our common stock on the Nasdaq Capital Market under the symbol “NXMD”.
The following table sets forth the high and low sales prices for our common stock for each quarterly period since September 27, 2017, which have been retroactively restated to give effect to the Reverse Stock Split.
| High | Low | |||||||
| Fiscal Year 2017 | ||||||||
| Third quarter (since September 27, 2017) | $ | 28.00 | $ | 28.00 | ||||
| Fourth quarter | $ | 35.00 | $ | 5.60 | ||||
| Fiscal Year 2018 | ||||||||
| First quarter | $ | 25.20 | $ | 7.70 | ||||
| Second quarter | $ | 20.00 | $ | 4.48 | ||||
| Third quarter | $ | 20.00 | $ | 6.50 | ||||
| Fourth quarter through December 4, 2018 | $ | 8.00 | $ | 3.00 | ||||
32
The last reported sales price of our common stock on the OTCQB on December 14, 2018 was $4.95. According to the records of our transfer agent, as of November 28, 2018, there were approximately 249 holders of record of our common stock. Quotes on the OTCQB may not be indicative of the market price of our common stock on a national securities exchange, including the Nasdaq Capital Market.
We have never declared or paid cash dividends on our capital stock. We currently intend to retain any future earnings for use in the operation of our business, and do not intend to declare or pay any cash dividends in the near future. Any further determination to pay dividends on our capital stock will be at the discretion of our board of directors, subject to applicable laws, and will depend on our financial condition, results of operations, capital requirements, general business conditions, and other factors that our board of directors consider relevant.
If you purchase Units in this offering, you will experience dilution to the extent of the difference between the price per Unit you pay in this offering and the net tangible book value per share of our common stock immediately after this offering. The net tangible book value of our common stock on September 30, 2018, was approximately $(694,693), or approximately $(0.35) per share. Net tangible book value per share is equal to the amount of our total tangible assets, less total liabilities, divided by the aggregate number of shares of our common stock outstanding.
After giving effect to the sale by us of the Units in this offering at an assumed public offering price of $4.85 per Unit, and after deducting the estimated underwriting discount and estimated offering expenses payable by us, our as adjusted net tangible book value as of September 30, 2018, would have been approximately $9,990,307, or approximately $2.25 per share. This represents an immediate increase in net tangible book value of approximately $2.60 per share to existing stockholders, and an immediate dilution of approximately $2.60 per share to new investors purchasing shares of our common stock and warrants to purchase shares of common stock in this offering. The following table illustrates this per share dilution:
| Assumed public offering price per Unit | $ | 4.85 | ||||||
| Net tangible book value per common share as of September 30, 2018 | $ | (0.35 | ) | |||||
| Increase in net tangible book per common share attributable to this offering | 2.60 | |||||||
| Adjusted net tangible book value per common share after this offering | 2.25 | |||||||
| Dilution in pro forma net tangible book value per common share to new investors | $ | 2.60 |
A $1.00 increase (decrease) in the assumed public offering price of $4.85 per Unit would increase (decrease) our as adjusted net tangible book value after this offering by approximately $2,274,000, or approximately $0.51 per share, and the dilution to new investors by approximately $0.49 per share, assuming that the number of Units offered by us, as set forth above, remains the same, and after deducting the estimated underwriting discount and estimated offering expenses payable by us. We may also increase or decrease the number of Units we are offering from the assumed number of shares set forth above. An increase (decrease) of 250,000 in the assumed number of Units sold in this offering would increase (decrease) our as adjusted net tangible book value after this offering by approximately $1,114,000, or approximately $0.12 per share, and decrease (increase) the dilution per share to new investors by approximately $0.12 per share, assuming that the assumed public offering price of $4.85 per share remains the same. The information discussed above is illustrative only, and will adjust based on the actual public offering price, the actual number of Units that we offer in this offering, and other terms of this offering determined at pricing.
33
If the underwriters exercise in full their option to purchase 371,134 additional shares of common stock and warrants to purchase an additional 742,268 shares of common stock in full at the assumed public offering price of $4.85 per Unit, the as adjusted net tangible book value of our common stock after this offering would be $2.42 per share, representing an immediate increase in net tangible book value of approximately $2.77 per share to existing stockholders, and an immediate dilution of $2.43 per share to the investors in this offering, after deducting the underwriting discount and estimated offering expenses payable by us.
This table does not take into account further dilution to new investors that could occur upon the exercise of outstanding options and warrants having a per share exercise price less than the public offering price per share in this offering. In addition, we may choose to raise additional capital due to market conditions or strategic considerations, even if we believe we have sufficient funds for our current or future operating plans. To the extent that additional capital is raised through the sale of equity or convertible debt securities, the issuance of these securities could result in further dilution to our stockholders.
The table and discussion above are based on 1,965,646 shares outstanding as of September 30, 2018 after giving effect to the Reverse Stock Split, and exclude as of that date:
| ● | 210,989 shares of our common stock issuable upon exercise of outstanding vested options at a weighted average exercise price of $14.17 per share, and 135,189 shares of our common stock issuable upon exercise of outstanding unvested options at a weighted average exercise price of $13.23 per share and 103,822 shares of common stock that remain available for issuance under the 2016 Plan; | |
| ● | 82,926 shares of our common stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $19.39 per share; and | |
| ● | 128,000 shares of our common stock issuable upon the conversion of the Leonite Capital, LLC senior secured convertible promissory note with an outstanding principal balance in the amount of $1,120,000 at a conversion price of $8.75 per share. |
The following table sets forth our capitalization as of September 30, 2018:
| ● | On an actual basis; | |
| ● | On an as adjusted basis to give effect to the issuance and sale of 2,474,227 Units in this offering at an assumed public offering price of $4.85 per Unit, and after deducting the estimated underwriting discount and estimated offering expenses payable by us. |
| As of September 30, 2018 | ||||||||
| Actual | As Adjusted | |||||||
| Long-term debt | $ | 2,327,666 | $ | 2,327,666 | ||||
| Stockholders’ equity | ||||||||
| Common stock, par value $0.001 | 1,966 | 4,440 | ||||||
| Additional paid-in capital | 15,838,654 | 26,521,180 | ||||||
| Equity instruments to be issued | 65,839 | 65,839 | ||||||
| Accumulated deficit | (6,810,662 | ) | (6,810,662 | ) | ||||
| Accumulated other comprehensive income | 43,157 | 43,157 | ||||||
| Total stockholders’ equity | 9,138,954 | 19,823,954 | ||||||
| Total capitalization | $ | 11,466,620 | $ | 22,151,620 | ||||
34
A $1.00 increase (decrease) in the assumed public offering price of $4.85 per Unit would increase (decrease) the as adjusted for this offering amount of each of additional paid-in capital, total stockholder’s equity, and total capitalization by approximately $2,274,000, assuming that the number of Units offered by us, as set forth above, remains the same, and after deducting the estimated underwriting discount and estimated offering expenses payable by us.
The table above and the number of shares of common stock that will be outstanding after the offering is based on 1,965,646 shares of common stock outstanding as of September 30, 2018, and exclude as of that date:
| ● | 210,989 shares of our common stock issuable upon exercise of outstanding vested options at a weighted average exercise price of $14.17 per share, and 135,189 shares of our common stock issuable upon exercise of outstanding unvested options at a weighted average exercise price of $13.23 per share and 103,822 shares of common stock that remain available for issuance under the 2016 Plan; | |
| ● | 82,926 shares of our common stock issuable upon exercise of outstanding warrants at a weighted average exercise price of $19.39 per share; and | |
| ● | 128,000 shares of our common stock issuable upon the conversion of the Leonite Capital, LLC senior secured convertible promissory note with an outstanding principal balance in the amount of $1,120,000 at a conversion split-adjusted price of $8.75 per share. |
MANAGEMENT’S DISCUSSION AND ANALYSIS
OF FINANCIAL CONDITION AND RESULTS OF OPERATIONS
The following discussion and analysis of financial condition and results of operations should be read together with our financial statements and accompanying notes appearing elsewhere in this prospectus. This Management’s Discussion and Analysis of Financial Condition and Results of Operations contains forward-looking statements that involve risks and uncertainties. Please see “Cautionary Note Regarding Forward-Looking Statements and Industry Data” set forth in the beginning of this prospectus, and see “Risk Factors” beginning on page 8 for a discussion of certain risk factors applicable to our business, financial condition, and results of operations. Operating results are not necessarily indicative of results that may occur in future periods.
Overview
Overview of Business
We are a medical device company focused on the development, manufacturing, and commercialization of neurostimulation technology for the treatment of various neurological disorders through electrical stimulation of neural tissues. We believe our neurostimulation technology platform has the potential to provide treatment to patients in several established neurostimulator markets, including deep brain stimulation (DBS), peripheral electrical nerve stimulation (PENS), sacral nerve stimulation (SNS), spinal cord stimulation (SCS), vagus nerve stimulation (VNS), and other emerging neurostimulator markets.
35
We anticipate that our first commercial application of our platform will be the Viant™ Deep Brain Stimulation System (or the Viant™ System). We plan to pursue regulatory approval of the Viant™ System for Parkinson’s disease, essential tremor, and dystonia in Europe in 2019, and for Parkinson’s disease in the United States in the second half of 2019. The Viant™ DBS device is designed to deliver best-in-class stimulation with the capability to collect local field potential (LFP) recordings. Using LFP surveys, neurologists will be able to quickly and confidently determine where to stimulate to take full advantage of directional leads. Moreover, our devices are designed to be non-invasively upgradable, enabling both physicians and patients to benefit from the latest technology as it is developed, without the need for implantable pulse generator replacement surgery to take advantage of new features.
The Viant™ System continues to meet critical milestones in its development program. Previous generations of the device have been CE Marked and were manufactured and sold to a joint venture between GlaxoSmithKline. We completed the ISO 13485 certification process, which is a pivotal hurdle, prior to regulatory submissions to the CE Mark authorities. Design verification, process validation, and testing requirements are nearly complete, and management expects we will complete the technical file in 2019 (with the exception of some longer-duration biocompatibility and shelf life tests). The Company expects to submit an application for a CE Mark in 2019. As related to the United States, we completed the pre-submission meetings with the FDA in early 2018 to determine scope of requirements for approval of the Viant™ System. As a result of that meeting, we intend to submit a PMA application without a clinical study. Although the FDA is allowing us to submit an application for a PMA that leverages the clinical data from a previously approved product in accordance with Section 216 of the Food and Drug Administration Modernization Act of 1997, no assurance can be given that our application which will be submitted without a clinical study will be sufficient for approval by the FDA. .
We have additional opportunities to license the neurostimulator platform to companies focused on enhancements to a comprehensive system offering for closed-loop, chronic disease therapeutics, including advanced computational biology, deep learning utilizing Internet of Medical Things technology, imaging solutions, e-health programs, and big data management and optimization, among others. Once we complete the development of the platform for our DBS application, we will be able to potentially license the platform to capitalize on hundreds of diseases of the nervous system that could be therapeutically addressed with neurostimulation.
We also operate our wholly owned subsidiary, Medi-Line, a Belgium company, which currently serves over 30 medical device customers in 16 countries, including multi-year contracts with Fortune 500 health care companies. The Belgian manufacturer owns state-of-the-art facilities, which feature two validated clean rooms (one assembly clean room Class ISO 7 or C, and one extrusion/injection molding clean room Class ISO 8 or D) and 600m2 of production space. Its capabilities will enable us to reduce risks associated with our commercial launch and speed the development of our neurostimulation products.
As of September 30, 2018, we had an accumulated deficit of $6,810,662. For the nine months ended September 30, 2018 and 2017, our net loss was $3,067,224 and $3,590,801 respectively.
We expect that we will continue to incur significant expenses and increasing operating losses relating to our ongoing activities, particularly as we continue to invest in research and development and initiate clinical trials required to receive regulatory approval for our medical devices in both the United States and the European Union. Additionally, when we initiate a launch of one or more of our products, we expect to incur substantial commercialization expenses related to the manufacture and distribution, as well as sales and marketing, of these products. In addition, we are subject to additional costs associated with operating as a public company. Accordingly, we may need to obtain additional funding to continue operations. Such financing may not be available to us on acceptable terms, or at all. In the event we require additional capital and are unable to secure such funding, we could be forced to delay, reduce, or eliminate our research and development activities, as well as any future commercialization of our products.
Prior to the acquisition of Medi-Line and NMB, we had not generated any revenues, and we financed our operations primarily with net proceeds from the private placements of our common stock, and from non-dilutive research and development grant awards. Our ability to generate revenues in addition to the Medi-Line manufacturing revenues will depend heavily on the successful completion of the requisite clinical trials and studies necessary to achieve approval to begin marketing our contemplated neurostimulation devices from the relevant regulatory authorities in the United States and the European Union.
36
Business Segments
The manufacturing segment includes the manufacturing operations of our wholly owned subsidiary Medi-Line, located in Angleur (Liege), Belgium. Medi-Line manufactures single-use medical devices for the medical and pharmaceutical sectors, including radiopharmacy technology, urology products, and sterilization cases and trays, and designs, develops, and offers worldwide production and supply-chain capabilities for these products to its customers.
The neurostimulation segment includes development, manufacturing, and commercialization of neurostimulation technology for the treatment of various neurological disorders through electrical stimulation of neural tissues. Our first commercial application will be the Viant™ Deep Brain Stimulation System. Operations for the neurostimulation segment are conducted in the United States, Puerto Rico, Belgium, and Germany.
Other items of revenue not directly related to manufacturing or neurostimulation revenues are categorized as other operating income. Other operating income and expenses not directly related to a specific segment are identified as “other” and not allocated to segments.
Results of Operations—Three and Nine Months Ended September 30, 2018 and 2017
Consolidated Sales Revenue
For the three months ended September 30, 2018:
| Three Months Ended September 30, | ||||||||
| 2018 | 2017 | |||||||
| Manufacturing | $ | 1,938,908 | $ | 816,325 | ||||
| Neurostimulation | 5,003 | 202,362 | ||||||
| Other | 33,718 | 20,992 | ||||||
| Consolidated total | $ | 1,977,629 | $ | 1,039,679 | ||||
Manufacturing segment: Manufacturing sales were $1,938,908. The prior period comparison for the three months ended September 30, 2017 includes only one month of sales as the acquisition of Medi-Line occurred on August 30, 2017.
Neurostimulation segment: Neurostimulation sales decreased by $197,359 from $202,362 for the three months ended September 30, 2017 to $5,003 for the three months ended September 30, 2018 as only a small sub-component part sale occurred for three months ended September 30, 2018 and no other deliveries were scheduled pursuant to any development, manufacturing and supply agreement with customers for the quarter.
Other: Our other operating revenues for the three months ended September 30, 2018 and 2017 were $33,718 and $20,992, respectively. The increase of 60.6% from 2017 to 2018 was primarily due to the inclusion of Medi-Line other operating income for the three months ended September 30, 2018 and only one month for the three months ended September 30, 2017.
For the nine months ended September 30, 2018:
Consolidated revenues increased to $7,666,827. The change in consolidated revenues consisted of the following segmented revenue activity:
| Nine Months Ended September 30, | ||||||||
| 2018 | 2017 | |||||||
| Manufacturing | $ | 7,119,557 | $ | 816,325 | ||||
| Neurostimulation | 400,055 | 292,936 | ||||||
| Other | 147,215 | 38,027 | ||||||
| Consolidated total | $ | 7,666,827 | $ | 1,147,288 | ||||
37
Manufacturing segment: Manufacturing sales were $7,119,557 for the nine months ended September 30, 2018. The prior period comparison for the nine months ended September 30, 2017 includes only one month of sales as the acquisition of Medi-Line occurred on August 30, 2017.
Neurostimulation segment: Neurostimulation sales for the nine months ended September 30, 2018 increased by $107,119 or 36.6% from the nine months ended September 30, 2017. Neurostimulation sales reflect the sale of neurostimulation devices and sub-components for operation of the devices for pre-clinical use to a single customer. The increase for the nine months ended September 30, 2018 over the nine months ended September 30, 2017 is due to scheduled deliveries pursuant to a development, manufacturing and supply agreement with the customer.
Other: Our other operating revenues for the nine months ended September 30, 2018 and 2017 were $147,215 and $38,027, respectively. The increase of 287.1% over the prior nine months was primarily due to the inclusion of Medi-Line other operating income for the nine months ended September 30, 2018. Other operating income consists primarily of Belgian government credits for employing staff in the research and development sector.
Cost of Revenue, Research and Development Expense, and Selling, General, and Administrative Expense
For the three months ended September 30, 2018:
Cost of revenue, research and development expense, and selling, general, and administrative expense as a percentage of revenue were as follows:
| Three Months Ended September 30, | ||||||||||||||||
| 2018 | % Rev | 2017 | % Rev | |||||||||||||
| Cost of product sold | $ | 1,384,335 | 70.0 | % | $ | 709,373 | 68.2 | % | ||||||||
| Research and development expenses | $ | 674,360 | 34.1 | $ | 287,229 | 27.6 | ||||||||||
| Selling, general, and administrative expenses | $ | 875,016 | 44.2 | % | $ | 651,556 | 62.7 | % | ||||||||
Cost of revenue: Consolidated costs of revenue increased to $1,384,335 for the three months ended September 30, 2018 from $709,373 for the three months ended September 30, 2017. The increase was driven by the addition of Medi-Line’s manufacturing activity and product mix for sales at Medi-Line for the three months ended September 30, 2018.
Research and development expenses: Consolidated research and development expenses increased $387,131 to $674,360 for the three months ended September 30, 2018 from $287,229 for the three months ended September 30, 2017. The net increase reflects the reduction of research and development credited to grants and the increase in research and development activity at Medi-Line.
It is expected that our research and development (“R&D”) activities and related expenses will increase significantly in the future as we increase the scope and rate of such efforts and begin more expensive development activities, including clinical trials and similar studies as required by the relevant regulatory authorities in our targeted jurisdictions (i.e., the United States and the European Union).
R&D expenses consist of the costs associated with our research and discovery efforts related to the design and development of our proposed medical devices. Primarily, R&D expenses are expected to include, but may not be limited to:
| ● | Facilities, laboratory supplies, equipment, and related expenses; | |
| ● | Employee-related expenses, which, among other things, includes salaries, benefits, travel, and stock-based compensation; | |
| ● | External R&D activities incurred under arrangements with third parties, such as contract research organizations, manufacturing organizations, consultants, and possibly a scientific advisory board; and | |
| ● | License fees and other costs associated with securing and protecting IP. |
Selling, general, and administrative expenses: Selling, general and administrative expenses increased $223,460 to $875,016 for the three months ended September 30, 2018 from $651,556 for the three months ended September 30, 2017. The net increase was driven by the increase in Medi-Line activity for the three months ended September 30, 2018 compared to one month of activity for Medi-Line for the three months ended September 30, 2017.
38
Selling, general and administrative expenses generally consist of salaries and similar costs associated with employees, including stock-based compensation expense. This category of expenses may also include facility costs and professional fees related to (i) legal and accounting services; (ii) capital formation; (iii) investor and public relations services; and (iv) general corporate consulting services.
It is expected that our selling, general, and administrative expenses will increase in the future as we expand our R&D activities in pursuit of regulatory approval for our contemplated medical devices, and as we increase sales and marketing expenses related to the sales of our neurostimulation devices.
For the nine months ended September 30, 2018:
Cost of revenue, research and development expense, and selling, general, and administrative expense as a percentage of revenue were as follows:
| Nine Months Ended September 30, | ||||||||||||||||
| 2018 | % Rev | 2017 | % Rev | |||||||||||||
| Cost of product sold | $ | 5,533,173 | 72.2 | % | $ | 737,664 | 64.3 | % | ||||||||
| Research and development expenses | 1,881,198 | 24.5 | 1,659,560 | 144.7 | ||||||||||||
| Selling, general, and administrative expenses | 2,724,960 | 35.5 | % | 1,817,218 | 158.4 | % | ||||||||||
Cost of revenue: Consolidated costs of revenue increased to $5,533,173 for the nine months ended September 30, 2018 from $737,664 for the nine months ended September 30, 2017. The increase was primarily driven by the addition of Medi-Line’s manufacturing activity for the nine months ended September 30, 2018 compared to one month of activity for Medi-Line for the nine months ended September 30, 2017.
Research and development expenses: Consolidated research and development expenses increased $221,638 to $1,881,198 for the nine months ended September 30, 2018 from $1,659,560 for the nine months ended September 30, 2017. The increase reflects the addition of research and development activity at Medi-Line and an increase in applicable grant related activity recorded as a reduction to research and development expense.
We have been awarded multiple grants and subsidies for our research and development activities, and receives these funds as advance payments and reimbursements for applicable project expenses. We recognize the amounts received in accordance with the contracts as a reduction of research and development expenses over the periods necessary to match the contract on a systematic basis to the costs that it is intended to compensate. We record, on the balance sheet, grants receivable (upon meeting the criteria discussed above) until cash is received. For the nine months ended September 30, 2018 and 2017, we recorded a credit to research and development expense in the amount of $1,054,765 and $1,631,034, respectively, related to these grants and subsidies.
Selling, general, and administrative expenses; Selling, general and administrative expenses increased $907,742 to $2,724,960 for the nine months ended September 30, 2018 from $1,817,218 for the nine months ended September 30, 2017. The net increase was driven by the addition of the Medi-Line activity for the nine months ended September 30, 2018 compared to one month of activity for Medi-Line for the nine months ended September 30, 2017.
Depreciation and Amortization
Depreciation and amortization expenses consist of amortization of acquired intangibles and depreciation of buildings, capital improvements, capitalized building lease, office equipment, and furniture and fixtures. Property, plant and equipment is depreciated using the straight-line method over the estimated useful lives of the assets.
39
During the year ended December 31, 2016, we acquired $6,120,000 in patents pertaining to the cardiovascular disease technology acquired in the merger with NXDE, and acquired $3,190,000 in patent licenses for the underlying patents referred to as the Siemens Patents. NMB holds patents and licenses totaling $1,053,097 related to our neurostimulation technology and devices. The amortization period for each of the individual patents depends on the legal terms for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. The patents and patent licenses are amortized using the straight-line method over the remaining time until expiration. The majority of these patents and patents underlying the license will expire between 2019 and 2036. Through the acquisition of Medi-Line, we acquired intangible assets with a fair value in the amount of $1,550,000 for trade secrets and know-how and $600,000 for customer relationships.
For the nine months ended September 30, 2018 and 2017, our depreciation expenses were $186,834 and $63,413, respectively. For the nine months ended September 30, 2018 and 2017, our amortization expenses were $908,241 and $861,377, respectively.
For the three months ended September 30, 2018 and 2017, our depreciation expenses were $61,021 and $38,112, respectively. For the three months ended September 30, 2018 and 2017, our amortization expenses were $302,081 and $288,680, respectively.
Interest Income (Expense)
For the three and nine months ended September 30, 2018, our interest expense was $77,873 and $235,869 respectively and for the three and nine months ended September 30, 2017, interest expense, net of interest income, was $26,537 and $23,751, respectively. Interest expense includes interest on bank loans and credit facilities, interest on leasing, interest on convertible debt, interest on shareholder notes, and non-cash amortizing interest for the original discount of convertible debt in the amount of $33,876 and $97,639 for the three and nine months ended September 30, 2018.
Other Income (Expense)
For the three and nine months ended September 30, 2018 we recorded other expense, net of other income, in the amount of $77,873 and $75,869, respectively. On May 22, 2018 we redeemed and cancelled 14,286 shares of our common stock from a former director as consideration for the purchase of certain intellectual property. These shares were valued at $160,000 and recorded as gain on disposition of patent. For the three and nine months ended September 30, 2017, we recorded other income, net of other expense, in the amount of $511,019 and $387,940, respectively. In the second quarter of 2017, we recorded a charge for the write-off of a loan in the amount of $171,946 and in the first quarter of 2017 recorded a charge of $37,788 for the exchange of stock held for investment and in the third quarter of 2017 we recorded a gain on bargain purchase for the acquisition of INGEST and Medi-Line in the amount of $624,211.
Provision for Income Taxes
For the nine months ended September 30, 2018, we recorded a provision for Belgian corporate income taxes in the amount of $11,928 based on the taxable income for Medi-Line. Our holding company subsidiary, NXEU, was assessed annual corporate taxes in the amount of $5,038 during three months ended September 30, 2018. For the nine months ended September 30, 2017 we recorded a benefit to provision for income tax in the amount of $13,203.
On December 27, 2017, NXPROC was granted a tax exemption pursuant to Act number 73-2008 (“ACT 73”) by the Government of Puerto Rico, Department of Economic Development and Commerce (“PRIDCO”). The exemption allows NXPROC to obtain tax credits in the amount of fifty percent (50%) of approved applicable research and development expenses of NXPROC. As of May 24, 2018, we have received all government approvals and certifications from PRIDCO and received tax credits in the amount of $732,340, for research and development activity in 2017, which had been posted to Departamento De Hacienda (the Puerto Rican Department of Finance) system and we have received $593,195 in net proceeds from the sale of all available tax credits realizing proceeds of 81% of the face value of the tax credits. For the nine months ended September 30, 2018, we recorded a benefit to provision for income taxes in the amount of $593,195.
40
Consolidated Earnings (Loss) Before Provision for Taxes on Income
For the three months ended September 30, 2018:
Consolidated loss before provision for taxes for the three months ended September 30, 2018, was $1,397,057, as compared to a loss of $424,252 for the three months ended September 30, 2017.
Income (Loss) Before Tax by Segment:
| Three Months Ended September 30, |
Percent | |||||||||||
| 2018 | 2017 | Change | ||||||||||
| Manufacturing | $ | 48,982 | $ | 114,720 | (57.3 | )% | ||||||
| Neurostimulation | (1,386,884 | ) | (1,070,982 | ) | 29.5 | |||||||
| Other (1) | (59,155 | ) | 532,010 | (111.1 | ) | |||||||
| Consolidated total | $ | (1,397,057 | ) | $ | (424,252 | ) | (229.30 | )% | ||||
| (1) | Amounts not allocated to segments include interest income (expense) and other income (expense), and amortization of acquisition intangible assets. |
Manufacturing segment: Manufacturing segment income before tax was $48,982. The prior period comparison for the three months ended September 30, 2017 includes only one month of sales as the acquisition of Medi-Line occurred on August 30, 2017.
Neurostimulation segment: For the three months ended September 30, 2018 the increase in loss before tax for the neurostimulation segment of $315,902 was primarily due to no device sales, only component parts sales, for the three months ended September 30, 2018 compared to $202,362 in device sales for the three months ended September 30, 2017.
For the nine months ended September 30, 2018:
Consolidated loss before provision for taxes for the nine months ended September 30, 2018, was $3,643,448, as compared to a loss of $3,604,004 for the nine months ended September 30, 2017.
Income (Loss) Before Tax by Segment:
| Nine Months Ended September 30, |
Percent | |||||||||||
| 2018 | 2017 | Change | ||||||||||
| Manufacturing | $ | 223,100 | $ | 114,720 | 94.5 | % | ||||||
| Neurostimulation | (3,892,894 | ) | (4,144,691 | ) | (6.1 | ) | ||||||
| Other (1) | 26,346 | 425,967 | (93.8 | ) | ||||||||
| Consolidated total | $ | (3,643,448 | ) | $ | (3,604,004 | ) | 1.09 | % | ||||
| (1) | Amounts not allocated to segments include interest income (expense) and other income (expense), and amortization of acquisition intangible assets. |
Manufacturing segment: Manufacturing segment income before tax was $223,100 for the nine months ended September 30,2018. The prior period comparison for the three months ended September 30, 2017 includes only one month of sales as the acquisition of Medi-Line occurred on August 30, 2017.
Neurostimulation segment: The neurostimulation segment loss before tax was $3,892,894 for the nine months ended September 30, 2018 which was a decrease from the neurostimulation segment loss for the nine months ended September 30, 2017 of $4,144,691. For the nine months ended September 30, 2018 the decrease in loss before tax for the neurostimulation segment was primarily due to $107,119 increase in device sales and a net decrease of $166,468 in combined research and development costs and general and administrative costs for the segment for the nine months ended September 30, 2018 as compared to the nine months ended September 30, 2017.
41
Year ended December 31, 2017 compared to December 31, 2016
Results of Operations
Consolidated Sales Revenue
In 2017, consolidated revenues increased 121% to $3,302,775 from $1,494,881 in 2016. The change in consolidated revenues consisted of the following segmented revenue activity:
Revenues by Segment
| 2017 | 2016 | % Change | ||||||||||
| Manufacturing | $ | 2,932,664 | $ | — | — | % | ||||||
| Neurostimulation | 291,750 | 1,456,038 | (80.0 | ) | ||||||||
| Other | 78,361 | 38,843 | 101.7 | |||||||||
| Revenue | $ | 3,302,775 | $ | 1,494,881 | 120.9 | % | ||||||
Manufacturing segment: For the year ended December 31, 2017, manufacturing sales were $2,932,664 for the period from September 1, 2017 to December 31, 2017, subsequent to acquisition of Medi-Line on August 30, 2017.
Neurostimulation segment: For the year ended December 31, 2017, neurostimulation sales decreased by $1,164,288 to $291,750 from $1,456,038 for the year ended December 31, 2016. The reduction in neurostimulation sales year over year primarily relates to a one-time option payment in the amount of $1,005,513 for the manufacture and supply of pre-clinical implantable neurostimulator devices in 2016.
Other: For the years ended December 31, 2017 and 2016, our other operating revenues were $78,361 and $38,843, respectively. Other operating income consists primarily of Belgian government credits for employing staff in the research and development sector.
Cost of Product Sold, Research and Development Expense, and Selling, General, and Administrative Expense
Cost of product sold, research and development expense, and selling, general, and administrative expense as a percentage of revenue were as follows:
| 2017 | % Rev | 2016 | % Rev | |||||||||||||
| Cost of product sold | $ | 2,321,756 | 70.3 | % | $ | 39,129 | 2.6 | % | ||||||||
| Research and development expenses | 2,942,981 | 89.1 | 759,502 | 50.8 | ||||||||||||
| Selling, general, and administrative expenses | 2,831,069 | 85.7 | % | 693,603 | 46.4 | % | ||||||||||
Cost of product sold: Consolidated costs of products sold for the year ended December 31, 2017 increased to $2,321,756 from $39,129 for the year ended December 31, 2016. The increase was driven by the addition of Medi-Line’s manufacturing activity in 2017, and the option payment of $1,005,513 for the manufacture and supply of pre-clinical implantable neurostimulator devices in 2016.
Research and development expenses: Consolidated research and development expenses for the year ended December 31, 2017 increased by $2,183,479 to $2,942,981 from $759,502 for the year ended December 31, 2016. The increase reflects the increased research and development activities for our neurostimulation platform, and for manufacturing setup and non-recurring engineering for the Viant™ Deep Brain Stimulation System.
42
We have been awarded multiple grants and subsidies for its research and development activities, and receives these funds as advance payments and reimbursements for applicable project expenses. We recognize the amounts received in accordance with the contracts as a reduction of research and development expenses over the periods necessary to match the contract on a systematic basis to the costs that it is intended to compensate. We record, on the balance sheet, grants receivable upon meeting the criteria discussed above until cash is received. For the years ended December 31, 2017 and 2016, we recorded a credit to research and development expense of $1,868,891 and $445,083 respectively related to these grants and subsidies.
For the year ended December 31, 2017, our selling, general, and administrative expenses increased to $2,831,069 from $693,603 for the year ended December 31, 2016. The change was driven by the addition of the Medi-Line activity, the addition of executive and sales staff salaries and wages, stock option expenses, increased public reporting and trading expenses, increased capital formation costs, and IR/PR costs.
Depreciation and Amortization
During the year ended December 31, 2016, we acquired $6,120,000 in patents pertaining to the cardiovascular disease technology acquired in the merger with NXDE, and acquired $3,190,000 in patent licenses for the underlying patents (the “Siemens Patents”). NMB holds patents and licenses totaling $1,053,097 related to our neurostimulation technology and devices. The amortization period for each of the individual patents depends on the legal terms for patents in the countries in which they are granted. In most countries, including the United States, the patent term is generally 20 years from the earliest claimed filing date of a non-provisional patent application in the applicable country. The patents and patent licenses are amortized using the straight-line method over the remaining time until expiration. The majority of these patents and patents underlying the license will expire between 2019 and 2036.
For the years ended December 31, 2017 and 2016, our depreciation expenses were $127,677 and $38,962, respectively. For the years ended December 31, 2017 and 2016, our amortization expenses were $1,170,033 and $597,959 respectively.
Interest Income (Expense)
For the year ended December 31, 2017, our interest expense, net of interest income, was $111,931, and for the year ended December 31, 2016, interest income, net of interest expense, was $5,311. For the year ended December 31, 2017, the net interest income (expense) includes interests on bank loans and credit facilities, interest on leasing, interest on mezzanine debt, interest on shareholder notes, and amortizing interest for the original discount of mezzanine debt in the amount of $45,711 in 2017. For the year ended December 31, 2016, net interest income (expense) includes interest income on related party loans, interest on leasing, interest on credit facilities, and interest on shareholder notes.
43
Other Income (Expense), net of Interest Income (Expense)
For the years ended December 31, 2017 and 2016, we recorded other income, net of other expenses, in the amount of $4,025,031, and recorded an expense in the amount of $173,500, respectively. For the year ended December 31, 2017, and based on the purchase price allocation and third-party valuations for Medi-Line’s real estate, land, and intangibles, management recorded a gain on bargain purchase for the acquisition of INGEST and Medi-Line in the amount of $4,311,554. We also recorded another expense in the amount of $37,788 for the loss on the exchange of stock (See Note 13 Related Party Transactions - January 6, 2017 Stock Exchange Agreement, to the Consolidated Financial Statements included herein), and an expense in the amount of $174,252 to record the bad debt on the write-off of a note receivable (See Note 13 Related Party Transactions - Nuviant Medical, GmbH Waiver of Debt Agreement, to the Consolidated Financial Statements included herein); and recorded an expense in the amount of $74,483 for the impairment of patents assets.
Provision for Income Taxes
For the years ended December 31, 2017 and 2016, we recorded no tax provision.
Consolidated Earnings (Loss) Before Provision for Taxes on Income
Consolidated loss before provision for taxes for the year ended December 31, 2017 was $2,177,641, as compared to a loss of $802,463 for the year ended December 31, 2016.
Income Before Tax by Segment
| 2017 | 2016 | % Change | ||||||||||
| Manufacturing | $ | (31,698 | ) | $ | — | — | % | |||||
| Neurostimulation | (6,117,404 | ) | (673,117 | ) | 808.8 | |||||||
| Other (1) | 3,971,461 | (129,346 | ) | (3,170.4 | ) | |||||||
| Income (loss) before taxes | $ | (2,177,641 | ) | $ | (802,463 | ) | 171.4 | % | ||||
(1) Amounts not allocated to segments include interest income (expense) and other income (expense) and amortization of acquisition intangible assets.
44
Manufacturing segment: In 2017, the manufacturing segment income before tax was $31,698, reflecting activity for the period of ownership of Medi-Line from September 1, 2017 to December 31, 2017, and therefore no comparison to 2016 is provided. During the period, Medi-Line incurred a charge in the amount of $168,933 related to a severance payment for a long-term employee that was terminated. In Belgium, severance payment requirements and accruals are administered by and mandated by the Belgian government, and accrue based on time in service.
Neurostimulation segment: In 2017, the neurostimulation segment loss before tax was $6,117,404 compared to a loss of $673,117 for 2016. The decrease in income before tax reflects increased research and development activities, increase in staff and corporate infrastructure, and a decrease in neurostimulation revenue year over year from 2016, which included a one-time option payment in the amount of $1,005,513.
Liquidity and Capital Resources
Prior to the acquisition of NMB and its subsidiary Medi-Line, we had not generated any revenues. We have financed our operations to date through private placements, National Institutes of Health awards for research and development projects, and loans from our largest shareholder, RS. As of September 30, 2018, we had cash on hand of $125,573. Based upon our budgeted burn rate, and along with expected grant funding, we currently have operating capital for approximately 2 months. We have historically relied on equity or debt financing and federal research and development subsidies to finance its ongoing operations.
2017 Private Placement & Common Stock Sale
On July 20, 2017, we closed on a private placement, pursuant to which we received $1,165,000 from the sale and issuance of 66,580 shares of restricted common stock. The shares of common stock were offered at $17.50 per share. In addition, on October 9, 2017, we issued 10,715 shares of restricted common stock pursuant to a common stock purchase agreement. The purchase price was $14.00 per share and we received $150,000.
Research and Development Grants
Our wholly owned subsidiary Pulsus Medical, LLC was awarded $751,000 of federal research grants applicable to Pulsus Medical, LLC’s products, and these funds became available to us beginning in the quarter ended September 30, 2017. We have been awarded a grant by the Cancer Prevention and Research Institute of Texas, which was approved in the amount of $392,156 and received $324,841 in December 2017 and $67,315 in January 2018.
NMB has been awarded subsidies from the Public Service of Wallonia - Department of Technology Development and the Research Programs Department. As of September 30, 2018, NMB currently has a total of $491,488, in remaining awarded funds to be received, including a $361,466 recorded as current accounts receivable and $130,042 to be reimbursed for future expenses for current projects. NMB has received approval for a grant application from the Public Service of Wallonia in the amount of $1,263,898. This grant funded research and development project is expected to commence in first quarter of 2019.
Medi-Line has been awarded subsidies from the Public Service of Wallonia - Department of Technology Development and the Research Programs Department. As of September 30, 2018, Medi-Line currently has a total of $761,839 in remaining awarded funds to be received, including a $266,320 recorded as current accounts receivable and $495,519 to be reimbursed for future expenses for current projects. Medi-Line has received approval for a grant application from the Public Service of Wallonia in the amount of $737,635. This grant funded research and development project is expected to commence in first quarter of 2019.
45
We have received a notice of award from the National Institute of Neurological Disorders and Stroke (NINDS) for the phase I portion in the amount of $838,241 for funding of an U44 Cooperative Agreement Award (U44NS108148) under the BRAIN Initiative. The award provides more than $1,554,475 through phase I and phase II of the project to support the development and clinical evaluation of a software tool to improve DBS programming through multi-modal mapping of disease-related neural signals, images/models, and device data. The project budget period began on September 30, 2018. The Puerto Rico Science, Technology, and Research Trust has awarded $100,000 in matching funds to support the first phase of the project.
2017 Tax Grant
On December 27, 2017, NXPROC was granted a tax exemption pursuant to Act number 73-2008 (“ACT 73”) by the Government of Puerto Rico, Department of Economic Development and Commerce (“PRIDCO”). The exemption allows NXPROC to obtain tax credits in the amount of fifty percent (50%) of approved applicable research and development expenses of NXPROC. These tax credits can be used to offset current year income taxes, or, if no income tax is due, can be sold to companies operating in Puerto Rico to offset their Puerto Rican income tax. In the case of a sale of the tax credits, the tax credits are typically sold at a discount to the dollar value of the credits.
As of May 24, 2018, we have received all government approvals and certifications from PRIDCO and received tax credits in the amount of $732,340, for research and development activity in 2017, which had been posted to Departamento De Hacienda (the Puerto Rican Department of Finance) system and we have received $593,195 in net proceeds from the sale of all available tax credits realizing proceeds of 81% of the face value of the tax credits.
Issuances for Services
During the fiscal years 2016 and 2017 we have issued an aggregate of 81,369 shares of common stock for certain research and development, legal, corporate structuring, software development, marketing and valuation consulting services rendered by third-party consultants. The foregoing shares were valued at $782,645.
During the nine months ended September 30, 2018, we have issued an aggregate of 8,892 shares of restricted common stock for certain software development, research and development, marketing and valuation services to third-party consultants. The foregoing services were valued at $112,561.
During the next 12 months, we may elect to issue additional debt or equity either by private placement or a registered offering. There can be no assurance that we will be successful in completing any new debt and/or equity financing, or receive assignments of grants. If the Company is unable to secure needed financing, or is unable to secure such financing on terms we find favorable, we may be forced to delay, limit, or terminate product development and/or future product commercialization.
Continued Operations
Until such time, if ever, as we can generate substantial revenues to cover the development and commercialization of our neurostimulation technology platform, we anticipate that we will need to finance our cash needs through a combination of future debt and equity financing, as well as expected non-dilutive research grant awards. Besides certain grant awards, as described above, we do not have any committed external source of funds. To the extent that we secure additional capital through the sale of equity securities or convertible debt, the ownership interest of our stockholders may be diluted, and the terms of any such securities we issue may include liquidation or other preferences that adversely affect the rights of common stockholders. In cases where we secure certain debt financing, if any such is available, we may become subject to certain covenants limiting or restricting our ability to take certain actions, such as incurring additional debt, making capital expenditures, or declaring dividends. In the event we are unable to secure needed financing, or are unable to secure such financing on terms we find favorable, we may be forced to delay, limit, or terminate product development and/or future commercialization of the same.
46
Off-Balance Sheet Arrangements
We have no off-balance sheet arrangements, including arrangements that would affect our liquidity, capital resources, market risk support and credit risk support, or other benefits.
Critical Accounting Policies
Our management’s discussion and analysis of our financial condition and results of operations is based on our consolidated financial statements, which were prepared in conformity with generally accepted accounting principles. The preparation of our consolidated financial statements requires us to establish accounting policies and make estimates and assumptions that affect our reported amounts of assets and liabilities at the date of the consolidated financial statements. These consolidated financial statements include some estimates and assumptions that are based on informed judgments and estimates of management. We evaluate our policies and estimates on an on-going basis, and discuss the development, selection, and disclosure of critical accounting policies with the board of directors. Predicting future events is inherently an imprecise activity, and as such requires the use of judgment. Our consolidated financial statements may differ based upon different estimates and assumptions.
Our significant accounting policies are described in more detail in the notes to our consolidated financial statements, above. See Note 2, Summary of Significant Accounting Policies, which we believe set forth the most critical accounting policies to aid you in fully understanding and evaluating our financial condition and results of operations.
New Accounting Pronouncements
Management does not believe that any recently issued but not yet effective accounting pronouncements, if adopted, would have a material effect on the accompanying consolidated financial statements
We are a medical device company focused on the development, manufacturing, and commercialization of neurostimulation technology for the treatment of various neurological disorders through electrical stimulation of neural tissues. Our neurostimulation technology platform has the potential to provide treatment to patients in several established neurostimulator markets, including deep brain stimulation (DBS), peripheral electrical nerve stimulation (PENS), sacral nerve stimulation (SNS), spinal cord stimulation (SCS), vagus nerve stimulation (VNS), and other emerging neurostimulator markets.
We expect that our first commercial application of our platform will be the Viant™ Deep Brain Stimulation System (or the Viant™ System). We plan to pursue regulatory approval of the Viant™ System for Parkinson’s disease, essential tremor, and dystonia in Europe in 2019, and for Parkinson’s disease in the United States in the second half of 2019. The Viant™ DBS device is designed to deliver best-in-class stimulation with the capability to collect local field potential (LFP) recordings. Using LFP surveys, neurologists will be able to quickly and confidently determine where to stimulate to take full advantage of directional leads. Moreover, our devices are designed to be non-invasively upgradable, enabling both physicians and patients to benefit from the latest technology as it is developed, without the need for IPG replacement surgery to take advantage of new features.
The Viant™ System continues to meet critical milestones in its development program. Previous generations of the device have been CE Marked and were manufactured and sold to GlaxoSmithKline plc, a British pharmaceutical company. We completed the ISO 13485 certification process, which is a pivotal hurdle, prior to regulatory submissions to the CE Mark authorities. Design verification, process validation, and testing requirements are nearly complete, and management expects we will complete the technical file in the first half of 2019. We expect to receive a CE Mark in early 2020. As related to the United States, we completed the pre-submission meetings with the FDA in early 2018 to determine scope of requirements for approval of the Viant™ System. As a result of that meeting, we intend to submit a PMA application without a clinical study. Although the FDA is allowing us to submit an application for a PMA that leverages the clinical data from a previously approved product in accordance with Section 216 of the Food and Drug Administration Modernization Act of 1997, no assurance can be given that our application which will be submitted without a clinical study will be sufficient for approval by the FDA.
47
We have additional opportunities to license the neurostimulator platform to companies focused on enhancements to a comprehensive system offering for closed-loop, chronic disease therapeutics, including advanced computational biology, deep learning utilizing Internet of Medical Things technology, imaging solutions, e-health programs, and big data management and optimization, among others. Once we complete the development of the platform for our DBS application, we will be able to potentially license the platform to capitalize on hundreds of diseases of the nervous system that might be therapeutically addressed with neurostimulation.
We also operate our wholly owned subsidiary, Medi-Line, a Belgium company. Medi-Line currently serves over 30 medical device customers in 16 countries, including multi-year contracts with Fortune 500 healthcare companies. The Belgian manufacturer owns state-of-the-art facilities, which feature two validated clean rooms (one assembly cleanroom Class ISO 7 or C, and one extrusion/injection molding cleanroom Class ISO 8 or D) and 600m2 of production space. Their capabilities will enable us to de-risk our commercial launch and speed the development of our neurostimulation products.
We were incorporated in the State of Nevada on December 7, 2015. Our principal corporate office is located at 1910 Pacific Avenue, Suite 20000, Dallas, Texas 75201, and our phone number is (844) 919-9990. Our website is www.nexeonmed.com.
Market Overview
The neurostimulation market is comprised of four large markets (SCS, SNS, DBS, and VNS), along with several smaller emerging markets, each focused on the treatment of a particular disease state through delivery of electrical stimulation to particular targeted sites in the body. We plan to compete in the DBS market with our Viant™ System, and we are evaluating our strategic options for competing in the PENS, SCS, SNS, and VNS markets. According to the Grandview Market Research report, the combined SCS, DBS, and SNS market sizes were approximately $3 billion for 2016.
Market
We will initially compete in the DBS market with the Viant™ DBS System for treatment of Parkinson’s disease, essential tremor, and dystonia. According to the Medtronic Registry, DBS therapy has been used worldwide in over 150,000 patients to treat chronic neurological diseases for over 20 years. We believe the demand for DBS devices is driven by the significant pool of patients with movement disorders such as Parkinson’s disease and essential tremor who do not respond to pharmacologic therapy and require alternative therapies to relieve debilitating symptoms.
According to market research and our internal estimates, the worldwide DBS market in 2016 was estimated at $795.64 million, and is expected to grow to $2.10 billion by 2025, a 11.9% compound annual growth rate. DBS therapy is estimated to have penetrated less than 10% of the United States market (96). We believe shortcomings of current DBS devices have limited adoption of DBS therapy in the United States.
We believe DBS market growth is tied to two advances in DBS technology: directional leads and LFP recording. We believe Boston Scientific and Abbott/St. Jude are rapidly gaining market share from Medtronic primarily due to their directional lead, which Medtronic does not offer. LFP research has increased exponentially in the past 50 years due to increased interest in identifying LFP-based biomarkers for Parkinson’s disease (Figure 1). We believe we will be able to gain market share due to the Viant™ System’ offering of directional leads and LFP recording capabilities. |
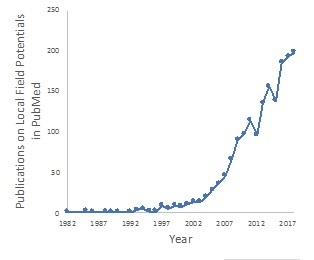 | |
| Figure 1: Deep Brain Stimulation field shows growing interest in LFPs as shown by increase in PubMed publications over the past 50 years (available at pubmed.gov, PubMed is a free resource that is developed and maintained by the National Center for Biotechnology Information (NCBI), at the U.S. National Library of Medicine (NLM), located at the National Institutes of Health (NIH). |
48
Customers
According to the Deep Brain Stimulation Market Report, there are up to 4,000 patients annually who obtain a DBS system for Parkinson’s disease (PD) or essential tremor in up to 250 U.S. Centers. In the top 10 countries for DBS research, there are approximately 430 centers and 7,500 DBS interventions each year. We believe that these numbers are likely to increase, as DBS therapy is expanding and technology is advancing. Neurologists and neurosurgeons at these DBS centers are potential customers of the Viant™ System and proposed programming tools. PD patients are also potential customers who will be attracted to the potentially improved outcomes of the Viant™ System due to more effective programming of our directional leads, and reduced programming time. We believe a new, effective, more efficient DBS therapy may help a substantial number of these PD patients to seek the Viant™ DBS therapy. We will initially target those neurologists and neurosurgeons who are interested in conducting DBS research, customizing therapy, and shortening programming time, and who are consequently attracted to our LFP recording capabilities.
The Need:
We believe many patients receive inadequate symptom control due to poorly placed DBS leads, current spread to unwanted structures, or non-optimized stimulation parameters. Neurologists report directional lead programming is time consuming (up to two hours/lead), sometimes over multiple visits. This is time-consuming for the neurologist and patient, and therapeutic efficacy is not always optimized. Although directional leads offer unprecedented therapeutic control, the time needed to explore all possible parameters is overwhelming.
Tools are needed to narrow the neurologists’ focus and facilitate the identification of correct programming settings on directional leads in a more efficient and efficacious manner. Neural sensing can assist neurologists with programming of directional leads by locating the pathological region of interest, or ROI. DBS current can then be steered in the direction of the ROI for optimized therapeutic outcome. With the addition of LFPs collected from a directional lead, there is a significant amount of new information that needs to be leveraged towards better therapeutic outcomes for patients. We believe there is a need to put this information into the context of patient anatomy, lead placement, and other patient information to allow neurologists to make efficient and effective programming decisions about directional leads.
Competing Technologies Fall Short:
There are no DBS programming tools on the market that use LFPs to guide programming of directional leads. This is due to the lack of available DBS devices that offer both directional leads for current steering and neural sensing capabilities. Currently, we believe the only FDA-approved DBS systems are stimulation-only devices. Neither system can sense or record neural signals such as LFPs. Recently, Medtronic developed a prototype DBS stimulation and recording platform called the Activa PC+S. Although not FDA-approved, the Medtronic prototype is used globally in investigational, proof-of-principle studies, in more than 20 centers. It is the only available implantable pulse generator for DBS that can record LFP, but it does not utilize a directional lead. The directional lead and the sensing capabilities are required in a single system to enable new, more effective and efficient methods of delivering DBS therapy.
49
Our Product – The Viant™ DBS System
We have developed the Viant™ DBS platform, which is designed to have the capability to perform current steering and LFP sensing with a directional DBS lead. The Viant™ DBS device is designed to deliver consistent, effective therapeutic results. It is designed to deliver best-in-class stimulation, with the capability to collect LFP recordings. Using LFP surveys, neurologists will be able to quickly and confidently determine where to stimulate to take full advantage of directional leads. Moreover, our devices are designed to be upgradable non-invasively, enabling both physicians and patients to benefit from the latest technology as it is developed, without the need for IPG replacement surgery to take advantage of new features. All components are summarized here.
The Viant™ System is designed to deliver DBS therapy for the treatment of Parkinson’s disease. It is comprised of implantable and external components (Fig. 2) that work synergistically to deliver well-controlled electrical pulses with specified parameters to target brain structures. Remote controls for the physician and for the patient enable communication with the implant. Each control offers different options. The energy source of the system is recharged through the skin after device implantation by use of the patient remote control in combination with the battery charger. The device previously received a CE Mark for Parkinson’s disease in 2012, but we have since developed our directional lead, wireless peripherals, and iOs external controllers. |
Figure 2: Components of the SynapseTM device. (Note: External Research System on PC Laptop, not pictured) |
50
Viant™ Implantable Components
Implantable pulse generator: The IPG contains a rechargeable battery and electronics that deliver low-level electrical pulses to the leads. The IPG has 16 independent current sources, and is connected to one or two leads via lead extension(s). It is a programmable device that delivers customized programs for each patient. The IPG is surgically implanted under the skin in the chest.
Directional leads: The leads are thin, insulated wires that conduct electrical pulses to brain structures (i.e., the subthalamic nucleus or globus pallidus internus) from the IPG. The directional lead has segmented electrode contacts arranged in a 1-3-3-1 pattern to facilitate current steering during DBS therapy (Fig. 3).
Viant™ External Components
Patient remote control (RC): The patient RC allows patients to adjust their stimulation within prescribed limits, and monitor their IPG battery charge levels. The RC design has a simple and intuitive interface developed from usability studies in people of all ages with movement disorders. The RC enables the patient to record LFPs at home, if desired, and acts as a relay to send signals from the IPG to the External Research System.
Battery charger (BC): The BC is connected to the RC and is used to charge the implanted IPG. To charge the IPG, the BC is placed over the implanted IPG in the chest. The RC continuously monitors charging voltage, current, and temperature in the IPG, and optimizes the inductive power generated by the BC for efficient charging.
Clinician programmer (CP): The CP allows the clinician to communicate with the IPG, and optimize stimulation parameters and current steering for each individual patient after optimal electrode contacts are chosen. |
Figure 3: Synapse directional DBS lead with 1-3-3-1 configuration |
Competition
The medical device industry is intensely competitive, subject to rapid change, and highly sensitive to the introduction of new products and other market activities of industry participants. We intend to compete in the DBS market for movement disorders. In the U.S. and European DBS market, the competitors include Abbott Laboratories, formerly known as St. Jude Medical (“Abbott”), Boston Scientific, and Medtronic. Abbott, Boston Scientific, and Medtronic are publicly traded companies or divisions of publicly traded companies, all of which have significantly greater resources than we have. In addition, these competitors also have established operations, long commercial histories, more extensive relationships with physicians, and wider product offerings within neuromodulation and other medical device product categories than we have. This may provide these competitors with greater negotiating power with customers and suppliers, and with more opportunities to interact with the stakeholders involved in purchasing decisions. Furthermore, although we have no current plans to enter the Chinese market, PINS Medical and SceneRay Corporation are established Chinese DBS companies that could begin to distribute their products in Europe and/or the United States. In addition, Aleva Neurotherapeutics has announced its intention to enter the European DBS market in late 2018, and other companies may likewise attempt to bring new products or therapies to this market. All of these companies will continue to develop new products that directly compete with our products, and their greater resources may allow them to respond more quickly to new technologies, new treatment indications, or changes in customer requirements. For all of these reasons, it may be more difficult to compete successfully against these or future competitors.
We believe the primary competitive factors in the neurostimulation market are:
| ● | Technological innovation, product enhancements, and speed of innovation; |
| ● | Pricing and reimbursement rates; |
| ● | Clinical studies and research; |
| ● | Sales force experience and access; and |
| ● | Integration into clinical flow. |
51
Competitive Advantage of the Viant™ System
The Viant™ DBS platform is designed to provide the most innovative capabilities currently available on the market, and to provide physicians and patients with improved solutions and tailored treatment options. It offers a combination of competitive DBS device features in a single platform (Figure 3).
We believe the key value propositions of the Viant™ System include:
Innovative core technology. The engine of our Viant™ System, the IPG, is based on more than 30 years of development. The custom-made chip was developed using our advanced engineering and design capabilities to have a broad spectrum of electrical outputs.
Directional lead for current steering. Our lead portfolio includes a directional lead for targeted DBS therapy, a methodology proven to increase efficacy and decrease side-effects of DBS therapy. With our directional lead, our Viant™ System is designed to deliver tailored therapy to a wide spectrum of PD patients.
Guided programming tools. Our software will enable the visualization of LFPs on directional leads to allow physicians to program DBS leads with greater efficacy and efficiency. Instead of time-intensive iterative and empirical programming approaches that require clinical observation, clinically relevant LFP bands can be used to identify the pathological region of interest within the patient’s brain. This will inform ideal contacts for programming to optimize DBS therapy.
Multiple independent current sources. Our Viant™ System is designed to deliver electrical energy through 16 independent current sources. This capability optimizes current delivery and improves field control, allowing for current steering and precision therapy.
Rechargeability. A majority of the neurostimulators on the market are not rechargeable, and require risky replacement surgeries when batteries die. Our Viant™ System is easily rechargeable to reduce the need for replacement surgeries and enable long-term recording with minimal impact of battery drain.
Upgradable technology enables next generation offerings. Viant™’s proprietary chipset and hardware is capable of being configured for use in next generation treatment offerings due to its flexible stimulation and recording capabilities. It can deliver higher frequencies and a broader array of stimulation patterns than devices currently on the market. Upgrades can be provided to already implanted patients via a noninvasive software or firmware upgrade. We believe these capabilities provide a solid foundation for new treatment options in DBS and other neuromodulation therapies. |
Figure 1: We offer features of competitors in a single platform |
52
Reimbursement
The DBS market for Parkinson’s disease is well-established. Most public and private health insurance companies, including Medicare, cover approved uses of DBS, including Parkinson’s treatment. According to the Centers for Medicare and Medicaid Services, DBS for Parkinson’s has established procedure codes and billing guidelines. Given clear reimbursement pathways for DBS, there are fewer regulatory and reimbursement hurdles relative to other emerging neurostimulation markets. Additionally, there is an established network of providers who currently use DBS therapy who are predicted to integrate our device into their practice.
Our Strategy
To successfully achieve our objectives, we are pursuing the following strategy:
| ● | Complete Viant™ development and transfer to manufacturing. We will continue to invest in completing the development and building the manufacturing capacity necessary to support the approval and commercial launch of the Viant™ DBS System. We will continue to pursue partnerships with contract manufacturers to utilize their manufacturing capabilities and achieve operating efficiencies. |
| ● | Submit Viant™ System for FDA approval and CE mark. Upon completion of the product dossier, we will submit to the FDA for a pre-market approval, and to the notified body, DEKRA, for CE Mark. |
| ● | Build our manufacturing, sales, and marketing organization. In 2019, we plan on building our international sales organization through a combination of distributors, independent sales agents, and a limited number of direct employees in anticipation of a 2020 launch in major European markets. Our representatives will target movement disorder specialists located primarily at large academic centers, as well as their referring networks of neurologists in countries with existing reimbursement coverage for these therapies. |
| ● | Invest in platform extensions to further drive product innovation and licensing opportunities. We are investing in pre-clinical and clinical research to demonstrate and further the innovation of our Viant™ DBS device. We anticipate this investment may result in further product innovations, expanded labeling, and new indications for the Viant™ DBS System. These innovations are expected to include next-generation IPG capabilities, additional lead offerings, and advancements in algorithmic programming. We also plan to license our platform to other companies developing therapies for nearly 600 diseases of the nervous system. |
Regulatory
In the European Economic Area (EEA), we choose to be subject to the requirements of the EU Active Implantable Medical Devices Directive 90/385/EEC (AIMDD), which mandates that our product receive a CE Mark prior to being placed on the market in the EEA. To obtain a CE Mark, we must prepare a technical file for our product, and undergo a conformity assessment by a notified body, which is an organization authorized under the AIMDD to conduct conformity assessments. Following successful completion of a conformity assessment, the notified body will grant a CE Mark. The AIMDD is repealed by Regulation (EU) 2017/745 (“Medical Device Regulation” or “MDR”), which came into effect in May of 2017 and will complete its transition in May 2020. We will be subject to the MDR in full as of May 2020.
We expect to complete the Viant™ DBS System technical file in the first half of 2019, and submit it for a conformity assessment with our notified body, DEKRA Certification B.V., shortly thereafter. While there have been some recent increases in the stringency of the relevant EU regulatory requirements, and there can be no assurances, absent unanticipated additional requirements or delays, we currently anticipate receiving the CE Mark for the Viant™ DBS System in early 2020.
53
The Viant™ DBS System is classified as a Class III medical device under the Food and Drug Act. The Food and Drug Act requires submission and FDA approval of a PMA application before marketing of a Class III device can begin. The PMA application process is considerably more demanding than the Class I and Class II 510(k) pre-market notification process. We have used the FDA’s pre-submission process to engage the FDA to clarify the path to ultimate product approval. At this time, we intend to submit to the FDA in 2019 for a PMA for the treatment of Parkinson’s disease without a clinical study.
The Viant™ System continues to meet critical milestones in its development program. Pursuant to a recently completed manufacturing program for a joint venture between GSK and Google, the Company obtained an ISO 13485 certificate, which is a pivotal hurdle prior to regulatory submissions to the CE Mark authorities, so that it could manufacture the product in compliance with GMP. The device has previously received a CE Mark, but we have upgraded the software and peripherals, and designed a 3D lead. Design verification, process validation, and testing requirements are nearly complete, and management expects we will complete the technical file in the second half of 2019. We expect to receive a CE Mark in early 2020. As related to the United States, we completed the pre-submission meetings with the United States Food and Drug Administration (FDA) in early 2018 to determine scope of requirements for approval of the Viant™ System. As a result of that meeting, we intend to submit a FDA PMA application without a clinical study. Although the FDA is allowing us to submit an application for a PMA that leverages the clinical data from a previously approved product in accordance with Section 216 of the Food and Drug Administration Modernization Act of 1997, no assurance can be given that our application which will be submitted without a clinical study will be sufficient for approval by the FDA.
Sales and Marketing
We are planning to launch our Viant™ device in the U.S. and Europe after regulatory approvals are achieved. We will initially launch in Germany, the largest European DBS market, after we achieve CE marking in 2020. Our strategy for European and U.S. sales and distribution will vary depending on region. We expect to hire a small, experienced sales force, all with prior experience of training 50 or more clinicians on active implantable devices. Each member of the sales force will be expected to have previous sales of at least $1 million annually, and a wealth of contacts in their regional areas.
Through market research, we have validated that our initial target will be key opinion leader neurosurgeons and neurologists in the U.S. and Europe who have the personnel, facilities, and desire to do DBS research, and who treat significant numbers of patients with DBS devices. We will prioritize selling to sites where both DBS research and clinical care are performed. We believe additional clinical data and testimonials from our early adopters will drive market growth.
Territories in the U.S. not targeted by our sales force will be covered using a distributor to reduce upfront costs and training. This approach will likely be altered if we form a strategic partnership with an existing medical device company prior to initial sales.
We currently have no sales force, as we are engaged in completing the product and preparing to make regulatory submissions. In 2017, we hired a vice president of sales and marketing to develop and execute our detailed commercial plan. We intend to build a US-based marketing team to promote awareness of our products by training and educating physicians, exhibiting at tradeshows, and conducting focused promotions.
We expect to begin to build our international sales organization in late 2019. To achieve operating efficiencies, we expect to use a combination of distributors, independent sales agents, and a limited number of direct employees. Our representatives will target movement disorder specialists located primarily at large academic centers, as well as their referring networks of neurologists in countries with existing reimbursement coverage for these therapies.
We have carefully selected our management team to ensure it is comprised of individuals with very specific and extensive experience in the neurostimulation and medical device industry. Our team has many years of experience (i) developing medical devices, (ii) eliciting early-stage funding to complete development, (iii) navigating the regulatory approval process in both the U.S. and the E.U., (iv) securing insurance reimbursement approval in both the U.S. and E.U., (v) fostering early-stage growth and development of startup companies, and (vi) designing and implementing a viable exit strategy or liquidity event for initial shareholders.
54
Product Development Pipeline
Nexeon MedSystems has developed a non-invasive auricular vagus nerve stimulator (aVNS) for the treatment of disease related to neurologic, inflammatory, and metabolic syndrome. Our initial clinical and commercial efforts are in atrial fibrillation (non-valvular paroxysmal) and opioid withdrawal. Our aVNS device is a non-invasive, battery-powered, self-administered device designed to transcutaneously stimulate the auricular branch of the vagus nerve (ABVN). The system is composed of three components: an earpiece, a handheld control unit, and a connector cable. The handheld control unit produces precise, current-controlled stimulation output. Electrical current is delivered through an earpiece with disposable hydrogel electrode contacts to maximize therapeutic potential and minimize the risk of patient discomfort. One electrode is designed to be placed into the cymba (which is innervated by the ABVN), and another electrode is placed in an area surrounding the outer ear (e.g., behind the ear). The handheld control unit and the earpiece are connected via a cable.

Clinical/regulatory: We have begun a single-site, 20-patient clinical trial at the Université Catholique de Louvain in Belgium, and are scheduled to complete this study in early 2019. The goal of this clinical study is to demonstrate that aVNS will significant reduce atrial fibrillation burden and system inflammation. Upon completion, we will discuss with the FDA and EU notified bodies what additional clinical data, if any, will be required in support of a de novo request (FDA) and CE Mark application (EU).
We have initiated a single-site, 15-patient clinical trial for the opioid withdrawal indication, including obtaining requisite IRB approval. The study is scheduled to be completed in early 2019. Upon completion, we will discuss with the FDA what additional clinical data, if any, is needed to file a de novo request for the opioid withdrawal indication.
Markets: According to the Grandview Research Report, the addressable market for our atrial fibrillation indication is $16 billion in unmet need. Additional addressable markets include severe inflammation ($30 billion), post-operative cognitive decline ($7 billion), and metabolic syndrome (~$35 billion).
The opioid epidemic is one of the most profound public health crises facing the United States. In 2016, an estimated 3.3 million people had an opioid use disorder related to prescription pain relievers and an estimated 626,000 had an opioid use disorder related to heroin use. We plan to initially market aVNS as an aid in reducing the symptoms of opioid withdrawal in the detoxification phase of treatment. We have filed an application with an institutional review board (IRB) for a 15-patient feasibility study to be conducted in Puerto Rico. This feasibility study is expected to be completed in late 2018 with results used to determine the magnitude of symptom reduction during the first hour of treatment. This data will then be used to work with the FDA on the design of a clinical study to support marketing approval through the de novo pathway. U.S. Market launch for opioid withdrawal will occur after the FDA grants market clearance, which we expect to occur in late 2019.
Commercial: U.S. market launch for atrial fibrillation will occur after the FDA grants the de novo request, which we expect to occur in mid-2019. Similarly, US market launch of the opioid withdrawal product will occur subsequent to the de novo request, which we expect to occur in mid-2019.
55
Research and Development
For the years ending December 31, 2017 and December 31, 2016, our research and development expenses were $2,942,981 and $759,502, respectively, primarily reflecting the costs associated with our research and discovery efforts related to the design and development of our proposed medical devices. It is expected that our research and development activities and related expenses will increase significantly in the future as we increase the scope and rate of such efforts, and begin more-expensive development activities, including clinical trials and similar studies as required by the relevant regulatory authorities in our targeted jurisdictions (i.e., the United States and European Union).
Original Equipment Manufacturer, Medi-Line S.A.
On September 1, 2017, we acquired NMB, which owns and operates Medi-Line. The Medi-Line medical device and is an OEM. Medi-Line provides high quality and efficiency in the development, engineering, and manufacturing of medical devices for the med-tech and pharmaceutical industries.
Product Line
Medi-Line currently manufactures radiopharmacy and urology products, and provides worldwide production and supply chain capabilities for these products to its customers. Medi-Line offers a wide range of services, including product and process development, validation and verification, technical and regulatory file writing, packaging, biocompatibility, and sterilization support services. Customers of Medi-Line are active in fields as diverse as urologic implants, neurosurgery, interventional gastroenterology, implantable neurostimulation, radiopharmacy, PET scan imagery, special high-added-value catheters, and microthin cuff catheters.
Sales and Marketing
Medi-Line sales and marketing efforts have been limited to date, with most new business coming in the form of referrals. We intend to grow our business by emphasizing our design and engineering expertise, internally developed products, manufacturing capabilities, international distribution network, and ability to provide customers with a comprehensive product offering. We present our products to customers in a concept, which offers the customer a collaborator for developing complete implant, instrument, and case solutions while working to create and respond to opportunities for any one of our product offerings. We believe there is an opportunity to leverage our existing relationships among our customer base to achieve greater penetration of our customized products.
Our Competitive Strengths
| ● | Comprehensive offerings. We believe we can support our customers’ new product offerings from product concept through market introduction, and thereafter by providing seamless design, engineering, prototyping, and manufacturing offerings. |
| ● | Single source for complete systems. We believe we can assist our customers in developing new implants, and we design and produce neurostimulator systems for specific neurological diseases. |
| ● | Precision manufacturing expertise. Our extensive expertise and know-how enables us to produce large volumes of specialized products to our customers’ precise standards, which we believe makes us a supplier of choice to the largest orthopedic companies, as well as addressing the broader needs of smaller customers. Our core production competencies include net-shaped forging, precision casting, thermo forming, precision sheet metal working, and machining/finishing. |
| ● | Over the past several years, we have developed high-precision machining capabilities believed to better serve the spine implant market. |
| ● | Quality and regulatory compliance. Our quality systems are based upon, and are in compliance with, International Organization for Standardization (ISO) requirements and, where applicable, FDA regulations. We continue to invest in this area to strengthen our leadership position. |
| ● | Customers. Our OEM Solutions business supplies products primarily to manufacturers in the medical device market. |
56
Quality Assurance
We maintain a comprehensive quality assurance and quality control program, which includes the control and documentation of all material specifications, operating procedures, equipment maintenance, and quality control methods. Our quality systems are based on FDA requirements and the ISO standards for medical device manufacturers. We believe that all our facilities are currently in substantial compliance with regulations applicable to them.
All aspects of the supply chain are integrated into our overall quality system. Our suppliers are evaluated and audited to assure compliance with all international trade compliance quality standards. Relative to our manufacturing processes, we maintain and adhere to specific standard operating procedures within our quality systems to ensure compliance with our customers’ requirements for their products. Our deep brain neurostimulator business likewise operates under a comprehensive quality system to ensure compliance with all product quality and customer obligations. The suppliers we utilize in the distribution process are evaluated and audited to assure compliance with all international trade compliance quality standards.
We are not aware of any significant quality issues or concerns, although if we experience a breakdown in our quality systems that result in the sale or manufacture of noncompliance products, we could incur costs and loss of business, recalls, lawsuits, or other adverse results.
Customers
Medi-Line sells to over 30 customers. Sales to our two largest customers represented 79.4% of sales revenue in 2017. Our largest customer has been a customer for nearly 15 years, and has a long-term contract lasting an additional 5 years. All sales are shipped directly to our customers.
Intellectual Property
We possess a strong patent portfolio, which is critical to successful commercialization of the Viant™ DBS System and all associated components. Intellectual property associated with the Viant™ System includes patents related to the Internet of Medical Things. Maintaining a focus in the Internet of Medical things allows us to utilize issued and pending patents for maximum commercial benefit and growth. Since its inception, we have actively pursued patent coverage of the proprietary systems and methods of delivering therapy. The patents have been deliberately written to provide broad and specific protection.
Our strategy is to protect proprietary positions by continuing to acquire and file U.S. and foreign patent applications related to this technology, inventions, and improvements to enhance the business and competitive advantages. Continuing to rigorously analyze competitive IP applications and their prosecution history will ensure that this freedom to operate position remains viable. As development of new products and prosecution of pending patent applications progresses, we will continue to strategically file additional applications, including continuations, continuations-in-part, and divisional applications, to protect the new developments and those already being prosecuted.
Temporal barrier to others: Even without patents, development of an approved class III system with a custom chip requires five to seven years to develop. We will have a large data set from the recording technology, which will enable therapy insights that will allow further improvements. In addition, we have thoroughly analyzed the patents, products, and therapies that may be considered competitive to the proposed system. We are committed to protecting its proprietary positions by continuing to file patent applications related to technology, inventions, and improvements to enhance their business and competitive advantages. As development of new products and prosecution of pending patent applications progresses, Nexeon MedSystems will continue to strategically file additional applications to protect these new developments.
In addition to seeking patent protection, we have utilized other available intellectual property rights to protect our developments. We utilize copyrighted software, manuals, and reports. We also maintain a number of trade secrets that are essential to our business. We have implemented procedures to maintain the appropriate secrecy required of such trade secrets. Finally, we have filed for and obtained several trademark registrations related to the branding of our products. This multifaceted approach provides us with the maximum protection available for our developments.
57
The following patents and applications relate to our technology for communicating between an implantable medical device and a remote computer system or health care provider.
| US 6385593 | Apparatus and method for automated invoicing of medical device systems | |
| EP 15853585 B1 | Seamless communication between an implantable medical device and a remote system |
The following patents and applications relate to our micro-perforated balloon catheter system for use in the treatment of restenosis associated with hemodialysis.
| NANOTUBE-REINFORCED BALLOONS FOR DELIVERING PATENTS | ||
| US 8187221 | Therapeutic agents within or beyond the wall of blood vessels, and methods of making and using same | |
| JP 5481479 | Therapeutic agents within or beyond the wall of blood vessels, and methods of making and using same | |
| APPARATUS AND METHODS FOR RENAL STENTING PATENTS | ||
| AU 2006244070 | Apparatus and methods for renal stenting | |
| SYSTEMS AND METHODS FOR ATRAUMATIC IMPLANTATION OF BIO-ACTIVE AGENTS PATENTS | ||
| US 7632262 | Systems and methods for atraumatic implantation of bio-active agents | |
| US 8377032 | Systems and methods for atraumatic implantation of bio-active agents | |
| EP 5773060.8 | Systems and methods for atraumatic implantation of bio-active agents | |
| EP 11150671.3 | Systems and methods for atraumatic implantation of bio-active agents | |
| AU 2005274775 | Systems and methods for atraumatic implantation of bio-active agents | |
| JP 4774403 | Systems and methods for atraumatic implantation of bio-active agents | |
| APPARATUS AND METHODS FOR TREATING TISSUE USING PASSIVE INJECTION SYSTEMS PATENTS | ||
| US 7338471 | Apparatus and methods for treating tissue using passive injection systems | |
| US 7862551 | Apparatus and methods for treating tissue using passive injection systems | |
| AU 2005274774 | Apparatus and methods for treating tissue using passive injection systems | |
| JP 4934029 | Apparatus and methods for treating tissue using passive injection systems | |
| GB & FR 1773440 | Apparatus and methods for treating tissue using passive injection systems | |
| DE 602005030269.7 | Apparatus and methods for treating tissue using passive injection systems | |
| METHODS AND APPARATUS FOR TREATING INFARCTED REGIONS OF TISSUE FOLLOWING PATENTS | ||
| US 7819856 | Acute myocardial infarction | |
| US 8062283 | Acute myocardial infarction | |
58
The following is a list of the Siemens Patents underlying the license for the intellectual property which relates to IOT technology as described by a system of interrelated computing devices, mechanical and digital machines, objects, animals, and/or people that have unique identifiers and a subsequent ability to transfer data over a network without requiring human-to-human or human-to-computer interaction. This technology can be utilized in a wide variety of medical device applications, most notably in hospitals, nursing facilities, or patients’ homes.
| Country | Patent # | Apln. # | Pub. # | |||
| AUSTRIA | AT521160 | 5109169.2 | EP1646185 | |||
| AUSTRIA | AT530961 | 3735054.3 | EP1470457 | |||
| AUSTRIA | AT615998 | 5108954.8 | EP1643324 | |||
| AUSTRIA | AT334459 | 4100499.5 | EP1494191 | |||
| BELGIUM | BE1494191 | 4100499.5 | EP1494191 | |||
| BRASIL | PI0710612 | BR0710612 | ||||
| BRASIL | PI0821881-1 | |||||
| CANADA | 2647652 | 2647652.0 | 2647652 | |||
| CANADA | 2662014 | 2662014.0 | 2662014 | |||
| CANADA | 2711225 | 2711225.0 | 2711225 | |||
| CANADA | 2836941 | 2836941.0 | 2836941 | |||
| CHINA | 200780032280.2 | CN101512976 | ||||
| CHINA | CN101422019 | 200780012887.4 | 101422019 | |||
| CHINA | CN101971568 | 200880123790.5 | 101971568 | |||
| CHINA | 201410392441.0 | CN104243248 | ||||
| EPO | EP1470456 | 3707570.2 | EP1470456 | |||
| EPO | EP1470457 | 3735054.3 | EP1470457 | |||
| EPO | EP1494191 | 4100499.5 | EP1494191 | |||
| EPO | EP1626532 | 5012617.6 | EP1626532 | |||
| EPO | EP1643324 | 5108954.8 | EP1643324 | |||
| EPO | EP1646185 | 5109169.2 | EP1646185 | |||
| EPO | EP2225854 | 8869509.3 | EP2225854 | |||
| EPO | 7101252.0 | EP1783959 | ||||
| EPO | 7774303.7 | EP2005713 | ||||
| EPO | 13172203.5 | EP2642696A3 | ||||
| EPO | 13172204.3 | EP2642697A3 | ||||
| FINLAND | FI1470456 | 3707570.2 | EP1470456 | |||
| FRANCE | FR1470456 | 3707570.2 | EP1470456 | |||
| FRANCE | FR1470457 | 3735054.3 | EP1470457 | |||
| FRANCE | FR1626532 | 5012617.6 | EP1626532 | |||
| FRANCE | FR1643324 | 5108954.8 | EP1643324 | |||
| FRANCE | FR1646185 | 5109169.2 | EP1646185 | |||
| FRANCE | FR2225854 | 8869509.3 | EP2225854 | |||
| FRANCE | FR1494191 | 4100499.5 | EP1494191 | |||
| GERMANY | 10317962.3 | |||||
| GERMANY | DE602005029532.1 | 5109169.2 | EP1646185 | |||
| GERMANY | DE602005039869.4 | 5108954.8 | EP1643324 | |||
| GERMANY | DE602005042990.5 | 5012617.6 | EP1626532 | |||
| GERMANY | DE602008030307.1 | 8869509.3 | EP2225854 | |||
| GERMANY | DE60330750.7 | 3707570.2 | EP1470456 | |||
| GERMANY | DE60338908.2 | 3735054.3 | EP1470457 | |||
| GERMANY | DE502004001017.2 | 4100499.5 | EP1494191 | |||
| GERMANY | 112007001804.0 | |||||
| GREECE | GR1494191 | 4100499.5 | EP1494191 | |||
| IRELAND | IE1494191 | 4100499.5 | EP1494191 | |||
| IRELAND | 1470456 | 3707570.2 | 1470456 | |||
| ITALY | IT1470457 | 3735054.3 | EP1470457 | |||
| ITALY | IT1626532 | 5012617.6 | EP1626532 | |||
| ITALY | IT1643324 | 5108954.8 | EP1643324 | |||
| ITALY | IT1646185 | 5109169.2 | EP1646185 | |||
| ITALY | IT2225854 | 8869509.3 | EP2225854 | |||
| ITALY | IT1494191 | 4100499.5 | EP1494191 | |||
| MEXICO | MX313282 | MX/a/2010/007211 | ||||
| MEXICO | MX322526 | MX/a/2013/010239 |
59
| Country | Patent # | Apln. # | Pub. # | |||
| NETHERLANDS | NL1470456 | 3707570.2 | EP1470456 | |||
| NETHERLANDS | NL1470457 | 3735054.3 | EP1470457 | |||
| NETHERLANDS | NL1626532 | 5012617.6 | EP1626532 | |||
| NETHERLANDS | NL1643324 | 5108954.8 | EP1643324 | |||
| NETHERLANDS | NL1646185 | 5109169.2 | EP1646185 | |||
| NETHERLANDS | NL1494191 | 4100499.5 | EP1494191 | |||
| SOUTH KOREA | 100989017 | 1020087024880.0 | ||||
| SOUTH KOREA | 101272384 | 1020107014107.0 | ||||
| SOUTH KOREA | KR101202914B1 | 1020107014718.0 | ||||
| SPAIN | ES2270271 | 4100499.5 | EP1494191 | |||
| SWEDEN | SE1470456 | 3707570.2 | EP1470456 | |||
| SWEDEN | SE1470457 | 3735054.3 | EP1470457 | |||
| SWEDEN | SE1626532 | 5012617.6 | EP1626532 | |||
| SWEDEN | SE1643324 | 5108954.8 | EP1643324 | |||
| SWEDEN | SE1646185 | 5109169.2 | EP1646185 | |||
| SWEDEN | SE2225854 | 8869509.3 | EP2225854 | |||
| SWEDEN | SE1494191 | 4100499.5 | EP1494191 | |||
| SWITZERLAND | CH1470456 | 3707570.2 | EP1470456 | |||
| SWITZERLAND | CH1470457 | 3735054.3 | EP1470457 | |||
| SWITZERLAND | CH1626532 | 5012617.6 | EP1626532 | |||
| SWITZERLAND | CH1643324 | 5108954.8 | EP1643324 | |||
| SWITZERLAND | CH1646185 | 5109169.2 | EP1646185 | |||
| SWITZERLAND | CH2225854 | 8869509.3 | EP2225854 | |||
| SWITZERLAND | CH1494191 | 4100499.5 | EP1494191 | |||
| UNITED KINGDOM | GB1470456 | 3707570.2 | EP1470456 | |||
| UNITED KINGDOM | GB1470457 | 3735054.3 | EP1470457 | |||
| UNITED KINGDOM | GB1626532 | 5012617.6 | EP1626532 | |||
| UNITED KINGDOM | GB1643324 | 5108954.8 | EP1643324 | |||
| UNITED KINGDOM | GB1646185 | 5109169.2 | EP1646185 | |||
| UNITED KINGDOM | GB2225854 | 8869509.3 | EP2225854 | |||
| UNITED KINGDOM | GB1494191 | 4100499.5 | EP1494191 | |||
| US | 60/352,452 | |||||
| US | 8131399 | 10/353,142 | ||||
| US | 8538589 | 13/366,095 | ||||
| US | 10/353,110 | |||||
| US | 10/672,527 | |||||
| US | 7860495 | 10/915,034 | ||||
| US | 12/629,548 | |||||
| US | 8200273 | 12/953,244 | ||||
| US | 7139239 | 10/958,770 | ||||
| US | 7437596 | 11/538,654 | ||||
| US | 7664573 | 10/952,705 | ||||
| US | 7746887 | 11/402,743 | ||||
| US | 60/823,788 | |||||
| US | 9030315 | 11/846,218 | ||||
| US | 60/823,909 | |||||
| US | 60/823,912 | |||||
| US | 8264371 | 11/969,111 | ||||
| US | 12/124,452 | |||||
| US | 61/037,739 | |||||
| US | 8224282 | 12/406,799 | ||||
| US | 8350691 | 12/269,136 | ||||
| US | 7363036 | 10/824,800 | ||||
| WO/PCT | PCT/US2003/002556 | WO/2003/064933 | ||||
| WO/PCT | PCT/US2003/002559 | WO/2003/065136 | ||||
| WO/PCT | PCT/US2007/007984 | WO/2007/123738 | ||||
| WO/PCT | PCT/US2007/077107 | WO/2008/027964 | ||||
| WO/PCT | PCT/US2008/013746 | WO/2009/088429 |
60
Employees
As of the date hereof, we have 48 full-time employees and 1 consultant at Medi-Line, and 7 employees and 1 consultant working in the neurostimulation business. None of our employees or consultants are represented by a labor union or covered by a collective bargaining agreement. We have employment agreements with all full-time employees. We consider our relationships with our employees to be good.
Properties
Our U.S. corporate headquarters is approximately 800 square feet of office space in Dallas, Texas, which we rent for $3,588 per year and renew on a monthly basis.
We have manufacturing facilities in Belgium. We continue to make investments to modernize our production facilities, improve our production processes, and develop superior technical skills that complement our manufacturing capabilities. These investments have allowed us to continue to improve the quality of our products, increase our manufacturing capacity, and improve our efficiency.
We do not own any real estate, with the exception of a portion of the excess unused land at the Medi-Line manufacturing facility. We lease the following properties:
The Medi-Line manufacturing plant and offices located in Angleur (Liege), Belgium, with an approximate area of 29,886 sq. ft. are financed under a capital lease for EUR €156,044 annually (approximately $186,919). The lease ends on October 25, 2021.
NXPROC leases approximately 221 sq. ft. of office space in Rio Piedres, Puerto Rico, for $5,640 per year, renewed on a monthly basis.
Legal Proceedings
On October 10, 2018, we received a petition and request for disclosure filed in the District Court of Dallas County, Texas naming Mr. Brian Blischak as the plaintiff and the Company as the defendant. The petition states Nexeon MedSystems Inc hired Mr. Blischak in December of 2016 as President and Chief Commercial Officer. Pursuant to the employment agreement between Mr. Blischak and us, we were required to pay Mr. Blischak salary and bonuses in exchange for Mr. Blischak’s performance as a corporate executive. Mr. Blischak seeks monetary relief in the amount of $376,985 for unpaid amounts pursuant to Mr. Blishcak’s employment agreement. Mr. Blishcak resigned his position as President and Chief Commercial Officer on November 1, 2018.
61
Executive Officers and Directors
The following table sets forth the names, ages, and positions of our current executive officers and directors.
| Name | Age | Position(s) | ||
| William Rosellini | 38 | Chairman and Chief Executive Officer | ||
| Christopher R. Miller | 49 | Chief Financial Officer | ||
| Kent J. George (1)(2)(3) | 58 | Director | ||
| Michael Neitzel (1)(2)(3) | 40 | Director | ||
| R. Wesley Dittmer II (1)(2)(3) | 55 | Director |
| (1) | Member of Audit Committee |
| (2) | Member of Compensation Committee |
| (3) | Member of Nominating & Governance Committee |
William Rosellini, JD, MBA
William Rosellini has served as chief executive officer and as a director of the Company since its inception. Since 2005, Mr. Rosellini has served as chairman of RS, which develops medical rehabilitation devices to support patients post-procedure. Rosellini, a former Minor League pitcher, holds five master’s degrees in addition to a law degree. He previously founded and led Lexington Technology Group, LLC, a database company commercializing a database solution that was sold to Document Security Systems, Inc. ; and Sarif Biomedical LLC, a stereotactic microsurgery company that was sold to Marathon Patent Group, Inc. . Mr. Rosellini became a board member of Marathon Patent Group, Inc. in 2013, and resigned in 2016. He started Emeritus Medical, Inc., a clinical engineering services company, in 2013, and sold it in 2016. Rosellini also founded Microtransponder in 2006. He left his position as CEO at Microtransponder in 2012. For a discussion of the conflicts of interest involved in Mr. Rosellini’s and other members of management’s other business endeavors, see “Conflicts of Interest” below. Mr. Rosellini holds an MBA from the University of Texas System; an MS in accounting from The University of Texas System; an MS in computational biology from Rutgers, The State University of New Jersey; an MS in neuroscience from The University of Texas; an MS of regulatory science from the University of Southern California; and a BA in economics from the University of Dallas. We believe that Mr. Rosellini is qualified to serve on our board of directors due to his role in founding the Company, and in the development of its business strategy, as well as his experience developing medical devices.
Christopher R. Miller
Mr. Miller served as interim chief financial officer of the Company commencing January 1, 2016, and was appointed as the chief financial officer on December 1, 2016. Since 2002, Mr. Miller has been providing financial and business development consulting and interim CFO services, with a focus on early-stage companies. From 2006 to 2008, Mr. Miller provided public company valuation, financial modeling, and due diligence services to Doherty & Company, LLC, a Los Angeles-based broker-dealer specializing in venture capital, private equity funding, mergers and acquisitions advisory, and valuations for early-stage companies. From June 2009 to June 2010, Mr. Miller served as a member of the board of directors of WindGen Energy, Inc. Other than the foregoing, Mr. Miller does not currently, and has not for the last five years, served as a member of the board of directors for any public companies. Mr. Miller holds a BS in finance from Arizona State University.
62
Kent J. George, JD
Mr. George has served as a director of the Company since January 1, 2017. Mr. George has been associated with Robinson & McElwee PLLC, a mid-Atlantic corporate law firm, since 1987. Mr. George served as the managing member from 1999 through 2014, and has served as the chief executive officer since 2014. With over three decades of experience in commercial transactions representing public and private companies, Mr. George has been recognized for his work in real estate by Chambers USA since 2014, and is AV peer-rated by Martindale-Hubbell. Mr. George’s practice focuses on business transactions, including mergers and acquisitions, business litigation, arbitration and dispute resolutions, and real estate transactions (retail, industrial, resort, lodging, and other commercial development projects). Mr. George holds a BA from Swarthmore College, a JD from the University of Chicago, and a BA and MA (law) from Oxford University. We believe Mr. George’s experience in business transactions qualifies him to serve on our board of directors.
Michael Neitzel, MBA
Mr. Neitzel has served as a director of the Company since January 1, 2017. Mr. Neitzel has been with Cambridge Homes (“Cambridge”) since April 1, 2017, and currently serves as director of acquisition, where he leverages his 15 years of experience in real estate acquisition and development to support the company’s growth initiatives. Cambridge is a privately held homebuilder headquartered in Plano, Texas. Prior to working at Cambridge, Mr. Neitzel served as a managing partner for DartPoints Holdings, LLC (“DartPoints”) from January 2014 to March 2017, a data center construction and management firm. Mr. Neitzel currently sits on the board of managers for DartPoints. Prior to DartPoints, Mr. Neitzel was with Gehan Homes from 2009 until 2014, a privately held homebuilder headquartered in Dallas, Texas. He has held management positions for both public and private, large-scale building companies, where his responsibilities included deal flow sourcing, acquisition, and development and delivery of subdivisions throughout Texas. Mr. Neitzel holds a BA in business administration from the University of Kansas and an MBA in finance from Southern Methodist University. We believe Mr. Neitzel’s managerial and business transactional experience qualifies him to serve on our board of directors.
R. Wesley Dittmer II, CPA
Mr. Dittmer has served as a director of the Company since April 24, 2018. Mr. Dittmer is an experienced financial executive with more than 20 years of finance and accounting experience with publicly traded companies, and an extensive background in capital formation, mergers and acquisitions (M&A), and operations. He is currently an independent consultant focused on M&A, capital raises, and supply chain optimization. He previously served as the CFO for the North American division of Parnell Inc. (2015-2017), AgJunction Inc. (2013-2015), and National Rural Telecommunications Cooperative (2010-2012), and was vice president of corporate development of Embarq Corp. from (1999-2009). Mr. Dittmer also served as a board member of Digital Bridge Communications (2011-2012) and GeoNav Group International (2010-2012). He is a certified public accountant, and received his BS in accounting from the University of Missouri, and an MBA in finance from Rockhurst College. We believe Mr. Dittmer’s accounting and finance experience qualifies him to serve on our board of directors.
Legal Proceedings
During the past 10 years, none of our directors, executive officers, promoters, control persons, or nominees has been:
| ● | The subject of any bankruptcy petition filed by or against any business of which such person was a general partner or executive officer, either at the time of the bankruptcy or within two years prior to that time; | |
| ● | Convicted in a criminal proceeding or subject to a pending criminal proceeding (excluding traffic violations and other minor offenses); |
| ● | Subject to any order, judgment, or decree, not subsequently reversed, suspended, or vacated, of any court of competent jurisdiction, or any federal or state authority, permanently or temporarily enjoining, barring, suspending, or otherwise limiting his involvement in any type of business, securities, or banking activities; | |
| ● | Found by a court of competent jurisdiction (in a civil action) or the Commodity Futures Trading Commission to have violated a federal or state securities or commodities law. |
63
| ● | The subject of, or a party to, any federal or state judicial or administrative order, judgment, decree, or finding not subsequently reversed, suspended, or vacated, relating to an alleged violation of (a) any federal or state securities or commodities law or regulation; (b) any law or regulation respecting financial institutions or insurance companies, including, but not limited to, a temporary or permanent injunction, order of disgorgement or restitution, civil money penalty, temporary or permanent cease and desist order, or removal or prohibition order; or (c) any law or regulation prohibiting mail or wire fraud, or fraud in connection with any business entity; or |
,
| ● | The subject of, or a party to, any sanction or order, not subsequently reversed, suspended, or vacated, of any self-regulatory organization (as defined in Section 3(a)(26) of the Exchange Act (15 U.S.C. 78c(a)(26))), any registered entity (as defined in Section 1(a)(29) of the Commodity Exchange Act (7 U.S.C. 1(a)(29))), or any equivalent exchange, association, entity, or organization that has disciplinary authority over its members or persons associated with a member. |
Code of Business Conduct and Ethics
We have adopted a Code of Business Conduct and Ethics that applies to our principal executive, financial, and accounting officers (or persons performing similar functions), a copy of which is filed as Exhibit 14.1 to the Company’s Form 10 Registration Statement filed with the SEC on July 6, 2016.
Board Composition and Election of Directors
Our board of directors is currently comprised of four members. Pursuant to our articles of incorporation and bylaws, directors shall hold office until the next annual meeting of the stockholders, and until his or her successor shall be elected and qualified. Directors may be removed, with or without cause and from time to time, as provided by Chapter 78 of the Nevada Revised Statues, then in effect. Our nominating & governance committee reviews director candidates and proposes director nominees.
Indemnification of Directors and Officers
Our officers and directors are indemnified as provided by the Nevada Revised Statutes (“NRS”) and our bylaws. Under the NRS, unless modified by a corporation’s articles of incorporation, a director is not liable to a corporation, its stockholders, or creditors for damages unless the director’s action or failure constituted a breach of fiduciary duty and such breach involved intentional misconduct, fraud, or a knowing violation of law. Our bylaws provide that we will indemnify our directors and officers to the fullest extent permissible under Nevada law if such person acted in good faith and in a manner that such person reasonably believed to be in, or not opposed to, the best interests of the Company, and, with respect to any criminal action, had no reasonable cause to believe such conduct was unlawful. We have entered into indemnification agreements with each of its directors, a copy of which is filed as Exhibit 10.07 of the Company’s Form 10 Registration Statement filed with the SEC on July 6, 2016.
We have purchased and maintains directors’ and officers’ liability insurance, and may make other financial arrangements on behalf of any individual entitled to indemnity. Our bylaws also provide that we will advance all expenses incurred to any person entitled to indemnity upon receipt of an undertaking by, or on behalf of, such person to repay said amounts should it be ultimately determined that the person was not entitled to indemnification.
Audit Committee, Compensation Committee, and Nominating & Governance Committee
We have audit, compensation, and nominating & governance committees. Each member of our committees is “independent” as such term is defined under and required by the federal securities laws and the rules of the Nasdaq Capital Market. The charters of each of the committees have been approved by our board, and are available on our website at www.nexeonmed.com.
64
Audit Committee
The director independence rules of the Nasdaq Capital Market require listed companies to have audit committees of at least three members, each of whom (in addition to satisfying other conditions) is an independent director. The audit committee currently consists of three independent directors: R. Wesley Dittmer (who serves as the committee chairperson), Kent J. George, and Michael Neitzel. Mr. Dittmer serves as “audit committee financial expert,” as defined in Item 407(d)(5)(ii) of Regulation S-K.
The audit committee’s duties include recommending to our board the engagement of independent auditors to audit our financial statements and to review our accounting and auditing principles. The audit committee reviews the scope, timing, and fees for the annual audit, and the results of audit examinations performed by independent public accountants, including their recommendations to improve our system of accounting and our internal control over financial reporting. The audit committee oversees the independent auditors, including their independence and objectivity. But the committee members are not acting as professional accountants or auditors, and their functions are not intended to duplicate or substitute for the activities of management and the independent auditors. The audit committee is empowered to retain independent legal counsel and other advisors as it deems necessary or appropriate to assist the audit committee in fulfilling its responsibilities, and to approve the fees and other retention terms of the advisors. Each of our audit committee members possesses an understanding of financial statements and generally accepted accounting principles.
Compensation Committee
The compensation committee consists of three independent directors: Michael Neitzel (who serves as the committee chairperson), Kent J. George, and R. Wesley Dittmer.
The compensation committee has certain duties and powers as described in its charter, including but not limited to periodically reviewing and approving our salary and benefits policies, compensation of executive officers, administering our stock option plans, and recommending and approving grants of stock options under such plans.
Nominating & Governance Committee
The nominating & governance committee consists of three independent directors: Kent J. George (who serves as the committee chairperson), Michael Neitzel and R. Wesley Dittmer. The nominating & governance committee selects or recommends nominees for directors. The director independence rules of the Nasdaq Capital Market require listed companies’ independent directors to select or recommend nominees for directors. Independent directors serving on our nominating & governance committee provide recommendations for directors. The nominating & governance committee considers and makes recommendations on matters related to the practices, policies, and procedures of the board, and takes a leadership role in shaping our corporate governance. As part of its duties, the nominating & governance committee assesses the size, structure, and composition of the board and its committees, and coordinates the evaluation of board performance. The nominating & governance committee also acts as a screening and nominating committee for candidates considered for election to the board.
Director Independence
As of the date hereof, our board of directors is composed of four members, three of whom (Kent J. George, Michael Neitzel and R. Wesley Dittmer) qualify as independent directors in accordance with the published listing requirements of the Nasdaq Capital Market. The Nasdaq Capital Market independence definition includes a series of objective tests, such as that the directors are not, and have not been for at least three years, one of our employees, and that neither the directors nor any of their family members have engaged in various types of business dealings with us.
Conflicts of Interest
We did not have an audit or compensation committee until January 1, 2017, thus providing for a potential conflict of interest in that our directors had the authority to determine issues concerning management compensation, nominations, and audit issues that may affect management decisions prior to the creation of such committees.
All potential or actual conflicts of interest for all of our officers and directors have been approved by the board of directors (with abstention by the conflicted director) pursuant to our Code of Business Conduct and Ethics. Such board approval for conflict of interest transactions is consistent with Nevada corporate law statutes.
65
Family Relationships
As of the date hereof, there are no family relationships of any kind among our executive officers, directors, or persons nominated or chosen by us to become executive officers or directors, except that Emily Hamilton, MD, serves as the director of emerging therapy for NXPROC. Dr. Hamilton is the wife of our CEO, William Rosellini.
Board Leadership Structure and Role in Risk Oversight
Although we have not adopted a formal policy on whether the chairman and chief executive officer positions should be separate or combined, we have traditionally determined that it is in the best interests of the Company and its shareholders to combine these roles. Due to our small size and early stage, we believe it is currently most effective to have the chairman and chief executive officer positions combined. In addition, having one person serve as both chairman and chief executive officer eliminates potential for confusion, and provides clear leadership for the Company, with a single person setting the tone and managing our operations. The board oversees specific risks, including but not limited to:
| ● | Appointing, retaining, and overseeing the work of the independent auditors, including resolving disagreements between the management and the independent auditors relating to financial reporting; | |
| ● | Approving all auditing and non-auditing services permitted to be performed by the independent auditors; |
| ● | Reviewing annually the independence and quality control procedures of the independent auditors; |
| ● | Reviewing, approving, and overseeing risks arising from proposed related party transactions; |
| ● | Discussing the annual audited financial statements with the management; |
| ● | Meeting separately with the independent auditors to discuss critical accounting policies, management letters, recommendations on internal controls, the auditor’s engagement letter and independence letter, and other material written communications between the independent auditors and the management; and | |
| ● | Monitoring the risks associated with management resources, structure, succession planning, development, and selection processes, including evaluating the effect the compensation structure may have on risk decisions. |
66
EXECUTIVE COMPENSATION
The tables below summarize all compensation awarded to, earned by, or paid to our executive officers for all services rendered in all capacities to us for the fiscal period(s) indicated.
Summary Executive Compensation
| Name and Position | Year | Salary ($) | Bonus ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Nonqualified Deferred Compensation Earnings ($) | All
Other Compensation(1) ($) | Total ($) | |||||||||||||||||||||||||
| (a) | (b) | (c) | (d) | (e) | (f) | (g) | (h) | (i) | (j) | |||||||||||||||||||||||||
| William Rosellini | 2017 | $ | 2 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 2 | |||||||||||||||||
| Chief Executive Officer | 2016 | $ | — | $ | 10,000 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 10,000 | |||||||||||||||||
| Brian Blischak | 2017 | $ | 250,000 | $ | 28,000 | $ | — | $ | — | $ | — | $ | — | $ | 15,956 | $ | 293,956 | |||||||||||||||||
| President & Chief Commercial Officer (resigned November 1, 2018) | 2016 | $ | 20,833 | $ | — | $ | — | $ | 365,343 | $ | — | $ | — | $ | — | $ | 386,176 | |||||||||||||||||
| Mark C. Bates | 2017 | $ | 27,000 | — | — | — | — | — | — | $ | 27,000 | |||||||||||||||||||||||
| Former Chief Innovation Officer (Resigned effective November 6, 2017) | 2016 | $ | 9,900 | $ | — | $ | — | $ | 47,056 | $ | — | $ | — | $ | — | $ | 56,956 | |||||||||||||||||
| Ronald Conquest | 2017 | $ | 155,000 | — | — | — | — | — | $ | 10,199 | $ | 165,199 | ||||||||||||||||||||||
| Former Executive Vice President of Finance (Resigned effective October 31, 2017) | 2016 | $ | 81,500 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 6,500 | $ | 88,000 | |||||||||||||||||
| Elizabeth Rosellini | 2017 | — | — | — | — | — | — | — | — | |||||||||||||||||||||||||
| Former Vice President of Clinical Affairs (Resigned effective December 31, 2016) | 2016 | $ | 20,000 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | 20,000 | |||||||||||||||||
| Christopher Miller | 2017 | $ | 125,000 | — | — | — | — | — | $ | 6,750 | $ | 131,750 | ||||||||||||||||||||||
| Chief Financial Officer | 2016 | $ | 21,577 | $ | $ | 252 | $ | 66,234 | $ | — | $ | — | $ | — | $ | 88,063 | ||||||||||||||||||
| Daniel Powell | 2017 | $ | 89,931 | — | $ | 33,392 | — | — | $ | 16,359 | $ | 139,686 | ||||||||||||||||||||||
| Vice President Sales and Marketing (terminated July 31, 2018) | 2016 | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | $ | — | |||||||||||||||||
| (1) | All other compensation represents reimbursement of health insurance premiums paid by executive. |
67
Summary Director Compensation
The following table provides information with respect to the total compensation granted to our directors for services as directors as of December 31, 2017.
| Name | Year | Fees Earned or Paid in Cash ($) | Stock Awards ($) | Option Awards ($) | Non-Equity Incentive Plan Compensation ($) | Non-Qualified Deferred Compensation Earnings ($) | All Other Compensation ($) | Total ($) | ||||||||||||||||||||||
| Mark Bates* | 2017 | $ | 9,000 | — | — | — | — | — | $ | 9,000 | ||||||||||||||||||||
| 2016 | — | — | — | — | — | — | — | |||||||||||||||||||||||
| Ron Conquest** | 2017 | — | — | — | — | — | — | — | ||||||||||||||||||||||
| 2016 | — | — | — | — | — | — | — | |||||||||||||||||||||||
| Kent J. George | 2017 | $ | 16,000 | $ | 20,679 | — | — | — | — | $ | 36,679 | |||||||||||||||||||
| 2016 | — | — | — | — | — | — | — | |||||||||||||||||||||||
| Michael Neitzel | 2017 | $ | 16,000 | $ | 20,679 | — | — | — | — | $ | 36,679 | |||||||||||||||||||
| 2016 | — | — | — | — | — | — | — | |||||||||||||||||||||||
| * | Resigned effective November 6, 2017 |
| ** | Resigned effective October 31, 2017 |
On January 1, 2017, we entered into a director services agreement with each of Kent J. George and Michael Neitzel. The director services agreements for Mr. George and Mr. Neitzel include the following terms:
Compensation
Director’s Fees. An annual director’s fee, payable in arrears in quarterly installments at the end of each calendar quarter during which a director has served as a director for the Company, shall be $3,000 per quarter. Notwithstanding the foregoing, our board unanimously approved a one-time $1,000 per quarter increase to the director’s fees payable to Mr. Dittmer in connection with this appointment.
Director’s Options. At the end of each three-month (3) period that the director serves as a director of the Company, we will grant to the director an option to purchase Eight Hundred Ninety Three (893) shares of the Company’s restricted common stock, at a price equal to Fourteen Dollars ($14.00) per share, or, in the alternative, the price per share of the Company’s then current market price per share. The term of each option shall be for a period of four (4) years from the date of issue of each option.
68
Expenses. We will reimburse the director for all reasonable out of pocket expenses incurred by the director in acting as director, subject to the director providing reasonable documentation and subject to our policies regarding such expenses, provided further that it is anticipated that such expenses shall primarily consist of travel expenses to board meetings, or such other expenses discussed and approved by our CEO and/or board of directors.
Assignment of Intellectual Property and Limited Non-Competition
Assignment of Intellectual Property. In connection with their appointments, directors agree to assign to us any and all rights, improvements, and copyrightable or patentable subject matter (and other intellectual property relating to our business) that such directors conceive or develop, either alone or with others, or which otherwise arise during the term of director providing management services to us, and for a period of six (6) months thereafter.
Limited Non-Competition. For a period of eighteen (18) months after the director ceases to be a director of the Company, the director shall not become, directly or indirectly, an employee of, or provide consulting services for, or have any ownership interest in, any other business entity that manufactures or sells “Competitive Products.” As used herein, “Competitive Products” means: any product that the Company develops or acquires the right to sell from time to time during the term of the Agreement.
Term. Director’s term shall be until either (i) the director resigns as a director of the Company, (ii) the majority of the members of the Company’s board of directors vote to remove director as a director of the Company, or (iii) a majority of the shareholders of the Company vote to elect a board of directors consisting of directors other than the director.
Employment Agreements
William Rosellini
On December 7, 2018, we entered into an employment agreement with William Rosellini pursuant to which he will continue to serve as our Chief Executive Officer and Chairman for a period of two years (the “Term”). On the second and subsequent anniversary dates of the Term, the employment agreement will automatically be extended for an additional year unless, not later than 90 calendar days prior to such anniversary date, the Company shall notify Mr. Rosellini of its intent to not extend the Term. The annual base salary under the agreement is $1.00. Pursuant to the employment agreement, the Company may terminate Mr. Rosellini’s employment for Cause (as such term is defined in the employment agreement) at any time or without cause on thirty (30) days’ written notice. If Mr. Rosellini’s employment is terminated without Cause or in connection with a Change in Control (as defined in the employment agreement), Mr. Rosellini will be entitled to a severance payment equal to a scaling percentage of his highest base salary and targeted bonus or incentive compensation, if any. Mr. Rosellini will have the right to terminate his employment at any time with Good Reason (as defined in the employment agreement) upon ninety (90) days’ written notice. The employment agreement provides for customary non-solicitation, confidentiality and invention assignment clauses.
Pursuant to the Contribution Agreement between us and RS, any compensation paid by us for Mr. Rosellini’s services will be made directly to his wholly owned company, RS.
Brian Blischak
Effective December 1, 2016, we executed an employment agreement with Brian Blischak in his capacity of president and chief commercial officer. The term of the employment agreement was four (4) years, which could be automatically be extended for one additional year. Upon expiration of the term of the employment agreement, Mr. Blischak would remain an “at will” employee of the Company, but still be subject to and bound by the terms of the employment agreement. The employment agreement provided that Mr. Blischak would have a minimum annual base salary of $250,000. The base salary did not include any benefits made available to Mr. Blischak, or any contributions or payments made on his behalf pursuant to any employee benefit plan or program of the Company, including any health, disability, or life insurance plan or program, 401(k) plan, cash bonus plan, stock incentive plan, retirement plan, or similar plan or program of any nature. Mr. Blischak resigned as president and chief commercial officer on November 1, 2018.
Stock options. Upon execution of the employment agreement, Mr. Blischak was granted an initial grant of 82,144 non-transferable stock options, to purchase shares of the Company’s common stock, consisting of 35,715 incentive stock options (“ISO”) and 46,429 non-qualified stock options (“NQSO”). With respect to the ISO options, 7,143 ISO options vested on December 1, 2016, and additional lots of 7,143 ISO options each would vest on January 2, 2017, 2018, 2019, and 2020. With respect to the NQSO options, 2,715 NQSO options vested on December 1, 2016, and 1,215 NQSO options vested on January 1, 2017. An average of 1,214 options would vest on the first day of each month thereafter until all NQSO options are fully vested on December 1, 2019. The exercise price of all options is $14.00 per share, and the options shall expire eight years from the grant date. All options shall vest immediately upon a Termination without Cause, Change in Control, or Termination for Good Reason, as set forth above. Mr. Blischak’s resignation was deemed for Good Reason such that all of his options vested upon his resignation.
69
Christopher R. Miller
Effective December 1, 2016, we entered into an employment agreement with Christopher R. Miller. The term of the agreement is for three (3) years. The employment agreement shall automatically renew for an additional one-year term. The employment agreement provides that Mr. Miller will have an annual base salary of (i) $125,000 per year from December 1, 2016 through December 31, 2017; (ii) $150,000 per year from January 1, 2018 through December 31, 2018; and (iii) $175,000 per year from January 1, 2019 through December 31, 2019.
Benefit programs. Mr. Miller shall be eligible to participate in various company benefit programs, as they become available, pursuant to the terms of our applicable benefit plans and policies available to other similarly situated employees of the Company.
Bonus. In addition to the annual base salary described above, Mr. Miller shall also be eligible for an annual performance-based bonus of 20% of his annual base salary, to be earned by satisfactorily meeting criteria established by our chief executive officer and approved by the compensation committee of the board of directors prior to March 1 each year. Mr. Miller will receive the full 20% bonus amount if such criteria are satisfactorily met. In the event that Mr. Miller’s performance exceeds this standard, he may be considered for a bonus in an amount larger than 20%. In the event that his performance falls short of this standard, he may receive less than the full bonus percentage. A minimum of 70% of the annual bonus compensation shall be paid in cash, and the balance shall be paid in unrestricted common stock of the Company, or such other mutually agreeable consideration. During the term of the Contract, the yearly annual bonus shall be paid within sixty (60) days of the calendar year end. In the event the employment agreement is terminated by us, or should Mr. Miller terminate his employment under the Employment Contract, Mr. Miller will earn the base salary prorated to the date of termination. The prorated base salary will be based on a thirty-day (30) calendar month.
Stock options. Upon execution of the employment agreement, Mr. Miller was granted stock options to purchase 21,858 shares of the Company’s restricted common stock, pursuant to our 2016 Omnibus Incentive Plan (the “2016 Plan”). The option shares vest at an average rate of 607 shares per month for a period of thirty-six (36) months. The option shares’ exercise price is $14.00 per share. Any option shares that have not yet been vested as of the date of the end of the term of the employment agreement shall be subject to forfeiture.
Stock award. In January 2016, we issued to Mr. Miller 18,000 shares, of the Company’s restricted common stock, for prior and then ongoing consulting services. On the effective date of the employment agreement, 10,572 shares were vested, and the remaining 7,428 shares vest at an average rate of 571 shares per month for a period of thirteen (13) months.
Termination.
Death or disability. In the event that Mr. Miller’s employment terminates due to his death, the employment agreement provides that his estate shall receive severance benefits equivalent to thirty (30) days of Mr. Miller’s base salary. In the event that Mr. Miller’s employment terminates due to his Disability (as defined in the employment agreement), the employment agreement provides that he will receive severance benefits equivalent to ninety (90) days of Mr. Miller’s base salary.
70
Termination for Cause. In the event that Mr. Miller’s employment is terminated by us for Cause (as defined in the employment agreement), Mr. Miller shall be entitled to all accrued compensation, including vested stock options, up through the date of termination, but shall not be entitled to additional severance payments.
Termination without Cause. In the event that Mr. Miller’s employment is terminated by us without Cause, Mr. Miller will receive the base salary then in effect, prorated to the date of termination. In addition, Mr. Miller will receive a severance payment equivalent to ninety (90) days of the base salary.
Termination for Good Reason. In the event that Mr. Miller terminates his employment with us for Good Reason (as defined in the employment agreement), he will receive a severance payment equivalent to ninety (90) days of base salary.
Release of claims. As a condition to receiving any severance, Mr. Miller must execute a full general release satisfactory to us, releasing all claims, known or unknown, that Mr. Miller may have against the Company, arising out of or in any way related to his employment or termination of employment with Company prior to receipt of the severance package.
Daniel Powell
Effective June 26, 2017, Mr. Powell accepted our offer of employment as vice president of sales and marketing. The employment was defined as “at-will” with regard to the term. The offer provided that Mr. Powell would have an annual base salary of $175,000 per year. Daniel Powell’s employment with the Company was terminated on July 31, 2018.
Stock options. Upon acceptance of the offer of employment, Mr. Powell was granted stock options to purchase 15,715 shares of the Company’s restricted common stock, pursuant to the Company’s 2016 Plan. The option to purchase shares were to vest at an average rate of 437 shares per month for a period of thirty-six (36) months. The option shares’ exercise price is $17.50 per share. Effective on the date that Mr. Powell would cease to be an employee of the Company or one if its subsidiaries, all unvested options would expire and be of no further force of effect.
Outstanding Equity Awards at Fiscal Year End
As of December 31, 2017, we had outstanding equity awards to purchase 119,717 shares of common stock made to our executives.
The following table sets forth information regarding each unexercised option held by each of our named executive officers as of December 31, 2017:
| Name | Number of Securities Underlying Unexercised Options Exercisable | Number of Securities Underlying Unexercised Options Unexercisable | Option Exercise Price | Option Expiration Date | ||||||||||||
| William Rosellini | — | — | $ | — | — | |||||||||||
| Brian Blischak (2) | 82,144 | — | $ | 14.00 | 12/01/2027 | |||||||||||
| Christopher R. Miller | 21,858 | — | $ | 14.00 | 12/01/2022 | |||||||||||
| Daniel Powell (3) | 15,715 | — | $ | 17.50 | 07/01/2023 | |||||||||||
| (1) | All option grants reflected in the table above were granted under the Company’s 2016 Plan. |
| (2) | Mr. Blischak resigned as president and chief commercial officer of the Company on November 1, 2018. |
| (3) | Mr. Powell’s position as vice president of sales of marketing was terminated on July 31, 2018. |
71
Securities Authorized for Issuance Under Equity Compensation Plans
We may, from time to time, issue certain equity awards pursuant to our 2016 Plan. The 2016 Plan was adopted by our board of directors on January 2, 2016, and was subsequently approved by our shareholders on January 2, 2016. As of December 31, 2017, incentive stock options to purchase an aggregate of 169,647 shares of common stock, and non-qualified options to purchase an aggregate of 93,231 shares of the Company’s common stock, were outstanding under the 2016 Plan, all with exercise prices ranging from a split-adjusted $14.00 to $28.00 per share. A total of 25,333 vested immediately upon grant, and the remaining vest in varying amounts ranging from 7,143 annually to monthly increments ranging from 90 to 1,215, based on individual stock option agreements. The options have terms as follows: 121,803 options have a three-year term starting on each date of vesting; 58,931 options have a four-year term starting on each date of vesting; and 82,144 have an eight-year term starting on the date of vesting.
The 2016 Plan is administered by a committee of two or more non-employee directors designated by the board. The committee currently determines to whom awards are made, the timing of any such awards, the type of securities, and the number of shares covered by each award, as well as the terms, conditions, performance criteria, restrictions, and other provisions of awards. The committee has the authority to cancel or suspend awards, accelerate the vesting, or extend the exercise period of any awards made pursuant to the 2016 Plan.
Shares Available Under the 2016 Plan
The maximum shares available for issuance under the 2016 Plan are 357,154 and subject to adjustment as set forth in the 2016 Plan. Any shares subject to an award that expires, is cancelled or forfeited, or is settled for cash shall, to the extent of such cancelation, forfeiture, expiration, or cash settlement, again become available for awards under the 2016 Plan. The committee can issue awards comprised of restricted stock, stock options, stock appreciation rights, stock units, and other awards, as set forth in the 2016 Plan. The Company anticipates effecting an increase to the amount of shares available under the 2016 Plan in the future.
The following table sets forth, as of December 31, 2017, (A) the number of securities to be issued upon the exercise of outstanding options, warrants, and rights issued under our equity compensation plans, (B) the weighted-average exercise price of such options, warrants, and rights, and (C) the number of securities remaining available for future issuance under our equity compensation plans (excluding those securities set forth in Item (A)).
| (a) | (b) | (c) | ||||||||||
| Plan Category | Number of Securities to be Issued Upon Exercise of Outstanding Options, Warrants, and Rights | Weighted-Average Exercise Price of Outstanding Options, Warrants, and Rights | Number of Securities Remaining Available for Future Issuance Under Equity Compensation Plans (Excluding Securities Reflected in Column (a)) | |||||||||
| Equity compensation plans approved by security holders | 262,878 | $ | 15.07 | 94,276 | ||||||||
| Equity compensation plans not approved by security holders | — | — | — | |||||||||
| Total | 262,878 | $ | 15.07 | 94,276 | ||||||||
72
BENEFICIAL OWNERS AND MANAGEMENT
The following table sets forth, as of December 4, 2018, certain information regarding beneficial ownership of our capital stock according to the information supplied to us, that were beneficially owned by (i) each person known by the Company to be the beneficial owner of more than 5% of each class of the Company’s outstanding voting stock, (ii) each director, (iii) each named executive officer identified in the Summary Compensation Table, and (iv) all named executive officers and directors as a group.
Beneficial ownership is determined in accordance with the rules and regulations of the SEC. In general, a person is deemed to be the beneficial owner of (i) any shares of the Company’s common stock over that such person has sole or shared voting power or investment power, plus (ii) any shares that such person has the right to acquire beneficial ownership of within 60 days of the above date, whether through the exercise of options, warrants, or otherwise. Applicable percentages are based on 1,965,646 shares of common stock outstanding on December 4, 2018, adjusted as required by rules promulgated by the SEC.
Unless otherwise indicated, the address of each beneficial owner listed in the table below is c/o Nexeon MedSystems Inc, 1910 Pacific Avenue, Suite 20000, Dallas, Texas 75201.
| Shares
Beneficially Owned Prior to Offering | ||||||||
| Name of Beneficial Owner | Number | Percent | ||||||
| Beneficial shareholders (greater than 5%): | ||||||||
| Rosellini Scientific Holdings, LLC | 671,027 | (1) | 34.1 | % | ||||
| Rosellini Family Irrevocable Trust UA Dated 09/24/2018 | 367,785 | (2) | 18.7 | % | ||||
| Jamie Tunnel | 367,785 | (3) | 18.7 | % | ||||
| Directors and Executive Officers: | ||||||||
| William Rosellini | 718,036 | (4) | 35.8 | % | ||||
| Brian Blischak | 88,383 | (5) | 4.3 | % | ||||
| Kent J. George | 9,281 | (6) | * | |||||
| Michael Neitzel | 50,239 | (7) | 2.6 | % | ||||
| Christopher R. Miller | 37,701 | (8) | 1.9 | % | ||||
| Daniel Powell | 7,044 | (9) | * | |||||
| Wesley Dittmer II | 1,786 | (10) | * | |||||
| All directors and officers as a group (seven persons) | 912,470 | 42.8 | % | |||||
| * | Less than 1%. |
| (1) | RSH is located at 10210 N. Central Expressway, Suite 105, Dallas, Texas. William Rosellini is the sole member and manager of RSH, and, in such capacity, has voting and dispositive power over the securities held by such entity. |
| (2) | The address of the shareholder is 425 PMB 693 ST 1, Dorado PR 00646. Jamie Tunnel is the trustee of the trust and has voting and dispositive power over the shares. Jamie Tunnel is the trustee of the trust and has voting and dispositive power over the shares. |
| (3) | Represents shares held by Rosellini Family Irrevocable Trust UA Dated 09/24/2018. Jamie Tunnel is the trustee of the trust and has voting and dispositive power over the shares. The address of the shareholder is 425 PMB 693 ST 1, Dorado PR 00646. |
73
| (4) | Represents (i) 671,027 shares of common stock held by Rosellini Scientific Holdings, LLC (“RSH”), (ii) an incentive stock option grant to purchase up to 8,929 shares of the common stock (which represents the vested portion, including shares vesting within 60 days) of an incentive stock option to purchase up to 17,858 shares of common stock pursuant to the 2016 Plan, (iii) a non-qualified stock option grant to purchase up to 32,148 shares of common stock (which represents the vested portion, including shares vesting within 60 days) of a non-qualified stock option to purchase up to 64,286 shares of common stock, and (iv) 5,932 shares held by Mr. Rosellini’s wife. Mr. Rosellini is the sole member and manager of RSH, and, in such capacity, has voting and dispositive power over the securities held by such entity. |
| (5) | Represents (i) an incentive stock option to purchase up to 35,715 shares of the Company’s common stock (which represents the vested portion, including shares vesting within 60 days) of an incentive stock option to purchase up to 35,715 shares of the Company’s common stock pursuant to the 2016 Plan, (ii) non-qualified stock option grants to purchase up to 50,537 shares of the Company’s common stock (which represents the vested portion, including shares vesting within 60 days) of a non-qualified stock options to purchase up to 50,537 shares of the Company’s restricted Common Stock, and (iii) 2,131 shares of common stock held by Mr. Blischak. Mr. Blischak resigned as president and chief commercial officer of the Company on November 1, 2018. |
| (6) | Represents (i) 6,251 shares of common stock (which represents the vested portion, including shares vesting within 60 days) of options to purchase up to 6,251 shares of common stock issued pursuant to the 2016 Plan, (ii) 416 shares of common stock held by George Brothers Investment Partnership (“George Brothers”), (iii) Warrants to purchase up to 416 shares of common stock held by George Brothers, and (iv) 2,198 shares of common stock held by Paragon Investment Group (“Paragon”). Mr. George is the managing partner of George Brothers and the manager of Paragon, and, in such capacities, has voting and dispositive power over the securities held by such entities. |
| (7) | Represents (i) 6,251 shares of common stock (which represents the vested portion, including shares vesting within 60 days) of options to purchase up to 6,251 shares of common stock issued pursuant to the 2016 Plan, (ii) 41,787 shares of common stock held by Yorkville MGB Investments, LLC (“Yorkville”), and (iii) 2,201 shares of common stock held by Mr. Neitzel. Mr. Neitzel is the manager of Yorkville, and, in such capacity, has voting and dispositive power over the securities held by such entity. |
| (8) | Represents (i) a grant of 18,000 shares of common stock, (ii) an incentive stock option grant to purchase up to 15,788 shares of common stock (which represents the vested portion, including shares vesting within 60 days) of an option to purchase up to 21,858 shares of common stock issued pursuant to the 2016 Plan, (iii) non-qualified stock option grant to purchase up to 2,143 shares of common stock (which represents the vested portion, including shares vesting within 60 days) of a non-qualified stock option to purchase up to 2,143 shares of common stock, and (iv) 1,770 shares of common stock held by Mr. Miller. |
| (9) | Represents (i) an incentive stock option grants to purchase 6,461 shares of common stock (which represents the vested portion, including shares vesting within 60 days) of options to purchase up to 16,501 shares of common stock issued pursuant to the 2016 Plan and (ii) 583 shares of common stock held by Mr. Powell. Mr. Powell’s employment with the Company was terminated on July 31, 2018. |
| (10) | Represents 1,786 shares of common stock (including shares vesting within 60 days) of an option to purchase up to 1,786 shares of common stock. The Option will be issued and become exercisable upon an increase in the number of shares reserved under the 2016 Plan. |
74
CERTAIN RELATIONSHIPS AND RELATED PARTY TRANSACTIONS
The following reflects certain relationships and related transactions since January 1, 2016 to the date hereof. All share amounts and per share amounts give effect to the Reverse Stock Split.
On January 2, 2016, we entered into a contribution agreement with RS, controlled by our CEO, William Rosellini, and RS’s wholly owned subsidiary Belltower Associates, LLC (collectively, RS and Belltower Associates, LLC are hereinafter referred to as “RS”). Under this agreement, we issued 942,858 shares of our common stock, in return for, among other consideration:
| i. | RS’s agreement to an assignment (subject to regulatory transfer approval) to us of Phase II, should it be granted, of the federal NIH/SBIR awarded grant #1R44HL129870-01; |
| ii. | 1,675,000 shares of common stock of Nuviant Medical, Inc. (“Nuviant”); |
| iii. | 167 shares of common stock of MicroTransponder, Inc., a Delaware corporation; and |
| iv. | 175 shares of common stock of Emeritus Clinical Solutions, Inc., a Delaware corporation. |
These transactions were valued based on the value of the contributed assets, as our shares had no ascertainable value as of the date of issuance of the shares. This was in accordance with ASC 845 Non-Monetary Transactions, whereby the value of a non-monetary assets acquired in exchange for another non-monetary asset is the fair value of the asset surrendered or received, whichever is more clearly evident. In this case, the value of the contributed assets were more ascertainable than the value of the shares issued. The value of the consideration to acquire these shares was $272,686.
In accounting for the contributions of assets regarding the transactions with William Rosellini and RS, we recorded the assets received at fair value in accordance with ASC 845, Non-Monetary Transactions. Prior to the contributions, William Rosellini and RS were not related parties of the Company. They became related parties through the issuance of the 942,858shares and a controlling interest in the Company. For the Nuviant and MicroTransponder, Inc. common stock, the amount at which the assets were acquired from the related persons were based on the fair market value as determined by an appraisal report establishing a fair market value for each private company’s common stock of $0.10 and $416 per share respectively. The valuation reports are prepared by a qualified third party independent appraiser in accordance with the AICPA’s Statement on Standards for Valuations No. 1 and the AICPA’s “Practice Aid”, Valuation of Privately-Held Company Equity Securities Issued as Compensation. The amount at which the Emeritus Clinical Solutions, Inc. stock was acquired was based on the most-recent third-party transaction of $204.08 per share. Assets acquired by the related persons within the last two years include the Nuviant common stock. The cost of the 1,675,000 common shares of Nuviant to the related party and acquired from RS was $1,675, or approximately $.001 per share. The cost of the 175 shares of Emeritus Clinical Solutions, Inc. common stock to the related party and acquired from RS was $0.175 at par value $.001.
In October 2016, prior to acquisition by us, we paid $124,870 to NMB (formerly Rosellini Scientific Benelux, SPRL) for research and development services for our intravascular drug delivery system technology platform. NMB was a company controlled by our CEO, William Rosellini.
On October 28, 2016, Michael Rosellini entered into a contribution agreement with the Company to acquire 86,930 shares of common stock of the Company, and 86,930 warrants to acquire another 86,930 shares of common stock of the Company as follows: (i) 44,072 shares of common stock and 44,072 warrants to purchase 44,072 shares of common stock at a strike price of $28.00 per share, with a term of 36 months are held by Michael Rosellini individually. Of such holdings, 44,072 shares of common stock and the 44,072 warrants were purchased from us pursuant to our private placement, which closed on December 2, 2016. As of December 6, 2018, 7,493 warrants have been exercised and 36,579 have been cancelled. (ii) 42,858 shares of common stock and 42,858 warrants to purchase 42,858 shares of common stock at a strike price of $28.00 per share with a term of 36 months are held by the IRA Resources, FBO Randy Michael Rosellini, ROTH IRA. Michael Rosellini has the sole power to vote and dispose of the shares held by the Roth IRA. The shares of common stock and the warrants were purchased from us pursuant to our private placement, which closed on December 2, 2016. As of December 6, 2018, 7,286 warrants have been exercised and 35,572 have been cancelled. Michael Rosellini is the father of William Rosellini, our chief executive officer.
Effective December 1, 2016, Brian Blischak, our former president and chief commercial officer, pursuant to the 2016 Plan, was granted an initial grant of 82,144 non-transferable stock options to purchase shares of the Company’s common stock, consisting of 35,715 ISOs and 46,429 NQSOs. With respect to the ISO options, 7,143 ISO options vested on the effective date, and 7,143 ISO options vested on January 1, 2017. Additional lots of 7,143 ISO options each shall vest on January 1, 2018, 2019 and 2020. With respect to the NQSO options, 2,715 NQSO options vested on the effective date, and 1,215 NQSO options vested on January 1, 2017. An average of 1,214 options shall vest on the first day of each month thereafter until all NQSO options are fully vested on December 1, 2019. The exercise price of all options is $14.00 per share, and the options shall expire eight years from the grant date. The fair value of the options was determined to be $365,342 using the Black-Scholes Option Pricing Model.
75
On December 15, 2016, pursuant to the terms of the License Agreement, William Rosellini sold, assigned, and transferred any and all of his right, title, and interest in and to the License owned by him related to the Siemens Patents to us pursuant to the Purchase Agreement filed as Exhibit 10.1 to our Current Report on Form 8-K filed with the SEC on December 20, 2016. As consideration for the transfer of the Siemens Patents and the License related thereto, we paid to Mr. Rosellini the sum of $140,000 in cash, and will issue to Mr. Rosellini 217,858 shares of the Company’s restricted common stock valued at $3,050,000. Mr. Rosellini, the CEO of the Company, is the sole member and manager of RS.
During the year ended December 31, 2016, RS, the largest shareholder in the Company, loaned $168,891 to us. The loan was non-interest bearing, with no set terms of repayment. As of December 31, 2016, the loan was repaid in full in cash.
On January 1, 2017, our board of directors appointed Emily Hamilton, MD to serve as the director of emerging therapy for NXPROC. Dr. Hamilton is the wife of our CEO, William Rosellini. Dr. Hamilton beneficially owns 5,932 shares of common stock of the Company, pursuant to our August 21, 2017 offer to Company employees, the opportunity to purchase shares of the Company’s restricted common stock for a discount through payroll deductions. These shares were valued at $51,882.
On May 19, 2017, NMB entered into a waiver of debt agreement to waive the outstanding loan balance and accrued interest outstanding pursuant to the September 21, 2015, loan agreement between NMB and Nuviant Medical, GmbH, an entity related to RS. The agreement waives the outstanding balance of the loan and accrued interest in the amount $171,946, and thereby waives any right or action in respect to this debt. An expense had been recorded as bad debt on the statement of comprehensive income in the amount $174,252. During the year ended December 31, 2017, and prior to the waiver of debt agreement, NMB loaned Nuviant Medical, GmbH $59,027.
On June 23, 2017, RS transferred 5,789 shares of restricted common stock to NMB. The shares were valued at $107,292. The loan is non-interest bearing, and will be re-paid to RS in restricted common stock issued by us through an intercompany loan with NMB. The 5,789 shares were exchanged for outstanding payables to vendors of NMB in the amount of $107,292. RS is also due $18,595 from a non-interest bearing loan to NMB in 2016. The total amount due to RS from these transactions is $125,887, and is recorded as due to related party on the balance sheet as of December 31, 2017
RS is a company wholly owned by Mr. Rosellini, which acquires interests in other companies such as Nexeon in exchange for RS assets. RS will not acquire any such properties in the future that are not first offered to us and voted on by its board of directors, with Mr. Rosellini abstaining.
RS functions as the personal holding company of Mr. Rosellini, who currently beneficially owns 34.1% of the Company’s common stock. In addition, RS has been the source of private funding, as well as federal and state grants, all of which benefit us. During the year ended December 31, 2016, RS, the largest shareholder in the Company, loaned $168,891 to us. The loan was non-interest bearing, with no set terms of repayment. As of December 31, 2016, the loan was repaid in full in cash and we currently do not have any loans outstanding with RS or Mr. Rosellini. Mr. Rosellini’s workweek averages 60 to 70 hours, and approximately 10% of this time (or 6 to 7 hours a week) are committed to the business of RS. Regardless, Mr. Rosellini is devoting, at a minimum, in excess of 40 hours a week to us. Mr. Rosellini is fully aware of his fiduciary responsibilities and to the principles of the Corporate Opportunity Doctrine as they relate to the Company. There can be no assurance that a material conflict of interest will not occur in the future. In the event a potential conflict should occur, it will be fully disclosed to our board of directors for a determination by the board as to the relevance and/or solution in order to avoid such potential conflict. As of the date of this filing, Mr. Rosellini, to the best of his knowledge and belief, is unaware of any material conflicts.
76
Director Independence
Although we are not listed on a national securities exchange, in determining whether the members of our board and its committees are independent, we have elected to use the definition of “independence” set forth by the Nasdaq Capital Market, and the standards for independence established by the Nasdaq Capital Market.
The director independence rules of the Nasdaq Capital Market require listed companies to have an audit committee of at least three members, each of whom (in addition to satisfying other conditions) is an independent director. Our audit committee is currently comprised of three independent directors, and would therefore meet this requirement.
The director independence rules of the Nasdaq Capital Market require that the compensation of the chief executive officer and other officers of a listed company be determined, or recommended to the board for determination, either by a compensation committee comprised of independent directors or by a majority of the independent directors on its board of directors. The compensation committee is currently comprised of three independent directors.
The director independence rules of the Nasdaq Capital Market require that board of director nominations must be either selected, or recommended for the board’s selection, by either a nominating committee comprised solely of independent directors or by a majority of the independent directors. Our nominating & governance committee is comprised of three independent directors, and we therefore believe we meet this requirement.
The following description summarizes the material terms and provisions of our capital stock. All share amounts and per share amounts have been adjusted to reflect the Reverse Stock Split.
General
Our authorized capital stock consists of 75,000,000 shares of common stock with a par value of $0.001. As of December 4, 2018, there were 1,965,646 shares of common stock outstanding. We have not authorized any shares of preferred stock.
Common Stock
Each share of our common stock is entitled to one vote at all meetings of our shareholders. Our shareholders are not permitted to cumulate votes in the election of directors. All shares of our common stock are equal with respect to liquidation rights and dividend rights. In the event of our liquidation, dissolution, or winding up, holders of our common stock will be entitled to receive, on a pro-rata basis, all our assets remaining. Except as otherwise required by Nevada law, holders of shares of our common stock do not have cumulative voting rights, which means that the holders of more than 50% of the outstanding shares, voting for the election of directors, can elect all the directors to be elected, if they so choose, and, in such event, the holders of the remaining shares will not be able to elect any of our directors.
Outstanding Warrants
As of December 4, 2018, we had outstanding warrants to purchase 82,926 shares of the Company’s common stock, all of which are exercisable, with a weighted average exercise price of $19.39 per share.
Warrants to be Offered
The following summary of certain terms and provisions of the warrants offered hereby is not complete, and is subject to, and qualified in its entirety by, the provisions of the form of the warrant, which is filed as an exhibit to the registration statement of which this prospectus is a part. Prospective investors should carefully review the terms and provisions set forth in the form of warrant.
77
Exercisability. The warrants are exercisable at any time up to the date that is five years from the closing of this offering. The warrants will be exercisable, at the option of each holder, in whole or in part, by delivering to us a duly executed exercise notice accompanied by payment in full for the number of shares of our common stock purchased upon such exercise (except in the case of a cashless exercise, as discussed below). Unless otherwise specified in the warrant, the holder will not have the right to exercise any portion of the Warrant if the holder (together with its affiliates) would beneficially own in excess of 4.99% of the number of shares of our common stock outstanding immediately after giving effect to the exercise, as such percentage ownership is determined in accordance with the terms of the warrants.
Cashless exercise. In the event that a registration statement covering shares of common stock underlying the warrants, or an exemption from registration, is not available for the resale of such shares of common stock underlying the warrants, the holder may, in its sole discretion, exercise the warrant in whole or in part, and, in lieu of making the cash payment otherwise contemplated to be made to us upon such exercise in payment of the aggregate exercise price, elect instead to receive upon such exercise the net number of shares of common stock determined according to the formula set forth in the warrant. In no event shall we be required to make any cash payments or net cash settlement to the registered holder in lieu of issuance of common stock underlying the warrants.
Exercise price. The initial exercise price per share of common stock purchasable upon exercise of the warrants is $ per share ( % of the public offering price of one Unit). The exercise price is subject to appropriate adjustment in the event of certain stock dividends and distributions, stock splits, stock combinations, reclassifications, or similar events affecting our common stock, and also upon any distributions of assets, including cash, stock, or other property, to our stockholders.
Transferability. Subject to applicable laws, the warrants may be transferred at the option of the holders upon surrender of the Warrants to us, together with the appropriate instruments of transfer.
Fundamental transaction. If, at any time while the warrants are outstanding, (1) we consolidate or merge with or into another corporation, and we are not the surviving corporation; (2) we sell, lease, license, assign, transfer, convey, or otherwise dispose of all or substantially all of our assets; (3) any purchase offer, tender offer, or exchange offer (whether by us or another individual or entity) is completed pursuant to which holders of our shares of common stock are permitted to sell, tender, or exchange their shares of common stock for other securities, cash, or property, and has been accepted by the holders of 50% or more of our outstanding shares of common stock; (4) we effect any reclassification or recapitalization of our shares of common stock, or any compulsory share exchange pursuant to which our shares of common stock are converted into or exchanged for other securities, cash, or property; or (5) we consummate a stock or share purchase agreement or other business combination with another person or entity whereby such other person or entity acquires more than 50% of our outstanding shares of common stock, each (a “Fundamental Transaction”), then, upon any subsequent exercise of the warrants, the holders thereof will have the right to receive the same amount and kind of securities, cash, or property as it would have been entitled to receive upon the occurrence of such Fundamental Transaction if it had been, immediately prior to such Fundamental Transaction, the holder of the number of warrant shares then issuable upon exercise of the warrant, and any additional consideration payable as part of the Fundamental Transaction.
In addition, in the event of a Fundamental Transaction (subject to certain exceptions), we or any successor entity shall, at the holder ’s option, purchase the holder’s warrants for an amount of cash equal to the value of the warrants as determined in accordance with the Black Scholes option pricing model.
Rights as a stockholder. Except as otherwise provided in the warrants or by virtue of such holder’s ownership of shares of our common stock, the holder of a warrant will not have the rights or privileges of a holder of our common stock, including any voting rights, until the holder exercises the warrant.
Governing Law; Exclusive Jurisdiction. The warrants are governed by, and construed in accordance with, the internal laws of the State of New York, without reference to the choice of law provisions thereof. The Company and (by acceptance) the holder each irrevocably submit to the exclusive jurisdiction of the courts of the State of New York located in New York County and the United States District Court for the Southern District of New York for purpose of any suit, action, proceeding or judgment relating to or arising out of the warrant or the transactions contemplated thereby.
78
Underwriter’s Warrants
Please see “Underwriting—Underwriter’s Warrants” for a description of the warrants we have agreed to issue to the representative of the underwriters in this offering, subject to the completion of the offering. The form of Underwriter’s warrants is included as an exhibit to the underwriting agreement.
Stock Options Under Equity Plans
As of December 4, 2018, there were 450,000 shares of common stock reserved for issuance under our stock option and equity plans. Of this number, approximately 345,736 shares are reserved for issuance upon exercise of outstanding options that were previously granted under our equity plans.
Anti-Takeover Provisions
In the future, we may become subject to Nevada’s control share law. A corporation is subject to Nevada’s control share law if it has more than 200 shareholders, at least 100 of whom are shareholders of record and residents of Nevada, conduct business in Nevada, or do so through an affiliated corporation. The law focuses on the acquisition of a “controlling interest,” which means the ownership of outstanding voting shares sufficient, but for the control share law, to enable the acquiring person to exercise the following proportions of the voting power of the corporation in the election of directors: (i) one-fifth or more, but less than one-third, (ii) one-third or more, but less than a majority, or (iii) a majority or more. The ability to exercise such voting power may be direct or indirect, as well as individual or in association with others.
The effect of the control share law is that the acquiring entity, and those acting in association with it, obtains only such voting rights in the control shares as are conferred by a resolution of the shareholders of the corporation, approved at a special or annual meeting of shareholders. The control share law contemplates that voting rights will be considered only once by the other shareholders. Thus, there is no authority to strip voting rights from the control shares of an acquiring entity once those rights have been approved. If the shareholders do not grant voting rights to the control shares acquired by an acquiring entity, then those shares do not become permanent non-voting shares. The acquiring entity is free to sell its shares to others.
If the buyers of those shares themselves do not acquire a controlling interest, then the control share law does not govern their shares. If control shares are accorded full voting rights and the acquiring entity has acquired control shares with a majority or more of the voting power, then any shareholders of record (other than an acquiring entity) who has not voted in favor of approval of voting rights is entitled to demand fair value for such shareholder’s shares. Nevada’s control share law may have the effect of discouraging takeovers of the Company.
In addition to the control share law, Nevada has a business combination law that prohibits certain business combinations between Nevada corporations and “interested shareholders” for three years after the “interested shareholders” first become “interested shareholders,” unless the corporation’s board of directors approves the combination in advance.
For purposes of Nevada law, an “interested shareholders” is any person who is: (i) the beneficial owner, directly or indirectly, of 10% or more of the voting power of the outstanding voting shares of the corporation, or (ii) an affiliate or associate of the corporation, and at any time within the three previous years was the beneficial owner, directly or indirectly, of 10% or more of the voting power of the then-outstanding shares of the corporation. The definition of the term “business combination” is sufficiently broad to cover virtually any kind of transaction that would allow a potential acquirer to use the corporation’s assets to finance the acquisition, or otherwise to benefit its own interests rather than the interests of the corporation and its other shareholders.
The effect of Nevada’s business combination law is to discourage parties potentially interested in taking control of our Company from doing so if it cannot obtain the approval of our board of directors.
79
Listing
In connection with this offering, we have applied to have our common stock listed on the Nasdaq Capital Market under the symbol “NXMD”. We intend to apply to have the warrants listed on the Nasdaq Capital Market under the symbol “NXMDW.” No assurance can be given that such listing will be approved or that a trading market will develop for the warrants. Without an active trading market, the liquidity of the warrants will be limited.
Transfer Agent and Registrar
The transfer agent and registrar for our common stock is Equity Stock Transfer, 237 W 37th St. Suite 602, New York, NY 10018; telephone number is (212) 575-5757. Equity Stock Transfer will serve as registrar for the warrants offered hereunder.
ThinkEquity, a division of Fordham Financial Management, Inc. is acting as representative of the underwriters of the offering. We have entered into an underwriting agreement dated , 2018 with the representative. Subject to the terms and conditions of the underwriting agreement, we have agreed to sell to each underwriter named below, and each underwriter named below has severally agreed to purchase, at the public offering price less the underwriting discounts set forth on the cover page of this prospectus, the number of units listed next to its name in the following table:
| Underwriter | Number of Units | |||
| ThinkEquity, a division of Fordham Financial Management, Inc. | ||||
| Dougherty & Company LLC | ||||
| Total | ||||
The underwriters are committed to purchase all the units offered by us, other than those covered by the over-allotment option to purchase additional shares of common stock and/or warrants described below, if they purchase any units. The obligations of the underwriters may be terminated upon the occurrence of certain events specified in the underwriting agreement. Furthermore, pursuant to the underwriting agreement, the underwriters’ obligations are subject to customary conditions, representations and warranties contained in the underwriting agreement, such as receipt by the underwriters of officers’ certificates and legal opinions.
We have agreed to indemnify the underwriters against specified liabilities, including liabilities under the Securities Act, and to contribute to payments the underwriters may be required to make in respect thereof.
The underwriters are offering the units, shares of common stock and warrants subject to prior sale, when, as and if issued to and accepted by them, subject to approval of legal matters by their counsel and other conditions specified in the underwriting agreement. The underwriters reserve the right to withdraw, cancel or modify offers to the public and to reject orders in whole or in part.
We have granted the underwriters an over-allotment option. This option, which is exercisable for up to 45 days after the date of this prospectus, permits the underwriters to purchase up to an aggregate of additional shares of common stock and/or warrants to purchase up to additional shares of common stock (equal to 15% of the common stock and warrants included in the units sold in the offering) in any combination thereof, at the public offering price per share and per warrant, respectively, less underwriting discounts and commissions, solely to cover over-allotments, if any. The purchase price to be paid per additional share of common stock shall be $ and the purchase price to be paid per additional warrant shall be $ . If this option is exercised in full, the total price to the public will be $ and the total net proceeds, before expenses, to us will be $ .
Discounts, Commissions and Reimbursement
The following table shows the public offering price, underwriting discount and proceeds, before expenses, to us. The information assumes either no exercise or full exercise by the underwriters of their over-allotment option.
| Per Unit | Total
with no Over-Allotment |
Total
with Over-Allotment |
||||||||||
| Public offering price | $ | $ | $ | |||||||||
| Underwriting discount (7%) | $ | $ | $ | |||||||||
| Non-accountable expense allowance (1%)(1) | $ | $ | $ | |||||||||
| Proceeds, before expenses, to us | $ | $ | $ | |||||||||
(1) We have agreed to pay a non-accountable expense allowance to the representative equal to 1.0% of the gross proceeds received in this offering (excluding any proceeds received from exercise of the underwriters’ over-allotment option).
80
We will also pay to the representative a commission of 6% of the gross proceeds we receive from any cash exercise of the warrants.
The underwriters propose to offer the units to the public at the public offering price set forth on the cover of this prospectus. In addition, the underwriters may offer some of the units to other securities dealers at such price less a concession not in excess of $ per unit. If all of the units offered by us are not sold at the public offering price, the representative may change the offering price and other selling terms by means of a supplement to this prospectus.
We have also agreed to pay certain expenses of the representative relating to the offering, including: (a) all filing fees and communication expenses associated with the review of this offering by the Financial Industry Regulatory Authority, Inc. (“FINRA”); (b) fees, expenses and disbursements relating to background checks of our officers and directors, in an amount not to exceed $15,000; (c) fees, expenses and disbursements relating to the registration, qualification or exemption of securities offered under the securities laws of such states and foreign jurisdictions designated by the representative; (d) fees and expenses of the representative’s legal counsel not to exceed $60,000; (e) the costs associated with bound volumes of the public offering materials as well as commemorative mementos and lucite tombstones; (f) $29,500 for fees and expenses for the underwriters’ use of book-building, prospectus tracking and compliance software for this offering; and (g) up to $20,000 of the representative’s actual accountable road show expenses for the offering, provided, that the total reimbursement the Company will owe to the underwriters under (d), (e), (f) and (g) will not exceed $95,000.
Pursuant to a letter agreement with National Securities Corporation (“National Securities”), dated November 9, 2018, we will also pay to National Securities, upon the closing of this offering, $100,000 as a financial advisory fee. Pursuant to such agreement, we have also paid to National Securities $25,000 and have agreed to pay to National Securities an additional $40,000 in satisfaction of our obligations under our letter agreement with National Securities dated April 27, 2018.
We estimate that the total expenses of the offering payable by us, excluding the total underwriting discount, will be approximately $ .
Underwriter Warrants
We have also agreed to issue to the representative or its designees, at the closing of this offering, warrants (the “Underwriter’s Warrants”) to purchase that number of our shares of common stock equal to six percent (6%) of the aggregate number of shares of common stock sold in the offering. The Underwriter’s Warrants will be exercisable at any time and from time to time, in whole or in part, during a period commencing six months from the effective date of this prospectus, and will expire five years after the effective date of this prospectus. The Underwriter’s Warrants will be exercisable at a price equal to 120% of the public offering price of the units in this offering. The Underwriter’s Warrants and the shares of common stock underlying the Underwriter’s Warrants have been deemed compensation by FINRA and are, therefore, subject to a 180-day lock-up pursuant to Rule 5110(g)(1) of FINRA. The underwriter or its permitted assignees under this Rule 5110(g)(1) shall not sell, transfer, assign, pledge or hypothecate the Underwriter’s Warrants or the shares of common stock underlying the Underwriter’s Warrants, nor engage in any hedging, short sale, derivative, put or call transaction that would result in the effective economic disposition of the Underwriter’s Warrants or the shares of common stock underlying the Underwriter’s Warrants, for a period of 180 days from the effective date of the registration statement, except that they may be assigned, in whole or in part, as specifically set forth in the underwriting agreement. The Underwriter’s Warrants will provide for cashless exercise and customary anti-dilution provisions (for share dividends, splits and recapitalizations and the like) consistent with FINRA Rule 5110.
Discretionary Accounts
The underwriters do not intend to confirm sales of the securities offered hereby to any accounts over which they have discretionary authority.
81
Lock-Up Agreements
Pursuant to “lock-up” agreements, we and our executive officers, directors and 5% or greater holders of outstanding common stock have agreed, subject to limited exceptions, without the prior written consent of the underwriters’ representative not to directly or indirectly offer to sell, sell, pledge or otherwise transfer or dispose of any of shares of (or enter into any transaction or device that is designed to, or could be expected to, result in the transfer or disposition by any person at any time in the future of) our common stock, enter into any swap or other derivatives transaction that transfers to another, in whole or in part, any of the economic benefits or risks of ownership of shares of our common stock, make any demand for or exercise any right or cause to be filed a registration statement, including any amendments thereto, with respect to the registration of any shares of common stock or securities convertible into or exercisable or exchangeable for common stock or any of our other securities or publicly disclose the intention to do any of the foregoing, subject to customary exceptions, for a period of 6 months from the date of this prospectus, in the case of our directors and officers, and for a period of 3 months from the date of this prospectus, in the case of us and any 5% or greater holder of outstanding common stock.
Right of First Refusal
We have granted the representative a right of first refusal, for a period of fifteen months from the consummation of this offering, to act as sole investment banker, book-runner and/or placement agent, at the representative’s sole discretion, for each and every future public and private equity offering, including all equity linked financings (each, a “Subject Transaction”), during such fifteen month period, of the Company, or any successor to or subsidiary of the Company, on terms and conditions customary to the representative for such Subject Transactions.
Electronic Offer, Sale and Distribution of Securities
A prospectus in electronic format may be made available on the websites maintained by one or more of the underwriters or selling group members. The representative may agree to allocate a number of securities to underwriters and selling group members for sale to its online brokerage account holders. Internet distributions will be allocated by the underwriters and selling group members that will make internet distributions on the same basis as other allocations. Other than the prospectus in electronic format, the information on these websites is not part of, nor incorporated by reference into, this prospectus or the registration statement of which this prospectus forms a part, has not been approved or endorsed by us, and should not be relied upon by investors.
Listing
Our common stock is presently quoted on the OTCQB tier of the OTCQB under the symbol “NXNN”. In connection with this offering we have applied to have our common stock listed on the Nasdaq Capital Market under the symbol “NXMD” and intend to apply to have the warrants listed on the Nasdaq Capital Market under the symbol “NXMDW.” No assurance can be given that such listing will be approved or that a trading market will develop for the warrants.
Stabilization
In connection with this offering, the underwriters may engage in stabilizing transactions, over-allotment transactions, syndicate-covering transactions, penalty bids and purchases to cover positions created by short sales.
Stabilizing transactions permit bids to purchase shares so long as the stabilizing bids do not exceed a specified maximum, and are engaged in for the purpose of preventing or retarding a decline in the market price of the shares while the offering is in progress.
Over-allotment transactions involve sales by the underwriters of shares in excess of the number of shares the underwriters are obligated to purchase. This creates a syndicate short position which may be either a covered short position or a naked short position. In a covered short position, the number of shares over-allotted by the underwriters is not greater than the number of shares that they may purchase in the over-allotment option. In a naked short position, the number of shares involved is greater than the number of shares in the over-allotment option. The underwriters may close out any short position by exercising their over-allotment option and/or purchasing shares in the open market.
82
Syndicate covering transactions involve purchases of shares in the open market after the distribution has been completed in order to cover syndicate short positions. In determining the source of shares to close out the short position, the underwriters will consider, among other things, the price of shares available for purchase in the open market as compared with the price at which they may purchase shares through exercise of the over-allotment option. If the underwriters sell more shares than could be covered by exercise of the over-allotment option and, therefore, have a naked short position, the position can be closed out only by buying shares in the open market. A naked short position is more likely to be created if the underwriters are concerned that after pricing there could be downward pressure on the price of the shares in the open market that could adversely affect investors who purchase in the offering.
Penalty bids permit the representative to reclaim a selling concession from a syndicate member when the shares originally sold by that syndicate member are purchased in stabilizing or syndicate covering transactions to cover syndicate short positions.
These stabilizing transactions, syndicate covering transactions and penalty bids may have the effect of raising or maintaining the market price of our shares of common stock or preventing or retarding a decline in the market price of our shares of common stock. As a result, the price of our common stock in the open market may be higher than it would otherwise be in the absence of these transactions. Neither we nor the underwriters make any representation or prediction as to the effect that the transactions described above may have on the price of our common stock. These transactions may be effected in the over-the-counter market or otherwise and, if commenced, may be discontinued at any time.
Passive market making
In connection with this offering, underwriters and selling group members may engage in passive market making transactions in our common stock on the Nasdaq Capital Market in accordance with Rule 103 of Regulation M under the Exchange Act, during a period before the commencement of offers or sales of the shares and extending through the completion of the distribution. A passive market maker must display its bid at a price not in excess of the highest independent bid of that security. However, if all independent bids are lowered below the passive market maker’s bid, then that bid must then be lowered when specified purchase limits are exceeded.
Other Relationships
Certain of the underwriters and their affiliates may in the future provide various investment banking, commercial banking and other financial services for us and our affiliates for which they may in the future receive customary fees.
Offer restrictions outside the United States
Other than in the United States, no action has been taken by us or the underwriters that would permit a public offering of the securities offered by this prospectus in any jurisdiction where action for that purpose is required. The securities offered by this prospectus may not be offered or sold, directly or indirectly, nor may this prospectus or any other offering material or advertisements in connection with the offer and sale of any such securities be distributed or published in any jurisdiction, except under circumstances that will result in compliance with the applicable rules and regulations of that jurisdiction. Persons into whose possession this prospectus comes are advised to inform themselves about and to observe any restrictions relating to the offering and the distribution of this prospectus. This prospectus does not constitute an offer to sell or a solicitation of an offer to buy any securities offered by this prospectus in any jurisdiction in which such an offer or a solicitation is unlawful.
Canada
The securities may be sold in Canada only to purchasers purchasing, or deemed to be purchasing, as principal that are accredited investors, as defined in National Instrument 45-106 Prospectus Exemptions or subsection 73.3(1) of the Securities Act (Ontario), and are permitted clients, as defined in National Instrument 31-103 Registration Requirements, Exemptions and Ongoing Registrant Obligations. Any resale of the securities must be made in accordance with an exemption from, or in a transaction not subject to, the prospectus requirements of applicable securities laws. Securities legislation in certain provinces or territories of Canada may provide a purchaser with remedies for rescission or damages if this prospectus (including any amendment thereto) contains a misrepresentation, provided that the remedies for rescission or damages are exercised by the purchaser within the time limit prescribed by the securities legislation of the purchaser’s province or territory. The purchaser should refer to any applicable provisions of the securities legislation of the purchaser’s province or territory for particulars of these rights or consult with a legal advisor. Pursuant to section 3A.3 of National Instrument 33-105 Underwriting Conflicts (NI 33-105), the underwriters are not required to comply with the disclosure requirements of NI 33-105 regarding underwriter conflicts of interest in connection with this offering.
83
The validity of the securities being offered by this prospectus has been passed upon for us by Sichenzia Ross Ference LLP, New York, New York. Sheppard, Mullin, Richter & Hampton LLP, New York, New York, has acted as counsel for the underwriters in connection with certain legal matters related to this offering.
The financial statements as of and for the fiscal years ended December 31, 2017 and 2016 appearing in this prospectus and registration statement have been audited by Paritz & Company, P.A., an independent registered public accounting firm, as stated in their report, appearing elsewhere herein, and are included in reliance upon such report, and upon the authority of such firm as experts in accounting and auditing.
WHERE YOU CAN FIND MORE INFORMATION
Federal securities laws require us to file information with the SEC concerning our business and operations. Accordingly, we file annual, quarterly, and special reports, and other information with the SEC.
The SEC maintains a web site (http://www.sec.gov) at which you can read or download our reports and other information.
We have filed with the SEC a registration statement on Form S-1 under the Securities Act with respect to the securities being offered hereby. As permitted by the rules and regulations of the SEC, this prospectus does not contain all the information set forth in the registration statement and the exhibits and schedules thereto. For further information with respect to the Company and the securities offered hereby, reference is made to the registration statement, and such exhibits and schedules, which may be accessed at the SEC’s web site.
84
INDEX TO CONSOLIDATED FINANCIAL STATEMENTS
F-1
REPORT OF INDEPENDENT REGISTERED PUBLIC ACCOUNTING FIRM
To the Board of Directors and Stockholders of
Nexeon Medsystems, Inc.
Opinion on the Consolidated Financial Statements
We have audited the accompanying consolidated balance sheets of Nexeon Medsystems, Inc. (the Company) as of December 31, 2017 and 2016, and the related consolidated statements of comprehensive income, stockholders’ equity, and cash flows for each of the two years in the period ended December 31, 2017, and the related notes (collectively referred to as the financial statements). In our opinion, the consolidated financial statements present fairly, in all material respects, the financial position of the Company as of December 31, 2017 and 2016, and the results of its operations and its cash flows for each of the two years in the period ended December 31, 2017, in conformity with accounting principles generally accepted in the United States of America.
Basis for Opinion
These consolidated financial statements are the responsibility of the Company’s management. Our responsibility is to express an opinion on the Company’s consolidated financial statements based on our audits. We are a public accounting firm registered with the Public Company Accounting Oversight Board (United States) (PCAOB) and are required to be independent with respect to the Company in accordance with the U.S. federal securities laws and the applicable rules and regulations of the Securities and Exchange Commission and the PCAOB.
We conducted our audits in accordance with the standards of the PCAOB. Those standards require that we plan and perform the audit to obtain reasonable assurance about whether the consolidated financial statements are free of material misstatement, whether due to error or fraud. The Company is not required to have, nor were we engaged to perform, an audit of its internal control over financial reporting. As part of our audits, we are required to obtain an understanding of internal control over financial reporting, but not for the purpose of expressing an opinion on the effectiveness of the Company’s internal control over financial reporting. Accordingly, we express no such opinion.
Our audits included performing procedures to assess the risks of material misstatement of the consolidated financial statements, whether due to error or fraud, and performing procedures that respond to those risks. Such procedures included examining, on a test basis, evidence regarding the amounts and disclosures in the consolidated financial statements. Our audits also included evaluating the accounting principles used and significant estimates made by management, as well as evaluating the overall presentation of the consolidated financial statements. We believe that our audits provide a reasonable basis for our opinion.
The accompanying consolidated financial statements have been prepared assuming that the Company will continue as a going concern. As discussed in Note 4 to the consolidated financial statements, The Company has sustained operating losses since inception and has an accumulated deficit of $3,743,438 at December 31, 2017. In addition, the Company does not have sufficient continuing revenue to cover its future operating expenses. The Company currently has limited liquidity and has not completed its efforts to establish an additional source of revenues sufficient to cover operating costs of the on-going neurostimulation research and development activities over an extended period of time. These factors, among others, raise substantial doubt regarding the Company’s ability to continue as a going concern. Management’s plans in regard to these matters are also described in Note 4 to the accompanying consolidated financial statements. The accompanying consolidated financial statements do not include any adjustments that might result from the outcome of this uncertainty.
| /s/ Paritz & Company, P.A. | |
| We have served as the Company’s auditor since 2016. | |
| Hackensack, New Jersey | |
| April 5, 2018 | |
F-2
AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
| December 31, 2017 | December 31, 2016 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 883,962 | $ | 2,124,795 | ||||
| Accounts receivable | 1,877,743 | 48,842 | ||||||
| Grants receivable | 804,152 | 69,391 | ||||||
| Inventory | 2,206,570 | — | ||||||
| Other current assets | 157,621 | 132,453 | ||||||
| Notes receivable – related party | — | 106,062 | ||||||
| Total Current Assets | $ | 5,930,048 | $ | 2,481,543 | ||||
| Property, plant and equipment, net | 3,569,832 | 69,354 | ||||||
| Investments | 112,072 | 148,860 | ||||||
| Intangible assets, net | 10,739,492 | 9,712,090 | ||||||
| Total Assets | $ | 20,351,444 | $ | 12,411,847 | ||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current Liabilities | ||||||||
| Accounts payable | 2,575,399 | 277,649 | ||||||
| Accrued liabilities | 503,751 | 113,019 | ||||||
| Current portion of long-term debt, net of original discount | 866,479 | 51,284 | ||||||
| Advance grant payments | 935,817 | 400,669 | ||||||
| Deferred liabilities | 174,230 | 12,401 | ||||||
| Due to related party | — | 81,008 | ||||||
| Accrued interest | 78,049 | — | ||||||
| Accrued interest payable – stockholders | — | 2,193 | ||||||
| Total Current Liabilities | 5,133,725 | 938,223 | ||||||
| Long-term Debt, net of original discount | 3,348,730 | 141,419 | ||||||
| Notes payable – stockholders | — | 10,000 | ||||||
| Total Liabilities | $ | 8,482,455 | $ | 1,089,642 | ||||
| Stockholders' Equity | ||||||||
| Common Stock - 75,000,000 shares authorized, $.001 par value; 27,591,441 and 21,711,953 issued and outstanding at December 31, 2017 and December 31, 2016, respectively | 27,591 | 21,712 | ||||||
| Additional paid-in capital | 15,497,986 | 9,759,560 | ||||||
| Equity instruments to be issued | 65,839 | 3,070,000 | ||||||
| Accumulated deficit | (3,743,438 | ) | (1,565,797 | ) | ||||
| Accumulated other comprehensive income | 21,011 | 36,730 | ||||||
| Total Stockholders' Equity | 11,868,989 | 11,322,205 | ||||||
| Total Liabilities and Stockholders' Equity | $ | 20,351,444 | $ | 12,411,847 | ||||
The accompanying notes are an integral part of the unaudited consolidated financial statements.
F-3
AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPRENSIVE INCOME
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Revenues | $ | 3,302,775 | $ | 1,494,881 | ||||
| Cost of revenue | 2,321,756 | 39,129 | ||||||
| Gross profit | 981,019 | 1,455,752 | ||||||
| Selling, general and administrative expenses | 2,831,069 | 693,603 | ||||||
| Research and development expenses – other | 2,942,981 | 751,434 | ||||||
| Research and development expenses – related party | — | 8,068 | ||||||
| Depreciation and amortization | 1,297,710 | 636,921 | ||||||
| (Loss) from operations | (6,090,741 | ) | (634,274 | ) | ||||
| Other Income (Expense) | ||||||||
| Interest income – related party | 2,036 | 19,049 | ||||||
| Gain on bargain purchase | 4,311,554 | — | ||||||
| Interest expense | (113,967 | ) | (13,738 | ) | ||||
| Loss on stock exchange | (37,788 | ) | — | |||||
| Write-off of loan of related party loan | (174,252 | ) | — | |||||
| Loss on impairment of asset | (74,483 | ) | (173,500 | ) | ||||
| Loss before provision (benefit) for taxes | (2,177,641 | ) | (802,463 | ) | ||||
| Provision (benefit) for taxes | — | — | ||||||
| Net loss | $ | (2,177,641 | ) | $ | (802,463 | ) | ||
| Other comprehensive income | ||||||||
| Foreign currency translation adjustment | (15,719 | ) | (23,411 | ) | ||||
| Comprehensive loss | (2,193,360 | ) | (825,874 | ) | ||||
| BASIC AND DILUTED PER SHARE DATA: | ||||||||
| Net loss per common share, basic and diluted | $ | (0.09 | ) | $ | (0.04 | ) | ||
| Weighted average common shares outstanding, basic and diluted | 24,861,237 | 19,044,803 | ||||||
The accompanying notes are an integral part of the unaudited consolidated financial statements.
F-4
AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF STOCKHOLDERS’ EQUITY
(Unaudited)
| Additional | Equity | Other Items of | ||||||||||||||||||||||||||
| Common Stock | Paid-In | Instruments | Accumulated | Comprehensive | ||||||||||||||||||||||||
| Shares | Amount | Capital | to be Issued | Deficit | Income | Total | ||||||||||||||||||||||
| Balances at December 31, 2015 | 500,000 | $ | 500 | $ | 367,553 | $ | — | $ | (763,334 | ) | $ | 60,141 | $ | (335,140 | ) | |||||||||||||
| Common stock issued for services | 423,500 | 423 | 171,329 | — | — | — | 171,752 | |||||||||||||||||||||
| Common stock issued for acquisition | 16,659,943 | 16,660 | 4,811,186 | 4,827,846 | ||||||||||||||||||||||||
| Common stock issued for patent license | — | — | — | 3,050,000 | — | — | 3,050,000 | |||||||||||||||||||||
| Common stock issued for conversion of notes payable and accrued interest | 1,287,564 | 1,288 | 1,389,335 | — | — | — | 1,390,623 | |||||||||||||||||||||
| Common stock issued for 2016 Private Placement for cash | 2,840,946 | 2,841 | 2,675,283 | 20,000 | — | — | 2,698,124 | |||||||||||||||||||||
Stock based compensation | — | — | 82,284 | — | — | — | 82,284 | |||||||||||||||||||||
| Warrants issued in 2016 Private Placement for cash | — | — | 162,823 | — | — | — | 162,823 | |||||||||||||||||||||
Warrants issued for conversion of notes payable and accrued interest | — | — | 99,767 | — | — | — | 99,767 | |||||||||||||||||||||
Net loss for the year ended December 31, 2016 | — | — | — | — | (802,463 | ) | — | (802,463 | ) | |||||||||||||||||||
| Other items of comprehensive loss | — | — | — | — | — | (23,411 | ) | (23,411 | ) | |||||||||||||||||||
| Balances at December 31, 2016 | 21,711,953 | 21,712 | 9,759,560 | 3,070,000 | (1,565,797 | ) | 36,730 | 11,322,205 | ||||||||||||||||||||
| Common stock issued for services | 715,667 | 716 | 610,177 | — | — | — | 610,893 | |||||||||||||||||||||
| Common stock issued for 2017 Private Placement for cash | 932,000 | 932 | 1,164,068 | — | — | — | 1,165,000 | |||||||||||||||||||||
| Common stock issued for cash | 150,000 | 150 | 149,850 | — | — | — | 150,000 | |||||||||||||||||||||
| Common stock issued for warrant exercise | 606,098 | 606 | 5,330 | — | — | — | 5,936 | |||||||||||||||||||||
| Common stock issued for 2016 Private Placement for cash | 24,000 | 24 | 23,976 | (20,000 | ) | — | — | 4,000 | ||||||||||||||||||||
| Common stock issued for patent license | 3,050,000 | 3,050 | 3,046,950 | (3,050,000 | ) | — | — | — | ||||||||||||||||||||
| Warrants issued for Leonite Capital Convertible Debt | — | — | 90,190 | — | — | — | 90,190 | |||||||||||||||||||||
| Common stock issued for Leonite Capital Convertible Debt | 100,000 | 100 | 99,900 | — | — | — | 100,000 | |||||||||||||||||||||
| Employee payroll stock purchases | 203,635 | 203 | 127,044 | — | — | — | 127,247 | |||||||||||||||||||||
| Common stock canceled per severance agreement | (56,000 | ) | (56 | ) | — | — | — | — | (56 | ) | ||||||||||||||||||
Stock based compensation | — | — | 319,057 | — | — | — | 319,057 | |||||||||||||||||||||
| Common stock issued for 2016 merger shares | (77,725 | ) | (78 | ) | (77,647 | ) | 77,725 | — | — | — | ||||||||||||||||||
| Stock issue to related party – repayment of loan | 219,927 | 220 | 167,657 | — | — | — | 167,877 | |||||||||||||||||||||
| Common stock issue for 2016 merger shares | 11,886 | 12 | 11,874 | (11,886 | ) | — | — | — | ||||||||||||||||||||
Net loss for the year ended December 31, 2017 | — | — | — | — | (2,177,641 | ) | — | (2,177,641 | ) | |||||||||||||||||||
| Other items of comprehensive loss | — | — | — | — | — | (15,719 | ) | (15,719 | ) | |||||||||||||||||||
| Balances at December 31, 2017 | 27,591,441 | $ | 27,591 | $ | 15,497,986 | $ | 65,839 | (3,743,438 | ) | $ | 21,011 | $ | 11,868,989 | |||||||||||||||
The accompanying notes are an integral part of the consolidated financial statements.
F-5
AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
| For the Years Ended | ||||||||
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net Loss | $ | (2,177,641 | ) | $ | (802,463 | ) | ||
| Adjustment to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 1,297,710 | 636,921 | ||||||
| Stock-based compensation | 1,260,942 | 254,036 | ||||||
| Loss on impairment of asset | 74,483 | 173,500 | ||||||
| Loss on exchange for stock | 37,788 | — | ||||||
| Gain on bargain purchase | (4,311,554 | ) | — | |||||
| Bad debt | 174,252 | — | ||||||
| Income tax benefit | — | — | ||||||
| Non-cash interest | 45,711 | — | ||||||
| Change in operating assets and liabilities: | ||||||||
| Accounts receivable | (491,940 | ) | (28,119 | ) | ||||
| Grants receivable | (511,127 | ) | 4,326 | |||||
| Inventory | (106,006 | ) | — | |||||
| Other current asset | 2,813 | (130,529 | ) | |||||
| Accounts payable | 1,175,745 | (27,628 | ) | |||||
| Accrued interest receivable – related party | — | (1,736 | ) | |||||
| Accrued liabilities | 9,658 | 67,206 | ||||||
| Advance grant payments | 484,819 | 198,859 | ||||||
| Accrued interest payable – other | (3,997 | ) | 208 | |||||
| Deferred liabilities | (74,474 | ) | (56,789 | ) | ||||
| Net cash provided by (used in) operating activities | (3,112,818 | ) | 287,792 | |||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Issuance of notes receivable – related party | (59,819 | ) | (912,392 | ) | ||||
| Cash paid for acquisitions net of cash acquired | (978,996 | ) | (140,000 | ) | ||||
| Additions to property plant and equipment | (61,667 | ) | (32,567 | ) | ||||
| Net cash used in investing activities | (1,100,482 | ) | (1,084,959 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from issuance of common stock and issuance of warrants | 1,324,936 | 2,860,946 | ||||||
| Proceeds from debt | 1,910,552 | 137,813 | ||||||
| Repayment of debt | (181,038 | ) | (52,731 | ) | ||||
| Repayment to related party | (87,369 | ) | (20,088 | ) | ||||
| Net cash provided by financing activities | 2,967,081 | 2,925,940 | ||||||
| Effects of exchange rate changes on cash | 5,386 | (3,978 | ) | |||||
| Net increase (decrease) in cash and cash equivalents | (1,240,833 | ) | 2,124,795 | |||||
| Cash and cash equivalents at beginning of year | 2,124,795 | — | ||||||
| Cash and cash equivalents at end of year | $ | 883.962 | $ | 2,124,795 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid during period for interest | $ | 59,546 | $ | 8,275 | ||||
| Cash paid during period for taxes | — | — | ||||||
| SUPPLEMENTAL DISCLOSURE OF NON-CASH ACTIVITIES: | ||||||||
| Original purchase discount on notes | $ | 154,266 | $ | — | ||||
| Common stock issued for acquisition | — | 4,505,486 | ||||||
| Common stock issued for conversion of shareholder notes and accrued interest | — | 1,287,564 | ||||||
| Common stock issued for investments | — | 322,360 | ||||||
| Settlement of notes receivable in exchange for intangible assets - related party | — | 805,204 | ||||||
| Equity instruments to be issued for acquisition of patent license | — | 3,050,000 | ||||||
The accompanying notes are an integral part of the unaudited consolidated financial statements.
F-6
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
NOTE 1 – BUSINESS – NATURE OR ORGANIZATION
Unless the context otherwise requires, references to “we,” “our,” “us,” “Nexeon” or the “Company” in these Notes mean Nexeon MedSystems Inc, a Nevada corporation, on a consolidated basis with its wholly-owned subsidiaries, as applicable.
Organization and Operations
Nexeon MedSystems Inc was incorporated in the State of Nevada on December 7, 2015. Nexeon MedSystems Inc is a neuromodulation medical device manufacturing company. As a development stage enterprise, the Company’s primary purposes are to develop and commercialize our neurostimulation technology platform for the treatment of various disorders via electrical stimulation of tissues associated with the nervous system. The neurostimulation technology platform was acquired through the acquisition of Nexeon Medsystems Belgium, SPRL (“NMB”). During 2016, the Company formed the following wholly owned subsidiaries: Nexeon Medsystems Europe, SARL (“Nexeon Europe”), Nexeon Medsystems Puerto Rico Operating Company Corporation (“NXPROC”), and Pulsus Medical LLC. Nexeon Europe is the holding company for NXPROC and Nexeon Medsystems Belgium, SPRL (“NMB”). NXPROC is focused on advanced computational biology and deep learning utilization associated with the Internet of Medical Things technology. Pulsus Medical, LLC conducts research and development related to cardiovascular disease technology acquired in the acquisition of NXDE. On September 1, 2017, through its wholly-owned subsidiary Nexeon Europe, the Company completed the acquisition of NMB, along with NMB’s wholly owned subsidiaries Medi-Line and its holding company INGEST, SPRL (“INGEST”), which are incorporated under the laws of Belgium. INGEST is the holding company for Medi-Line. Medi-Line provides the medical device manufacturing expertise and experience needed to scale our business. Medi-Line is a leading global source of innovative medical device solutions with existing customers that include Fortune 50 companies, neurostimulator companies, and the Company. On September 27, 2017 Nexeon Medsystems Inc began trading on the OTCQB stock exchange under the symbol NXNN.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Management Estimates and Assumptions
The preparation of the Company’s financial statements are in conformity with accounting principles generally accepted in the United States of America which requires management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements and the reported amounts of expenses during the reporting periods. Management makes these estimates using the best information available at the time the estimates are made; however, actual results could differ materially from these estimates.
Principals of Consolidation
The consolidated financial statements include the accounts of the Company, and its wholly-owned subsidiaries. All material inter-company accounts, transactions, and profits have been eliminated in consolidation.
Cash and Cash Equivalents
The Company considers those short-term, highly liquid investments with maturities of three months or less as cash and cash equivalents. The Company currently has no cash equivalents.
Long-lived Assets
Long-lived assets such as property, equipment and identifiable intangibles are reviewed for impairment whenever facts and circumstances indicate that the carrying value may not be recoverable. When required, impairment losses on assets to be held and used are recognized based on the fair value of the asset. The fair value is determined based on estimates of future cash flows, market value, of similar assets, if available, or independent appraisals, if required. If the carrying amount of the long-lived asset is not recoverable from its undiscounted cash flows, an impairment loss is recognized for the difference between the carrying amount and fair value of the asset. When fair values are not available, the Company estimates fair value using the expected future cash flows discounted at a rate commensurate with the risk associated with the recovery of the assets. The Company recognized impairment losses in the amount of $74,483 during the year ended December 31, 2017.
F-7
Property and Equipment
Property and equipment are stated at cost. Equipment is depreciated using the straight-line method over the estimated useful lives of the assets. Leasehold improvements are amortized based upon the lesser of the term of the lease or the useful life of the asset and such expense is included in depreciation expense. Repair and maintenance costs are expensed as incurred. The Company capitalizes all furniture and equipment with cost greater than $1,000 and benefiting more than one accounting period in the period purchased.
Net Income (Loss) Per Share
The Company calculates net income (loss) per share as required by Accounting Standards Codification subtopic 260-10, “Earnings per Share” (“ASC 260-10”). Basic earnings (loss) per share is calculated by dividing net income (loss) by the weighted average number of common shares outstanding for the period. Diluted earnings per share is calculated by dividing net income (loss) by the weighted average number of common shares and dilutive common stock equivalents outstanding. During the periods when they are anti-dilutive, common stock equivalents, if any, are not considered in the computation. Basic and diluted earnings per share were the same for the years ended December 31, 2017 and 2016, respectively as the Company has no dilutive securities.
Revenue Recognition
Revenues currently consist of single use medical devices for the medical and pharmaceutical sectors at Med-Line and pre-clinical neurostimulation device sales at NMB. We recognize revenue when all of the following conditions are satisfied: (1) there is persuasive evidence of an arrangement; (2) the product or service has been provided to the customer; (3) the amount of fees to be paid by the customer is fixed or determinable; and (4) the collection of our fees is probable. The Company will record revenue when it is realizable and earned and the services have been rendered to the customers. Additionally, the Company will record revenue from the sale of its manufactured products and medical devices when the product is delivered to the customer.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes in accordance with ASC Topic 740, “Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year and (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if, based on the weight of the available positive and negative evidence, it is more likely than not some portion or all of the deferred tax assets will not be realized.
ASC Topic 740.10.30 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740.10.40 provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The Company has no material uncertain tax positions for any of the reporting periods presented.
All tax positions are first analyzed to determine if the weight of available evidence indicates that it is more likely than not that the position will be sustained under audit, including resolution of any related appeals or litigation processes. After the initial analysis, the tax benefit is measured as the largest amount that is more than 50% likely of being realized upon ultimate settlement.
F-8
If the Company is required to pay interest on the underpayment of income taxes, the Company recognizes interest expense in the first period the interest becomes due according to the provisions of the relevant tax law.
If the Company is subject to payment of penalties, the Company recognizes an expense for the amount of the statutory penalty in the period when the position is taken on the income tax return. If the penalty was not recognized in the period when the position was initially taken, the expense is recognized in the period when the Company changes its judgment about meeting minimum statutory thresholds related to the initial position taken.
Research and Development Expenses
Research and development expenses are charges to expense as incurred. Research and development expenses include, but are not limited to, product development, clinical and regulatory expenses, payroll and other personnel expenses, materials, supplies, consulting costs, and non-recurring engineering costs. These expenses are assigned to the research, development and clinical projects to develop the Company’s implantable neurostimulation, sensing, and recording technology for a variety of clinical therapeutic applications and for manufacturing product development.
The Company has been awarded grants subsidies for on-going research and development projects from the National Institutes of Health Department of Health and Human Services, through the Public Service of Wallonia - Department of Technology Development and the Research Programs Department (the Wallonia region is located in South Brussels, in Belgium) and the Cancer Prevention and Research Institute of Texas to support our research projects with potential for commercialization. The Company receives the funding in a combination of advance payments at commencement of a project and through reimbursement requests, invoices, for applicable research and development expenses as expenses are incurred. These grants and subsidies provide non-dilutive funds that do not include a repayment obligation. Participation by the granting agency typically accounts for 50% to 100% of the project costs in grants or subsidies.
The Company recognizes the amounts receivable in regard to the grants contracts at fair value when there is reasonable assurance that the contract amount will be received and that all the conditions of the specific contract will be complied with in order to properly match the reimbursements with the specific expenditures that the specific contract intends to reimburse. The Company recognizes the amounts received in accordance with the contracts as a reduction of research and development expenses over the periods necessary to match the contract on a systematic basis to the costs that it is intended to compensate. The Company records, on the balance sheet, Grants receivable upon meeting the criteria discussed above until cash is received. Where the Company receives payments in advance it is recorded as Advance grant payments on the balance sheet and relieved against research and development expense as the associated costs are incurred.
As of December 31, 2017, the Company has $804,152 in Grants receivable for project expenses invoiced and to be invoiced, but not yet paid which have been recorded as a reduction of research and development expense in the accompanying statement of operations and $935,817 in Advance payments received and yet to be expended.
Foreign Currency Translation and Transactions
The Company’s reporting currency is the U.S. dollar. The Company’s operations in Belgium use their local currencies as their functional currency. The financial statements in foreign currency are translated into U.S. Dollars, “USD,” in accordance with ASC Topic 830, Foreign Currency Translation. All assets and liabilities are translated at the year-end currency exchange rate, stockholders’ equity items are translated at the historical rates and income statement items are translated at the average exchange rate prevailing during the year. Translation adjustments resulting from this process are reported under other comprehensive income (“OCI”) in accordance with ASC Topic 220, Reporting Comprehensive Income as a Component of Stockholders’ Equity. Foreign exchange transaction gains and losses are reflected in the statement of comprehensive income.
F-9
Fair Value Measurements
The Company adopted the provisions of ASC Topic 820, “Fair Value Measurements and Disclosures,” which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring fair value and expands disclosure of fair value measurements.
The estimated fair value of certain financial instruments, including cash and cash equivalents are carried at historical cost basis, which approximates their fair values because of the short-term nature of these instruments.
ASC 820 defines “fair value” as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs that may be used to measure fair value:
Level 1 — quoted prices in active markets for identical assets or liabilities
Level 2 — quoted prices for similar assets and liabilities in active markets or inputs that are observable
Level 3 — inputs that are unobservable (for example cash flow modeling inputs based on assumptions)
The Company currently has no assets or liabilities valued at fair value on a recurring basis.
Investments in Non-Consolidated Subsidiaries
Investments in non-consolidated entities are accounted for using the equity method or cost basis depending upon the level of ownership and/or the Company's ability to exercise significant influence over the operating and financial policies of the investee. When the equity method is used, investments are recorded at original cost and adjusted periodically to recognize the Company's proportionate share of the investees' net income or losses after the date of investment. When net losses from an investment accounted for under the equity method exceed its carrying amount, the investment balance is reduced to zero and additional losses are not provided for. The Company resumes accounting for the investment under the equity method if the entity subsequently reports net income and the Company's share of that net income exceeds the share of net losses not recognized during the period the equity method was suspended. Investments are written down only when there is clear evidence that a decline in value that is other than temporary has occurred. The Company accounts for its investment in MicroTransponder, Inc. under the cost method due to the lack of significant influence.
Leases
Leases are reviewed and classified as capital or operating at their inception in accordance with ASC Topic 840, Accounting for Leases. For leases that contain rent escalations, the Company records monthly rent expense equal to the total amount of the payments due in the reporting period over the lease term. The difference between rent expense recorded and the amount paid is credited or charged to deferred rent account, when presented on balance sheet.
Acquired Intangibles
Acquired intangibles include patents and patent licenses acquired by the Company, which are recorded at fair value, assigned an estimated useful life, and are amortized on a straight-line basis over their estimated useful lives ranging from 3 to 19 years. The Company periodically evaluates whether current facts or circumstances indicate that the carrying values of its acquired intangibles may not be recoverable. If such circumstances are determined to exist, an estimate of the undiscounted future cash flows of these assets, or appropriate asset groupings, is compared to the carrying value to determine whether an impairment exists. If the asset is determined to be impaired, the loss is measured based on the difference between the carrying value of the intangible asset and its fair value, which is determined based on the net present value of estimated future cash flows.
F-10
Common Stock Purchase Warrants and Other Derivative Financial Instruments
The Company classifies as equity any contracts that require physical settlement or net-share settlement or provide us a choice of net-cash settlement or settlement in our own shares (physical settlement or net-share settlement) provided that such contracts are indexed to our own stock as defined in ASC 815-40 “Contracts in Entity's Own Equity.” We classify as assets or liabilities any contracts that require net-cash settlement (including a requirement to net cash settle the contract if an event occurs and if that event is outside our control) or give the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement). We assess classification of our common stock purchase warrants at each reporting date to determine whether a change in classification between assets and liabilities is required.
Stock-Based Compensation
ASC 718 requires companies to measure all stock compensation awards using a fair value method and recognize the related compensation cost in its financial statements. Beginning with the Company’s quarterly period that began on January 1, 2016, the Company adopted the provisions of FASB ASC 718 and expenses the fair value of employee stock options and similar awards in the financial statements. The Company accounts for share-based payments in accordance with ASC 718, “Compensation - Stock Compensation,” which requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based on the grant date fair value of the award. In accordance with ASC 718-10-30-9, “Measurement Objective – Fair Value at Grant Date,” the Company estimates the fair value of the award using the Black-Scholes option pricing model for valuation of the share-based payments. The Company believes this model provides the best estimate of fair value due to its ability to incorporate inputs that change over time, such as volatility and interest rates, and to allow for actual exercise behavior of option holders. The simplified method is used to determine compensation expense since historical option exercise experience is limited relative to the number of options issued. The compensation cost is recognized ratably using the straight-line method over the expected vesting period.
The Company accounts for stock-based compensation to other than employees in accordance with FASB ASC 505-50. Equity instruments issued to other than employees are valued at the earlier of a commitment date or upon completion of the services, based on the fair value of the equity instruments, and is recognized as expense over the service period.
During the years ended December 31, 2017 and 2016, the Company recognized stock-based compensation expense aggregating $319,057 and $82,284, respectively for common stock options issued to Company personnel, directors and consultants. Stock-based compensation consisting of restricted common stock issued to employees aggregating $127,247 and $0, respectively, and paid stock-based compensation consisting of restricted common stock issued to non-employees aggregating $610,893 and $171,500, respectively. During the years ended December 31, 2017 and 2016, the Company paid stock-based compensation, to affiliates aggregating $0 and $252, respectively.
Recently Issued Accounting Pronouncements
Management does not believe that any recently issued, but not yet effective accounting pronouncements, if adopted, would have a material effect on the accompanying financial statements.
Other recent accounting pronouncements issued by the FASB (including its Emerging Issues Task Force), the AICPA, and the SEC during the current reporting period did not, or are not believed by management to have a material impact on the Company’s present or future consolidated financial statements.
F-11
NOTE 3 – BUSINESS COMBINATIONS
On September 1, 2017 (the “Acquisition Date”), the Company, through its wholly-owned subsidiary Nexeon Europe, completed the acquisition of NMB pursuant to the Acquisition Agreement entered into on January 10, 2017, between Rosellini Scientific, LLC (“RS”), a Texas limited liability company controlled by our Chief Executive Officer, William Rosellini, and Nexeon Europe (the “Acquisition”). RS was the sole shareholder of NMB owning 107,154 shares (the “Shares”). Pursuant to the Acquisition Agreement, RS granted to Nexeon Europe the exclusive and irrevocable right to purchase the Shares upon the terms and conditions set forth in the Acquisition Agreement (the “Right to Purchase”). The consideration for the Right to Purchase was US $1,000 (the “Acquisition Price”). Upon Nexeon Europe exercising the Right to Purchase, the Agreement was automatically deemed converted into and considered a share transfer agreement for the purchase of the Shares and the Acquisition Price became the Purchase Price of the Shares and was deemed to have been satisfied by Nexeon Europe to RS as of the date of the Acquisition Agreement.
Due to RS controlling both the Company and NMB, the acquisition has been recorded as a combination of entities under common control and the results of NMB for the years ended December 31, 2017 and 2016 are reported retrospectively on a consolidated basis in the Company’s financial statements.
Included in the acquisition of NMB, are its wholly-owned subsidiaries, Medi-Line and its holding company INGEST. On August 30, 2017, NMB acquired INGEST and Medi-Line for $1,648,240 (payable as €1,450,000 EUR cash) or $977,996 (€891,496 EUR) net of cash acquired. As part of the transaction, and prior to the acquisition, Nexeon Europe loaned NMB $970,400 (€818,075 EUR) pursuant to the existing loan agreement and promissory note, NMB secured a credit facility in the amount of $330,319 (€275,000 EUR) and Medi-Line loaned NMB $540,032 (€450,000 EUR). Payment of the purchase price included the settlement of a note payable in the amount of $120,007 (€100,000 EUR) and a dividend payable in the amount of $9,901 (€8,250 EUR) to the sellers of INGEST. The balance of the loan and all accrued interest related to the loan agreement and promissory note between Nexeon Europe and NMB along with the $540,032 (€450,000 EUR) loan from Medi-Line to NMB is eliminated through consolidation in the financial statements.
Medi-Line provides the medical device manufacturing expertise and experience needed to scale our business. Medi-Line is a leading global source of innovative medical device solutions with existing customers that include Fortune 50 companies, neurostimulator companies, and the Company. Medi-Line provides high quality and efficiency in the development, engineering, and manufacturing of medical devices for the med-tech and pharmaceutical industries.
The acquisition of INGEST and Medi-Line was accounted for using the acquisition method and, accordingly, the results of operations of INGEST and Medi-Line were reported in the Company's financial statements beginning on August 30, 2017, the date of acquisition. The Revenue and Net loss reported in the financial statements for the combined INGEST and Medi-Line for the year ending December 31, 2017 were $2,972,993 and $116,974 respectively. Medi-Line incurred a charge in the amount of $168,933 related to a severance payment for a long-term employee that was terminated. In Belgium, severance payment requirements and accruals are administered by and mandated by the Belgian government and accrue based on time in service.
F-12
The following table presents the fair value of assets acquired and liabilities assumed as of the acquisition date on August 30, 2017, based on management’s preliminary allocation as well as the adjustments made up to December 31, 2017, based on the certified valuations:
| Preliminary | Final | |||||||
| August 30, 2017 | December 31, 2017 | |||||||
| Purchase price | $ | 1,740,102 | $ | 1,740,102 | ||||
| Cash and cash equivalents | 670,244 | 670,244 | ||||||
| Inventory | 2,224,907 | 2,100,668 | ||||||
| Accounts receivable | 1,384,957 | 1,384,957 | ||||||
| Grants receivable | 190,002 | 190,002 | ||||||
| Other current assets | 21,819 | 21,819 | ||||||
| Property, plant and equipment | 1,728,151 | 3,633,826 | ||||||
| Software licenses | 35,513 | — | ||||||
| Note receivable | 540,032 | 540,032 | ||||||
| Intangible assets | 445,585 | 2,150,000 | ||||||
| Total Assets Acquired | 7,241,210 | 10,691,548 | ||||||
| Current liabilities | 2,452,166 | 2,225,735 | ||||||
| Deferred charges | 12,244 | 12,244 | ||||||
| Non-current liabilities | 2,401,913 | 2,401,913 | ||||||
| Total Liabilities Assumed | 4,866,323 | 4,639,892 | ||||||
| Net Assets Acquired | 2,374,887 | 6,015,656 | ||||||
| Goodwill | $ | (634,785 | ) | $ | (4,311,554 | ) | ||
The acquisition of INGEST and Medi-Line resulted in approximately $4,311,554 of negative goodwill based on third-party valuations. The negative goodwill is recorded as a bargain purchase in other income on the statements of comprehensive income. The income related to the bargain purchase is not expected to be applicable for tax purposes.
Unaudited Pro forma Consolidated Results
The following table provides unaudited pro forma results of operations for the years ended December 31, 2017 and 2016, as if INGEST and Medi-line had been acquired as of January 1, 2016. The pro forma results include the effect of certain purchase accounting adjustments such as the estimated changes in depreciation and amortization expense on the acquired tangible and intangible assets and the recognition of grant subsidies. However, pro forma results do not include any anticipated cost savings or other effects of the planned integration of INGEST and Medi-Line. Accordingly, such amounts are not necessarily indicative of the results if the acquisition had occurred on the dates indicated or which may occur in the future.
| December 31, | ||||||||
| 2017 | 2016 | |||||||
| Revenues | $ | 7,880,467 | $ | 8,496,687 | ||||
| Net income (loss) | (1,837,096 | ) | (504,113 | ) | ||||
| Net income (loss) per common share, basic and diluted | $ | (0.07 | ) | $ | (0.03 | ) | ||
F-13
NOTE 4 – GOING CONCERN
The accompanying financial statements have been prepared in conformity with generally accepted accounting principles, which contemplate continuation of the Company as a going concern. The Company has sustained operating losses since inception and has an accumulated deficit of $3,743,438 at December 31, 2017. In addition, the Company does not have sufficient continuing revenue to cover its future operating expenses. The Company currently has limited liquidity and has not completed its efforts to establish an additional source of revenues sufficient to cover operating costs of the on-going neurostimulation research and development activities over an extended period of time. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts and classification of liabilities should the Company be unable to continue as a going concern. The Company is seeking additional financing and for continue operations, but there is no guarantee such financing will be available or on terms favorable to the Company.
NOTE 5 – LOANS AND LEASES
Loans and leases consist of the following as of December 31, 2017:
Notes Payable
12.00% Senior Secured Convertible Promissory Note:
On August 21, 2017, Company entered into a securities purchase agreement with Leonite Capital, LLC (“LC”), a Delaware limited liability company to provide the Company with additional resources to conduct its business. Pursuant to the securities purchase agreement, LC purchased a unit consisting of (i) a note in the principal amount of $1,120,000 at an original issue discount of $120,000, (ii) warrants to purchase 500,000 shares of the Company’s common stock, and (iii) the commitment shares equaling 100,000 shares of the Company’s restricted common stock valued at $100,000 (the “Commitment Shares”). Interest is at the rate of 12.00% per annum and the maturity date is 24 months from the date of issue. The note is a senior secured obligation of the Company, with priority over all future indebtedness of the Company. LC shall have the right at any time at LC’s option to convert all or any part of the outstanding and unpaid principal amount and accrued and unpaid interest of the note into fully paid and non-assessable shares of common stock or any shares of capital stock or other securities of the Company into which such common stock shall hereafter be changed or reclassified with a limitation of owning a maximum of 4.99% of outstanding common stock of the Company at time of conversion. The conversion price shall be, at the option of LC, $1.75, subject to a one-time re-pricing 275 days after the closing, or (ii) 80% multiplied by the price per share paid by the investors in a subsequent Equity Financing. An amount of $274,266 was recorded on the balance sheet as an original discount to the consist of $120,000 original discount, $100,000 in restricted common stock and $54,266 as the fair value of the warrants issued in the transaction. The $274,266 will be expensed as interest expense over the 24-month term of the loan. For the LC loan, $373,333 is recorded as Loans and lease payable- current portion and $746,667 is recorded as Loan and lease payable – long-term on the balance sheet. $137,136 of the original discount is recorded as Loans and lease payable- current portion and $91,419 is recorded as Loan and lease payable – long-term on the balance sheet.
1.27% Secured bank Loan:
On August 29, 2017, Medi-Line entered into a credit contract with CBC Banque in the original amount of approximately $2,036,362 (€1,700,000 EUR). The loan is secured by a mortgage on the Medi-Line manufacturing facility and carries an interest rate of 1.27% per annum with a seven-year term having monthly payments of interest and principal of approximately $23,365 (€21,175 EUR). $281,013 of the outstanding balance is recorded as Loans and lease payable – current portion and $1,662,505 is recorded as Loan and lease payable – long-term on the balance sheet.
1.27% Secured Bank Loan:
On August 29, 2017, NMB entered into a credit contract with CBC Banque in the original amount of approximately $329,412 (€275,000 EUR). The loan carries an interest rate of 1.27% per annum with a seven-year term having monthly payments of interest and principal of approximately $4,103 (€ 3,425 EUR). The loan is secured by the shares of NMB. $45,458 of the outstanding balance is recorded as Loans and lease payable – current portion and $268,935 is recorded as Loan and lease payable – long-term on the balance sheet.
F-14
0.72% Secured Bank Loan:
On May 7, 2016, Medi-Line entered into a credit contract with CBC Banque in the original amount of approximately $68,781 (€57,420 EUR). The loan carries an interest rate of 0.72% per annum with a 48-month term having monthly payments of interest and principal of approximately $1,454 (€ 1,214 EUR). The loan is secured by the assets of Medi-Line. Proceeds of the loan were used to acquire manufacturing equipment. The loan is secured by the shares of NMB. $17,195 of the outstanding balance is recorded as Loans and lease payable- current portion and $26,028 is recorded as Loan and lease payable – long-term on the balance sheet.
Loan Subsidy:
NMB was awarded a loan subsidy through the Public Service of Wallonia in the amount of $598,665 (€499,779 EUR). Of the total amount awarded, $179,600 (€149,934 EUR) is categorized as loan with repayment amounts ranging from $5,986 and $23,947 annually from 2018 through 2032. The current portion of the liability is recorded as Loans and leases payable in the amount of $5,987 and $173,613 is included in long-term debt on the balance sheet. The award amounts in excess of the loan amount are invoiced for reimbursement and recorded as a credit to applicable research and development expenses.
Revolving Credit:
The Company has a revolving credit card with BB&T Financial with an outstanding balance of $13,313 as of December 31, 2017, a credit limit of $60,000 and a current APR of 25.4%, and a revolving credit card with Comerica Bank with an outstanding balance of $9,860 as of December 31, 2017, a credit limit of $11,000 and a current APR of 0%.
KBC Accounts Receivable Factoring Facility:
Medi-Line has an accounts receivable discounting agreement with KBC Commercial Finance for up to 85% of Medi-Line’s customer accounts receivables. The fee for the advances on receivables is the 2-month LIBOR plus 1.5% on annual basis. As of December 31, 2017, the outstanding balance on the credit facility was $49,018 and is recorded as Loans and lease payable- current portion on the balance sheet.
0.72% Secured Line of Credit:
Medi-line also has an unsecured line of credit with CBC Banque in the amount of approximately $89,840 (€75,000 EUR). The outstanding balance of this credit line as of December 31, 2017 is $0. The line of credit is secured by the assets of Medi-Line.
Capital Leases
Building Lease:
Medi-Line has a capital lease payable in the amount of $749,337 to KBC Vendor Lease. On December 13, 2005, Medi-Line entered into a capital lease facility for the financing of the manufacturing facility construction in the amount of $3,425,880 (€2,860,000 EUR) with a 15- year term. Quarterly lease payments excluding VAT are $46,730 (€39,011 EUR). The Company has the right to purchase the building at the end of the lease term for three percent (3%) of the original lease amount. $186,936 of the outstanding balance is recorded as Loans and lease payable- current portion and $562,401 is recorded as Loan and lease payable – long-term on the balance sheet.
F-15
Equipment Lease:
NMB has a capital lease payable in the amount of $21,502, which is record as Loans and lease payable- current portion, to Biotech Coaching S.A. On February 4, 2015, the Company entered into a sale-leaseback transaction with Biotech Coaching S.A. for the sale and lease in the original amount of $131,765 (€110,000 EUR) for medical and clean-room equipment. In March 2015, the Company commenced leasing the equipment with a 36-month term. Monthly lease payments excluding VAT are $3,824 (€3,192 EUR). The Company has the right to purchase the equipment at the end of the lease term for a residual value of $1,298 (€1,318 EUR).
| Carrying Amount | ||||
| Long-Term Debt | ||||
| 12.00% Senior Convertible Secured Note, amortization begins 2018, 2019 maturity | $ | 1,120,000 | ||
| 1.27% Secured Bank Loan, monthly amortization, 2024 maturity | 1,943,518 | |||
| 1.27% Secured Bank Loan, monthly amortization, 2024 maturity | 314,393 | |||
| 0.72% Secured Bank Loan, monthly amortization, 2020 maturity | 43,223 | |||
| Loan Subsidy, amortization begins 2018, 2032 maturity | 179,600 | |||
| Revolving Credit | 23,173 | |||
| KBC Accounts Receivable Factoring | 49,018 | |||
| Capitalized Building lease | 749,337 | |||
| Capitalized Equipment lease | 21,502 | |||
| Less: original purchase discount, net of amortization | (228,555 | ) | ||
| Total debt | 4,215,209 | |||
| Less: current portion of debt, net of original discount current portion | (866,479 | ) | ||
| Total long-term debt | $ | 3,348,730 | ||
Aggregate maturities of long-term obligations commencing in 2018 are:
| 2018 | 2019 | 2020 | 2021 | 2022 | After 2022 | |||||||||||||||||||
| Loans and notes | $ | 658,041 | 1,009,136 | 349,620 | 351,253 | 355,518 | 720,802 | |||||||||||||||||
| Capital leases | 208,438 | 187,201 | 187,467 | 187,733 | — | — | ||||||||||||||||||
| Total | $ | 866,479 | 1,196,337 | 537,087 | 538,986 | 355,518 | 720,802 | |||||||||||||||||
NOTE 6 – INCOME TAXES
The Company is incorporated in the United States of America and is subject to United States federal taxation. No provisions for income taxes have been made, as the Company had no U.S. taxable income for the year ended December 31, 2017 and 2016. The effective income tax rate for the Company for both of the years ended December 31, 2017 and 2016 were 34% and 40%, respectively. Some of our subsidiaries generated income and we accrued income tax according to the Belgian corporate income tax rate, but some had a loss and no tax provision was recorded. The Belgium corporate income tax (“CIT”) is levied at a rate of 33% plus a 3% crisis tax, which is a sur-tax on the CIT amount, implying an effective rate of 33.99%. No state, region or municipal income tax is levied.
The Belgian government enacted in December 2017 a significant tax reform law. The new tax legislation contains several key tax provisions including the reduction of the corporate income tax rate from the current 33.99% to 29.58% in 2018 and 2019 and 25% from 2020. Additionally, the use of net operating losses which could previously offset 100% of taxable income is now limited to offset only 70% of taxable income. The Company will not have to pay additional current tax due to the enacted changes.
F-16
The reconciliation of income tax provision (benefit) at the U.S. statutory rate of 34% for the years ended December 31, 2017 and 2016 to the Company’s effective tax rate is as follows:
| Years Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Income tax (benefit) at U.S. statutory rate | $ | (740,000 | ) | $ | (636,000 | ) | ||
| Tax rate difference between Belgium and U.S. | — | — | ||||||
| Permanent difference | (1,526,000 | ) | — | |||||
| Change in valuation allowance | 2,266,000 | 636,000 | ||||||
| Tax provision (benefit) at effective tax rate | $ | — | $ | — | ||||
The provision for income taxes are summarized as follows:
| Years Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Federal | ||||||||
| Current | $ | — | $ | — | ||||
| Deferred | — | — | ||||||
| Total income tax provision (benefit) | $ | — | $ | — | ||||
The tax effects of temporary differences that give rise to the Company’s net deferred tax asset as of December 31, 2017 and 2016 are as follows:
| Years Ended December 31, | ||||||||
| 2017 | 2016 | |||||||
| Net loss carryforward U.S. – tax effect | $ | 1,295,000 | $ | — | ||||
| Net loss carryforwards Belgium – tax effect | 406,000 | 636,000 | ||||||
| Less valuation allowance | (1,701,000 | ) | (636,000 | ) | ||||
| Deferred tax asset, net of allowance | $ | — | $ | — | ||||
As of December 31, 2017, the Company has approximately $7,789,555 of net operating losses (“NOL”) carryovers to offset taxable income, if any, in future years which expire in fiscal 2036. In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income and tax planning strategies in making this assessment. Based on the assessment, management has established a full valuation allowance against all of the deferred tax assets relating to the NOL period because it is more likely than not that all of the deferred tax assets will not be realized.
The Company’s deferred tax assets and liabilities were remeasured to reflect the reduction in the U.S. corporate income tax rate from 35% to 21%, and a reduction in the Belgian tax rate from 34% to 25%, resulting in a deferred tax expense of approximately $1,202,000 for the year ended December 31, 2017 that is still fully valued against as of December 31, 2017. This expense is attributable to the Company being in a net deferred tax asset position at the time of remeasurement. As the company maintains a full valuation allowance, this amount can be seen on the rate reconciliation as an adjustment to deferred tax asset and corresponding valuation allowance.
On December 22, 2017, the Tax Cuts and Jobs Act of 2017 (the “Tax Act”) was signed into law making significant changes to the Internal Revenue Code. Changes include, but are not limited to, a federal corporate tax rate decrease from 35% to 21% for tax years beginning after December 31, 2017, the transition of U.S international taxation from a worldwide tax system to a territorial system, and a one-time transition tax on the mandatory deemed repatriation of foreign earnings. We have estimated our provision for income taxes in accordance with the Tax Act and guidance available as of the date of this filing but have kept the full valuation allowance. As a result, we have recorded no income tax expense in the fourth quarter of 2017, the period in which the legislation was enacted.
On December 22, 2017, Staff Accounting Bulletin No. 118 ("SAB 118") was issued to address the application of US GAAP in situations when a registrant does not have the necessary information available, prepared, or analyzed (including computations) in reasonable detail to complete the accounting for certain income tax effects of the Tax Act. The deferred tax expense recorded in connection with the remeasurement of deferred tax assets is a provisional amount and a reasonable estimate at December 31, 2017 based upon the best information currently available. The ultimate impact may differ from these provisional amounts, possibly materially, due to, among other things, additional analysis, changes in interpretations and assumptions the Company has made, additional regulatory guidance that may be issued, and actions the Company may take as a result of the Tax Act. Any subsequent adjustment to these amounts will be recorded to current tax expense in the quarter of 2018 when the analysis is complete. The accounting is expected to be complete when the 2017 U.S. corporate income tax return is filed in 2018.
F-17
NOTE 7 – PROPERTY PLANT and EQUIPMENT
Property Plant and equipment at cost and accumulated depreciation as of December 31, 2017 and 2016 were:
| Estimated useful lives | December 31, 2017 | December 31, 2016 | ||||||||
| Land | $ | 96,884 | $ | — | ||||||
| Capitalized building | 39 years | 3,017,552 | — | |||||||
| Machinery and equipment | 5 to 15 years | 677,734 | 149,204 | |||||||
| Total property plant and equipment – gross | 3,792,170 | 149,204 | ||||||||
| Less: accumulated depreciation | (222,338 | ) | (79,850 | ) | ||||||
| Total property plant and equipment – net | $ | 3,569,832 | $ | 69,354 | ||||||
Property plant and equipment depreciation expense for the years ended December 31, 2017 and 2016 was $127,677 and $38,962, respectively.
NOTE 8 – INTANGIBLE ASSETS
Intangible assets that have finite useful lives are amortized over their estimated useful lives. Intangible assets as of December 31, 2017 and 2016 are as follows:
| Estimated useful lives | December 31, 2017 | December 31, 2016 | ||||||||
| Intangible assets with definitive lives: | ||||||||||
| Patents, licenses and intellectual property | 4 to 20 years | $ | 10,363,097 | $ | 10,313,660 | |||||
| Fair value of customer relationships at acquisition | 10 years | 600,000 | — | |||||||
| Less: accumulated amortization | (1,773,605 | ) | (601,570 | ) | ||||||
| Patents, licenses and intellectual property – net | 9,189,492 | 9,712,090 | ||||||||
| Intangible assets with indefinite lives: | ||||||||||
| Fair value of trade secrets and know-how at acquisition | 1,550,000 | — | ||||||||
| Total intangible assets – net | $ | 10,739,492 | $ | 9,712,090 | ||||||
Intangible asset amortization expense for the years ended December 31, 2017 and 2016 was $1,170,032 and $597,960, respectively.
NOTE 9 – INVENTORIES
Inventory balances as of December 31, 2017 and 2016 are as follow:
| December 31, 2017 | December 31, 2016 | |||||||
| Raw materials and supplies | $ | 1,811,749 | $ | — | ||||
| Work in process | 334,322 | — | ||||||
| Finished goods | 60,499 | — | ||||||
| Total inventories | $ | 2,206,570 | $ | — | ||||
NOTE 10 – SEGMENTS OF BUSINESS
The Company operates in two distinct business segments within the medical device industry; manufacturing and neurostimulation. The manufacturing segment includes the manufacturing operations of our wholly-owned subsidiary Medi-Line located in Angleur (Liege) Belgium. The neurostimulation segment includes development, manufacturing, and commercialization of neurostimulation technology. Operations for the neurostimulation segment are conducted in the United States, Puerto Rico, Belgium and Germany. Other items of revenue, not directly related to manufacturing or neurostimulation revenues are categorized as other operating income. Other operating income and expenses not directly related to a specific segment are identified as Income (expense) not allocated to segments.
| Revenue | Long Lived Assets | |||||||||||||||
| 2017 | 2016 | 2017 | 2016 | |||||||||||||
| Manufacturing | $ | 2,932,664 | $ | — | $ | 3,535,516 | $ | — | ||||||||
| Neurostimulation | 291,750 | 1,456,038 | 8,643,118 | 9,780,556 | ||||||||||||
| Other | 78,361 | 38,843 | 2,130,690 | 888 | ||||||||||||
| Consolidated total | $ | 3,302,775 | $ | 1,494,881 | $ | 14,309,324 | $ | 9,781,444 | ||||||||
F-18
| Income Before Tax | Identifiable Assets | |||||||||||||||
| 2017 | 2016 | 2017 | 2016 | |||||||||||||
| Manufacturing | $ | (31,698 | ) | $ | — | $ | 7,803,409 | $ | — | |||||||
| Neurostimulation | (6,117,404 | ) | (673,117 | ) | 9,421,311 | 10,141,459 | ||||||||||
| Other | 3,971,461 | (129,346 | ) | 3,126,724 | 2,270,388 | |||||||||||
| Consolidated total | $ | (2,177,641 | ) | $ | (802,463 | ) | $ | 20,351,444 | $ | 12,411,847 | ||||||
| Additions to Property Plant and Equipment | Depreciation and Amortization | |||||||||||||||
| 2017 | 2016 | 2017 | 2016 | |||||||||||||
| Manufacturing | $ | 50,495 | $ | — | $ | 74,344 | $ | — | ||||||||
| Neurostimulation | 11,172 | 31,581 | 1,203,169 | 636,822 | ||||||||||||
| Other | — | 986 | 20,197 | 99 | ||||||||||||
| Consolidated total | $ | 61,667 | $ | 32,567 | $ | 1,297,710 | $ | 636,921 | ||||||||
NOTE 11 – EQUITY
Common Stock Issuances
During the year ended December 31, 2017, the Company issued an aggregate of 715,667 shares of the Company’s restricted common stock for certain legal, corporate structuring and research and development consulting services rendered by third-party consultants. The foregoing shares were valued at $610,893.
On March 17, 2017, the Company offered to current warrant holders who participated in the 2016 Private Placement which closed on December 2, 2016, the opportunity to convert their warrants into common stock of the Company on the following terms (the “Warrant Conversion Offer”). The offer terms included the exercise of seventeen (17) warrants for seventeen (17) shares of the Company’s common stock at an exercise price of $0.01 per share for every one hundred (100) warrants owned. The remaining eighty-three (83) warrants per hundred warrants owned would be cancelled. The offer was on an all-or-nothing basis to convert all warrants held by each warrant holder (the “Warrant Conversion Offer”). Pursuant to the offer, 593,598 warrants have been exercised for an aggregate of 593,598 shares of the Company’s common stock and 2,898,151 warrants were cancelled in connection with the Warrant Conversion Offer. The total proceeds from the exercise of the 593,598 warrants pursuant to the Warrant Conversion Offer were $5,936.
F-19
The Company conducted the 2017 Private Placement, which closed on July 20, 2017 for up to 2,000,000 shares of common stock to accredited investors only. Pursuant to which it would receive up to $2,500,000 in proceeds. The shares of common stock were offered at $1.25 per share. The Company received $1,165,000 from the sale of common stock and issued 932,000 shares of common stock.
On December 15, 2016, Mr. Rosellini sold, assigned, and transferred all his right, title, and interest in and to the license owned by him related to the Siemens Patents to the Company pursuant to a Patent License Asset Purchase Agreement (the “Purchase Agreement”). Pursuant to the terms of the Purchase Agreement, during the year ended December 31, 2017, 3,050,000 shares of the Company’s restricted common stock were issued to Mr. Rosellini valued at $3,050,000. See Note 13 – Related Party Transactions, Patent License Agreement (Siemens Patents) and Patent License Asset Purchase Agreement to the Consolidated Financial Statements included herein.
On August 21, 2017, the Company entered into a securities purchase agreement with LC to provide the Company with additional resources to conduct its business. Pursuant to the SPA, the Company issued to LC the Commitment Shares as consideration for entering into the SPA with the Company. The shares were valued at $100,000.
On September 28, 2017, the Company issued 24,000 shares of common stock to an individual subscriber of the 2016 Private Placement. $20,000 of the subscription was previously recorded in Equity Instruments to be Issued. The remaining $4,000 of the subscription was deposited and the shares were transferred to common stock and Additional paid in capital on the balance sheet. The shares were valued at $24,000.
On October 9, 2017, the Company issued 150,000 shares of the Company’s restricted common stock through a subscription of the shares for cash to Henri Decloux following NMB’s acquisition of INGEST and Medi-Line. Henri Decloux was one of the two previous owners and sellers of INGEST to NMB. HD Resources, SPRL (“HD”) is owned by Henri Decloux and Medi-Line has contracted with HD for the management of Medi-Line.
On October 9, 2017, the Company issued 81,035 shares of the Company’s restricted common stock to RS, a company controlled by our Chief Executive Officer, William Rosellini, as repayment for 81,035 shares of the Company’s restricted common stock RS loaned to NMB for payment of outstanding vendor invoices. On December 29, 2017 an additional 61,884 shares of the Company’s restricted common stock were issued to RS in settlement for a cash loan to NMB from RS and for an adjustment to the market value of the 81,035 shares described above per the debt repayment agreement dated December 29, 2017. In total, the 142,919 shares were valued $119,746.
On November 22, 2017, Michael Rosellini exercised his right to purchase 200,000 shares of the Company’s restricted common stock. The cashless exercise pursuant to the warrant was elected and the Company issued 12,500 shares of restricted common stock pursuant to the exercise.
On December 7, 2017, 56,000 shares originally issued to Ron Conquest were cancelled pursuant to a Resignation and Release agreement dated November 7, 2017.
On December 29, 2017, the Company issued to Michael Rosellini 77,008 shares of the Company’s restricted common stock as repayment for a loan to the Company per the loan agreement dated December 29, 2017. The shares were valued at $48,130. Michael Rosellini is a shareholder of the Company and father of our Chief Executive Officer.
On August 21, 2017, the Company offered to current employees the opportunity to purchase shares of the Company’s restricted common stock for a discount through payroll deductions. As of the date of this filing, 203,635 shares of the Company’s restricted common stock were issued. The shares were valued at $127,247.
F-20
Related to the Merger with NXDE, 1,659,943 shares of the Company’s common stock were recorded as issued as of the closing of the merger. The Company had been unsuccessful in contacting five NXDE preferred stock holders and issuing 77,725 shares of the Company’s stock. In 2017, these shares are valued at $77,725 and have been adjusted from Common stock and Additional paid in capital to Equity instruments to be issued until these shares can be issued. On October 25, 2017, the Company issued 11,886 shares of the Company’s common stock to one of the five beneficial owners. The value of these shares is $11,886. As of December 31, 2017, 65,839 shares remain unissued and recorded as Equity instruments to be issued.
The issuance of the above securities was deemed to be exempt from the registration requirements of the Securities Act, by Section 4(a)(2) thereof, as a transaction by an issuer not involving a public offering.
Warrants
On February 1, 2016, the Company initiated a private placement for the sale of up to 5,500,000 units at $1.00 per Unit (the “2016 Private Placement”). Each Unit consists of one share of restricted common stock and one warrant to purchase one additional share of restricted common stock. The 2016 Private Placement was closed on December 2, 2016. The warrants have an exercise price of $2.00 per share and expire 36 months from the date of issue. The warrants have limited transferability to an affiliate of the holder only, cannot be sold as a warrant and do not contain cashless exercise provisions.
On March 17, 2017, the Company offered to current warrant holders who participated in the 2016 Private Placement the opportunity to convert their warrants into common stock of the Company on the following terms (the “Warrant Conversion Offer”). The offer terms included the exercise of seventeen (17) warrants for seventeen (17) shares of the Company’s common stock at an exercise price of $0.01 per share for every one hundred (100) warrants owned. The remaining eighty-three (83) warrants per hundred warrants owned would be cancelled. The offer was on an all-or-nothing basis to convert all warrants held by each warrant holder.
As of December 31, 2017, 593,598 warrants have been exercised for an aggregate of 593,598 shares of the Company’s common stock and 2,898,151 warrants were cancelled in connection with the Warrant Conversion Offer and 660,761 warrants are outstanding related to the 2016 Private Placement. 24,000 of the outstanding 660,761 warrants were issued in the year ended December 31, 2017 upon completion of the subscription agreement by an individual subscriber of the 2016 Private Placement.
On August 21, 2017, the Company entered into a securities purchase agreement with LC to provide the Company with additional resources to conduct its business. Pursuant to the SPA, the Company issued to LC warrants to purchase 250,000 shares of the Company’s common stock with an exercise price of $2.50 per share purchased and having a 2-year term and issued warrants to purchase 250,000 shares of the Company’s common stock with an exercise price of $3.00 per share purchased and having a 5-year term. The options were valued at $54,266 using the Black-Scholes option pricing model using a risk-free rate of 1.33%, expected volatility of 44.45%, expected life of 2 and 5 years, far value of the Company’s common stock of $1.25 with no expected dividends.
In connection with the securities purchase agreement with LC, Michael Rosellini, a shareholder in the Company and father of the Company’s CEO William Rosellini, provided a personal guarantee in the amount of $1,120,000 to induce LC to make the loan to the Company and accept the promissory note in the amount of $1,120,000. As consideration for providing the personal guarantee, the Company issued Michael Rosellini warrants to purchase 200,000 shares of the Company’s common stock with an exercise price of $1.50 per share purchased and having a 2-year term. The options were valued at $35,924 using the Black-Scholes option pricing model using a risk-free rate of 1.33%, expected volatility of 44.45%, expected life of 2 years, far value of the Company’s common stock of $1.25 with no expected dividends. Note 13 –Related Party Transactions Warrants Issued for Personal Guarantee.
On November 22, 2017, Michael Rosellini exercised his right to purchase 200,000 shares of the Company’s restricted common stock. The cashless exercise pursuant to the warrant was elected and the Company issued 12,500 shares of restricted common stock pursuant to the exercise.
F-21
As of December 31, 2017, 793,598 warrants have been exercised and 2,898,151 warrants have been cancelled.
As of December 31, 2017, a total of 1,160,761 shares of common stock of the Company have been reserved for issuance upon exercise of the warrants.
Options Grants – 2016 Plan
The Company may, from time to time, issue certain equity awards pursuant to our 2016 Plan. The 2016 Plan was adopted by our Board of Directors on January 2, 2016 and was subsequently approved by our shareholders. The Company reserved 5,000,000 shares of common stock for issuance pursuant option grants under the 2016 Plan.
During the year ended December 31, 2017, the Company issued stock options to purchase a total of 1,895,200 shares of the Company’s common stock under the 2016 Plan, with exercise prices ranging from $1.00 to $2.00 per share and cancelled 677,000 shares with an exercise price of $1.00, as follows:
| (i) | Granted to the Chief Science Officer of Nexeon Medsystems Puerto Rico Operating Company Corporation, a wholly owned subsidiary of the Company, 100,000 incentive stock options to purchase 100,000 shares of common stock, with an exercise price of $1.00 per share. The options vest in monthly increments of 8,333 options per month for 11 months and 8,337 options shall vest in the 12th month, with the three-year term for each option beginning upon each date of vesting. And granted 325,000 nonqualified stock options to purchase 325,000 shares of common stock, with an exercise price of $1.00 per share. The options vest in monthly increments of 27,083 options per month for 11 months and 27,087 options shall vest in the 12th month, with the three-year term for each option beginning upon each date of vesting. The fair value of the options was determined to be $113,073 using the Black-Scholes Option Pricing Model. The total 425,000 options were cancelled as of September 28, 2017 pursuant to the amended employment agreement with the executive. |
| (ii) | Granted to a Director appointed to the Board of Directors nonqualified stock options to purchase a total of 50,000 shares of common stock, in four grants of 12,500 each, with a weighted average exercise price of $1.45 per share. The options are immediately exercisable, and each option grant expires four years from the date of grant. The fair value of the options was determined to be $20,679 using the Black-Scholes Option Pricing Model. |
| (iii) | Granted to a second Director appointed to the Board of Directors nonqualified stock options to purchase a total of 50,000 shares of common stock, in three grants of 12,500 each, with a weighted average exercise price of $1.45 per share. The options are immediately exercisable, and each option grant expires four years from the date of grant. The fair value of the options was determined to be $20,679 using the Black-Scholes Option Pricing Model. |
| (iv) | Granted to our Vice President, Sales and Marketing, an initial grant of 220,000 nontransferable incentive stock options to purchase 220,000 shares of common stock, with an exercise price of $1.25 per share. Each option shall expire 36 months from the date of vesting. The options shall vest at the rate of 6,111 options per month for a period of 35 months and 6,115 options shall vest in the 36th month. Vesting commences on the first day of the month following the Grant Date. The fair value of the options was determined to be $33,392 using the Black-Scholes Option Pricing Model. |
| (v) | Granted to a consultant of the Company nonqualified stock options to purchase a total of 150,000 shares of common stock, with an exercise price of $1.00 per share. The options shall vest at the rate of 50,000 options per year beginning on January 2, 2018, 50,000 options on January 2, 2019 and 50,000 options on January 2, 2020 and each option grant expires three years from the date of vesting. The fair value of the options was determined to be $35,917 using the Black-Scholes Option Pricing Model. The total 150,000 options were cancelled upon resignation of the consultant prior to vesting of the options. |
F-22
| (vi) | Granted to a consultant of the Company nonqualified stock options to purchase a total of 380,000 shares of common stock, with an exercise price of $1.00 per share. 84,448 options vested immediately and the remaining 295,552 options vest over 28 months at approximately 10,556 options per month from the grant date and each option grant expires three years from the date of vesting. The fair value of the options was determined to be $128,778 using the Black-Scholes Option Pricing Model. |
| (vii) | Three non-executive employees of NMB were granted stock options upon the acquisition of NMB by the Company. Stock options to purchase a total of 725,000 shares of common stock with an exercise price of $1.25 were granted. 161,104 options vested immediately and the remaining 563,896 will vest over 28 months from the grant date at approximately 20,138 per month and each option grant expires four years from the date of vesting. The fair value of the options was determined to be $204,532 using the Black-Scholes Option Pricing Model. |
| (viii) | Granted to a consultant of NMB nonqualified stock options to purchase a total of 25,200 shares of common stock, with an exercise price of $1.00 per share. The options vest at a rate of 2,100 per month and vesting commences on the first day of the month following the Grant Date and each option expires three years from the date of vesting. The fair value of the options was determined to be $6,706 using the Black-Scholes Option Pricing Model. |
| (ix) | On November 6th, 2017, Mark Bates resigned from his position as Chief Innovation Officer and member of the Board of Directors. Pursuant to the severance agreement between Dr. Bates and the Company, of the original grant of 252,000 options on April 1, 2016, 102,000 options were cancelled, and 150,000 options became fully vested. |
The options were valued at $563,754 using the Black-Scholes option pricing model with the following weighted average assumptions:
| Risk-free interest rate | 1.56 | % | ||
| Expected life | 3.41 years | |||
| Expected dividends | 0.00 | % | ||
| Expected volatility | 46.51 | % | ||
| Fair value of the Company's common stock | $ | 1.14 |
Aggregate options expense recognized for the year ended December 31, 2017 was $319,057.
As of December 31, 2017, there were 1,319,800 shares available for grant under the 2016 Plan, excluding the 3,680,200 options outstanding.
As of December 31, 2017, there were 2,375,200 incentive stock options outstanding to purchase an aggregate of 3,680,200 shares of common stock and 1,305,200 non-qualified options outstanding to purchase an aggregate of 1,305,200 shares of the Company's common stock and 1,449,800 shares available for grant under the 2016 Plan.
Stock option activity, both within and outside the 2016 Plan, and warrant activity for the year ended December 31, 2017, are as follows:
| Stock Options | Stock Warrants | |||||||||||||||
| Weighted | Weighted | |||||||||||||||
| Average | Exercise | |||||||||||||||
| Shares | Price | Shares | Price | |||||||||||||
| Outstanding December 31, 2016 | 2,332,000 | $ | 1.00 | 4,128,510 | $ | 2.00 | ||||||||||
| Granted | 2,025,200 | 1.14 | 724,000 | 2.38 | ||||||||||||
| Canceled | (677,000 | ) | 1.00 | (2,898,151 | ) | 2.00 | ||||||||||
| Expired | — | — | — | — | ||||||||||||
| Exercised | — | — | (793,598 | ) | 0.39 | |||||||||||
| Outstanding at December 31, 2017 | 3,680,200 | $ | 1.08 | 1,160,761 | $ | 2.32 | ||||||||||
| Exercisable at December 31, 2017 | 1,480,400 | $ | 1.07 | 1,160,761 | $ | 2.32 | ||||||||||
F-23
The range of exercise prices and remaining weighted average life of the options outstanding at December 31, 2017 were $1.00 to $2.00 and 2.56 to 6.92 years, respectively.
The range of exercise prices and remaining weighted average life of the warrants outstanding at December 31, 2017 were $1.00 to $3.00 and 1.10 to 4.67 years, respectively.
NOTE 12 – 2016 OMNIBUS INCENTIVE PLAN
The 2016 Plan was adopted by our Board of Directors on January 2, 2016 and was subsequently approved by our shareholders. As of December 31, 2017, options to purchase a total of 3,680,200 shares of the Company's common stock were issued under the 2016 Plan, 2,685,200 with an exercise price of $1.00 per share and 945,000 with an exercise price of $1.25 per share and 25,000 with an exercise price of $1.80 per share and 25,000 with an exercise price of $2.00 per share. 454,656 options vested immediately upon grant and the remaining vest in varying amounts ranging from 100,000 annually to monthly increments ranging from 2,083 to 17,000 based on individual stock option agreements. The options have terms as follows: 1,705,200 options have a three-year term starting on each date of vesting, 825,000 options have a four-year term starting on each date of vesting, and 1,150,000 have an eight-year term starting on the date of vesting.
The 2016 Plan is administered by a committee of two or more non-employee independent directors designated by the Board. The committee shall perform the requisite duties with respect to awards granted. The committee currently determines to whom awards are made, the timing of any such awards, the type of securities, and number of shares covered by each award, as well as the terms, conditions, performance criteria, restrictions, and other provisions of awards. The committee has the authority to cancel or suspend awards, accelerate the vesting, or extend the exercise period of any awards made pursuant to the 2016 Plan.
Shares Available under the 2016 Plan
The maximum shares available for issuance under the 2016 Plan are 5,000,000 shares, subject to adjustment as set forth in the 2016 Plan. Any shares subject to an award that expires, is cancelled or forfeited or is settled for cash shall, to the extent of such cancelation, forfeiture, expiration or cash settlement, again become available for awards under the 2016 Plan. The committee can issue awards comprised of restricted stock, stock options, stock appreciation rights, stock units and other awards, as set forth in the 2016 Plan.
Transferability
Except as otherwise provided in the 2016 Plan, (i) during the lifetime of a participant, only the participant or the participant’s guardian or legal representative may exercise an option or stock appreciation right, or receive payment with respect to any other award and (ii) no award may be sold, assigned, transferred, exchanged or encumbered, voluntarily or involuntarily, other than by will or the laws of descent and distribution.
Change in Control
In the event of a merger, the surviving or successor entity (or its parent) may continue, assume or replace outstanding awards as of the date of the relevant transaction and such awards or replacements therefore shall remain outstanding and be governed by their respective terms. Such awards or replacements can be executed in part on the condition that the contractual obligations represented by the award are expressly assumed by the surviving or successor entity (or its parent) with appropriate adjustments to the number and type of securities subject to the award and the exercise price thereof so as to preserve the intrinsic value of the award existing at the time of the relevant transaction. Alternatively, the surviving or successor entity (or its parent) could issue to a participant a comparable equity-based award that preserves the intrinsic value of the original award existing at the time of the relevant transaction and contains terms and conditions that are substantially similar to those of the award.
F-24
If and to the extent that outstanding awards under the 2016 Plan are not continued, assumed or replaced in connection with a merger or relevant corporate transaction, then all outstanding awards shall become fully vested and exercisable for such period of time prior to the effective date of the relevant transaction as is deemed fair and equitable by the committee and shall terminate at the effective date of said transaction.
NOTE 13 – RELATED PARTY TRANSACTIONS
During the year ended December 31, 2017, the Company had the following transactions with related parties.
Patent License Agreement (Siemens Patents) and Patent License Asset Purchase Agreement Shares Issued
During the year ended December 31, 2017, the Company issued to Mr. Rosellini 3,050,000 shares of the Company’s restricted common stock valued at $3,050,000 in connection with the Patent License Agreement. On September 29, 2016, William Rosellini, our Chief Executive Officer, a director and a majority shareholder of the Company, entered into a patent license agreement (the “License Agreement”) with Magnus IP GmbH, German corporation (“Magnus”). Pursuant to the terms of the License Agreement, Magnus granted to Mr. Rosellini, and his affiliates, a non-exclusive, non-transferable, non-assignable without the right to sublicense worldwide license to a portfolio of 86 patents, referred to herein as the “Siemens Patents”.
The intellectual property relates to IOT technology as described by a system of interrelated computing devices, mechanical and digital machines, objects, animals, and/or people that have unique identifiers and a subsequent ability to transfer data over a network without requiring human-to-human or human-to-computer interaction. This technology can be utilized in a wide variety of medical device applications, most notably in hospitals, nursing facilities, or patients’ homes.
On December 15, 2016, Mr. Rosellini sold, assigned, and transferred all his right, title, and interest in and to the license owned by him related to the Siemens Patents to the Company pursuant to the Purchase Agreement. As consideration for the transfer of the Siemens Patents and the license related thereto, the Company paid to Mr. Rosellini the sum of $140,000 in cash and 3,050,000 shares of the Company’s restricted common stock valued at $3,050,000.
January 6, 2017 Stock Exchange Agreement
On January 6, 2017, the Company and RS, a company controlled by our CEO William Rosellini, entered into a stock exchange agreement. Subject to the terms and conditions set forth the stock exchange agreement, on the Effective Date, RS sold, transferred, and assigned to Nexeon all of its right, title and interest in and to 100 shares of common stock of MicroTransponder Inc., a Delaware corporation (the “MTI Shares”) in exchange for 389 shares of common stock of Emeritus Clinical Solutions, Inc. (formerly Telemend, Inc.), a Texas corporation, owned by Nexeon and Nexeon sold, transferred and assigned to RS 389 shares of common stock of Emeritus Clinical Solutions, Inc. in exchange for the 100 MTI Shares.
January 10, 2017 Acquisition Agreement
On January 10, 2017, RS, a company controlled by our CEO, William Rosellini, and Nexeon Europe, which is a wholly-owned subsidiary of the Company, and in the presence of NMB, entered into an acquisition agreement. RS is the sole shareholder of NMB owning 107,154 shares (the “NMB Shares”).
Pursuant to the acquisition agreement, RS granted to Nexeon Europe the exclusive and irrevocable right to purchase the NMB Shares upon the terms and conditions set forth in the acquisition agreement (the “Right to Purchase”). The consideration for the Right to Purchase is US $1,000 (the “Acquisition Price”). Nexeon Europe shall have the right to exercise the Right to Purchase commencing from the date of the acquisition agreement and terminating on December 31, 2017 (the “Acquisition Period”). In the event Nexeon Europe exercises the Right to Purchase, the acquisition agreement shall be automatically deemed converted into and considered a share transfer agreement for the purchase of the NMB Shares and the Acquisition Price shall be considered the purchase price of the NMB Shares and shall be deemed to have been satisfied by Nexeon Europe to RS as of the date of the acquisition agreement. If Nexeon Europe elects not to exercise the Right to Purchase on or before December 31, 2017, then the acquisition agreement shall become null and void and of no further force and effect.
F-25
On September 1, 2017, through its wholly-owned subsidiary Nexeon Europe, the Company completed the acquisition of NMB, along with NMB’s wholly owned subsidiaries Medi-Line and its holding company INGEST, which are incorporated under the laws of Belgium. INGEST is the holding company for Medi-Line. The option price of $1,000 is due and payable to RS and is recorded as Due to related party on the balance sheet as of December 31, 2017.
Warrant Conversion Offer
On March 21, 2017, the Company offered to current warrant holders who participated in the 2016 Private Placement which closed on December 2, 2016, the opportunity to convert their warrants into common stock of the Company on the following terms. The offer terms included the exercise of seventeen (17) warrants for seventeen (17) shares of the Company’s common stock at an exercise price of $0.01 per share for every one hundred (100) warrants owned. The remaining eighty-three (83) warrants per hundred warrants owned would be cancelled. The offer was on an all-or-nothing basis to convert all warrants held by each warrant holder. During the year ended December 31, 2017, the following officers, directors and related parties have converted warrants pursuant to the Warrant Exchange Offer, as follows:
Mark C. Bates, previously our Chief Innovation Officer and a Director, held 370,000 warrants and pursuant to the terms of the Warrant Conversion Offer, converted 62,900 warrants into 62,900 shares of common stock, with 307,100 warrants being cancelled. The 62,900 shares of common stock were value at $629.
Dr. Michael Rosellini, the father of William Rosellini, our Chief Executive Officer, held 617,000 warrants individually and 600,000 warrants under the Michael Rosellini ROTH IRA. Dr. Rosellini, pursuant to the terms of the Warrant Conversion Offer, converted 206,890 warrants into 206,890 shares of common stock, with 1,010,110 warrants being cancelled. The 206,890 shares of common stock are held as follows: 104,890 shares by Dr. Rosellini individually and 102,000 shares by the Michael Rosellini ROTH IRA. The 206,890 shares of common stock were value at $2,069.
Michael Neitzel, a Director, held 500,000 warrants through Yorkville MGB Investments, LLC (“Yorkville”). Mr. Neitzel is the Managing Partner of Yorkville. Mr. Neitzel converted 85,000 warrants into 85,000 shares of common stock pursuant to the terms of the Warrant Conversion Offer, with 415,000 warrants being cancelled. The 85,000 shares of common stock were value at $850.
Nuviant Medical, GmbH Waiver of Debt Agreement
On May 19, NMB entered into a Waiver of debt agreement to waive the outstanding loan balance and accrued interest outstanding pursuant to the September 21, 2015, loan agreement between NMB and Nuviant Medical, GmbH, a related entity to RS. The agreement waives the outstanding balance of the loan and accrued interest in the amount $171,946 and thereby waiving any right or action in respect to this debt. An expense had been recorded as bad debt on the statement of comprehensive income in the amount $174,252. During the year ended December 31, 2017 and prior to the waiver of debt agreement, NMB loaned Nuviant Medical, GmbH $59,027.
F-26
RS Loan of Nexeon MedSystems Inc Common Stock and Debt Repayment Agreement Dated December 29, 2017
On June 23, 2017, RS transferred 81,035 shares of restricted common stock of the Company to NMB. The shares were valued at $107,292. The loan is non-interest bearing and is to be re-paid to RS in Nexeon MedSystems Inc restricted common stock issued by the Company through an intercompany loan with NMB. The 81,035 shares were exchanged for outstanding payables to vendors of NMB in the amount of $107,292.
On October 9, 2017, the Company issued 81,035 shares of the Company’s restricted common stock to RS, a company controlled by our Chief Executive Officer, William Rosellini as repayment for 81,035 shares of the Company’s restricted common stock RS loaned to NMB for payment of outstanding vendor invoices. On December 29, 2017 an additional 61,884 shares of the Company’s restricted common stock were issued to RS in settlement for a cash loan to NMB from RS and for an adjustment to the market value of the 81,035 shares described above per the debt repayment agreement dated December 29, 2017. In total, the 142,919 shares were valued $119,746. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act, by Section 4(a)(2) thereof, as a transaction by an issuer not involving a public offering.
Warrants Issued for Personal Guarantee
In connection with the securities purchase agreement with LC, Michael Rosellini, a shareholder in the Company and father of the Company’s CEO William Rosellini, provided a personal guarantee in the amount of $1,120,000 to induce LC to make the loan to the Company and accept the promissory note in the amount of $1,120,000. As consideration for providing the personal guarantee, the Company issued Michael Rosellini warrants to purchase 200,000 shares of the Company’s common stock with an exercise price of $1.50 per share purchased and having a 2-year term.
On November 22, 2017, Michael Rosellini exercised his right to purchase 200,000 shares of the Company’s restricted common stock. The cashless exercise pursuant to the warrant was elected and the Company issued 12,500 shares of restricted common stock pursuant to the exercise.
Share Loan Agreement Dated December 29, 2017
On December 29, 2017, the Company issued 77,008 shares of restricted common stock pursuant to the share loan agreement with Michael Rosellini as repayment for a loan of 53,214 shares of registered common stock borrowed by the Company and used to pay certain vendors of the Company. The 77,008 restricted shares were valued at $48,130. Michael Rosellini is a shareholder of the Company and father of our Chief Executive Officer.
NOTE 14 – COMMITMENTS AND CONTINGENCIES
The Company is subject to a patent royalty agreement that requires 3% of Net Product Sales received from commercialization of the 35 patents or other intellectual property acquired in the Merger with NXDE to be paid to NXDE, LLC. No sales have been generated from any of the acquired patents or intellectual property.
The Company acquired a non-exclusive license to a portfolio of 86 patents and is subject to a 6% royalty to Magnus IP GmbH of the Net Sales of all licensed products sold, licensed, leased or otherwise disposed of pursuant to the license. No sales have been generated from the licensed intellectual property.
NOTE 15 – CONCENTRATION
For the year ended December 31, 2017, two of our customers accounted for approximately 45% and 23% of sales. For the year ended December 31, 2016, one of our customer accounted for approximately 94% of sales.
For the year ended December 31, 2017, the company purchased approximately 13% of its products from one distributor, as compared to 2016 where no distributor accounted for more than 10% of product purchased.
For the year ended December 31, 2017, three of our customers accounted for 54%, 15% and 15% of accounts receivable, as compared to 2016 where no customer accounted for more than 10% of accounts receivable.
F-27
NOTE 16 – SUBSEQUENT EVENTS
On February 28, 2018, pursuant to the 2016 Plan, the Compensation Committee of the Board of Director’s approved the following stock options grants:
| (i) | Granted to the Company’s Chief Executive Officer, incentive stock option to purchase up to 250,000 shares of common stock with an exercise price of $0.76 per share. 125,000 options vested immediately and the remaining 125,000 options vest on the anniversary of the grant date. Granted non-qualified stock options to purchase up to 900,000 shares of common stock with an exercise price of $0.76 per share. These options vest in equal monthly amounts of 37,500 beginning on March 1, 2018. Each option grant expires three years from the date of vesting. The fair value of the options was determined to be $226,009 using the Black-Scholes Option Pricing Model. |
| (ii) | Granted to the Company’s Chief Commercialization officer non-qualified stock options to purchase up to 57,000 shares of common stock with an exercise price of $0.76 per share. These options vested immediately on the grant date. The options expire eight years from the date of vesting. The fair value of the options was determined to be $12,855 using the Black-Scholes Option Pricing Model. |
| (iii) | Granted to the Company’s Chief Financial Officer, non-qualified stock options to purchase up to 40,000 shares of common stock with an exercise price of $0.76 per share. These options vested immediately upon grant date. Each option grant expires three years from the date of vesting. The fair value of the options was determined to be $9,021 using the Black-Scholes Option Pricing Model. |
| (iv) | Granted to the Vice President Sales and Marketing incentive stock options to purchase up to 11,000 shares of common stock with an exercise price of $0.76 per share. These options vested immediately on the grant date. The options expire three years from the date of vesting. The fair value of the options was determined to be $2,481 using the Black-Scholes Option Pricing Model. |
| (v) | Granted to non-executive employees incentive stock options to purchase up to 46,200 shares of common stock with an exercise price of $0.76 per share. 31,200 of these options vested immediately on the grant date and 15,000 vest in equal monthly amounts of 2,500 beginning on March 1, 2018. The options expire three years from the date of vesting. The fair value of the options was determined to be $10,420 using the Black-Scholes Option Pricing Model. |
On February 23, 2018, Medi-Line’s line of credit with CBC bank was amended to increase the advance amount to €300,000 approximately $368,982 and structure the financing as a straight loan with an interest rate of 1.25% above the EURIBOR rate for the period the funds are drawn down. The €300,000 will be available for drawdown through April 30, 2018 at which point the facility will be reduced to €200,000 and further reduced €100,000 on May 31, 2018. The security includes a pledge of Medi-Line business assets in the amount of €300,000.
On March 8, 2018, the Company issued an aggregate of 23,744 shares of the Company’s restricted common stock for certain sales and marketing and software consulting services rendered by third-party consultants. The foregoing shares were valued at $14,840. 8,160 of these shares were issued to Daniel Powell, the Company’s Vice President Sales and Marketing. These shares were issued for services provided by Mr. Powell prior to his employment by the Company. The issuance of these securities was deemed to be exempt from the registration requirements of the Securities Act, by Section 4(a)(2) thereof, as a transaction by an issuer not involving a public offering.
On March 31, 2018, pursuant to the 2016 Plan, the Company issued the following stock options per director’s agreements:
| (i) | Granted to a director appointed to the Board of Directors nonqualified stock options to purchase a total of 12,500 shares of common stock with an exercise price of $0.865 per share. The options are immediately exercisable, and each option grant expires four years from the date of grant. The fair value of the options was determined to be $3,596 using the Black-Scholes Option Pricing Model. |
| (ii) | Granted to a second director appointed to the Board of Directors nonqualified stock options to purchase a total of 12,500 shares of common stock with an exercise price of $0.865 per share. The options are immediately exercisable, and each option grant expires four years from the date of grant. The fair value of the options was determined to be $3,596 using the Black-Scholes Option Pricing Model. |
F-28
AND SUBSIDIARIES
CONSOLIDATED BALANCE SHEETS
(Unaudited)
| As of | ||||||||
| September 30, 2018 | December 31, 2017 | |||||||
| Assets | ||||||||
| Current Assets | ||||||||
| Cash and cash equivalents | $ | 125,573 | $ | 883,962 | ||||
| Accounts receivable | 1,709,915 | 1,877,743 | ||||||
| Grants receivable | 819,615 | 804,152 | ||||||
| Inventory | 2,420,988 | 2,206,570 | ||||||
| Other current assets | 106,614 | 157,621 | ||||||
| Total Current Assets | 5,182,705 | 5,930,048 | ||||||
| Property, plant and equipment, net | 3,449,467 | 3,569,832 | ||||||
| Investments | 112,072 | 112,072 | ||||||
| Intangible assets, net | 9,833,647 | 10,739,492 | ||||||
| Total Assets | $ | 18,577,891 | $ | 20,351,444 | ||||
| Liabilities and Stockholders’ Equity | ||||||||
| Current Liabilities | ||||||||
| Accounts payable | 2,822,693 | 2,575,399 | ||||||
| Accrued liabilities | 1,558,501 | 503,751 | ||||||
| Current portion of long-term debt, net of original discount | 1,673,667 | 866,479 | ||||||
| Advance grant payments | 568,844 | 935,817 | ||||||
| Deferred liabilities | 401,478 | 174,230 | ||||||
| Accrued interest | 86,088 | 78,049 | ||||||
| Total Current Liabilities | 7,111,271 | 5,133,725 | ||||||
| Long-term debt, net of original discount | 2,327,666 | 3,348,730 | ||||||
| Total Liabilities | $ | 9,438,937 | $ | 8,482,455 | ||||
| Stockholders’ Equity | ||||||||
| Common stock - 75,000,000 shares authorized, $.001 par value; 1,965,646 and 1,970,915 issued and outstanding at September 30, 2018 and December 31, 2017, respectively | 1,966 | 1,971 | ||||||
| Additional paid-in capital | 15,838,654 | 15,523,606 | ||||||
| Equity instruments to be issued | 65,839 | 65,839 | ||||||
| Accumulated deficit | (6,810,662 | ) | (3,743,438 | ) | ||||
| Accumulated other comprehensive income | 43,157 | 21,011 | ||||||
| Total Stockholders’ Equity | 9,138,954 | 11,868,989 | ||||||
| Total Liabilities and Stockholders’ Equity | $ | 18,577,891 | $ | 20,351,444 | ||||
The accompanying notes are an integral part of the unaudited consolidated financial statements.
F-29
AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF COMPRENSIVE INCOME
(Unaudited)
| For the Three Months Ended | For the Nine Months Ended | |||||||||||||||
| September 30, | September 30, | |||||||||||||||
| 2018 | 2017 | 2018 | 2017 | |||||||||||||
| Revenues | $ | 1,977,629 | $ | 1,039,679 | $ | 7,666,827 | $ | 1,147,288 | ||||||||
| Cost of revenue | 1,384,335 | 709,373 | 5,533,173 | 737,664 | ||||||||||||
| Gross profit | 593,294 | 330,306 | 2,133,654 | 409,624 | ||||||||||||
| Selling, general, and administrative expenses | 875,016 | 651,556 | 2,724,960 | 1,817,218 | ||||||||||||
| Research and development expenses | 674,360 | 287,229 | 1,881,198 | 1,659,560 | ||||||||||||
| Depreciation and amortization | 363,102 | 326,792 | 1,095,075 | 924,790 | ||||||||||||
| (Loss) from operations | (1,319,184 | ) | (935,271 | ) | (3,567,579 | ) | (3,991,944 | ) | ||||||||
| Other Income (Expense) | ||||||||||||||||
| Interest income | — | 3,041 | — | 3,216 | ||||||||||||
| Interest income – related party | — | — | — | 2,009 | ||||||||||||
| Interest expense | (77,873 | ) | (26,792 | ) | (235,869 | ) | (31,762 | ) | ||||||||
| Gain on bargain purchase | — | 624,211 | — | 624,211 | ||||||||||||
| Loss on stock exchange | — | — | — | (37,788 | ) | |||||||||||
| Bad debt | — | — | — | (171,946 | ) | |||||||||||
| Gain on sale of patent | — | — | 160,000 | — | ||||||||||||
| Foreign exchange loss | — | (89,441 | ) | — | — | |||||||||||
| Loss before provision (benefit) for taxes | (1,397,057 | ) | (424,252 | ) | (3,643,448 | ) | (3,604,004 | ) | ||||||||
| Provision (benefit) for taxes | (5,510 | ) | — | (576,224 | ) | (13,203 | ) | |||||||||
| Net (loss) | $ | (1,391,547 | ) | $ | (424,252 | ) | $ | (3,067,224 | ) | $ | (3,590,801 | ) | ||||
| Other comprehensive income | ||||||||||||||||
| Foreign currency translation adjustment | 56,704 | (81,264 | ) | 22,147 | 5,002 | |||||||||||
| Comprehensive loss | (1,334,843 | ) | (505,516 | ) | (3,045,077 | ) | (3,585,799 | ) | ||||||||
| BASIC AND DILUTED PER SHARE DATA: | ||||||||||||||||
| Net Loss per common share, basic and diluted | $ | (0.71 | ) | $ | (0.22 | ) | $ | (1.56 | ) | $ | (2.08 | ) | ||||
| Weighted average common shares outstanding, basic and diluted | 1,965,646 | 1,915,138 | 1,969,719 | 1,726,426 | ||||||||||||
The accompanying notes are an integral part of the unaudited consolidated financial statements.
F-30
AND SUBSIDIARIES
CONSOLIDATED STATEMENTS OF CASH FLOWS
(Unaudited)
| For the Nine Months Ended | ||||||||
| September 30, | ||||||||
| 2018 | 2017 | |||||||
| CASH FLOWS FROM OPERATING ACTIVITIES: | ||||||||
| Net Loss | $ | (3,067,224 | ) | $ | (3,590,801 | ) | ||
| Adjustment to reconcile net loss to net cash used in operating activities: | ||||||||
| Depreciation and amortization | 1,095,076 | 924,786 | ||||||
| Stock-based compensation | 475,043 | 217,605 | ||||||
| Loss on exchange for stock | — | 37,788 | ||||||
| Gain on sale of patent | (160,000 | ) | — | |||||
| Bad debt | — | 171,946 | ||||||
| Gain on bargain purchase | — | (624,211 | ) | |||||
| Provision for income taxes | — | (13,203 | ) | |||||
| Non-cash interest | 102,850 | 11,428 | ||||||
| Change in operating assets and liabilities: | ||||||||
| Accounts receivable | 111,726 | (436,504 | ) | |||||
| Grants receivable | 20,023 | (734,790 | ) | |||||
| Inventory | (291,940 | ) | (153,562 | ) | ||||
| Other current asset | 49,460 | 103,858 | ||||||
| Accounts payable | 328,254 | 495,987 | ||||||
| Accrued liabilities | 1,122,339 | (103,443 | ) | |||||
| Advance grant payments | (369,221 | ) | (440,739 | ) | ||||
| Accrued interest payable | 8,039 | (2,556 | ) | |||||
| Deferred liabilities | 184,679 | 340 | ||||||
| Net cash (used in) operating activities | (390,896 | ) | (4,136,071 | ) | ||||
| CASH FLOWS FROM INVESTING ACTIVITIES: | ||||||||
| Issuance of notes receivable – related party | — | (59,027 | ) | |||||
| Cash paid for acquisitions net of cash acquired | — | (957,182 | ) | |||||
| Additions to property plant and equipment | (63,083 | ) | (13,920 | ) | ||||
| Net cash used in investing activities | (63,083 | ) | (1,030,129 | ) | ||||
| CASH FLOWS FROM FINANCING ACTIVITIES: | ||||||||
| Proceeds from issuance of common stock | — | 1,773,288 | ||||||
| Proceeds from debt | 358,906 | 1,839,387 | ||||||
| Proceeds from related party | — | 34,438 | ||||||
| Proceeds from grant advance | (656,256 | ) | 235,349 | |||||
| Repayment of debt | — | (57,541 | ) | |||||
| Net cash (used in) financing activities | (297,350 | ) | 3,824,921 | |||||
| Effects of exchange rate changes on cash | (7,060 | ) | (18,717 | ) | ||||
| Net (decrease) in cash and cash equivalents | (758,389 | ) | (1,359,996 | ) | ||||
| Cash and cash equivalents at beginning of period | 883,962 | 2,124,795 | ||||||
| Cash and cash equivalents at end of period | $ | 125,573 | $ | 764,799 | ||||
| SUPPLEMENTAL DISCLOSURE OF CASH FLOW INFORMATION: | ||||||||
| Cash paid during period for interest | $ | 122,803 | $ | 17,708 | ||||
| Cash paid during period for taxes | 3,452 | — | ||||||
| SUPPLEMENTAL DISCLOSURE OF NON-CASH ACTIVITIES: | ||||||||
| Redemption of stock for patent sale | $ | 160,000 | $ | — | ||||
| Original purchase discount on notes | 274,266 | |||||||
The accompanying notes are an integral part of the unaudited consolidated financial statements.
F-31
AND SUBSIDIARIES
NOTES TO CONSOLIDATED FINANCIAL STATEMENTS
(Unaudited)
NOTE 1 – BUSINESS – NATURE OR ORGANIZATION
Unless the context otherwise requires, references to “we,” “our,” “us,” “Nexeon,” or the “Company” in these Notes mean Nexeon MedSystems Inc, a Nevada corporation, on a consolidated basis with its wholly owned subsidiaries, as applicable.
Organization and Operations
Nexeon MedSystems Inc was incorporated in the State of Nevada on December 7, 2015. Nexeon MedSystems Inc is a neuromodulation medical device manufacturing company. As a development-stage enterprise, the Company’s primary purpose is to develop and commercialize its neurostimulation technology platform for the treatment of various disorders via electrical stimulation of tissues associated with the nervous system. The neurostimulation technology platform was acquired through the acquisition of Nexeon MedSystems Belgium, SPRL (“NMB”). During 2016, the Company formed the following wholly owned subsidiaries: Nexeon MedSystems Europe, SARL (“Nexeon Europe”), Nexeon MedSystems Puerto Rico Operating Company Corporation Inc. (“NXPROC”), and Pulsus Medical LLC. Nexeon Europe is the holding company for NXPROC and Nexeon MedSystems Belgium, SPRL (“NMB”). NXPROC is focused on advanced computational biology and deep learning utilization associated with the Internet of Medical Things technology. Pulsus Medical, LLC conducts research and development related to cardiovascular disease technology acquired in its merger with Nexeon MedSystems, Inc., a private Delaware corporation (“NXDE”). On September 1, 2017, through its wholly owned subsidiary Nexeon Europe, the Company completed the acquisition of NMB, along with NMB’s wholly owned subsidiaries Medi-Line, S.A. (“Medi-Line”) and its holding company INGEST, SPRL (“INGEST”), which are incorporated under the laws of Belgium. INGEST is the holding company for Medi-Line. The Company believes Medi-Line provides the medical device manufacturing expertise and experience needed to scale its business. Medi-Line is a leading global source of innovative medical device solutions, with existing customers that include Fortune 50 companies and neurostimulator companies. On September 27, 2017 Nexeon MedSystems Inc began trading on the OTCQB platform under the symbol “NXNN”.
NOTE 2 – SUMMARY OF SIGNIFICANT ACCOUNTING POLICIES
Basis of Presentation
The accompanying unaudited consolidated financial statements include the accounts of Nexeon MedSystems Inc and its wholly owned subsidiaries NXPROC, Nexeon Europe, Pulsus Medical, LLC, and NMB as of September 30, 2018 and December 31, 2017, and for the three and nine months ended September 30, 2018 and 2017. The financial statements include the accounts of Medi-Line and INGEST as of September 30, 2018 and December 31, 2017 and for the three and nine months ended September 30, 2018 and the month of September 2017 for the three and nine months ended September 30, 2017. The Company’s unaudited consolidated financial statements have been prepared in accordance with accounting principles generally accepted in the United States of America for interim financial statements, and with the instructions for Form 10-Q and Article 8 of Regulation S-X of the United States Securities and Exchange Commission (“SEC”). Accordingly, they do not contain all information and footnotes required by accounting principles generally accepted in the United States of America for annual financial statements. These unaudited consolidated financial statements should be read in conjunction with the audited financial statements of the Company, and related notes thereto, which are included in the Company’s Annual Report on Form 10-K as of and for the year ended December 31, 2017. In the opinion of the Company’s management, the accompanying unaudited financial statements contain all the adjustments necessary (consisting only of normal recurring accruals) to present the financial position of the Company as of September 30, 2018, and the results of operations and cash flows for the periods presented. The results of operations for interim periods are not necessarily indicative of the operating results for the full fiscal year or any future period. All significant intercompany accounts and transactions have been eliminated in consolidation.
Management Estimates and Assumptions
The preparation of the Company’s financial statements are in conformity with accounting principles generally accepted in the United States of America, which require management to make estimates and assumptions that affect the reported amounts of assets and liabilities and disclosure of contingent assets and liabilities at the date of the financial statements, and the reported amounts of expenses during the reporting periods. Management makes these estimates using the best information available at the time the estimates are made; however, actual results could differ materially from these estimates.
Cash and Cash Equivalents
The Company considers those short-term, highly liquid investments with maturities of three months or less as cash and cash equivalents. The Company currently has no cash equivalents.
F-32
Long-Lived Assets
Long-lived assets such as property, equipment, and identifiable intangibles are reviewed for impairment whenever facts and circumstances indicate that the carrying value may not be recoverable. When required, impairment losses on assets to be held and used are recognized based on the fair value of the assets. The fair value is determined based on estimates of future cash flows, market value of similar assets, if available, or independent appraisals, if required. If the carrying amount of the long-lived asset is not recoverable from its undiscounted cash flows, an impairment loss is recognized for the difference between the carrying amount and fair value of the asset. When fair values are not available, the Company estimates fair value using the expected future cash flows discounted at a rate commensurate with the risk associated with the recovery of the assets.
Property and Equipment
Property and equipment are stated at cost. Equipment is depreciated using the straight-line method over the estimated useful lives of the assets. Leasehold improvements are amortized based upon the lesser of the term of the lease or the useful life of the asset, and such expense is included in depreciation expense. Repair and maintenance costs are expensed as incurred. The Company capitalizes all furniture and equipment with cost greater than $1,000 and benefiting more than one accounting period in the period purchased.
Inventories
The value of inventories, comprised solely of finished goods, are stated at the lesser of net realizable value or cost, determined using the first-in, first-out (“FIFO”) method. To value inventory, management must estimate excess or obsolete inventory, as well as inventory that is not of saleable quality. This valuation involves an inherent level of risk and uncertainty due to the unpredictability of trends in the industry and customer demand for the Company’s products. In assessing the ultimate realization of inventories, management must make judgments as to future demand requirements, and compare those with the current or committed inventory levels. Reserve requirements generally increase as demand decreases due to market conditions and technological and product life-cycle changes. Write-downs of excess and obsolete inventories were $0 and $0 in the nine months ended September 30, 2018 and 2017, respectively. Future events and variations in valuation methods or assumptions may cause significant fluctuations in this estimate, and could have a material impact on the Company’s results.
Net Income (Loss) Per Share
The Company calculates net income (loss) per share as required by Accounting Standards Codification subtopic 260-10, “Earnings per Share” (“ASC 260-10”). Basic earnings (loss) per share is calculated by dividing net income (loss) by the weighted average number of common shares outstanding for the period. Diluted earnings per share is calculated by dividing net income (loss) by the weighted average number of common shares and dilutive common stock equivalents outstanding. During the periods when they are anti-dilutive, common stock equivalents, if any, are not considered in the computation. Basic and diluted earnings per share were the same for the three and nine months ended September 30, 2018 and 2017, respectively, as the Company has no dilutive securities.
Revenue Recognition
Revenues currently consist of single-use medical devices for the medical and pharmaceutical sectors at Medi-Line and pre-clinical neurostimulation device sales at NMB.
In May 2014, the FASB issued Accounting Standards Update (ASU) No. 2014-09, Revenue from Contracts with Customers (Topic 606), which supersedes all existing revenue recognition requirements, including most industry specific guidance. This new standard requires a company to recognize revenues when it transfers goods or services to customers in an amount that reflects the consideration that the company expects to receive for those goods or services. The FASB subsequently issued the following amendments to ASU No. 2014-09 that have the same effective date and transition date: ASU No. 2016-08, Revenue from Contracts with Customers (Topic 606): Principal versus Agent Considerations; ASU No. 2016-10, Revenue from Contracts with Customers (Topic 606): Identifying Performance Obligations and Licensing; ASU No. 2016-12, Revenue from Contracts with Customers (Topic 606): Narrow-Scope Improvements and Practical Expedients; and ASU No. 2016-20, Technical Corrections and Improvements to Topic 606, Revenue from Contracts with Customers. The Company adopted these amendments with ASU 2014-09 (collectively, the new revenue standards).
The new revenue standards became effective for the Company on January 1, 2018, and were adopted using the modified retrospective method. The adoption of the new revenue standards as of January 1, 2018 did not change the Company’s revenue recognition as the majority of its revenues continue to be recognized when the customer takes control of its product. As the Company did not identify any accounting changes that impacted the amount of reported revenues with respect to its product revenues, no adjustment to retained earnings was required upon adoption.
F-33
Under the new revenue standards, the Company recognizes revenues when its customer obtains control of promised goods or services, in an amount that reflects the consideration which it expects to receive in exchange for those goods. The Company recognizes revenues following the five-step model prescribed under ASU No. 2014-09: (i) identify contract(s) with a customer; (ii) identify the performance obligations in the contract; (iii) determine the transaction price; (iv) allocate the transaction price to the performance obligations in the contract; and (v) recognize revenues when (or as) we satisfy the performance obligation.
Revenues from product sales are recognized when the customer obtains control of the Company’s product, which occurs at a point in time, typically upon delivery to the customer. The Company expenses incremental costs of obtaining a contract as and when incurred if the expected amortization period of the asset that it would have recognized is one year or less or the amount is immaterial.
Income Taxes
The Company uses the asset and liability method of accounting for income taxes in accordance with ASC Topic 740, “Income Taxes.” Under this method, income tax expense is recognized for the amount of: (i) taxes payable or refundable for the current year and (ii) deferred tax consequences of temporary differences resulting from matters that have been recognized in an entity’s financial statements or tax returns. Deferred tax assets and liabilities are measured using enacted tax rates expected to apply to taxable income in the years in which those temporary differences are expected to be recovered or settled. The effect on deferred tax assets and liabilities of a change in tax rates is recognized in the results of operations in the period that includes the enactment date. A valuation allowance is provided to reduce the deferred tax assets reported if, based on the weight of the available positive and negative evidence, it is more likely than not that some portion or all of the deferred tax assets will not be realized.
ASC Topic 740.10.30 clarifies the accounting for uncertainty in income taxes recognized in an enterprise’s financial statements, and prescribes a recognition threshold and measurement attribute for the financial statement recognition and measurement of a tax position taken or expected to be taken in a tax return. ASC Topic 740.10.40 provides guidance on de-recognition, classification, interest and penalties, accounting in interim periods, disclosure, and transition. The Company has no material uncertain tax positions for any of the reporting periods presented.
All tax positions are first analyzed to determine if the weight of available evidence indicates that it is more likely than not that the position will be sustained under audit, including resolution of any related appeals or litigation processes. After the initial analysis, the tax benefit is measured as the largest amount that is more than 50% likely of being realized upon ultimate settlement.
If the Company is required to pay interest on the underpayment of income taxes, the Company recognizes interest expense in the first period the interest becomes due according to the provisions of the relevant tax law.
If the Company is subject to payment of penalties, the Company recognizes an expense for the amount of the statutory penalty in the period when the position is taken on the income tax return. If the penalty was not recognized in the period when the position was initially taken, the expense is recognized in the period when the Company changes its judgment about meeting minimum statutory thresholds related to the initial position taken.
Research and Development Expenses
Research and development expenses are charges to expense as incurred. Research and development expenses include, but are not limited to, product development, clinical and regulatory expenses, payroll and other personnel expenses, materials, supplies, consulting costs, and non-recurring engineering costs. These expenses are assigned to the research, development, and clinical projects to develop the Company’s implantable neurostimulation, sensing, and recording technology for a variety of clinical therapeutic applications, and for manufacturing product development.
The Company has been awarded grants subsidies for ongoing research and development projects from the National Institutes of Health Department of Health and Human Services, through the Public Service of Wallonia - Department of Technology Development and the Research Programs Department (the Wallonia region is located in South Brussels, in Belgium), the Cancer Prevention and Research Institute of Texas and the Puerto Rico Science, Technology and Research Trust to support our research projects with potential for commercialization. The Company receives the funding in a combination of advance payments at commencement of a project and through reimbursement requests. Invoices for applicable research, and development expenses as expenses are incurred. These grants and subsidies provide non-dilutive funds that do not include a repayment obligation. Participation by the granting agency typically accounts for 50% to 100% of the project costs in grants or subsidies.
F-34
The Company recognizes the amounts receivable in regard to the grant contracts at fair value when there is reasonable assurance that the contract amount will be received and that all the conditions of the specific contract will be complied with in order to properly match the reimbursements with the specific expenditures that the specific contract intends to reimburse. The Company recognizes the amounts received in accordance with the contracts as a reduction of research and development expenses over the periods necessary to match the contract on a systematic basis to the costs that it is intended to compensate. The Company records, on the balance sheet, grants receivable (upon meeting the criteria discussed above) until cash is received. Where the Company receives payments in advance, it is recorded as advance grant payments on the balance sheet, and relieved against research and development expense as the associated costs are incurred.
As of September 30, 2018, the Company has $819,615 in grants receivable for project expenses invoiced and to be invoiced, but not yet paid, which have been recorded as a reduction of research and development expense in the accompanying statement of operations, and $568,844 in advance payments received and yet to be expended.
Foreign Currency Translation and Transactions
The Company’s reporting currency is the U.S. dollar. The Company’s operations in Belgium use their local currencies as their functional currency. The financial statements in foreign currency are translated into U.S. Dollars (“USD”) in accordance with ASC Topic 830, Foreign Currency Translation. All assets and liabilities are translated at the period-end currency exchange rate. Stockholders’ equity items are translated at the historical rates, and income statement items are translated at the average exchange rate prevailing during the period. Translation adjustments resulting from this process are reported under other comprehensive income (“OCI”) in accordance with ASC Topic 220, Reporting Comprehensive Income as a Component of Stockholders’ Equity. Foreign exchange transaction gains and losses are reflected in the statement of comprehensive income.
Fair Value Measurements
The Company adopted the provisions of ASC Topic 820, “Fair Value Measurements and Disclosures,” which defines fair value as used in numerous accounting pronouncements, establishes a framework for measuring fair value, and expands disclosure of fair value measurements.
The estimated fair value of certain financial instruments, including cash and cash equivalents, are carried at historical cost basis, which approximates their fair values because of the short-term nature of these instruments.
ASC 820 defines “fair value” as the exchange price that would be received for an asset or paid to transfer a liability (an exit price) in the principal or most advantageous market for the asset or liability in an orderly transaction between market participants on the measurement date. ASC 820 also establishes a fair value hierarchy, which requires an entity to maximize the use of observable inputs and minimize the use of unobservable inputs when measuring fair value. ASC 820 describes three levels of inputs that may be used to measure fair value:
Level 1 — Quoted prices in active markets for identical assets or liabilities
Level 2 — Quoted prices for similar assets and liabilities in active markets, or inputs that are observable
Level 3 — Inputs that are unobservable (for example, cash flow modeling inputs based on assumptions)
The Company currently has no assets or liabilities valued at fair value on a recurring basis.
Investments in Non-Consolidated Subsidiaries
Investments in non-consolidated entities are accounted for using the equity method or cost basis, depending upon the level of ownership and/or the Company’s ability to exercise significant influence over the operating and financial policies of the investee. When the equity method is used, investments are recorded at original cost, and adjusted periodically to recognize the Company’s proportionate share of the investees’ net income or losses after the date of investment. When net losses from an investment accounted for under the equity method exceed its carrying amount, the investment balance is reduced to zero and additional losses are not provided for. The Company resumes accounting for the investment under the equity method if the entity subsequently reports net income and the Company’s share of that net income exceeds the share of net losses not recognized during the period the equity method was suspended. Investments are written down only when there is clear evidence that a decline in value that is other than temporary has occurred. The Company accounts for its investment in MicroTransponder, Inc. under the cost method due to the lack of significant influence.
Leases
Leases are reviewed and classified as capital or operating at their inception in accordance with ASC Topic 840, Accounting for Leases. For leases that contain rent escalations, the Company records monthly rent expense equal to the total amount of the payments due in the reporting period over the lease term. The difference between rent expense recorded and the amount paid is credited or charged to deferred rent account when presented on balance sheet.
F-35
Acquired Intangibles
Acquired intangibles include patents, patent licenses, trade secrets and know-how, and customer relationships acquired by the Company, which are recorded at fair value and are assigned an estimated useful life, and amortized on a straight-line basis over their estimated useful lives (ranging from 3 to 19 years) for assets with definitive lives. The Company periodically evaluates whether current facts or circumstances indicate that the carrying values of its acquired intangibles may not be recoverable. If such circumstances are determined to exist, an estimate of the undiscounted future cash flows of these assets, or appropriate asset groupings, is compared to the carrying value to determine whether an impairment exists. If the asset is determined to be impaired, the loss is measured based on the difference between the carrying value of the intangible asset and its fair value, which is determined based on the net present value of estimated future cash flows.
Common stock Purchase Warrants and Other Derivative Financial Instruments
The Company classifies as equity any contracts that require physical settlement or net-share settlement or provide us a choice of net-cash settlement or settlement in our own shares (physical settlement or net-share settlement), provided that such contracts are indexed to our own stock, as defined in ASC 815-40 “Contracts in Entity’s Own Equity.” We classify as assets or liabilities any contracts that require net-cash settlement (including a requirement to net-cash settle the contract if an event occurs and if that event is outside our control) or give the counterparty a choice of net-cash settlement or settlement in shares (physical settlement or net-share settlement). We assess classification of our common stock, par value $0.001 per share (“Common stock”) purchase warrants at each reporting date to determine whether a change in classification between assets and liabilities is required.
Stock-Based Compensation
ASC 718 requires companies to measure all stock compensation awards using a fair value method, and to recognize the related compensation cost in its financial statements. Beginning with the Company’s quarterly period that began on January 1, 2016, the Company adopted the provisions of FASB ASC 718, and expenses the fair value of employee stock options and similar awards in the financial statements. The Company accounts for share-based payments in accordance with ASC 718, “Compensation - Stock Compensation,” which requires all share-based payments to employees, including grants of employee stock options, to be recognized in the financial statements based on the grant date fair value of the award. In accordance with ASC 718-10-30-9, “Measurement Objective – Fair Value at Grant Date,” the Company estimates the fair value of the award using the Black-Scholes option pricing model for valuation of the share-based payments. The Company believes this model provides the best estimate of fair value due to its ability to incorporate inputs that change over time, such as volatility and interest rates, and to allow for actual exercise behavior of option holders. The simplified method is used to determine compensation expense since historical option exercise experience is limited relative to the number of options issued. The compensation cost is recognized ratably using the straight-line method over the expected vesting period.
The Company accounts for stock-based compensation to other than employees in accordance with FASB ASC 505-50. Equity instruments issued to other than employees are valued at the earlier of a commitment date or upon completion of the services, based on the fair value of the equity instruments, and is recognized as expense over the service period.
During the nine months ended September 30, 2018 and 2017, the Company recognized stock-based compensation expense aggregating $362,482 and $217,605, respectively, for Common stock options issued to Company personnel, directors, and consultants. During the nine months ended September 30, 2018 and 2017, the Company paid stock-based compensation consisting of restricted Common stock to non-employees consultants and issued or recorded as Equity instruments to be issued an aggregate of $112,561 and $0, respectively.
Recently Issued Accounting Pronouncements
Management does not believe that any recently issued but not yet effective accounting pronouncements, if adopted, would have a material effect on the accompanying financial statements.
Other recent accounting pronouncements issued by the FASB (including its Emerging Issues Task Force), the AICPA, and the SEC during the current reporting period did not have, or are not believed by management to have, a material impact on the Company’s present or future consolidated financial statements.
F-36
NOTE 3 – BUSINESS COMBINATIONS
On September 1, 2017 (the “Acquisition Date”), the Company, through its wholly owned subsidiary Nexeon Europe, completed the acquisition of NMB pursuant to an acquisition agreement (the “Acquisition Agreement”) entered into on January 10, 2017, between Rosellini Scientific, LLC (“RS”), a Texas limited liability company controlled by our chief executive officer, William Rosellini, and Nexeon Europe (the “Acquisition”). RS was the sole shareholder of NMB, owning 107,154 shares (the “NMB Shares”). Pursuant to the Acquisition Agreement, RS granted to Nexeon Europe the exclusive and irrevocable right to purchase the NMB Shares upon the terms and conditions set forth in the Acquisition Agreement (the “Right to Purchase”). The consideration for the Right to Purchase was USD $1,000 (the “Acquisition Price”). Upon Nexeon Europe exercising the Right to Purchase, the Acquisition Agreement was automatically deemed converted into and considered a share transfer agreement for the purchase of the NMB Shares, and the Acquisition Price became the purchase price of the NMB Shares and was deemed to have been satisfied by Nexeon Europe to RS as of the date of the Acquisition Date.
Due to RS controlling both the Company and NMB, the acquisition has been recorded as a combination of entities under common control, and the results of NMB for the three and nine months ended September 30, 2018 and 2017 are reported retrospectively on a consolidated basis in the Company’s financial statements.
Included in the acquisition of NMB are its wholly owned subsidiaries, Medi-Line and its holding company INGEST. On August 30, 2017, NMB acquired INGEST and Medi-Line for $1,648,240 (payable as €1,450,000 EUR cash), or $977.996 (€891,496 EUR) net of cash acquired. As part of the transaction, and prior to the acquisition, Nexeon Europe loaned NMB $970,400 (€818,075 EUR) pursuant to the existing loan agreement and promissory note, NMB secured a credit facility in the amount of $330,319 (€275,000 EUR), and Medi-Line loaned NMB $540,032 (€450,000 EUR). Payment of the purchase price included the settlement of a note payable in the amount of $120,007 (€100,000 EUR) and a dividend payable in the amount of $9,901 (€8,250 EUR) to the sellers of INGEST. The balance of the loan and all accrued interest related to the loan agreement and promissory note between Nexeon Europe and NMB, along with the $540,032 (€450,000 EUR) loan from Medi-Line to NMB, is eliminated through consolidation in the financial statements.
We believe Medi-Line provides the medical device manufacturing expertise and experience needed to scale our business. Medi-Line is a leading global source of innovative medical device solutions, with existing customers that include Fortune 50 companies and neurostimulator companies. Medi-Line seeks to provide high quality and efficiency in the development, engineering, and manufacturing of medical devices for the med-tech and pharmaceutical industries.
The acquisition of INGEST and Medi-Line was accounted for using the acquisition method, and, accordingly, the results of operations of INGEST and Medi-Line were reported in the Company’s financial statements beginning on August 30, 2017, the date of acquisition.
Unaudited Pro Forma Consolidated Results
The following table provides unaudited pro forma results of operations for the three and nine months ended September 30, 2018 and 2017, as if INGEST and Medi-line had been acquired as of January 1, 2017. The pro forma results include the effect of certain purchase accounting adjustments, such as the estimated changes in depreciation and amortization expense on the acquired tangible and intangible assets, and the recognition of grant subsidies. Pro forma results do not include any anticipated cost savings or other effects of the planned integration of INGEST and Medi-Line. Accordingly, such amounts are not necessarily indicative of the results if the acquisition had occurred on the dates indicated, or which may occur in the future.
| For the Three Months Ended | For the Nine Months Ended | |||||||||||||||
| September 30, | September 30, | |||||||||||||||
| 2018 | 2017 | 2018 | 2017 | |||||||||||||
| Revenues | $ | 1,977,629 | $ | 1,985,067 | $ | 7,666,827 | $ | 5,716,007 | ||||||||
| Net income (loss) | (1,391,547 | ) | (438,503 | ) | (3,067,224 | ) | (3,408,754 | ) | ||||||||
| Net income (loss) per common share, basic and diluted | (0.71 | ) | (0.23 | ) | (1.56 | ) | (1.97 | ) | ||||||||
NOTE 4 – GOING CONCERN
The accompanying financial statements have been prepared in conformity with generally accepted accounting principles, which contemplate continuation of the Company as a going concern. The Company has sustained operating losses since inception, and has an accumulated deficit of $6,810,662 and a working capital deficit of $1,928,566 at September 30, 2018. In addition, the Company does not have sufficient continuing revenue to cover its future operating expenses. The Company currently has limited liquidity, and has not completed its efforts to establish an additional source of revenues sufficient to cover all of the projected operating costs of the ongoing neurostimulation research and development activities over an extended period of time. These factors, among others, raise substantial doubt about the Company’s ability to continue as a going concern. The accompanying consolidated financial statements do not include any adjustments relating to the recoverability and classification of recorded asset amounts and classification of liabilities should the Company be unable to continue as a going concern. The Company will need to seek additional financing for continued operations, but there is no guarantee such financing will be available or on terms favorable to the Company. In the third quarter of 2018, the Company began to consolidate its manufacturing facilities in Niel, Belgium with its Medi-Line operations in Liege, Belgium. These operations include a facility, equipment, research and development staff, general and administrative. There is no guarantee these reductions by the Company will alleviate the going concern.
F-37
NOTE 5 – LOANS AND LEASES
Loans and leases consist of the following as of September 30, 2018:
Notes Payable
12.00% Senior Secured Convertible Promissory Note:
On August 21, 2017, the Company entered into a securities purchase agreement with Leonite Capital, LLC (“LC”), a Delaware limited liability company, to provide the Company with additional resources to conduct its business. Pursuant to the securities purchase agreement, LC purchased a unit consisting of (i) a note in the principal amount of $1,120,000 at an original issue discount of $120,000, (ii) warrants to purchase 35,716 shares of the Company’s Common stock, and (iii) commitment shares equaling 7,143 shares of the Company’s restricted common stock, valued at $100,000. Interest is at the rate of 12.00% per annum, and the maturity date is 24 months from the date of issue. The note is a senior secured obligation of the Company, with priority over all future indebtedness of the Company. LC shall have the right at any time, at LC’s option, to convert all or any part of the outstanding and unpaid principal amount and accrued and unpaid interest of the note into fully paid and non-assessable shares of Common stock, or any shares of capital stock, subject to beneficial ownership limitations of a maximum of 4.99% of outstanding Common stock of the Company at time of conversion. The conversion price shall be, at the option of LC, $24.75, subject to a one-time re-pricing 275 days after the closing, or (ii) 80% multiplied by the price per share paid by the investors in a subsequent equity financing. An amount of $274,266 was recorded on the balance sheet as an original discount including a $120,000 original discount, $100,000 in restricted common stock and $54,266 as the fair value of the warrants issued in the transaction. The $274,266 will be expensed as interest expense over the 24-month term of the loan. For the LC loan, $1,120,000 is recorded as Current portion of long-term debt, net of original discount. $(125,705) of the original discount is recorded as Current portion of long-term debt, net of original discount on the balance sheet.
1.27% Secured bank Loan:
On August 29, 2017, Medi-Line entered into a credit contract with CBC Banque SA (“CBC Banque”) in the original amount of approximately $2,036,362 (€1,700,000 EUR). The loan is secured by a mortgage on the Medi-Line manufacturing facility, and carries an interest rate of 1.27% per annum, with a seven-year term having monthly payments of interest and principal of approximately $23,365 (€21,175 EUR). $297,507 of the outstanding balance is recorded as Current portion of long-term debt, net of original discount on the balance sheet, and $1,456,086 is recorded as Long-term debt, net of original discount on the balance sheet.
1.27% Secured Bank Loan:
On August 29, 2017, NMB entered into a credit contract with CBC Banque in the original amount of approximately $329,412 (€275,000 EUR). The loan carries an interest rate of 1.27% per annum, with a seven-year term having monthly payments of interest and principal of approximately $4,103 (€ 3,425 EUR). The loan is secured by the shares of NMB. $14,562 of the outstanding balance is recorded as Current portion of long-term debt, net of original discount portion on the balance sheet, and $285,061 is recorded as Long-term debt, net of original discount on the balance sheet.
0.72% Secured Bank Loan:
On May 7, 2016, Medi-Line entered into a credit contract with CBC Banque in the original amount of approximately $68,781 (€57,420 EUR). The loan carries an interest rate of 0.72% per annum, with a 48-month term having monthly payments of interest and principal of approximately $1,454 (€ 1,214 EUR). The loan is secured by the assets of Medi-Line. Proceeds of the loan were used to acquire manufacturing equipment. The loan is secured by the shares of NMB. $16,745 of the outstanding balance is recorded as Current portion of long-term debt, net of original discount on the balance sheet, and $13,458 is recorded as Long-term debt, net of original discount on the balance sheet.
Loan Subsidy:
NMB was awarded a loan subsidy through the Public Service of Wallonia in the amount of $598,665 (€499,779 EUR). Of the total amount awarded, $179,600 (€149,934 EUR) is categorized as loan, with repayment amounts ranging from $5,986 to $23,947 annually from 2018 through 2032. The current portion of the liability is recorded as Current portion of long-term debt, net of original discount on the balance sheet in the amount of $5,987, and $173,613 is included as Long-term debt, net of original discount on the balance sheet. The award amounts in excess of the loan amount are invoiced for reimbursement and recorded as a credit to applicable research and development expenses.
Revolving Credit:
The Company has a revolving credit card with BB&T Financial with an outstanding balance of $12,847 as of September 30, 2018, a credit limit of $60,000, and a current APR of 25.4%; and a revolving credit card with Comerica Bank with an outstanding balance of $11,390 as of September 30, 2018, a credit limit of $11,000, and a current APR of 0%.
F-38
Floating Rate Secured Line of Credit:
On February 23, 2018, Medi-Line’s line of credit with CBC Banque was amended to increase the advance amount to €300,000 ($369,561) and to structure the financing as a straight loan with an interest rate of 1.25% above the EURIBOR rate for the period the funds are drawn down. The €300,000 was be available for drawdown through April 30, 2018, at which point the facility was reduced to €200,000, and further reduced €100,000 on May 31, 2018. The security includes a pledge of Medi-Line business assets in the amount of €300,000. The outstanding balance as of September 30, 2018 in the amount of $116,012 is recorded as Current portion of long-term debt, net of original discount on the balance sheet.
Capital Leases
Building Lease:
On December 13, 2005, Medi-Line entered into a capital lease facility for the financing of the manufacturing facility construction in the amount of $3,425,880 (€2,860,000 EUR), with a 15-year term. Quarterly lease payments excluding VAT are $46,730 (€39,202 EUR). The Company has the right to purchase the building at the end of the lease term for three percent (3%) of the original lease amount. $181.239 of the outstanding balance is recorded as Current portion of long-term debt, net of original discount, and $426,448 is recorded as Long-term debt, net of original discount on the balance sheet.
Equipment Lease:
On February 4, 2015, the Company entered into a sale-leaseback transaction with Biotech Coaching S.A. for the sale and lease in the original amount of $131,765 (€110,000 EUR) for medical and clean-room equipment. In March 2015, the Company commenced leasing the equipment, with a 36-month term. Monthly lease payments excluding VAT are $3,824 (€3,192 EUR). The Company has the right to purchase the equipment at the end of the lease term for a residual value of $1,579 (€1,318 EUR). The remaining balance of the lease in the amount of $23,083 is recorded as Current portion of long-term debt, net of original discount on the balance sheet.
| Carrying Amount | ||||
| Long-Term Debt | ||||
| 12.00% Senior Convertible Secured Note, amortization begins 2018, 2019 maturity | 1,120,000 | |||
| 1.27% Secured Bank Loan, monthly amortization, 2024 maturity | 1,753,593 | |||
| 1.27% Secured Bank Loan, monthly amortization, 2024 maturity | 272,623 | |||
| 0.72% Secured Bank Loan, monthly amortization, 2020 maturity | 30,203 | |||
| Floating Rate Secured Line of Credit | 116,012 | |||
| Loan Subsidy, amortization begins 2018, 2032 maturity | 179,600 | |||
| Revolving Credit | 24,237 | |||
| Capitalized Building Lease | 607,687 | |||
| Capitalized Equipment Lease | 23,083 | |||
| Less: Original purchase discount, net of amortization | (125,705 | ) | ||
| Total Debt | 4,001,333 | |||
| Less: Current portion of debt, net of original discount current portion | (1,673,667 | ) | ||
| Total Long-Term Debt | $ | 2,327,666 | ||
KBC Accounts Receivable Discounting Agreement:
Medi-Line has an accounts receivable discounting agreement with KBC Commercial Finance, NV (“KBC ComFin”) for up to 35% of Medi-Line’s customer accounts receivables with no concentration limits per customer. Pursuant to the discounting agreement, Medi-Line will transfer title to KBC ComFin for all receivables that fall under the scope of agreement. The fee for the advance portion of the receivables transferred to KBC ComFin is the two-month LIBOR plus 1.5% on annual basis. As KBC ComFin holds the title to the receivables and assumes the insolvency risk for receivables that are transferred and fall under the scope of the agreement, invoices transferred per the agreement are reduced from Medi-line’s customer accounts receivable upon transfer and recorded to a KBC ComFin accounts receivable sub-account and netted against advances and final payments received per the agreement.
NOTE 6 – INCOME TAXES
The Company is incorporated in the United States of America, and is subject to United States federal taxation. No provisions for United States income taxes have been made, as the Company had no U.S. taxable income for the nine months ended September 30, 2018 and 2017. The effective income tax rate for the Company for the three months ended September 30, 2018 and 2017 were 21% and 34%, respectively. One of our subsidiaries generated income, and as of September 30, 2018 we accrued income tax in the amount of $11,928 according to the Belgian corporate income tax rate and the other operating subsidiaries reported a loss and no tax provision was recorded. Beginning in 2018, the corporate income tax (“CIT”) levied in Belgium has been reduced to an effective rate of 29.58%. No state, region, or municipal income tax is levied.
F-39
During nine months ended September 30, 2018, the Company’s holding company subsidiary, NXEU, was assessed annual corporate taxes in the amount of $5,038.
As of September 30, 2018, the Company has approximately $8,485,737 of net operating losses (“NOL”) carryovers to offset taxable income, if any, in future years, which expire in fiscal 2036. In assessing the realization of deferred tax assets, management considers whether it is more likely than not that some portion or all of the deferred tax assets will be realized. The ultimate realization of deferred tax assets is dependent upon the generation of future taxable income during the periods in which those temporary differences become deductible. Management considers the scheduled reversal of deferred tax liabilities, projected future taxable income, and tax planning strategies in making this assessment. Based on the assessment, management has established a full valuation allowance against all of the deferred tax assets relating to the NOL period because it is more likely than not that all of the deferred tax assets will not be realized.
On December 22, 2017, the Tax Cuts and Jobs Act of 2017 (the “Tax Act”) was signed into law, making significant changes to the Internal Revenue Code. Changes include, but are not limited to, a federal corporate tax rate decrease from 35% to 21% for tax years beginning after December 31, 2017; the transition of U.S international taxation from a worldwide tax system to a territorial system; and a one-time transition tax on the mandatory deemed repatriation of foreign earnings. We have estimated our provision for income taxes in accordance with the Tax Act and guidance available as of the date of this filing, but have kept the full valuation allowance. As a result, we have recorded no United States income tax expense in the nine months ended September 30, 2018.
The Belgian government enacted in December 2017 a significant tax reform law. The new tax legislation contains several key tax provisions, including the reduction of the corporate income tax rate from the current 33.99% to 29.58% in 2018 and 2019, and 25% from 2021. Additionally, the use of net operating losses (which could previously offset 100% of taxable income) is now limited to offset only 70% of taxable income.
On December 27, 2017, NXPROC was granted a tax exemption pursuant to Act number 73-2008 (“ACT 73”) by the Government of Puerto Rico, Department of Economic Development and Commerce (“PRIDCO”). The exemption allows NXPROC to obtain tax credits in the amount of fifty percent (50%) of approved applicable research and development expenses of NXPROC. As of July 23, 2018, the Company has received all government approvals and certifications from PRIDCO and received tax credits in the amount of $732,340, for research and development activity in 2017, which had been posted to Departamento De Hacienda (the Puerto Rican Department of Finance) system and the Company has received $593,195 in net proceeds from the sale of all available tax credits realizing proceeds of 81% of the face value of the tax credits. For the nine months ended September 30, 2018, the Company recorded a benefit to provision for income taxes in the amount of $593,195.
NOTE 7 – PROPERTY PLANT and EQUIPMENT
Property plant and equipment at cost and accumulated depreciation as of September 30, 2018 and December 31, 2017 were:
| Estimated useful lives | September 30, 2018 | December 31, 2017 | ||||||||
| Land | $ | 96,884 | $ | 96,884 | ||||||
| Capitalized building | 39 years | 3,017,552 | 3,017,552 | |||||||
| Machinery and equipment | 5 to 15 years | 739,088 | 677,734 | |||||||
| Total property plant and equipment – gross | 3,853,524 | 3,792,170 | ||||||||
| Less: accumulated depreciation | (404,057 | ) | (222,338 | ) | ||||||
| Total property plant and equipment – net | $ | 3,449,467 | $ | 3,569,832 | ||||||
Property plant and equipment depreciation expense for the nine months ended September 30, 2018 was $186,834, and for the nine months ended September 3, 2017 was $63,413.
NOTE 8 – INTANGIBLE ASSETS
Intangible assets that have finite useful lives are amortized over their estimated useful lives. Intangible assets as of September 30, 2018 and December 31, 2017 are as follows:
| Estimated useful lives | September 30, 2018 | December 31, 2017 | ||||||||
| Intangible assets with definitive lives: | ||||||||||
| Patents, licenses, and intellectual property | 4 to 20 years | $ | 10,363,097 | $ | 10,363,097 | |||||
| Fair value of customer relationships at acquisition | 10 years | 600,000 | 600,000 | |||||||
| Less: accumulated amortization | (2,679,450 | ) | (1,773,605 | ) | ||||||
| Patents, licenses, and intellectual property – net | 8,283,647 | 9,189,492 | ||||||||
| Intangible assets with indefinite lives: | ||||||||||
| Fair value of trade secrets and know-how at acquisition | 1,550,000 | 1,550,000 | ||||||||
Intangible asset amortization expense for the nine months ended September 30, 2018 was $909,241, and for the nine months ended September 30, 2017 was $861,377.
F-40
NOTE 9 – INVENTORIES
Inventory balances as of September 30, 2018 and December 31, 2017 are as follow:
| September 30, 2018 | December 31, 2017 | |||||||
| Raw materials and supplies | $ | 1,688,415 | $ | 1,811,749 | ||||
| Work in process | 732,573 | 334,322 | ||||||
| Finished goods | — | 60,499 | ||||||
| Total inventories | $ | 2,420,988 | $ | 2,206,570 | ||||
NOTE 10 – SEGMENTS OF BUSINESS
The Company operates in two distinct business segments within the medical device industry: manufacturing and neurostimulation.
The manufacturing segment includes the manufacturing operations of our wholly owned subsidiary Medi-Line, located in Angleur (Liege), Belgium. Medi-Line manufactures single-use medical devices for the medical and pharmaceutical sectors, including radiopharmacy technology, urology products, and sterilization cases and trays, and designs, develops, and offers worldwide production and supply-chain capabilities for these products to its customers.
The neurostimulation segment includes development, manufacturing, and commercialization of neurostimulation technology for the treatment of various neurological disorders through electrical stimulation of neural tissues. Our first commercial application of the platform will be the Viant™ Deep Brain Stimulation System. Operations for the neurostimulation segment are conducted in the United States, Puerto Rico, Belgium, and Germany.
Other items of revenue not directly related to manufacturing or neurostimulation revenues are categorized as other operating income. Other operating income and expenses not directly related to a specific segment are identified as “other,” and not allocated to segments.
An analysis and reconciliation of the Company’s business segments and geographic information to the respective information in the Condensed Consolidated Financial Statements follows. Revenue by geographic area are presented by allocating revenue from external customers based on where the products are shipped or services are rendered:
Revenue by Segment:
| For the Three Months Ended | For the Nine Months Ended | |||||||||||||||
| September 30, | September 30, | |||||||||||||||
| 2018 | 2017 | 2018 | 2017 | |||||||||||||
| Manufacturing | $ | 1,938,908 | $ | 816,325 | $ | 7,119,557 | $ | 816,325 | ||||||||
| Neurostimulation | 5,003 | 202,362 | 400,055 | 292,936 | ||||||||||||
| Other | 33,718 | 20,992 | 147,215 | 38,027 | ||||||||||||
| Consolidated total | $ | 1,977,629 | $ | 1,039,679 | $ | 7,666,827 | $ | 1,147,288 | ||||||||
Income (Loss) Before Income Tax by Segment:
| For the Three Months Ended | For the Nine Months Ended | |||||||||||||||
| September 30, | September 30, | |||||||||||||||
| 2018 | 2017 | 2018 | 2017 | |||||||||||||
| Manufacturing | $ | 48,982 | $ | 114,720 | $ | 223,100 | $ | 114,720 | ||||||||
| Neurostimulation | (1,386,884 | ) | (1,070,982 | ) | (3,892,894 | ) | (4,144,691 | ) | ||||||||
| Other (1) | (59,155 | ) | 532,010 | 26,346 | 425,967 | |||||||||||
| Consolidated total | $ | (1,397,057 | ) | $ | (424,252 | ) | $ | (3,643,448 | ) | $ | (3,604,004 | ) | ||||
(1) Amounts not allocated to segments include interest income (expense) and other income (expense), and amortization of acquisition intangible assets.
F-41
Sales by Geographic Area:
| For the Three Months Ended | For the Nine Months Ended | |||||||||||||||
| September 30, | September 30, | |||||||||||||||
| 2018 | 2017 | 2018 | 2017 | |||||||||||||
| Sales Non-domestic locations | ||||||||||||||||
| United Kingdom | $ | 886,886 | $ | 519,364 | $ | 3,530,395 | $ | 609,938 | ||||||||
| Belgium | 557,079 | 229,817 | 2,286,767 | 229,817 | ||||||||||||
| Switzerland | 166,674 | 59,253 | 563,641 | 59,253 | ||||||||||||
| Netherlands | 184,161 | 85,763 | 533,329 | 85,763 | ||||||||||||
| Norway | 142,470 | 120,094 | 487,640 | 120,094 | ||||||||||||
| Rest of world | 6,641 | 4,396 | 117,840 | 4,396 | ||||||||||||
| Consolidated sales | 1,943,911 | 1,018,687 | 7,519,612 | 1,109,261 | ||||||||||||
| Other operating revenue | 33,718 | 20,992 | 147,215 | 38,027 | ||||||||||||
| Consolidated revenue | $ | 1,977,629 | $ | 1,039,679 | $ | 7,666,827 | $ | 1,147,288 | ||||||||
Long-Lived Assets:
| September 30, | December 31, | |||||||
| 2018 | 2017 | |||||||
| Manufacturing | $ | 3,422,963 | $ | 3,535,516 | ||||
| Neurostimulation | 7,774,609 | 8,643,118 | ||||||
| Other | 2,085,542 | 2,130,690 | ||||||
| Consolidated total | $ | 13,283,114 | $ | 14,309,324 | ||||
NOTE 11 – EQUITY
We effected a 1-for-14 reverse stock split of our outstanding Common stock, or, the “Reverse Stock Split”, on June 25, 2018 and, unless otherwise indicated, all per share amounts set forth herein have been retroactively restated to reflect the Reverse Stock Split.
The Company issued the following securities during the nine months ended September 30, 2018:
Common Stock Issuances
On March 6, 2018, we issued an aggregate of 1,697 shares of restricted common stock for certain sales and marketing and software consulting services rendered by third-party consultants. The foregoing shares were valued at $14,840. 583 of these shares were issued to Daniel Powell, the Company’s vice president of sales and marketing at the time of issuance. These shares were issued for services provided by Mr. Powell prior to his employment by the Company.
On April 19, 2018, we issued an aggregate of 7,195 shares of restricted common stock for certain research and development and valuation services provided by third-party consultants. The foregoing shares were valued at $97,721
Common Stock Redemption
On May 22, 2018, the Company redeemed and cancelled 14,286 shares of its common stock from a former director as consideration for the purchase of certain intellectual property.
Warrants
The Company issued no Warrants for the nine months ended September 30, 2018.
Options Grants – 2016 Plan
The Company may, from time to time, issue certain equity awards pursuant to our 2016 Omnibus Incentive Plan (the “2016 Plan”). The 2016 Plan was adopted by our board of directors on January 2, 2016, and was subsequently approved by our shareholders. On July 8, 2018, the Board of Directors of the Company approved an increase in the number of shares of common stock reserved for issuance pursuant to option grants under the 2016 Plan to 450,000 shares of common stock .
F-42
During the nine months ended September 30, 2018, the Company issued stock options to purchase a total of 138,198 shares of the Company’s common stock under the 2016 Plan, with exercise prices ranging from $8.00 to $20.00 per share, and cancelled stock options to purchase a total of 54,898 shares of the Company’s common stock under the 2016 Plan, with exercise prices ranging from $11.00 to $17.50 per share, as follows:
| (i) | On February 28, 2018 and as compensation for service to the Company as chief executive officer, the Company granted to William Rosellini an incentive stock option to purchase up to 17,858 shares of the Company’s restricted common stock with an exercise price of $10.64. The option to purchase 8,929 shares of common stock was immediately exercisable, and the option to purchase the remaining 8,929 shares of common stock vests on the anniversary of the grant date. The Company also granted a non-qualified stock option to purchase up to 64,286 shares of common stock with an exercise price of $10.64 per share. The option to purchase 2,679 common shares vests in equal monthly amounts beginning on March 1, 2018. The option to purchase the Company’s common stock expires three (3) years from the date they become exercisable pursuant to the grant vesting schedule. The fair value of the options was determined to be $226,009 using the Black-Scholes Option Pricing Model. |
| (ii) | On February 28, 2018 and as compensation for service to the Company as president and chief commercial officer, the Company granted to Brian Blischak a non-qualified stock option to purchase up to 4,108 shares of the Company’s restricted common stock with an exercise price of $10.64 per share. The option was immediately exercisable at date of issue. The term of the option shall be for a period of eight (8) years from the date of issue. The fair value of the option was determined to be $12,855 using the Black-Scholes Option Pricing Model. |
| (iii) | On February 28, 2018 and as compensation for service to the Company as chief financial officer, the Company granted to Christopher Miller a non-qualified stock option to purchase up to 2,143 shares of the Company’s restricted common stock with an exercise price of $10.64 per share. The option was immediately exercisable at date of issue. The term of the option shall be for a period of three (3) years from the date of issue. The fair value of the option was determined to be $6,766 using the Black-Scholes Option Pricing Model. |
| (iv) | On February 28, 2018 and as compensation for service to the Company as vice president sales and marketing, the Company granted to Daniel Powell an incentive stock option to purchase up to 786 shares of the Company’s restricted common stock with an exercise price of $10.64 per share. The option was immediately exercisable at date of issue. The term of the option shall be for a period of three (3) years from the date of issue. The fair value of the option was determined to be $2,481 using the Black-Scholes Option Pricing Model. |
| (v) | On February 28, 2018 and as compensation for their service to the Company, the Company granted to non-executive employees incentive stock options to purchase up to 3,301 shares of the Company’s restricted common stock with an exercise price of $10.64 per share. The option to purchase 2,229 shares was immediately exercisable at date of issue, and the option to purchase 1,072 shares of common stock vests in equal monthly amounts beginning on March 1, 2018. The option to purchase the Company’s common stock expires three (3) years from the date they become exercisable pursuant to the grant vesting schedule. The fair value of these options was determined to be $10,420 using the Black-Scholes Option Pricing Model. |
| (vi) | As compensation for service as a director of the Company, the Company granted to Kent J. George non-qualified stock options to purchase 893 shares of the Company’s restricted common stock on each date of March 31, 2018, June 30, 2018 and September 30, 2018 with exercise prices of $12.11, $20.00, and $8.00 per share respectively. The options were immediately exercisable at date of issue. The term of the options shall be for four (4) years from the date of issue. The fair value of the options granted were determined to be $12,034 using the Black-Scholes Option Pricing Model. |
| (vii) | As compensation for service as a director of the Company, the Company granted to Michael Neitzel non-qualified stock options to purchase 893 shares of the Company’s restricted common stock on each date of March 31, 2018, June 30, 2018 and September 30, 2018 with exercise prices of $12.11, $20.00, and $8.00 per share respectively. The options were immediately exercisable at date of issue. The term of the options shall be for four (4) years from the date of issue. The fair value of the options granted were determined to be $12,034 using the Black-Scholes Option Pricing Model. |
| (viii) | As compensation for service as a director of the Company, the Company granted to Wes Dittmer non-qualified stock options to purchase 893 shares of the Company’s restricted common stock common stock on each date of June 30, 2018 and on September 30, 2018 with exercise prices of $20.00 and $8.00 per share respectively. The options were immediately exercisable at date of issue. The term of the options shall be for four (4) years from the date of issue. The term of the options shall be for four (4) years from the date of issue. The fair value of the options granted were determined to be $8,439 using the Black-Scholes Option Pricing Model. |
F-43
| (ix) | On July 22, 2018, the Company granted to a new employee at NXPROC incentive stock options to purchase up to 38,572 shares of the Company’s restricted common stock with an exercise price of $11.00 per share with vesting over 48 months. The term of the option was for a period of four (4) years from the date of vesting. The fair value of the option was determined to be $92,789 using the Black-Scholes Option Pricing Model. Prior to vesting the option to purchase 38,572 shares was cancelled. |
| (x) | During the three months ended September 30, 2018, incentive stock options to purchase 16,326 shares of the Company’s restricted common stock were cancelled pursuant to the 2016 Plan for employee separations from the Company. The range of exercise prices for these cancelled options ranged from $14.00 to $17.50. |
Unless otherwise stated, the issuance of the above securities were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act or Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or contracts relating to compensation as provided under Rule 701.
The granted options were valued at $383,828 using the Black-Scholes option pricing model, with the following weighted average assumptions:
| Risk-free interest rate | 2.44% | |
| Expected life | 3.48 years | |
| Expected dividends | 0.00% | |
| Expected volatility | 56.12% | |
| Fair value of the Company’s Common stock | $10.89 |
Aggregate options expense recognized for the nine months ended September 30, 2018, was $362,482.
As of September 30, 2018, there were 103,822 shares available for grant under the 2016 Plan, excluding the 346,178 options outstanding.
As of September 30, 2018, there were incentive stock options outstanding to purchase an aggregate of 175,266 shares of common stock, and non-qualified options outstanding to purchase an aggregate of 170,912 shares of the Company’s common stock option activity, both within and outside the 2016 Plan, and Warrant activity for the nine months ended September 30, 2018, are as follows:
| Stock Options | Stock Warrants | |||||||||||||||
| Weighted | Weighted | |||||||||||||||
| Average | Exercise | |||||||||||||||
| Shares | Price | Shares | Price | |||||||||||||
| Outstanding December 31, 2017 | 262,878 | $ | 15.07 | 82,926 | $ | 19.39 | ||||||||||
| Granted | 138,198 | 10.89 | — | — | ||||||||||||
| Canceled | (54,898 | ) | 12.53 | — | — | |||||||||||
| Expired | — | — | — | — | ||||||||||||
| Exercised | — | — | — | — | ||||||||||||
| Outstanding at September 30, 2018 | 346,178 | $ | 13.80 | 82,926 | $ | 19.39 | ||||||||||
| Exercisable at September 30, 2018 | 210,989 | $ | 14.17 | 82,926 | $ | 19.39 | ||||||||||
The range of exercise prices and remaining weighted average life of the options outstanding at September 30, 2018, were $8.00 to $28.00 and 1.44 to 7.44 years, respectively.
The range of exercise prices and remaining weighted average life of the Warrants outstanding at September 30, 2018, were $8.00 to $28.00 and 1.17 to 3.89 years, respectively.
Unless otherwise stated, the issuance of the above securities were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act or Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or contracts relating to compensation as provided under Rule 701.
F-44
NOTE 12 – 2016 OMNIBUS INCENTIVE PLAN
The 2016 Plan was adopted by our board of directors on January 2, 2016 and was subsequently approved by our shareholders. On July 8, 2018, the Board of Directors of the Company approved an increase in the number of shares of common stock reserved for issuance pursuant to option grants under the 2016 Plan to 450,000 shares of common stock. As of September 30, 2018, options to purchase a total of 346,178 shares of the Company’s common stock were outstanding under the 2016 Plan with the following exercise prices and terms at grant date:
| As of September 30, 2018 | ||||||||||||||
| Exercise | Options to | Options to | ||||||||||||
| Price | Purchase Shares | Term (yrs) | Purchase Shares | |||||||||||
| $ | 8.00 | 2,679 | 3 | 193,851 | ||||||||||
| 10.64 | 92,482 | 4 | 66,075 | |||||||||||
| 12.11 | 1,786 | 8 | 86,252 | |||||||||||
| 14.00 | 185,518 | |||||||||||||
| 17.50 | 57,462 | |||||||||||||
| 20.00 | 2,679 | |||||||||||||
| 25.20 | 1,786 | |||||||||||||
| 28.00 | 1,786 | |||||||||||||
| Total Shares | 346,178 | 346,178 | ||||||||||||
The 2016 Plan is administered by the compensation committee which currently consists of three independent directors. The committee performs the requisite duties with respect to awards granted. The committee currently determines to whom awards are made, the timing of any such awards, the type of securities, and number of shares covered by each award, as well as the terms, conditions, performance criteria, restrictions, and other provisions of awards. The committee has the authority to cancel or suspend awards, accelerate the vesting, or extend the exercise period of any awards made pursuant to the 2016 Plan.
Shares Available Under the 2016 Plan
The maximum shares available for issuance under the 2016 Plan are 450,000 shares, subject to adjustment as set forth in the 2016 Plan. Any shares subject to an award that expires, is cancelled or forfeited, or is settled for cash shall, to the extent of such cancelation, forfeiture, expiration, or cash settlement, again become available for awards under the 2016 Plan. The committee can issue awards comprised of restricted stock, stock options, stock appreciation rights, stock units, and other awards, as set forth in the 2016 Plan.
Transferability
Except as otherwise provided in the 2016 Plan, (i) during the lifetime of a participant, only the participant or the participant’s guardian or legal representative may exercise an option or stock appreciation right, or receive payment with respect to any other award, and (ii) no award may be sold, assigned, transferred, exchanged, or encumbered, voluntarily or involuntarily, other than by will or the laws of descent and distribution.
Change in Control
In the event of a merger, the surviving or successor entity (or its parent) may continue, assume, or replace outstanding awards as of the date of the relevant transaction, and such awards or replacements therefore shall remain outstanding and be governed by their respective terms. Such awards or replacements can be executed in part on the condition that the contractual obligations represented by the award are expressly assumed by the surviving or successor entity (or its parent), with appropriate adjustments to the number and type of securities subject to the award and the exercise price thereof so as to preserve the intrinsic value of the award existing at the time of the relevant transaction. Alternatively, the surviving or successor entity (or its parent) could issue to a participant a comparable equity-based award that preserves the intrinsic value of the original award existing at the time of the relevant transaction and contains terms and conditions that are substantially similar to those of the award.
If and to the extent that outstanding awards under the 2016 Plan are not continued, assumed, or replaced in connection with a merger or relevant corporate transaction, then all outstanding awards shall become fully vested and exercisable for such period of time prior to the effective date of the relevant transaction as is deemed fair and equitable by the committee, and shall terminate at the effective date of said transaction.
F-45
NOTE 13 – RELATED PARTY TRANSACTIONS
During the nine months ended September 30, 2018, the Company had the following transactions with related parties.
Common stock Issuance
On March 8, 2018, the Company issued 583 shares of the Company’s restricted Common stock to Daniel Powell, the Company’s vice president sales and marketing, for certain sales and marketing consulting services rendered by Mr. Powell prior to his employment by the Company. The foregoing shares were valued at $5,100.
Options Grants – 2016 Plan
On February 28, 2018, the Company issued the following stock options under the 2016 Plan:
| (i) | As compensation for service to the Company as chief executive officer, the Company granted to William Rosellini an incentive stock option to purchase up to 17,858 shares of the Company’s restricted common stock with an exercise price of $10.64. The option to purchase 8,929 shares of common stock was immediately exercisable, and the option to purchase the remaining 8,929 shares of common stock vests on the anniversary of the grant date. The Company also granted a non-qualified stock option to purchase up to 64,286 shares of common stock with an exercise price of $10.64 per share. The option to purchase 2,679 common shares vests in equal monthly amounts beginning on March 1, 2018. The option to purchase the Company’s common stock expires three (3) years from the date they become exercisable pursuant to the grant vesting schedule. The fair value of the options was determined to be $226,009 using the Black-Scholes Option Pricing Model. |
| (ii) | As compensation for service to the Company as chief commercialization officer, the Company granted to Brian Blischak a non-qualified stock option to purchase up to 4,108 shares of the Company’s restricted common stock with an exercise price of $10.64 per share. The option was immediately exercisable at date of issue. The term of the option shall be for a period of eight (8) years from the date of issue. The fair value of the option was determined to be $12,855 using the Black-Scholes Option Pricing Model. |
| (iii) | As compensation for service to the Company as chief financial officer, the Company granted to Christopher Miller a non-qualified stock option to purchase up to 2,143 shares of the Company’s restricted common stock with an exercise price of $10.64 per share. The option was immediately exercisable at date of issue. The term of the option shall be for a period of three (3) years from the date of issue. The fair value of the option was determined to be $6,766 using the Black-Scholes Option Pricing Model. |
| (iv) | As compensation for service to the Company as vice president sales and marketing, the Company granted to Daniel Powell an incentive stock option to purchase up to 786 shares of the Company’s restricted common stock with an exercise price of $10.64 per share. The option was immediately exercisable at date of issue. The term of the option shall be for a period of three (3) years from the date of issue. The fair value of the option was determined to be $2,481 using the Black-Scholes Option Pricing Model. |
On March 31, 2018, the Company issued the following stock options under the 2016 Plan:
| (i) | As compensation for service as a director of the Company, the Company granted to Kent J. George a non-qualified stock option to purchase a total of 893 shares of the Company’s restricted common stock with an exercise price of $12.11 per share. The option was immediately exercisable at date of issue. The term of the option shall be for four (4) years from the date of issue. The fair value of the option was determined to be $3,596 using the Black-Scholes Option Pricing Model. |
| (ii) | As compensation for service as a director of the Company, the Company granted to Michael Neitzel a non-qualified stock option to purchase a total of 893 shares of the Company’s restricted Common stock with an exercise price of $12.11 per share. The option was immediately exercisable at date of issue. The term of the option shall be for four (4) years from the date of issue. The fair value of the option was determined to be $3,596 using the Black-Scholes Option Pricing Model. |
On June 30, 2018, the Company issued the following stock options under the 2016 Plan:
| (i) | As compensation for service as a director of the Company, the Company granted to Kent J. George a non-qualified stock option to purchase a total of 893 shares of the Company’s restricted common stock with an exercise price of $20.00 per share. The option was immediately exercisable at date of issue. The term of the option shall be for four (4) years from the date of issue. The fair value of the option was determined to be $5,778 using the Black-Scholes Option Pricing Model. |
| (ii) | As compensation for service as a director of the Company, the Company granted to Michael Neitzel a non-qualified stock option to purchase a total of 893 shares of the Company’s restricted common stock with an exercise price of $20.00 per share. The option was immediately exercisable at date of issue. The term of the option shall be for four (4) years from the date of issue. The fair value of the option was determined to be $5,778 using the Black-Scholes Option Pricing Model. |
F-46
| (iii) | As compensation for service as a director of the Company, the Company granted to Wes Dittmer a non-qualified stock option to purchase a total of 893 shares of the Company’s restricted common stock with an exercise price of $20.00 per share. The option was immediately exercisable at date of issue. The term of the option shall be for four (4) years from the date of issue. The fair value of the option was determined to be $5,778 using the Black-Scholes Option Pricing Model. |
On July 31, 2018 options to purchase 10,040 shares of the of the Company’s restricted common stock granted to Daniel Powell, the vice president of sales and marketing, with an exercise price of $17.50 were cancelled pursuant the employees separation from the Company and the 2016 Plan.
On September 30, 2018, the Company issued the following stock options under the 2016 Plan:
| (i) | As compensation for service as a director of the Company, the Company granted to Kent J. George a non-qualified stock option to purchase a total of 893 shares of the Company’s restricted common stock with an exercise price of $8.00 per share. The option was immediately exercisable at date of issue. The term of the option shall be for four (4) years from the date of issue. The fair value of the option was determined to be $2,661 using the Black-Scholes Option Pricing Model. |
| (ii) | As compensation for service as a director of the Company, the Company granted to Michael Neitzel a non-qualified stock option to purchase a total of 893 shares of the Company’s restricted common stock with an exercise price of $8.00 per share. The option was immediately exercisable at date of issue. The term of the option shall be for four (4) years from the date of issue. The fair value of the option was determined to be $2,661 using the Black-Scholes Option Pricing Model. |
| (iii) | As compensation for service as a director of the Company, the Company granted to Wes Dittmer a non-qualified stock option to purchase a total of 893 shares of the Company’s restricted common stock with an exercise price of $8.00 per share. The option was immediately exercisable at date of issue. The term of the option shall be for four (4) years from the date of issue. The fair value of the option was determined to be $2,661 using the Black-Scholes Option Pricing Model. |
Unless otherwise stated, the issuance of the above securities were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act or Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or contracts relating to compensation as provided under Rule 701.
NOTE 14 – COMMITMENTS AND CONTINGENCIES
The Company is subject to a patent royalty agreement that requires 3% of net product sales received from commercialization of the 35 patents or other intellectual property acquired in the merger with NXDE to be paid to NXDE, LLC. NXDE, LLC is special purpose entity formed at the time of merger for the purpose of receiving the above-mentioned royalty payments, if any, and is not an affiliate of the Company or NXDE. No sales have been generated from any of the acquired patents or intellectual property.
The Company acquired a non-exclusive license to a portfolio of 86 patents, and is subject to a 6% royalty to Magnus IP GmbH of the net sales of all licensed products sold, licensed, leased, or otherwise disposed of pursuant to the license. No sales have been generated from the licensed intellectual property.
NOTE 15 – CONCENTRATION
For the nine months ended September 30, 2018, two of our customers accounted for approximately 51.6% and 26.7% of sales. For the nine months ended September 30, 2017, three of our customers accounted for approximately 39.4%, 25.9%, and 18.2% of sales
For the nine months ended September 30, 2018, the Company purchased approximately 18.5% of its products from one distributor, as compared to the nine months ended September 30, 2017, the Company purchased approximately 12.5% of its products from one distributor.
For the nine months ended September 30, 2018, three of our customers accounted for 50.0%, 22.8%, and 10.5% of accounts receivable, as compared to the nine months ended September 30, 2017, where two of our customers accounted for 45.2% and 10.6% of accounts receivable.
For the three months ended September 30, 2018, two of our customers accounted for approximately 66.0% and 29.7% of sales. For the three months ended September 30, 2017, three of our customers accounted for approximately 42.9%, 19.8%, and 17.5% of sales.
For the three months ended September 30, 2018, the company did not purchase more than 10% of its products from any single distributor, as compared to the three months ended September 30, 2017, the Company purchased approximately 16.7% and 12.0% respectively of its products from two distributors.
NOTE 16 - SUBSEQUENT EVENTS
None.
F-47
2,474,227 Units

Each Unit Consisting of One Share of Common Stock and
Warrants to Purchase Two Shares of Common Stock
PROSPECTUS
Sole Book-Running Manager
ThinkEquity
a division of Fordham Financial Management, Inc.
Co-Manager
Dougherty & Company
, 2018
PART II
INFORMATION NOT REQUIRED IN PROSPECTUS
Item 13. Other Expenses of Issuance and Distribution
The following table sets forth the fees and expenses to be incurred in connection with the registration of the securities being registered hereby, all of which will be borne by the registrant. Except for the SEC registration fee, all amounts are estimates.
| Description | Amount | |||
| SEC registration fee | $ | 5,791 | ||
| FINRA filing fee | 7,668 | |||
| NASDAQ listing fee | 54,580 | |||
| Accounting fees and expenses | 5,000 | |||
| Legal fees and expenses | 375,000 | |||
| Transfer agent and registrar fees and expenses | 830 | |||
| Miscellaneous expenses | 26,131 | |||
| Total expenses | $ | 475,000 | ||
Item 14. Indemnification of Directors and Officers
Our officers and directors are indemnified as provided by the Nevada Revised Statutes (“NRS”) and our bylaws. Under the NRS, unless modified by a corporation’s articles of incorporation, a director is not liable to a corporation, its shareholders, or creditors for damages unless the director’s action or failure constituted a breach of fiduciary duty and such breach involved intentional misconduct, fraud, or a knowing violation of law. Our bylaws provide that we will indemnify our directors and officers to the extent fully permissible under Nevada law if such person acted in good faith and in a manner that such person reasonably believed to be in, or not opposed to, the best interests of the Company, and, with respect to any criminal action, had no reasonable cause to believe such conduct was unlawful.
The Company has purchased and maintains directors’ and officers’ liability insurance, and may make other financial arrangements on behalf of any individual entitled to indemnity. Our bylaws also provide that we will advance all expenses incurred to any person entitled to indemnity upon receipt of an undertaking by, or on behalf of, such person to repay said amounts should it be ultimately determined that the person was not entitled to indemnification.
Item 15. Recent Sales of Unregistered Securities
Set forth below is an enumeration of all securities issued by the Company since December 7, 2015 (inception) that have not been registered under the Securities Act. All share amounts and per share amounts have been adjusted to reflect the Reverse Stock Split.
2015 & 2016 Management Issuances
On December 15, 2015, we issued 35,715 shares of common stock to our chief operating officer, Ron Conquest, for the sum of $500. 15,143 shares of the 35,715 shares became vested upon issue, and the remaining 20,572 shares shall vest over a 36-month period at an average rate of 571 shares per shares per month. Vesting began January 1, 2016.
On January 1, 2016, we issued 18,000 shares of restricted common stock to Christopher Miller for certain accounting and budget-related services rendered, as well as for serving as interim chief financial officer until such time as a permanent chief financial officer was hired. The shares vest over a 24-month period.
II-1
On January 2, 2016, we issued 128,572 shares of common stock to our vice president of clinical affairs, Dr. Elizabeth Rosellini DDS (the sister of our CEO), in return for 214 shares of common stock of Emeritus Clinical Solutions, Inc., a Delaware corporation, and 60,000 shares of common stock of Nuviant Medical, Inc., a Nevada corporation. The value of the consideration to acquire these shares was $49,673.
January 2016 Contribution Agreement
On January 2, 2016, we entered into a contribution agreement with RS. Under this agreement, we issued 942,858 shares of common stock in return for, among other consideration:
i. RS’s agreement to an assignment (subject to regulatory transfer approval) to us of Phase II, should it be granted, of the federal NIH/SBIR awarded grant #1R44HL129870-01;
ii. 1,675,000 shares of common stock of Nuviant Medical, Inc., a Nevada corporation;
iii. 167 shares of common stock of MicroTransponder, Inc., a Delaware corporation; and
iv. 175 shares of common stock of Emeritus Clinical Solutions, Inc., a Delaware corporation.
The value of the consideration to acquire these shares was $272,686.
These transactions were valued based on the value of the contributed assets, as our shares had no ascertainable value as of the date of issuance of the shares. This was in accordance with ASC 845 Non-Monetary Transactions, whereby non-monetary assets acquired in exchange for another non-monetary asset are valued at the fair value of the asset surrendered or received, whichever is more clearly evident. In this case, the value of the contributed assets were more ascertainable than the value of the shares issued.
Prior to the contribution, William Rosellini was not a related party of ours, but became a related party on January 2, 2016, through the issuance of the 942,858 shares and a controlling interest in the Company. Mr. Rosellini, our CEO, is the sole member and manager of RS.
2016 Plan Issuances
On April 1, 2016, pursuant to the 2016 Plan, we issued non-qualified options to purchase 18,000 shares of common stock, with an exercise price of $14.00 and a term of three years to each of the following: Dr. Mark Bates MD; Dr. Elizabeth Rosellini DDS; and Sheneka Rains (a consultant) for services rendered to the Company since inception, as well as ongoing services to be provided from time to time. The options vest in monthly increments of 500 shares, with a three-year term for each option beginning upon each date of vesting. The fair value of the options was determined to be $141,168 using the Black-Scholes Option Pricing Model. Dr. Rosellini resigned as our officer effective November 6, 2017, and these options for Dr. Rosellini were cancelled.
On June 1, 2016, pursuant to the 2016 Plan, we issued Dr. Melanie McWade, PhD incentive stock options to purchase 18,000 shares of common stock with an exercise price of $14.00. The options vest in monthly increments of 500 shares, with a three-year term for each option beginning upon each date of vesting. The fair value of the options was determined to be $42,113 using the Black-Scholes Option Pricing Model.
Effective December 1, 2016, Brian Blischak, our previous president and chief commercial officer, pursuant to the 2016 Plan, received an initial grant of 82,144 non-transferable stock options to purchase shares of the Company’s common stock, consisting of 35,715 ISOs and 46,429 NQSOs. With respect to the ISO options, 7,143 ISO options vested on the effective date, and 7,143 ISO options vested on January 1, 2017. Additional lots of 7,1443 ISO options each were to vest on January 1, 2018, 2019, and 2020. With respect to the NQSO options, 2,715 NQSO options vested on the effective date, and 1,215 NQSO options vested on January 1, 2017. An average of 1,214 options were to vest on the first day of each month thereafter until all NQSO options were to be fully vested on December 1, 2019. The exercise price of all options is $14.00 per share. The fair value of the options was determined to be $365,342 using the Black-Scholes Option Pricing Model.
II-2
Effective December 1, 2016, Christopher R. Miller, our chief financial officer, was granted incentive stock options to purchase 21,858 shares of common stock. The options vest in monthly increments over 36 months at an average rate of 607 shares per month, with the three-year term for each option beginning upon each date of vesting. The exercise price of all options is $14.00 per share. The fair value of the options was determined to be $66,234 using the Black-Scholes Option Pricing Model.
On December 1, 2016, Navid Khodaparast, our director of clinical research, was granted 8,572 incentive stock options to purchase 8,572 shares of common stock. The options vest in monthly increments over 36 months at an average rate of 238 shares per month, with the three-year term for each option beginning upon each date of vesting. As of December 6, 2018, options to purchase 6,786 shares of common stock vested and options to purchase 1,786 shares of common stock have been cancelled, but none exercised. The exercise price of all options is $14.00 per share. The fair value of the options was determined to be $25,974 using the Black-Scholes Option Pricing Model.
February 2016 Merger
On February 17, 2016, we merged with NXDE. We were the surviving entity. We converted 100% of NXDE’s issued and outstanding common and preferred stock into 118,549 shares of our common stock. As of the date of this filing, we have been unsuccessful in contacting four beneficial owners of 4,705 of the above-mentioned shares, and these shares are recorded in Equity instruments to be issued until such time as we are able to issue these shares.
In addition to the conversion of NXDE shares into our common stock, we shall pay a limited liability company formed by the former shareholders of NXDE for the purpose of receiving a royalty equal to three percent (3%) of net product sales received by the Company, its affiliates, and licensees derived from the commercialization of patents or other intellectual property owned by NXDE prior to the merger. Our former chief innovation officer and director, Dr. Mark Bates, was the chief executive officer of NXDE.
This merger was unanimously approved at a meeting of NXDE’s common and preferred shareholders, all of whom are “Accredited Investors” as defined by Rule 501 of SEC Regulation D. Based on these facts, we believe the issuance of Company common stock to the NXDE common and preferred shareholders qualifies as a Section 4(a)(2) exemption from registration.
As part of the merger agreement with NXDE (“Merger Agreement”), Dr. Bates, our former chief innovation officer and director, received a total of 27,587 shares of the Company’s common stock upon conversion of the NXDE preferred shares, and converted $370,000 of debt owed to him by NXDE into 26,429 shares of our common stock and warrants to purchase 26,429 additional shares of common stock at a strike price of $28.00 per share, with a term of 36 months. In addition, Dr. Bates contributed $202,825 of accrued interest on his debt, which were reflected as additional paid-in capital to the Company during the first quarter of 2016. On November 7, 2017, Dr. Bates formally resigned from his positions as chief innovation officer and as a member of the board of directors. As of December 6, 2018, 4,493 of these warrants have been exercised for 4,493 shares of the Company’s common stock, and 21,936 warrants have been cancelled.
As part of the Merger Agreement with NXDE, Mr. Ralph Ballard, who was a co-founder and director of NXDE, received a total of 8,841 shares of the Company’s common stock upon conversion of the NXDE preferred shares, which were divided as follows: 100 shares to Mr. Ballard personally, 550 shares to a custodial IRA FBO Ralph Ballard, and 8,191 shares to Ballard Investments, Inc. In addition, as part of the Merger Agreement with NXDE, Mr. Ballard converted $451,482 of debt owed to him by NXDE, and received 32,249 shares of our common stock, and warrants to purchase 32,249 shares at a strike price of $28.00 per share with a term of 36 months, which shares and warrants were issued to Ballard Investments, Inc. Mr. Ballard has the power to vote and dispose of the shares held by his IRA and Ballard Investments, Inc. In addition, three trusts representing three of Mr. Ballard’s children converted a total of $431,821 of debt and received 30,846 shares of our common stock, and warrants to purchase 30,846 shares of common stock at a strike price of $28.00 per share with a term of 36 months, divided between and issued to the three trusts. The three trusts are irrevocable trusts managed by an arm’s-length third-party professional fiduciary. Mr. Ballard disclaims any beneficial ownership in the common stock and warrants issued to the three trusts. As of December 6, 2018, 10,727 of these warrants have been exercised for 10,727 shares of the Company’s common stock, and 52,368 warrants have been cancelled.
II-3
Subsequent to the Merger, three additional NXDE shareholders converted a total of $34,261 in debt for 2,449 shares of our common stock, and warrants to purchase 2,449 shares of our common stock at a strike price of $28.00 per share with a term of 36 months. As of December 6, 2018, none of these warrants have been exercised.
2016 Private Placement
On December 2, 2016, we closed the 2016 Private Placement, pursuant to which we received $2,864,946 in net cash proceeds from the issuance of 204,659 units, and converted $1,287,564 in shareholder debt to 91,973 units in the private placement. Each unit consisted of one share of restricted common stock and one warrant to purchase one additional share of restricted common stock. As of December 6, 2018, 42,411 warrants have been exercised for an aggregate of 42,411 shares of the Company’s common stock, and 206,987 warrants have been cancelled.
2016 Patent License Asset Purchase
On December 15, 2016, Mr. Rosellini sold, assigned, and transferred all his right, title, and interest in and to the license owned by him related to the Siemens Patents to the Company pursuant to a Patent License Asset Purchase Agreement (the “Purchase Agreement”). Pursuant to the terms of the Purchase Agreement, during the year ended December 31, 2017, 217,858 shares of the Company’s restricted common stock were issued to Mr. Rosellini valued at $3,050,000. (See Note 13 – Related Party Transactions, Patent License Agreement (Siemens Patents) and Patent License Asset Purchase Agreement to the Consolidated Financial Statements included herein.)
2016 & 2017 Issuances for Services
For the year ended December 31, 2016, we issued an aggregate of 12,251 shares of common stock for certain legal, corporate structuring, and research and development consulting services rendered by third-party consultants. The foregoing shares were valued at $171,500.
On December 9, 2016, Nexeon MedSystems Puerto Rico, a wholly owned subsidiary of the Company, entered into a services agreement with Adaptive Business Solutions, LLC to provided corporate structuring consulting services in exchange for $60,000 in restricted shares of the Company’s common stock. No stock was issued at the time the agreement was entered into in 2016. As of the date of this filing, 3,910 shares of the Company’s common stock have been issued in exchange for services provided.
On February 14, 2017, we entered into a services agreement with ACORN Management Partners, LLC to provide strategic business outreach and strategic relations services in exchange for a monthly fee of $7,500, and $125,000 in restricted shares of our common stock. The initial term of the agreement is six months. As of the date of this filing, 8,929 shares of the Company’s common stock have been issued in exchange for services provided.
On February 23, 2017, we entered into a services agreement with Sichenzia Ross Ference LLP to provide certain legal services for a flat rate of $40,000. The fee shall be paid $20,000 in cash and $20,000 in restricted shares of the Company’s common stock. 1,429 shares of the Company’s common stock have been issued in exchange for services provided.
For the year ended December 31, 2017, we issued an aggregate of 51,132 shares of common stock for certain legal, corporate structuring, and research and development consulting services rendered by third-party consultants. The foregoing shares were valued at $610,893.
2017 Warrant Conversion Offer
On March 17, 2017, we offered to current warrant holders who participated in the 2016 Private Placement (which closed on December 2, 2016) the opportunity to convert their warrants into common stock of the Company on the following terms (the “Warrant Conversion Offer”). The offer terms included the exercise of seventeen (17) warrants for seventeen (17) shares of the Company’s common stock at an exercise price of $0.01 per share for every one hundred (100) warrants owned. The remaining eighty-three (83) warrants per hundred warrants owned would be cancelled. The offer was on an all-or-nothing basis to convert all warrants held by each warrant holder. Pursuant to the offer, 42,411 warrants have been exercised for an aggregate of 42,411 shares of the Company’s common stock, and 207,022 warrants were cancelled in connection with the Warrant Conversion Offer. The total proceeds from the exercise of the 42,411 warrants pursuant to the Warrant Conversion Offer were $5,936.
II-4
2017 Private Placement
We conducted the 2017 Private Placement (which closed on July 20, 2017) for up to 142,858 shares of common stock to accredited investors only, pursuant to which we could receive up to $2,500,000 in proceeds. The shares of common stock were offered at $17.50 per share. We received $1,165,000 from the sale of common stock, and issued 66,580 shares of common stock.
2017 Loan to NMB
On April 1, 2017, we issued 12,500 shares of the Company’s common stock in exchange for an increase of $175,000 in loan to NMB. The shares were issued to AtidTek, LLC for certain project management and engineering services provided to NMB. The shares were valued at $175,000.
On October 9, 2017, we issued 5,789 shares of the Company’s restricted common stock to RS, a company controlled by our chief executive officer, William Rosellini, as repayment for 5,789 shares of our restricted common stock that RS loaned to NMB for payment of outstanding vendor invoices. On December 29, 2017, an additional 4,421 shares of our restricted common stock were issued to RS in settlement for a cash loan to NMB from RS, and for an adjustment to the market value of the 5,789 shares described above per the debt repayment agreement dated December 29, 2017. In total, the 10,210 shares were valued at $119,746.
2017 Employee Purchase Plan
On August 21, 2017, we offered to current employees the opportunity to purchase shares of our restricted common stock for a discount through payroll deductions. As of the date of this filing, 14,556 shares of our restricted common stock were issued. The shares were valued at $127,247.
2017 Private Offering to Leonite Capital, LLC
On August 21, 2017, we entered into a securities purchase agreement with Leonite Capital, LLC, a Delaware limited liability company, pursuant to which Leonite Capital, LLC purchased a unit consisting of (i) a note in the principal amount of $1,120,000 at an original issue discount of $120,000, (ii) warrants to purchase 35,716 shares of our common stock, and (iii) 7,143 shares of our common stock. The funds from the purchase were received by the Company on August 24, 2017. As of December 6, 2018, none of these warrants have been exercised.
2017 Private Offering to Henri Decloux
On October 9, 2017, we issued 10,715 shares of our restricted common stock through a subscription of the shares for cash to Henri Decloux following NMB’s acquisition of INGEST and Medi-Line. Henri Decloux was one of the two previous owners and sellers of INGEST to NMB. HD Resources, SPRL (“HD”) is owned by Henri Decloux, and Medi-Line has contracted with HD for the management of Medi-Line. These shares were valued at $150,000.
2017 Cancellation of Shares
On December 7, 2017, 4,000 shares originally issued to Ron Conquest were cancelled pursuant to a resignation and release agreement dated November 7, 2017.
2017 Loan to Michael Rosellini
On December 29, 2017, we issued to Michael Rosellini 5,501 shares of our restricted common stock as repayment for a loan of 3,801 shares of registered common stock borrowed by the Company and used to pay certain vendors of the Company pursuant to a loan agreement dated December 29, 2017. The shares were valued at $48,130.
II-5
On December 29, 2017, we issued 893 shares of our restricted common stock to Michael Rosellini, pursuant to the exercise notice from Mr. Rosellini dated November 22, 2017. Mr. Rosellini exercised his right to purchase 14,286 shares of the Company’s restricted common stock. The cashless exercise pursuant to the warrant was elected, and we issued 893 shares of restricted common stock pursuant to the exercise. The shares were valued at $20,000.
2017 & 2018 Plan Issuances
For the year ended December 31, 2017, we issued, pursuant to our 2016 Plan, 144,662 options grants to purchase 144,662 common shares to directors, employees, and non-employee contractors. 77,160 options were granted as non-qualified stock options, and 67,502 options were granted as incentive stock options. The range of exercise prices for these options were between $14.00 and $28.00. The term of these options at grant date ranged from three to four years. The fair value of the options was determined to be $563,754 using the Black-Scholes Option Pricing Model. During the year ended December 31, 2017, 54,088 options to purchase 54,088 common shares of the Company were cancelled. (See Note 11 – EQUITY to the Consolidated Financial Statements included herein.)
On February 28, 2018, pursuant to the 2016 Plan, the compensation committee of the board of director’s approved incentive stock option grants to purchase up to 21,945 shares of common stock with an exercise price of $10.64 per share. 11,944 options vested immediately, and the remaining 10,001 options vest over the following 12 months. The compensation committee granted non-qualified stock options to purchase up to 70,537 shares of common stock with an exercise price of $10.64 per share. 6,251 options vested immediately, and the remaining 64,286 options vest over the following 24 months. The fair value of the options was determined to be $258,531 using the Black-Scholes Option Pricing Model.
On March 31, 2018, we granted to two directors previously appointed to the board of directors non-qualified stock options to purchase a total of 1,786 shares of common stock with an exercise price of $12.11 per share. The fair value of the options was determined to be $7,191 using the Black-Scholes Option Pricing Model.
On June 30, 2018, we granted to three directors previously appointed to the board of directors non-qualified stock options to purchase a total of 2,679 shares of common stock with an exercise price of $20.00 per share. The fair value of the options was determined to be $17,334 using the Black-Scholes Option Pricing Model.
On September 30, 2018, we granted to three directors previously appointed to the board of directors non-qualified stock options to purchase a total of 2,679 shares of common stock with an exercise price of $8.00 per share. The fair value of the options was determined to be $7,983 using the Black-Scholes Option Pricing Model.
During the three months ended September 30, 2018, the Company issued stock options to purchase a total of 41,251 shares of the Company’s common stock and cancelled stock options to purchase a total of 54,898 shares of the Company’s common stock under the 2016 Plan.
On July 22, 2018, the Company granted to a new employee at NXPROC incentive stock options to purchase up to 38,572 shares of the Company’s restricted common stock with an exercise price of $11.00 per share with vesting over 48 months. The term of the option was for a period of four (4) years from the date of vesting. The fair value of the option was determined to be $92,789 using the Black-Scholes Option Pricing Model. Prior to vesting the option to purchase 38,572 shares was cancelled.
From July 31, 2018 through October 1, 2018 incentive stock options to purchase 16,768 shares of the Company’s restricted common stock were cancelled pursuant to the 2016 Plan for employee separations from the Company. The range of exercise prices for these cancelled options ranged from $14.00 to $17.50.
2018 Issuances for Services
On March 8, 2018, we issued an aggregate of 1,697 shares of restricted common stock for certain sales and marketing and software consulting services rendered by third-party consultants. The foregoing shares were valued at $14,840. 583 of these shares were issued to Daniel Powell, the Company’s vice president of sales and marketing. These shares were issued for services provided by Mr. Powell prior to his employment by the Company.
On April 19, 2018, we issued an aggregate of 7,195 shares of restricted common stock for certain research and development and valuation services provided by third-party consultants. The foregoing shares were valued at $96,308.
2018 Common Stock Redemption
On May 22, 2018, the Company redeemed and cancelled 14,286 shares of its common stock from a former director as consideration for the purchase of certain intellectual property.
Unless otherwise stated, the issuance of the above securities were deemed to be exempt from registration under the Securities Act in reliance upon Section 4(a)(2) of the Securities Act, or Regulation D promulgated thereunder, or Rule 701 promulgated under Section 3(b) of the Securities Act as transactions by an issuer not involving any public offering or contracts relating to compensation, as provided under Rule 701.
II-6
Item 16. Exhibits and Financial Statement Schedules
| (a) | Exhibits. |
II-7
| * | Filed herewith. |
| ** | Previously filed. |
| (1) | Incorporated by reference to the Company’s Form 10 filed with the Securities and Exchange Commission on July 6, 2016. |
| (2) | Incorporated by reference to the Company’s Amendment No. 1 to the Form 10 filed with the Securities and Exchange Commission on August 16, 2016. |
| (3) | Incorporated by reference to the Company’s Amendment No. 2 to the Form 10 filed with the Securities and Exchange Commission on September 9, 2016. |
| (4) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 20, 2016. |
| (5) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 29, 2016. |
| (6) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on January 17, 2017. |
| (7) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on January 19, 2017. |
| (8) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on February 28, 2017. |
| (9) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on June 28, 2017. |
| (10) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on August 25, 2017. |
| (11) | Incorporated by reference to the Company’s Annual Report on Form 10-K filed with the Securities and Exchange Commission on April 5, 2018. |
| (12) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on November 20, 2018. |
| (13) | Incorporated by reference to the Company’s Current Report on Form 8-K filed with the Securities and Exchange Commission on December 7, 2018. |
II-8
Item 17. Undertakings.
The undersigned registrant hereby undertakes:
(1) To file, during any period in which offers or sales are being made, a post-effective amendment to this registration statement:
| (i) | To include any prospectus required by Section 10(a)(3) of the Securities Act; | |
| (ii) | To reflect in the prospectus any facts or events arising after the effective date of the registration statement (or the most recent post-effective amendment thereof) that, individually or in the aggregate, represent a fundamental change in the information set forth in the registration statement. Notwithstanding the foregoing, any increase or decrease in volume of securities offered (if the total dollar value of securities offered would not exceed that which was registered) and any deviation from the low or high end of the estimated maximum offering range may be reflected in the form of a prospectus filed with the Securities and Exchange Commission pursuant to Rule 424(b) if, in the aggregate, the changes in volume and price represent no more than a 20 percent change in the maximum aggregate offering price set forth in the “Calculation of Registration Fee” table in the effective registration statement; and | |
| (iii) | To include any material information with respect to the plan of distribution not previously disclosed in the registration statement, or any material change to such information in the registration statement. |
(2) That, for the purpose of determining any liability under the Securities Act, each such post-effective amendment shall be deemed to be a new registration statement relating to the securities offered therein, and the offering of the securities at that time shall be deemed to be the initial bona fide offering thereof.
(3) To remove from registration by means of a post-effective amendment any of the securities being registered that remain unsold at the termination of the offering.
(4) That, for the purpose of determining liability of the undersigned registrant under the Securities Act to any purchaser in the initial distribution of the securities:
The undersigned registrant undertakes that in a primary offering of securities of the undersigned registrant pursuant to this registration statement, regardless of the underwriting method used to sell the securities to the purchaser, if the securities are offered or sold to such purchaser by means of any of the following communications, the undersigned registrant will be a seller to the purchaser, and will be considered to offer or sell such securities to such purchaser:
| (i) | Any preliminary prospectus, or prospectus of the undersigned registrant relating to the offering required to be filed pursuant to Rule 424 (§ 230.424 of this chapter); | |
| (ii) | Any free writing prospectus relating to the offering prepared by or on behalf of the undersigned registrant, or used or referred to by the undersigned registrant; | |
| (iii) | The portion of any other free writing prospectus relating to the offering containing material information about the undersigned registrant or its securities provided by or on behalf of the undersigned registrant; and | |
| (iv) | Any other communication that is an offer in the offering made by the undersigned registrant to the purchaser. |
Insofar as indemnification for liabilities arising under the Securities Act may be permitted to directors, officers, and controlling persons of the registrant pursuant to the foregoing provisions, or otherwise, the registrant has been advised that, in the opinion of the Securities and Exchange Commission, such indemnification is against public policy as expressed in the Act, and is, therefore, unenforceable.
In the event that a claim for indemnification against such liabilities (other than the payment by the registrant of expenses incurred or paid by a director, officer, or controlling person in the successful defense of any action, suit, or proceeding) is asserted by such director, officer, or controlling person in connection with the securities being registered, the registrant will, unless in the opinion of its counsel the matter has been settled by controlling precedent, submit to a court of appropriate jurisdiction the question whether such indemnification by it is against public policy as expressed in the Act, and will be governed by the final adjudication of such issue.
For the purpose of determining liability under the Securities Act to any purchaser, each prospectus filed pursuant to Rule 424(b) as part of a registration statement relating to an offering, other than registration statements relying on Rule 430B or other than prospectuses filed in reliance on Rule 430A (§ 230.430A of this chapter), shall be deemed to be part of and included in the registration statement as of the date it is first used after effectiveness—provided, however, that no statement made in a registration statement or prospectus that is part of the registration statement or made in a document incorporated or deemed incorporated by reference into the registration statement or prospectus that is part of the registration statement will, as to a purchaser with a time of contract of sale prior to such first use, supersede or modify any statement that was made in the registration statement or prospectus that was part of the registration statement or made in any such document immediately prior to such date of first use.
II-9
SIGNATURES
Pursuant to the requirements of the Securities Act of 1933, the registrant has duly caused this registration statement to be signed on its behalf by the undersigned, thereunto duly authorized, in the City of Dallas, State of Texas, on December 17, 2018.
| NEXEON MEDSYSTEMS INC | ||
| By: | /s/ William Rosellini | |
| William Rosellini | ||
Chief Executive Officer (Principal Executive Officer) | ||
| By: | /s/ Christopher R. Miller | |
| Christopher R. Miller | ||
Chief Financial Officer (Principal Financial and Accounting Officer) | ||
Pursuant to the requirements of the Securities Act of 1933, this registration statement has been signed by the following persons in the capacities and on the date indicated.
| Name | Title | Date | ||
| /s/ William Rosellini | Chief Executive Officer, Director | December 17, 2018 | ||
| William Rosellini | (Principal Executive Officer) | |||
| /s/ * | Chief Financial Officer | December 17, 2018 | ||
| Christopher R. Miller | (Principal Financial and Accounting Officer) | |||
| /s/ * | Director | December 17, 2018 | ||
| Kent J. George | ||||
| /s/ * | Director | December 17, 2018 | ||
| Michael Neitzel | ||||
| /s/ * | Director | December 17, 2018 | ||
| R. Wesley Dittmer II |
| *By | /s/ William Rosellini | |
| William Rosellini | ||
| Attorney-in-fact |
II-10