Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - EYEGATE PHARMACEUTICALS INC | tv509033_8k.htm |
Exhibit 99.1

NASDAQ: EYEG Corporate Presentation Two Versatile Platforms Moving Towards Commercialization December 2018

Some of the matters discussed in this presentation contain forward - looking statements that involve significant risks and uncertainties, including statements relating to the prospects for the Company’s OBG and EGP - 437 product candidates , for the timing and outcome of the Company’s clinical trials, the potential approval to market OBG and EGP - 437 , and the Company’s capital needs . Actual events could differ materially from those projected in this presentation and the Company cautions investors not to rely on the forward - looking statements contained in, or made in connection with, the presentation . Among other things, the Company’s clinical trials may be delayed or may eventually be unsuccessful . The Company may consume more cash than it currently anticipates and faster than projected . Competitive products may reduce or eliminate the commercial opportunities of the Company’s product candidates . If the U . S . Food and Drug Administration or foreign regulatory agencies determine that the Company’s product candidates do not meet safety or efficacy endpoints in clinical evaluations, they will not receive regulatory approval and the Company will not be able to market them . Operating expense and cash flow projections involve a high degree of uncertainty, including variances in future spending rate due to changes in corporate priorities, the timing and outcomes of clinical trials, regulatory and developments and the impact on expenditures and available capital from licensing and strategic collaboration opportunities . If the Company is unable to raise additional capital when required or on acceptable terms, it may have to significantly alter, delay, scale back or discontinue operations . Additional risks and uncertainties relating to the Company and its business can be found in the “Risk Factors” section of the Company’s Annual Report on Form 10 - K filed with the SEC on March 02 , 2018 . The Company undertakes no duty or obligation to update any forward - looking statements contained in this presentation as a result of new information, future events or changes in the Company’s expectations, except as required by applicable law . The Company uses its website ( www . EyeGatePharma . com ), Facebook page ( https : //www . facebook . com/ EyeGatePharma / ), corporate Twitter account ( https : //twitter . com/EyeGatePharma ), and LinkedIn page ( https : //www . linkedin . com/company/ 135892 / ) as channels of distribution of information about the Company and its product candidates . Such information may be deemed material information, and the Company may use these channels to comply with its disclosure obligations under Regulation FD . Therefore, investors should monitor the Company’s website and its social media accounts in addition to following its press releases, SEC filings, public conference calls, and webcasts . The social media channels that the Company intends to use as a means of disclosing the information described above may be updated from time to time as listed on the Company’s investor relations website . Forward Looking Statements 2

Two ophthalmic platform technologies: CMHA - S and Iontophoresis drug delivery CMHA - S platform (cross - linked Hyaluronic Acid) – first product is an eye drop formulation (OBG) OBG eye drop regulated as a device, accelerating the product development plan and time to market, with FDA filing expected in 2019 OBG is the first and only prescription Hyaluronic Acid eye drop in the U.S. OBG: recently announced positive data from two clinical studies (PRK surgery and PE) Iontophoresis (EGP - 437) Phase 3 Uveitis trial misses primary endpoint, data under review to assess strategic options Company Highlights (NASDAQ: EYEG) 3

CMHA - S Platform Ocular Bandage Gel (OBG) Eye Drop
5 OBG: A Differentiated Product on a Rapid Path to Approval Proprietary Formulation of a Known and Trusted Substance Creates a Unique and Differentiated Product » Following a medical device pathway, filing expected in 2019 » Unique crosslinked HA produces a preservative - free, high concentration (0.75%) eye drop that resists degradation and adheres to the ocular surface without blurring vision » Hydrating, protectant and lubricant that facilitates management of corneal re - epithelialization » Addresses continued unmet medical need and research supports favorable economics » Positive results in multiple clinical trials: two for PRK and one for PE (dry eye)

6 Hyaluronic Acid (HA) HA is a Naturally Occurring Molecule that Possesses Beneficial Properties Hyaluronic Acid » Non - immunogenic: does not illicit an immune response » Binds up to 1,000 times its volume in water weight providing hydration and lubrication ideal for the ocular surface » Contributes to cellular proliferation and migration during the wound healing process » Approved in the U.S. for wound and burn management as well as osteoarthritis » A low concentration formulation (0.1% to 0.4%) is the Standard of Care in Europe and Asia for dry eye » Well known and trusted substance in US ophthalmic care
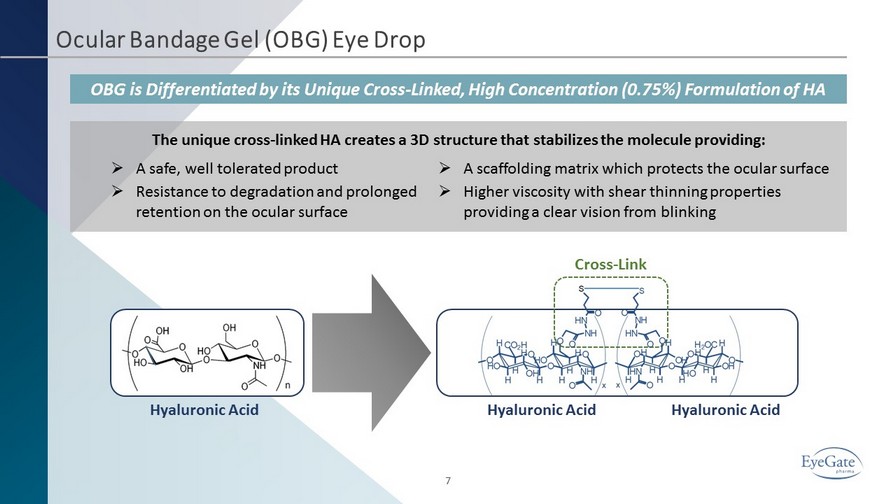
Ocular Bandage Gel (OBG) Eye Drop OBG is Differentiated by its Unique Cross - Linked, High Concentration (0.75%) Formulation of HA The unique cross - linked HA creates a 3D structure that stabilizes the molecule providing: Hyaluronic Acid Hyaluronic Acid Hyaluronic Acid 7 » A scaffolding matrix which protects the ocular surface » Higher viscosity with shear thinning properties providing a clear vision from blinking » A safe, well tolerated product » Resistance to degradation and prolonged retention on the ocular surface Cross - Link

8 » A clear, viscous, preservative - free eye drop containing a high concentration (0.75%) of crosslinked hyaluronic acid (CMHA - S) » A long - acting lubricant that protects the corneal surface to promote healing and reduce staining associated with Punctate Epitheliopathies in dry eye patients » Demonstrated ability to manage re - epithelialization in first clinical study for PRK patients » Combining with therapeutics: initial 3 classes are antibiotics, corticosteroids and antihistamines Ocular Bandage Gel (OBG) Eye Drop Efficacy of CMHA - S has Been Demonstrated in Various Animal Pathologic Conditions A B Molly: 12 year old cat with a non - healing corneal defect A. Non - healing at 42 days B. Ulcer healing after 12 days of using 0.75% CMHA - S » Post traumatic corneal stromal ulcers (real world dogs and cats) » Dry eye (veterinary dogs who failed topical cyclosporine) » Corneal abrasion and alkali burn injuries (rabbit models)

PE (non - dry eye) ~45.0 million Epithelial injury (exposure) ~16.0 million Ocular trauma ~1.8 million Surgery ~1.0 million Broad Market Opportunity 9 Wounds: Surgery • Refractive surgeries (PRK) • LASIK • Collagen cross - linking Wounds: Trauma • Injuries / Abrasions • Chemical burns • Difficult to heal (PCED) PE: Dry Eye • Episodic / mild • Moderate • Severe PE: non - Dry Eye • Pre - Cataract Surgery • Contact lens wear • Ocular irritants Over 60 Million Potential Patients with Corneal Wounds or Epitheliopathies in the U.S.

10 Increasing Prevalence of Dry Eye Disease with Favorable Economics 1. Gayton JL. Clin Ophthalmol . 2009;3:405 - 12.; 2. Report of the international dry eye workshop (DEWS). Ocul Surf. 2007;5(2):65 - 204.; 3. Allergan Dry Eye Survey, American Optometric Association, November 6, 2011. Dry Eye Disease is One of the Most Common Chronic Ophthalmologic Diseases 1,2,3 Payer Research, which Anticipates Generic Restasis, Supports Pricing in the Range of $125 - $225 Nearly half of all U.S. adults (48%) experience one or more dry eye symptoms regularly Half of all women (52%) experience one or more dry eye symptoms regularly 2 in 5 women age 45−54 who suffer from dry eye symptoms (42%) experience blurred vision 30% of men 55 and older have experienced dry eye symptoms for more than 10 years Primary focus is on the punctate epitheliopathy (dry eye) market » Patients are currently not adequately managed on artificial tears and/or adjunctive to Restasis and Xiidra » Physician research supports the need for additional treatment options and strong support for OBG profile in dry eye and wound management

Near - Term Milestones 11 Program Disease Area Q4 2018 Q1 2019 OBG Eye Drop Cross - linked Hyaluronic Acid Large Corneal Wounds: Photorefractive Keratectomy (PRK) 2 nd Clinical Study Completed Pre - Submission Meeting with FDA (CDRH) Punctate Epitheliopathies (PE): e.g. Dry Eye, Contact Lens Wear, etc. 1 st Clinical Study Completed

Positive Clinical Trial Results 12 Completed First Human Clinical Trial in PRK Patients Healed Wound on Day 3 Day 1 Day 3 Number of Subjects Per Arm Number Percent Horizontal* Vertical* Horizontal Vertical Arm 1 Ocular Bandage Gel 12 10 83.3% 4.1 4.5 0.1 0.2 Arm 2 Ocular Bandage Gel + Bandage Contact Lens 14 9 64.3% 6.3 6.5 0.3 0.3 Arm 3 (Standard of Care) Bandage Contact Lens + Artificial Tears 13 7 53.8% 6.4 6.2 0.6 0.6 54.8% 35.9% 27.4% 83.3% 66.7% Ocular Bandage Gel: % Better than Bandage Contact Lens *Length in mm Re - epithelialization Wound Healing Study 1 : OBG vs. Standard of Care (Bandage Contact Lens + Artificial Tears) x Approximately 55% more patients treated with OBG healed by Day 3 x As early as Day 1 (24 hr. post - op) average wound size was around 36% smaller and 83.3% smaller by Day 3 with OBG alone 1. Open - label, unmasked study. Durrie et al. J Cataract Refract Surg. 2018 Mar;44(3):369 - 375.

13 Second PRK Pilot Study

Photorefractive Keratectomy (PRK) Study Design Schematic Objective is to demonstrate re - epithelialization of large corneal epithelial defects 14 Additional study for patients that have undergone bilateral Photorefractive Keratectomy (PRK) • 45 subjects (3 arms): OBG (2 dosing regimens) vs standard of care (bandage contact lens + artificial tears) • Evaluated by a masked reading center (Tufts) using digital photography of fluorescein stained slit lamp photos
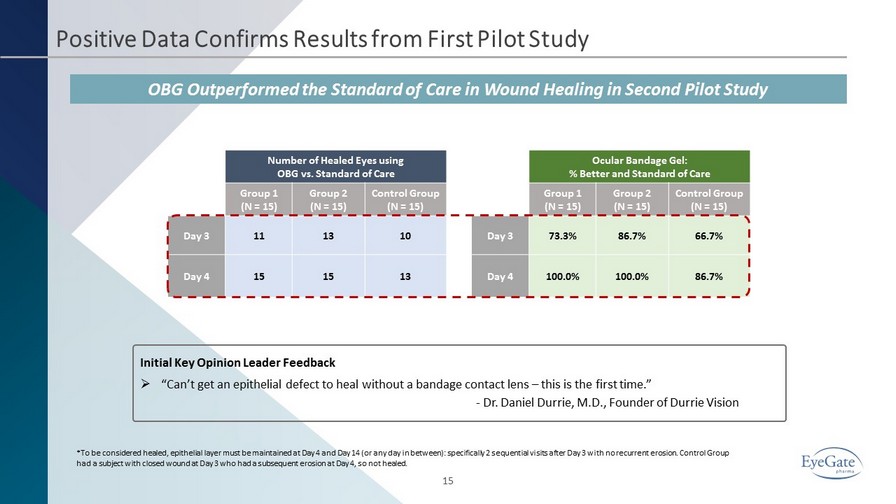
Positive Data Confirms Results from First Pilot Study 15 OBG Outperformed the Standard of Care in Wound Healing in Second Pilot Study Number of Healed Eyes using OBG vs. Standard of Care Group 1 (N = 15) Group 2 (N = 15) Control Group (N = 15) Day 3 11 13 10 Day 4 15 15 13 Ocular Bandage Gel: % Better and Standard of Care Group 1 (N = 15) Group 2 (N = 15) Control Group (N = 15) Day 3 73.3% 86.7% 66.7% Day 4 100.0% 100.0% 86.7% *To be considered healed, epithelial layer must be maintained at Day 4 and Day 14 (or any day in between): specifically 2 seq uen tial visits after Day 3 with no recurrent erosion. Control Group had a subject with closed wound at Day 3 who had a subsequent erosion at Day 4, so not healed. Initial Key Opinion Leader Feedback » “Can’t get an epithelial defect to heal without a bandage contact lens – this is the first time.” - Dr. Daniel Durrie, M.D., Founder of Durrie Vision

PRK Wound Size Dramatically Improved using OBG 16 Group 1 (N = 15) Group 2 (N = 15) Control Group (N = 15) Day 2 3.07 3.91 4.15 Day 3 0.10 0.19 0.16 Day 4 0.00 0.00 0.33 Day 14 0.00 0.00 0.00 Average Wound Size (mm 2 ) Day 2 (48 hours post surgery) average wound size 26% and 6% smaller than the Control Group for OBG Group 1 and 2 respectively Group 1 (N = 15) Group 2 (N = 15) Control Group (N = 15) Day 2 7.97 12.20 23.95 Day 3 0.99 1.98 1.91 Maximum Wound Size (mm 2 ) Day 2 (48 hours post surgery) maximum wound size 67% and 49% smaller than the Control Group for OBG Group 1 and 2 respectively *To be considered healed, epithelial layer must be maintained at Day 4 and Day 14 (or any day in between): specifically 2 seq uen tial visits after Day 3 with no recurrent erosion. Control Group had a subject with closed wound at Day 3 who had a subsequent erosion at Day 4, so not healed. No safety concerns

17 PE Pilot Study
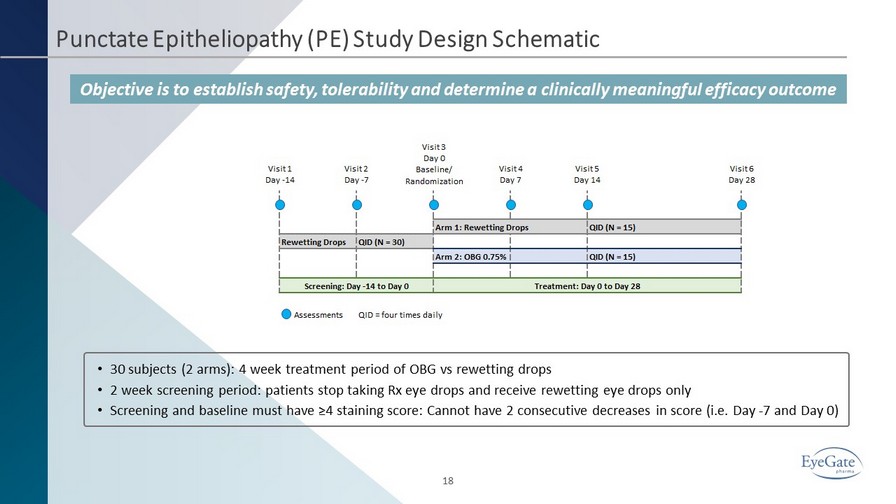
Punctate Epitheliopathy (PE) Study Design Schematic Objective is to establish safety, tolerability and determine a clinically meaningful efficacy outcome 18 • 30 subjects (2 arms): 4 week treatment period of OBG vs rewetting drops • 2 week screening period: patients stop taking Rx eye drops and receive rewetting eye drops only • Screening and baseline must have ≥4 staining score: Cannot have 2 consecutive decreases in score (i.e. Day - 7 and Day 0)

Punctate Epitheliopathy (PE) Study Showed Positive Data Statistically significant improvement in symptoms** by Day 7 19 * Wilcoxon Two - Sample Test OBG achieved a 30% decrease from baseline vs only 4% for control OBG demonstrates fast onset with SS p - Value at Day 7 and continues through Day 28 ** Symptomology assessed using the SPEED Questionnaire: Max Score = 28 p - Value* = 0.0343 p - Value* = 0.0573 p - Value* = 0.0464

Punctate Epitheliopathy (PE) Pilot Study Results: Staining 20 Central Cornea 1 Quickly Improves and Continues to Perform 1) Central Cornea important for visual field 2) Combined Data = Study eye + non - study eye » This data supports and correlates with symptomology due to high concentration of nerve endings being located in the central cornea » SPEED Questionnaire is answered based on comfort in both eyes 0.0% - 30.0% - 40.0% - 25.0% 0.0% - 13.6% - 22.7% - 22.7% -45.0% -40.0% -35.0% -30.0% -25.0% -20.0% -15.0% -10.0% -5.0% 0.0% Day 0 Day 7 Day 14 Day 28 Combined: Central Cornea OBG Rewetting Drops

Iontophoresis Platform EyeGate® II Delivery System and EGP - 437

EyeGate® II Delivery System and EGP - 437 22 A Non - Invasive Method of Propelling Charged Active Compounds into Ocular Tissues EGP - 437 , a reformulated corticosteroid, Dexamethasone Sodium Phosphate is delivered into the ocular tissues through EyeGate’s proprietary innovative drug delivery system, the EyeGate® II Delivery System A. Applicator B. Small electrical current at electrode C. Charged drug product (in applicator) D. Active product propelled into the eye E. Eye receiving drug product noninvasively Dose is controlled by current strength and application time Drug Product A. C. B. E. D.

Exclusive Licensing Agreement with Bausch Health (Formerly Valeant Pharmaceuticals) 23 Worldwide License to Manufacture, Sell, Distribute, and Commercialize EGP - 437 with the EyeGate® II Delivery System for Uveitis and Post - Operative Ocular Inflammation and Pain $136.5 million in potential payments, including up - front, development and commercial milestones Anterior Uveitis • $1 million up - front payment • Up to $32.5 million development and commercials milestones Cataract Surgery • $4 million up - front payment • Up to $99 million development and commercials milestones High Single Digit Royalties based on net sales with upward adjustment to double - digit for cataract surgery EyeGate is responsible for the completion of clinical development and FDA filing for both indications. Bausch holds no rights to “use of system” with other drugs.
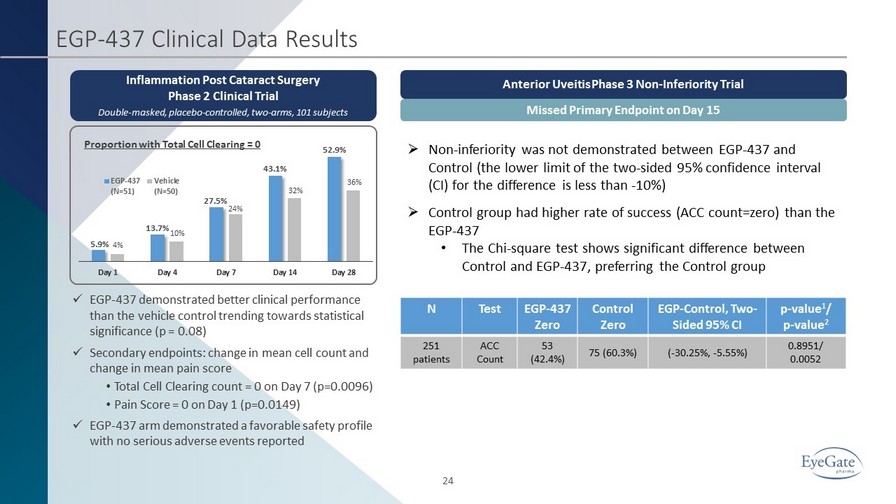
24 EGP - 437 Clinical Data Results Inflammation Post Cataract Surgery Phase 2 Clinical Trial Double - masked, placebo - controlled, two - arms, 101 subjects 5.9% 13.7% 27.5% 43.1% 52.9% 4% 10% 24% 32% 36% Day 1 Day 4 Day 7 Day 14 Day 28 EGP-437 (N=51) Vehicle (N=50) Proportion with Total Cell Clearing = 0 x EGP - 437 demonstrated better clinical performance than the vehicle control trending towards statistical significance (p = 0.08) x Secondary endpoints: change in mean cell count and change in mean pain score • Total Cell Clearing count = 0 on Day 7 (p=0.0096) • Pain Score = 0 on Day 1 (p=0.0149) x EGP - 437 arm demonstrated a favorable safety profile with no serious adverse events reported » Non - inferiority was not demonstrated between EGP - 437 and Control (the lower limit of the two - sided 95% confidence interval (CI) for the difference is less than - 10%) » Control group had higher rate of success (ACC count=zero) than the EGP - 437 • The Chi - square test shows significant difference between Control and EGP - 437, preferring the Control group Anterior Uveitis Phase 3 Non - Inferiority Trial Missed Primary Endpoint on Day 15 N Test EGP - 437 Zero Control Zero EGP - Control, Two - Sided 95% CI p - value 1 / p - value 2 251 patients ACC Count 53 (42.4%) 75 (60.3%) ( - 30.25%, - 5.55%) 0.8951/ 0.0052

Two ophthalmic platform technologies: CMHA - S and Iontophoresis drug delivery CMHA - S platform (cross - linked Hyaluronic Acid) – first product is an eye drop formulation (OBG) OBG eye drop regulated as a device, accelerating the product development plan and time to market, with FDA filing expected in 2019 OBG is the first and only prescription Hyaluronic Acid eye drop in the U.S. OBG: recently announced positive data from two clinical studies (PRK surgery and PE) Iontophoresis (EGP - 437) Phase 3 Uveitis trial misses primary endpoint, data under review to assess strategic options Company Highlights (NASDAQ: EYEG) 25

Thank You NASDAQ: EYEG Contact Us Joseph Green Edison Advisors Investor Relations Tel: (646) 653 - 7030 E - mail: jgreen@edisongroup.com
