Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - IDERA PHARMACEUTICALS, INC. | a18-41620_1ex99d2.htm |
| 8-K - 8-K - IDERA PHARMACEUTICALS, INC. | a18-41620_18k.htm |
2 Forward Looking Statements and Other Important Cautions This presentation contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933, as amended, and Section 21E of the Securities Exchange Act of 1934, as amended. All statements, other than statements of historical fact, included or incorporated in this presentation, including statements regarding the Company's strategy, future operations, collaborations, intellectual property, cash resources, financial position, future revenues, projected costs, prospects, plans, and objectives of management, are forward-looking statements. The words "believes," "anticipates," "estimates," "plans," "expects," "intends," "may," "could," "should," "potential," "likely," "projects," "continue," "will," and "would" and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. Idera cannot guarantee that it will actually achieve the plans, intentions or expectations disclosed in its forward-looking statements and you should not place undue reliance on the Company's forward-looking statements. There are a number of important factors that could cause Idera's actual results to differ materially from those indicated or implied by its forward-looking statements. Factors that may cause such a difference include: whether interim results from a clinical trial will be predictive of the final results of the trial, whether results obtained in preclinical studies and clinical trials will be indicative of the results that will be generated in future clinical trials, including in clinical trials in different disease indications; whether products based on Idera's technology will advance into or through the clinical trial process on a timely basis or at all and receive approval from the United States Food and Drug Administration or equivalent foreign regulatory agencies; whether, if the Company's products receive approval, they will be successfully distributed and marketed; and such other important factors as are set forth under the caption "Risk Factors" in the Company's Annual Report and on Form 10-K for the period ended December 31, 2017 and on Form 10-Q for the period ended September 30, 2018. Although Idera may elect to do so at some point in the future, the Company does not assume any obligation to update any forward-looking statements and it disclaims any intention or obligation to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise.

Featured Speakers 3 Vincent Milano Idera CEO Joanna Horobin, M.B., Ch.B. Idera CMO Adi Diab, M.D. – Lead Trial Investigator, Assistant Professor, Department of Melanoma Medical Oncology, Division of Cancer Medicine, University of Texas, MD Anderson Cancer Center

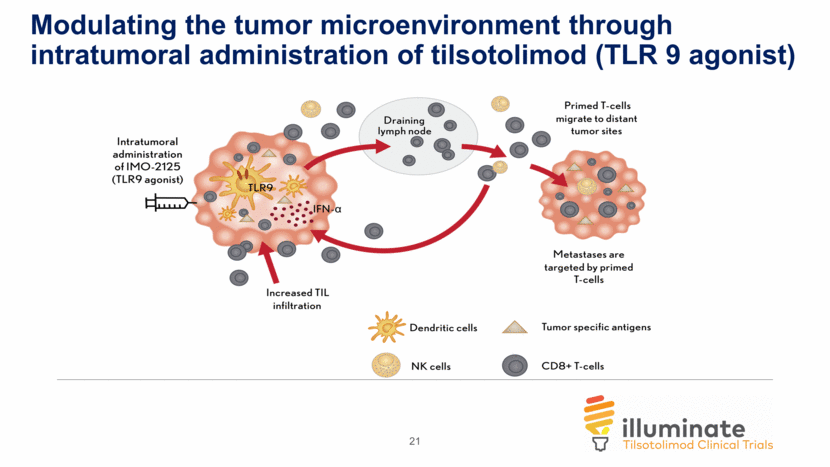
Background: Tilsotolimod (IMO-2125) is an investigational synthetic oligonucleotide which binds to TLR9, altering the tumor microenvironment by improving antigen presentation of dendritic cells and macrophages with subsequent proliferation of antigen specific cytotoxic T lymphocytes (CD8+ T-cells) in both injected and uninjected tumors resulting in tumor cell death (Haymaker, SITC 2017); There is a high unmet medical need in metastatic melanoma for patients who progress after PD-1 inhibitors, as treatment options are very limited; Post PD-1 inhibitor failure, standard of care (single agent ipilimumab) offers only modest benefit (10-16% ORR) (Long, Society for Melanoma Research 2016), (Bowyer S, et al. Br. J Cancer, 2016), (Zimmer L, et al. Eur J Cancer 75, 47-55); Initial clinical experience with 8 mg tilsotolimod + ipi is promising. This report is an analysis of the first 37 subjects (34 evaluable for disease assessment) in a multi-center study who received 1+ doses of the treatment combination and at least one disease assessment. 4
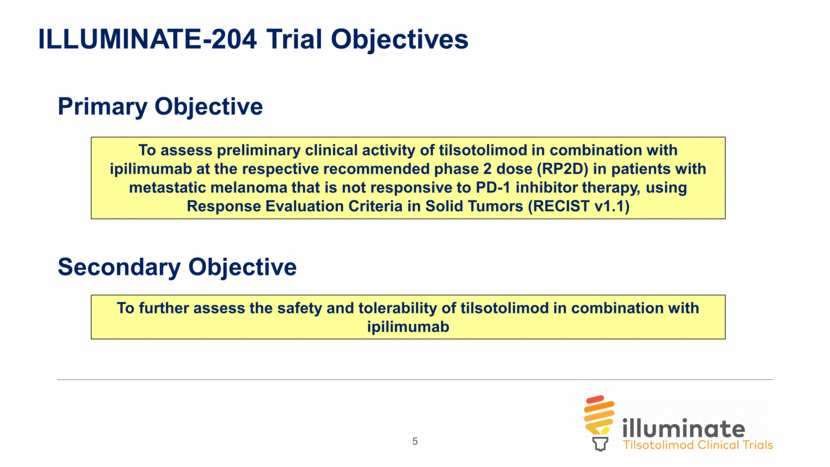
5 ILLUMINATE-204 Trial Objectives To assess preliminary clinical activity of tilsotolimod in combination with ipilimumab at the respective recommended phase 2 dose (RP2D) in patients with metastatic melanoma that is not responsive to PD-1 inhibitor therapy, using Response Evaluation Criteria in Solid Tumors (RECIST v1.1) To further assess the safety and tolerability of tilsotolimod in combination with ipilimumab Primary Objective Secondary Objective
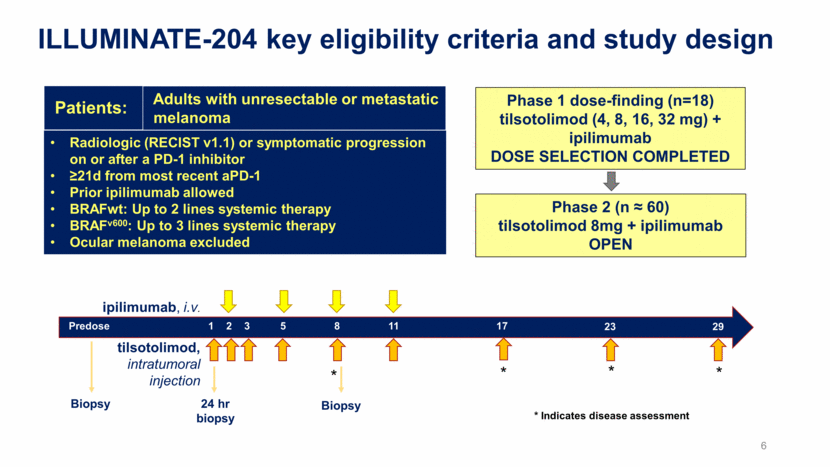
ILLUMINATE-204 key eligibility criteria and study design Radiologic (RECIST v1.1) or symptomatic progression on or after a PD-1 inhibitor >21d from most recent aPD-1 Prior ipilimumab allowed BRAFwt: Up to 2 lines systemic therapy BRAFv600: Up to 3 lines systemic therapy Ocular melanoma excluded Adults with unresectable or metastatic melanoma Patients: Phase 1 dose-finding (n=18) tilsotolimod (4, 8, 16, 32 mg) + ipilimumab DOSE SELECTION COMPLETED Phase 2 (n 60) tilsotolimod 8mg + ipilimumab OPEN 1 2 3 5 8 11 17 23 29 Predose 24 hr biopsy Biopsy Biopsy * ipilimumab, i.v. tilsotolimod, intratumoral injection * * * * Indicates disease assessment 6
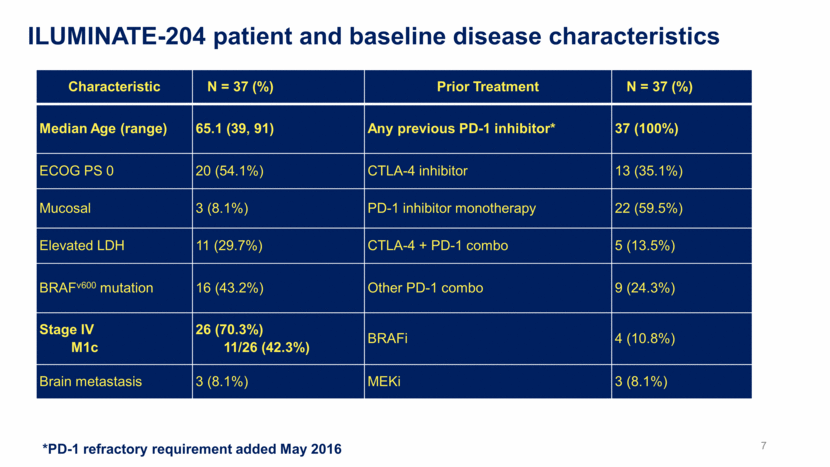
ILUMINATE-204 patient and baseline disease characteristics *PD-1 refractory requirement added May 2016 Characteristic N = 37 (%) Prior Treatment N = 37 (%) Median Age (range) 65.1 (39, 91) Any previous PD-1 inhibitor* 37 (100%) ECOG PS 0 20 (54.1%) CTLA-4 inhibitor 13 (35.1%) Mucosal 3 (8.1%) PD-1 inhibitor monotherapy 22 (59.5%) Elevated LDH 11 (29.7%) CTLA-4 + PD-1 combo 5 (13.5%) BRAFv600 mutation 16 (43.2%) Other PD-1 combo 9 (24.3%) Stage IV M1c 26 (70.3%) 11/26 (42.3%) BRAFi 4 (10.8%) Brain metastasis 3 (8.1%) MEKi 3 (8.1%) 7
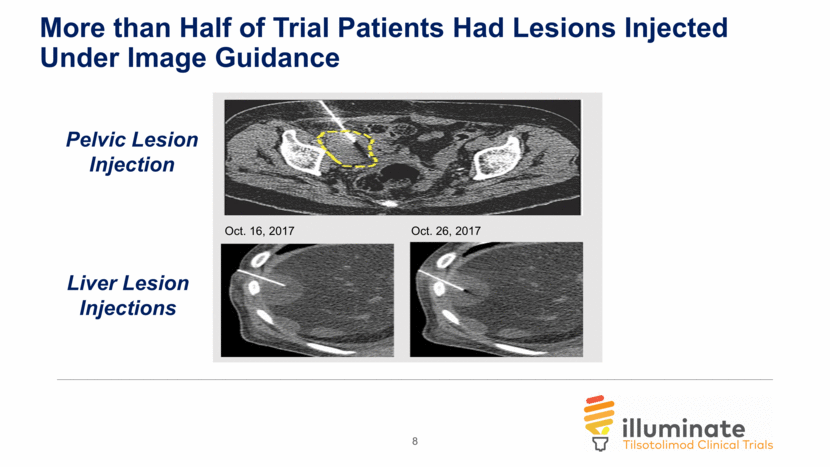
More than Half of Trial Patients Had Lesions Injected Under Image Guidance 8 Oct. 16, 2017 Oct. 26, 2017 Liver Lesion Injections Pelvic Lesion Injection
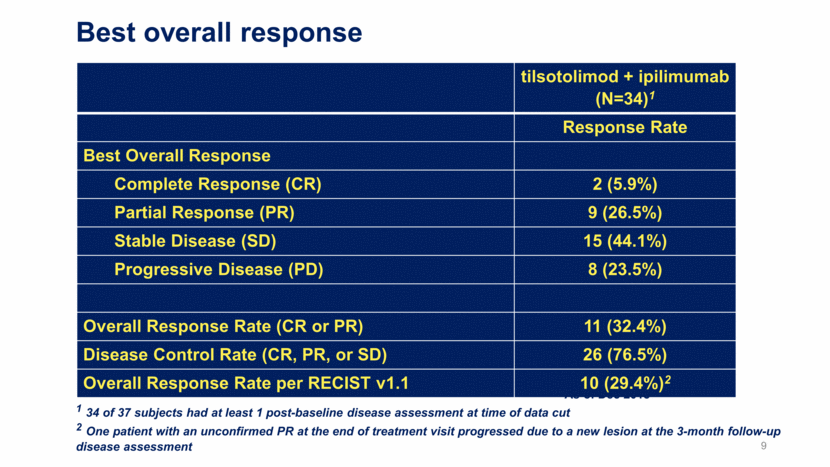
Best overall response tilsotolimod + ipilimumab (N=34)1 Response Rate Best Overall Response Complete Response (CR) 2 (5.9%) Partial Response (PR) 9 (26.5%) Stable Disease (SD) 15 (44.1%) Progressive Disease (PD) 8 (23.5%) Overall Response Rate (CR or PR) 11 (32.4%) Disease Control Rate (CR, PR, or SD) 26 (76.5%) Overall Response Rate per RECIST v1.1 10 (29.4%)2 As of Dec 2018 1 34 of 37 subjects had at least 1 post-baseline disease assessment at time of data cut 2 One patient with an unconfirmed PR at the end of treatment visit progressed due to a new lesion at the 3-month follow-up disease assessment 9
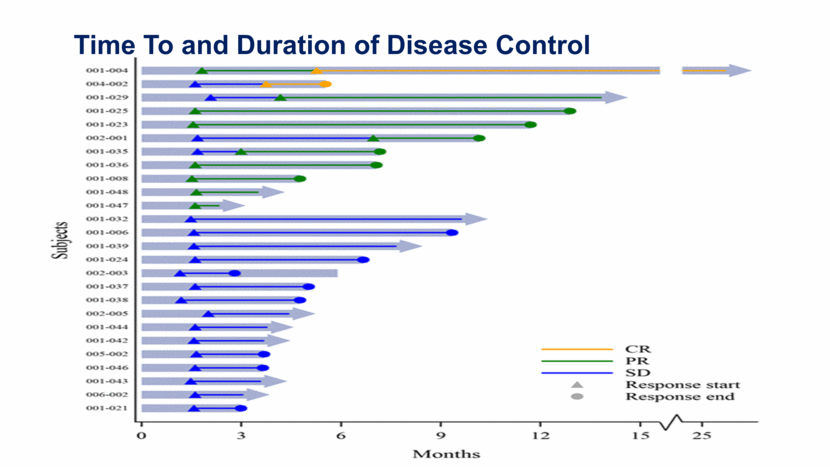
10 Time To and Duration of Disease Control
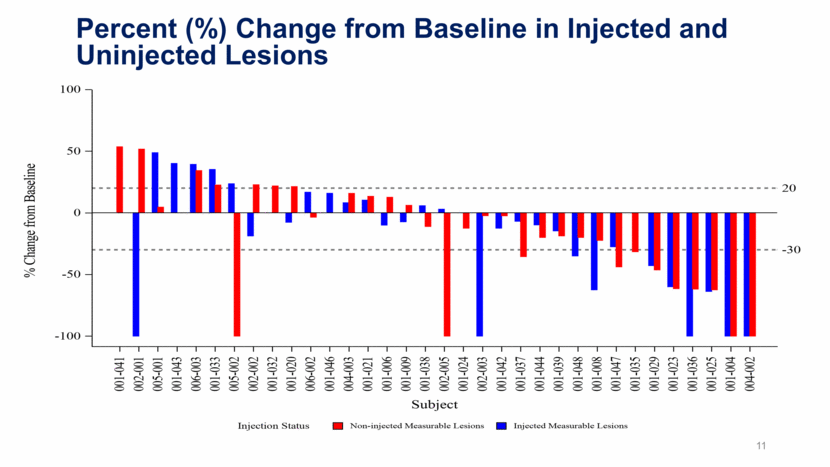
11 Percent (%) Change from Baseline in Injected and Uninjected Lesions
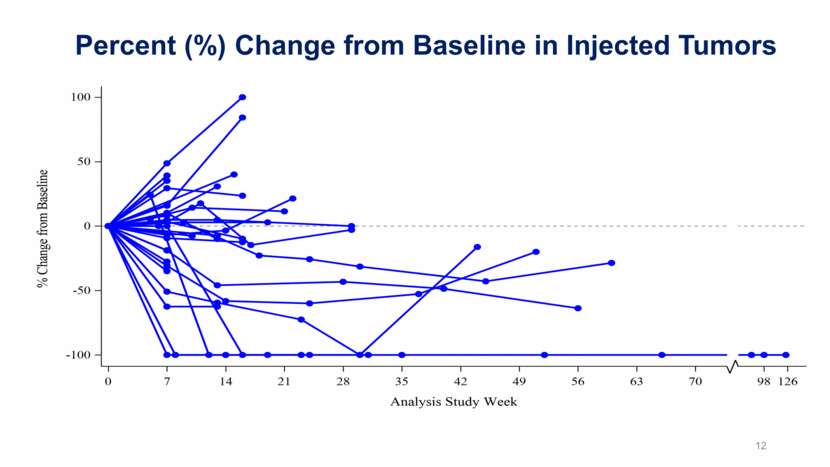
12 Percent (%) Change from Baseline in Injected Tumors
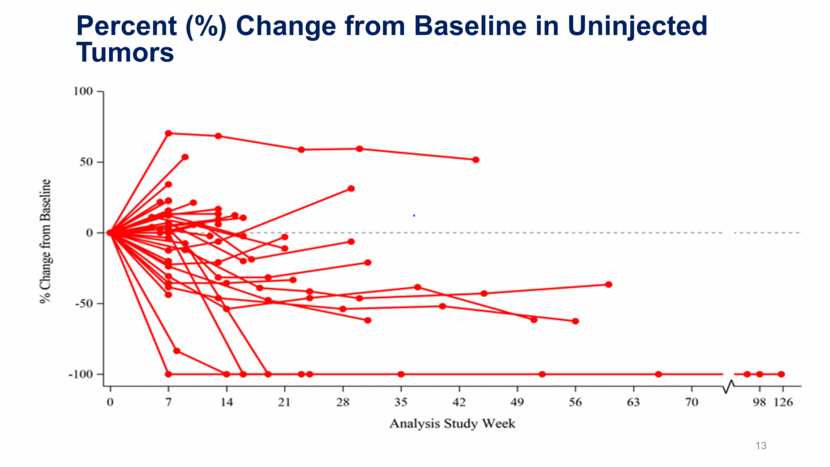
13 Percent (%) Change from Baseline in Uninjected Tumors
Safety Analysis Subjects treated with tilsotolimod + ipilimumab (N=37) At Least One AE 35 (94.6%) At Least One Serious AE 11 (29.7%) At Least One Grade >3 AE 19 (51.4%) AE Leading to Treatment Withdrawal 4 (10.8%) AE Leading to Study Discontinuation 0 (0.0%) Death 0 (0.0%) Maximum Severity Grade 1 2 (5.4%) Grade 2 14 (37.8%) Grade 3 17 (45.9%) Grade 4 2 (5.4%) Grade 5 0 (0.0%) Relationship to Study Drugs Related 31 (83.8%) Unrelated 4 (10.8%) 14 No safety events associated with deep injections (liver, adrenal)
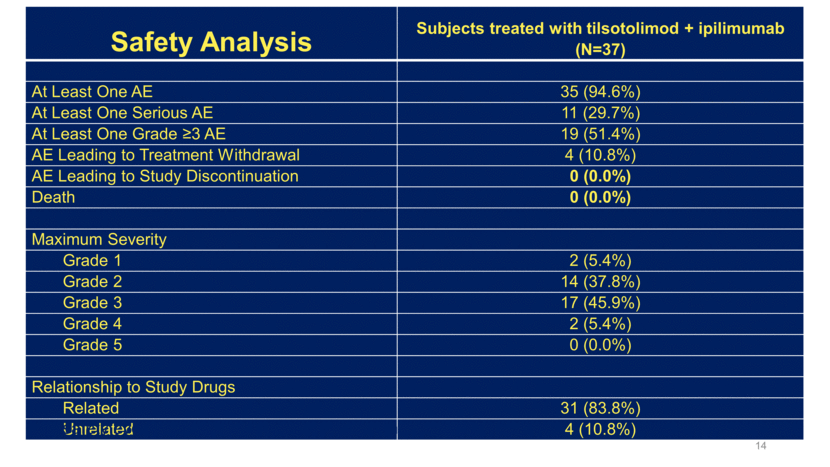
Immune-Related AEs Consistent with Ipilimumab 15 AE preferred term 8 mg tilsotolimod/ipilimumab N=37 Patients Reporting at Least One Adverse Event 9 (24.3%) Hypophysitis 4 (10.8%) Colitis 3 (8.1%) Autoimmune hepatitis 2 (5.4%) Adrenal insufficiency 1 (2.7%) Enterocolitis 1 (2.7%) Guillain-Barre syndrome 1 (2.7%)
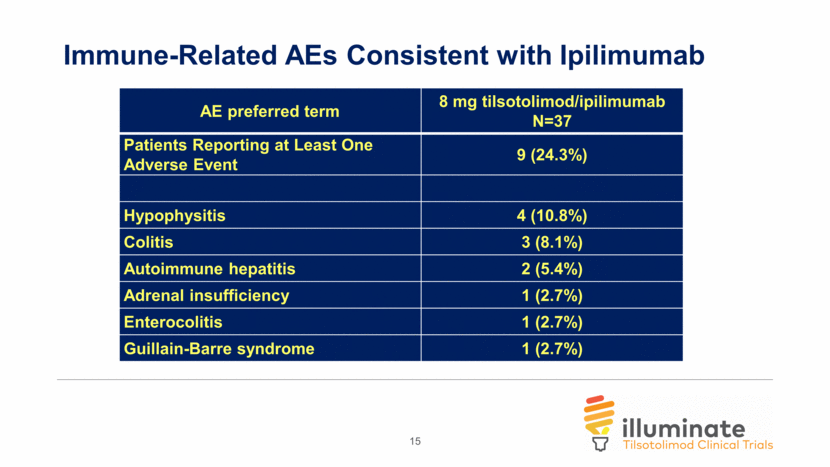
Illuminate-204 Data Update Conclusions 37 patients dosed with 8 mg of tilsotolimod in combination with ipilimumab were evaluated for this update; 34 patients were evaluable for efficacy; All patients were evaluable for safety; and Accrual is ongoing, with an additional 4 patients dosed Responses, including 2 Complete Responses (CR), were observed in 11 of the 34 evaluable patients (32.4%); Duration of response is ranging from 1+ month to 30+ months, with 36% of responses ongoing Per RECIST v1.1, the Overall Response Rate (ORR) is 29.4%; one patient with an unconfirmed Partial Response (uPR) at the end of treatment visit progressed due to a new lesion at the 3-month follow-up disease assessment; Overall, 26 patients out of 34 evaluable for efficacy (76.5%) experienced disease control (CR, PR, or Stable Disease (SD)); 16
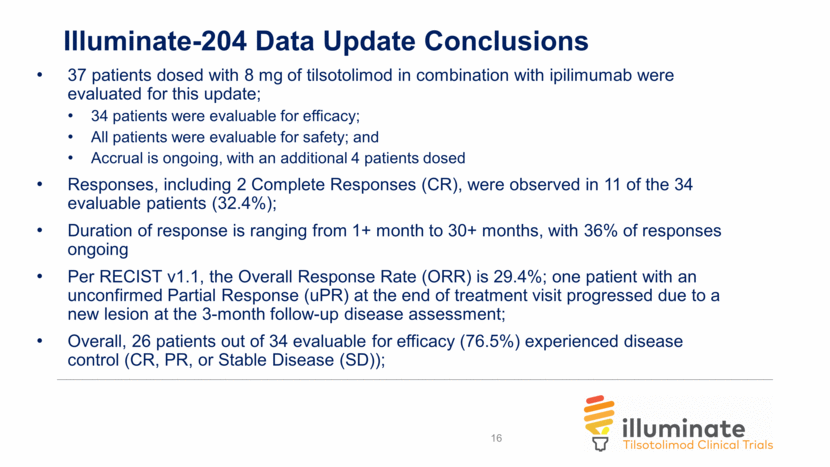
Illuminate-204 Data Update Conclusions Analysis of spider plots show tumor shrinkage in both injected and uninjected lesions, indicating an abscopal effect; Responding subjects include one patient with mucosal melanoma and one patient with acral melanoma, two forms of melanoma that are particularly difficult to treat; 2 of 5 patients with prior ipilimumab experience achieved responses, further demonstrating a signal that tilsotolimod has the potential to help overcome prior ipilimumab resistance; The combination regimen continues to be generally well tolerated. 9/37 subjects (24.3%) had immune-related toxicities indicating that tilsotolimod + ipilimumab does not appear to add immune-related toxicity versus ipilimumab alone; Injection-related toxicities were grade 1-2 transient fever and flu-like symptoms lasting <48 hours; and, More than half of the trial patients had lesions injected under image-guidance. 17
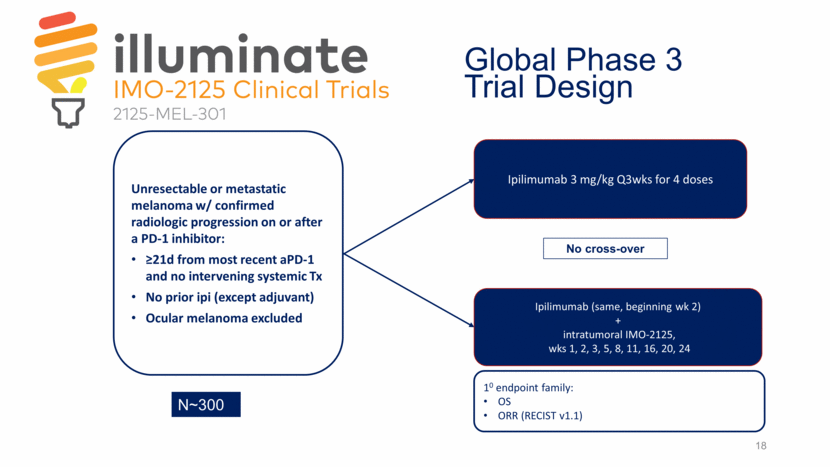
18 Ipilimumab 3 mg/kg Q3wks for 4 doses Ipilimumab (same, beginning wk 2) + intratumoral IMO-2125, wks 1, 2, 3, 5, 8, 11, 16, 20, 24 Unresectable or metastatic melanoma w/ confirmed radiologic progression on or after a PD-1 inhibitor: >21d from most recent aPD-1 and no intervening systemic Tx No prior ipi (except adjuvant) Ocular melanoma excluded 10 endpoint family: OS ORR (RECIST v1.1) No cross-over Global Phase 3 Trial Design N~300
Questions & Answers

Appendix
Modulating the tumor microenvironment through intratumoral administration of tilsotolimod (TLR 9 agonist) 21
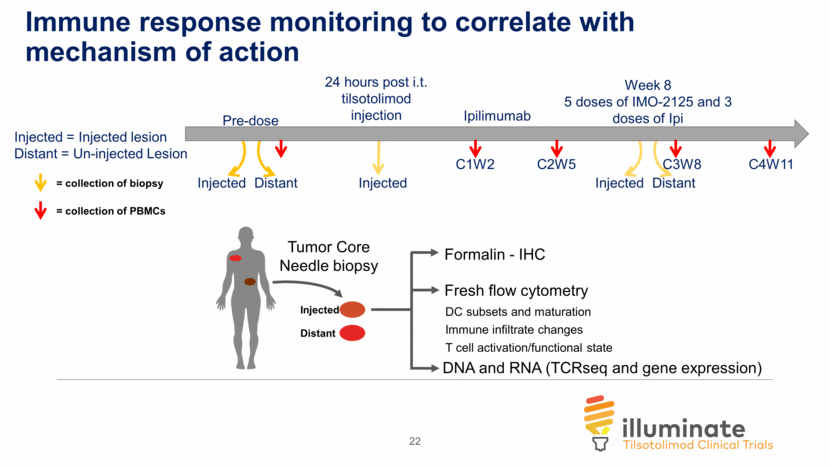
DNA and RNA (TCRseq and gene expression) DC subsets and maturation Immune infiltrate changes T cell activation/functional state = collection of biopsy Injected = Injected lesion Distant = Un-injected Lesion = collection of PBMCs Immune response monitoring to correlate with mechanism of action Fresh flow cytometry Formalin - IHC Tumor Core Needle biopsy Injected Distant Pre-dose 24 hours post i.t. tilsotolimod injection Week 8 5 doses of IMO-2125 and 3 doses of Ipi Injected Distant C1W2 C2W5 C4W11 Injected Distant Injected Ipilimumab C3W8 22
23
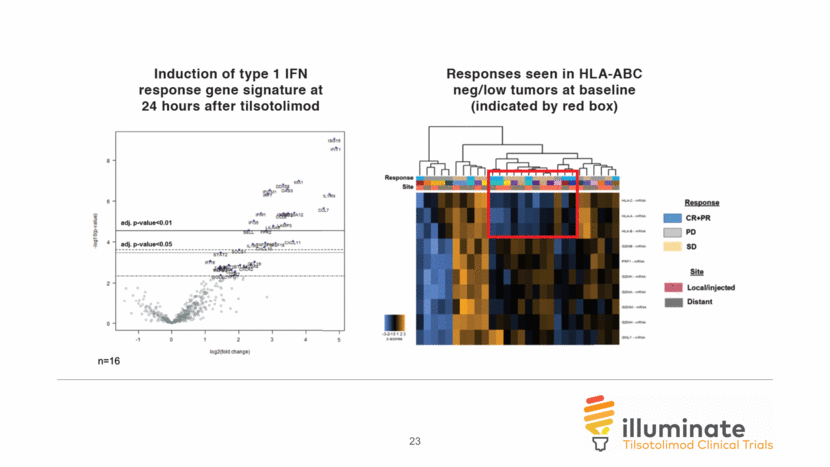
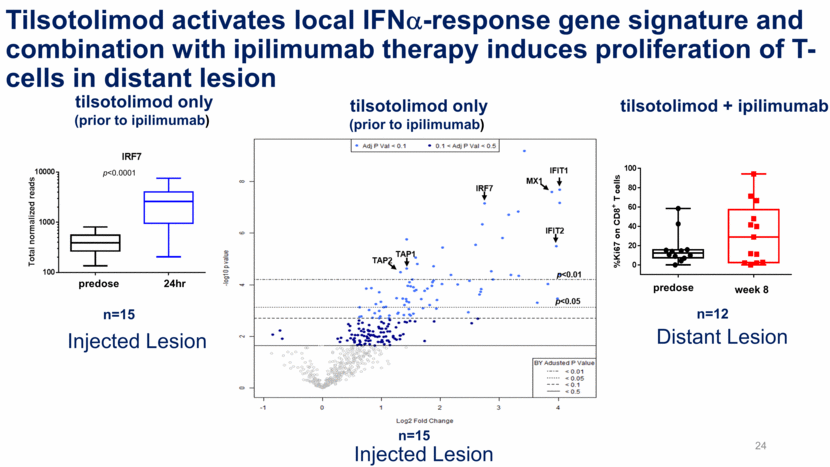
Tilsotolimod activates local IFNa-response gene signature and combination with ipilimumab therapy induces proliferation of T-cells in distant lesion n=15 p<0.0001 24hr predose 28 n=12 tilsotolimod only (prior to ipilimumab) tilsotolimod + ipilimumab tilsotolimod only (prior to ipilimumab) week 8 predose Injected Lesion Injected Lesion Distant Lesion n=15 24
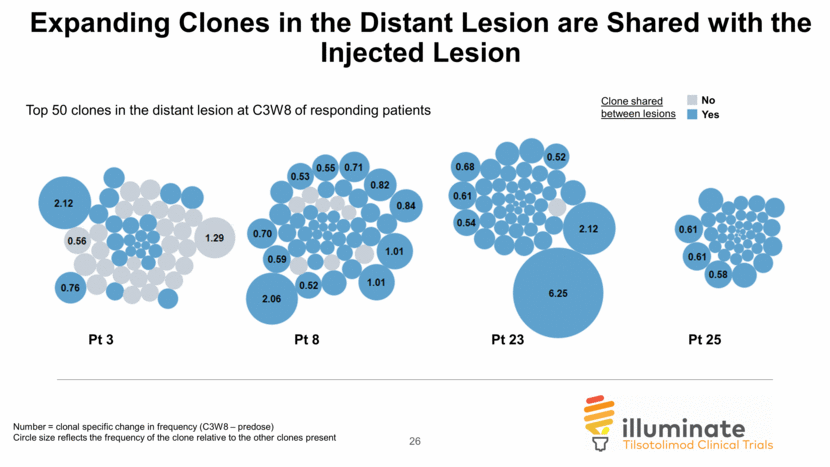
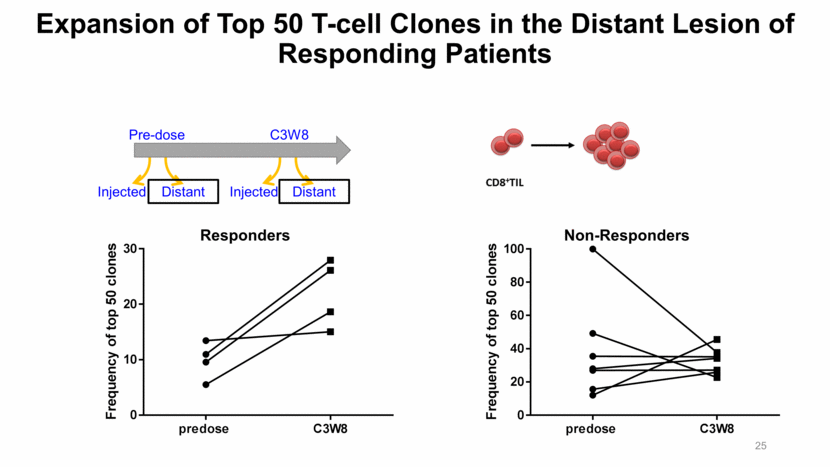
Expansion of Top 50 T-cell Clones in the Distant Lesion of Responding Patients Injected Pre-dose Distant C3W8 Injected Distant Responders Non-Responders 25 predose C3W8 0 10 20 30 F r e q u e n c y o f t o p 5 0 c l o n e s predose C3W8 0 20 40 60 80 100 F r e q u e n c y o f t o p 5 0 c l o n e s
Expanding Clones in the Distant Lesion are Shared with the Injected Lesion Pt 23 Number = clonal specific change in frequency (C3W8 – predose) Circle size reflects the frequency of the clone relative to the other clones present Pt 25 Pt 8 Pt 3 Clone shared between lesions Top 50 clones in the distant lesion at C3W8 of responding patients Yes No 26