Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - SUPERNUS PHARMACEUTICALS, INC. | a18-41352_1ex99d2.htm |
| EX-99.1 - EX-99.1 - SUPERNUS PHARMACEUTICALS, INC. | a18-41352_1ex99d1.htm |
| 8-K - 8-K - SUPERNUS PHARMACEUTICALS, INC. | a18-41352_18k.htm |
1 Supernus Pharmaceuticals SPN-812 Phase III Topline Data Investor Webcast – December 2018
Safe Harbor Statement This presentation and other matters discussed today or answers that may be given to questions asked include forward-looking statements within the meaning of the federal securities laws. These statements, among other things, relate to Supernus’ business strategy, goals and expectations concerning its product candidates, future operations, prospects, plans and objectives of management. The words “anticipate”, “believe”, “could”, “estimate”, “expect”, “intend”, “may”, “plan”, “predict“, “project”, “will“, and similar terms and phrases are used to identify forward-looking statements in this presentation. Supernus’ operations involve risks and uncertainties, many of which are outside its control, and any one of which, or a combination of which, could materially affect its results of operations and whether the forward-looking statements ultimately prove to be correct. Supernus assumes no obligation to update any forward-looking statements except as required by applicable law. Supernus has filed with the U.S. Securities and Exchange Commission (SEC) reports and other documents required by Section 13 or 15(d) of the Securities Exchange Act of 1934, as amended. Before you purchase any Supernus securities, you should read such reports and other documents to obtain more complete information about the company’s operations and business and the risks and uncertainties that it faces in implementing its business plan. You may get these documents for free by visiting EDGAR on the SEC website at http://www.sec.gov. 2

Viloxazine hydrochloride Norepinephrine reuptake inhibitor, selective serotonin activity New Chemical Entity (NCE) with five year market exclusivity Previously marketed outside the U.S. as an antidepressant Building strong IP with expirations from 2029-2033 Clinical data point to a well-differentiated ADHD product Targeted NDA filing 2H 2019, and if approved, launch 2H 2020 3 SPN-812 Novel Non-Stimulant ADHD Product Candidate

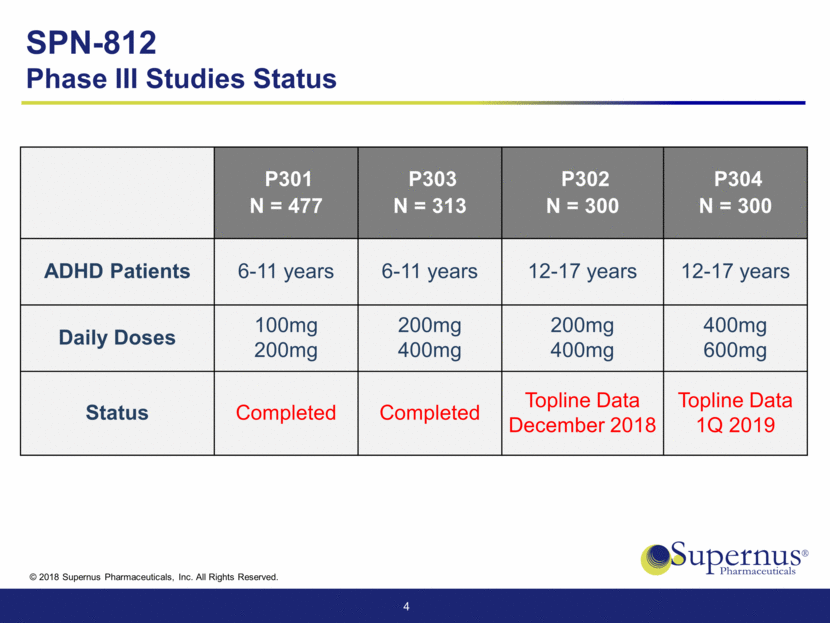
SPN-812 Phase III Studies Status P301 N = 477 P303 N = 313 P302 N = 300 P304 N = 300 ADHD Patients 6-11 years 6-11 years 12-17 years 12-17 years Daily Doses 100mg 200mg 200mg 400mg 200mg 400mg 400mg 600mg Status Completed Completed Topline Data December 2018 Topline Data 1Q 2019 4
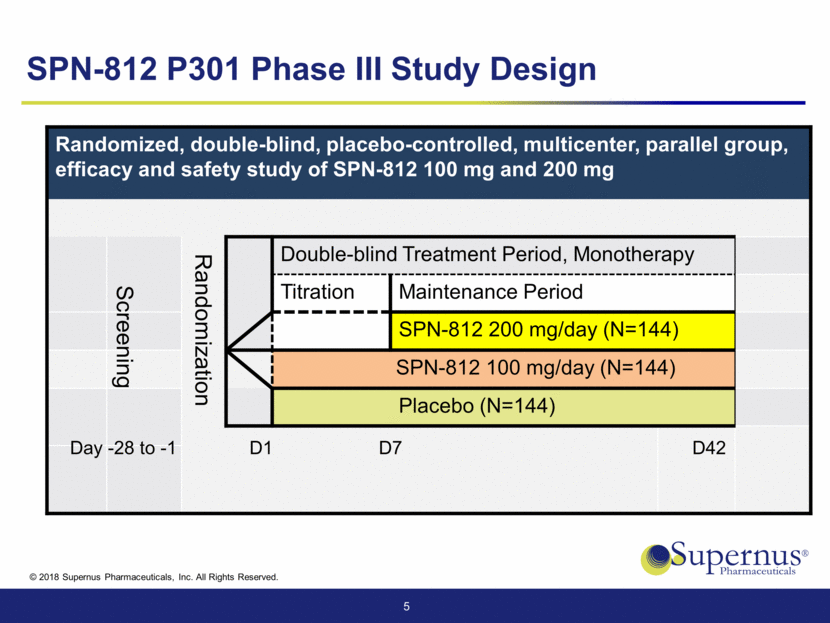
Randomized, double-blind, placebo-controlled, multicenter, parallel group, efficacy and safety study of SPN-812 100 mg and 200 mg Randomization Double-blind Treatment Period, Monotherapy Titration Maintenance Period SPN-812 200 mg/day (N=144) SPN-812 100 mg/day (N=144) Placebo (N=144) 5 D7 D42 Day -28 to -1 D1 Screening SPN-812 P301 Phase III Study Design
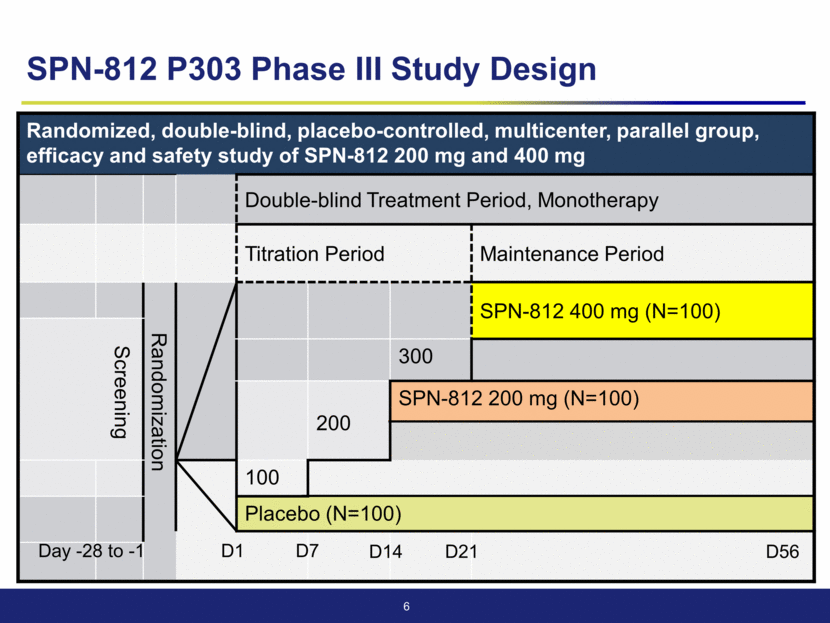
Randomized, double-blind, placebo-controlled, multicenter, parallel group, efficacy and safety study of SPN-812 200 mg and 400 mg Double-blind Treatment Period, Monotherapy Titration Period Maintenance Period Randomization SPN-812 400 mg (N=100) Screening 300 200 SPN-812 200 mg (N=100) 100 Placebo (N=100) 6 D7 D14 D56 D1 D21 Day -28 to -1 SPN-812 P303 Phase III Study Design
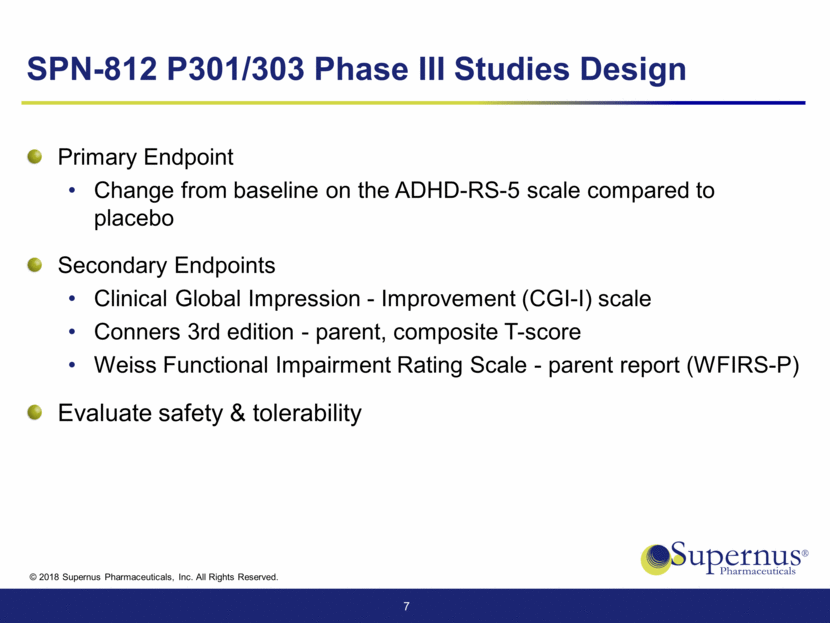
SPN-812 P301/303 Phase III Studies Design Primary Endpoint Change from baseline on the ADHD-RS-5 scale compared to placebo Secondary Endpoints Clinical Global Impression - Improvement (CGI-I) scale Conners 3rd edition - parent, composite T-score Weiss Functional Impairment Rating Scale - parent report (WFIRS-P) Evaluate safety & tolerability 7
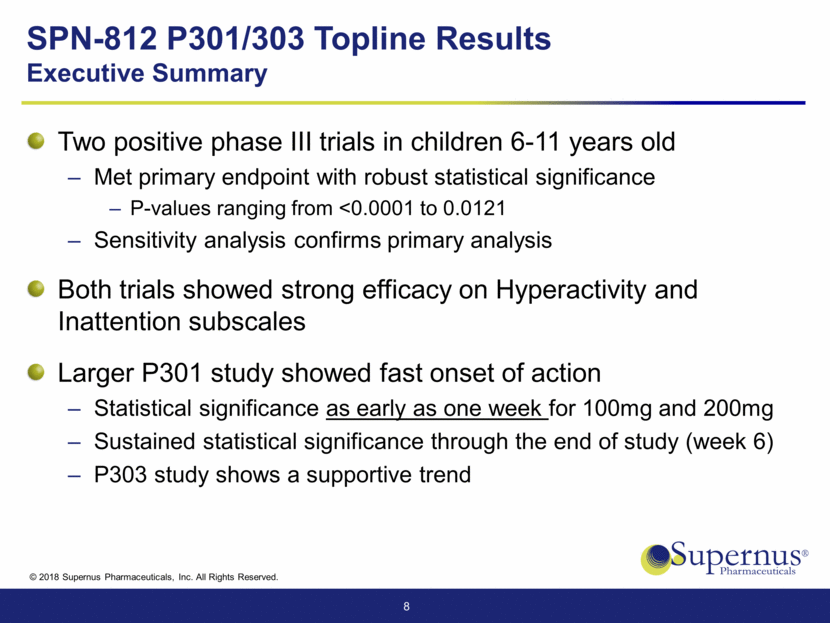
SPN-812 P301/303 Topline Results Executive Summary Two positive phase III trials in children 6-11 years old Met primary endpoint with robust statistical significance P-values ranging from <0.0001 to 0.0121 Sensitivity analysis confirms primary analysis Both trials showed strong efficacy on Hyperactivity and Inattention subscales Larger P301 study showed fast onset of action Statistical significance as early as one week for 100mg and 200mg Sustained statistical significance through the end of study (week 6) P303 study shows a supportive trend 8
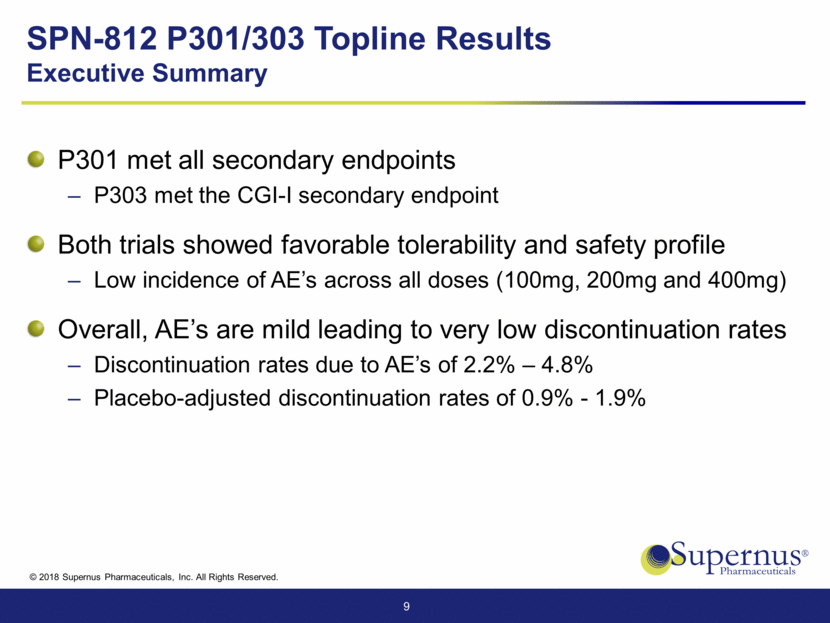
SPN-812 P301/303 Topline Results Executive Summary P301 met all secondary endpoints P303 met the CGI-I secondary endpoint Both trials showed favorable tolerability and safety profile Low incidence of AE’s across all doses (100mg, 200mg and 400mg) Overall, AE’s are mild leading to very low discontinuation rates Discontinuation rates due to AE’s of 2.2% – 4.8% Placebo-adjusted discontinuation rates of 0.9% - 1.9% 9
SPN-812 P301 Study Topline Results

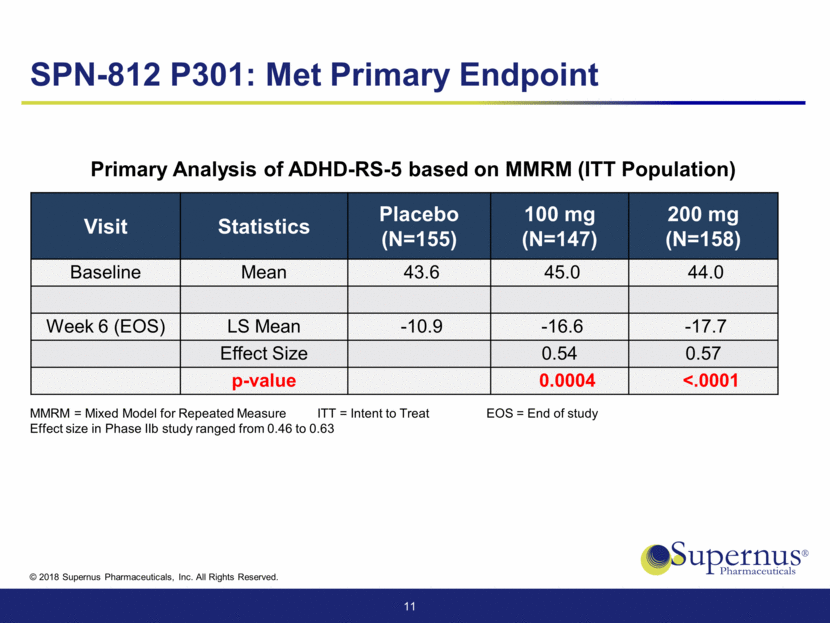
SPN-812 P301: Met Primary Endpoint Visit Statistics Placebo (N=155) 100 mg (N=147) 200 mg (N=158) Baseline Mean 43.6 45.0 44.0 Week 6 (EOS) LS Mean -10.9 -16.6 -17.7 Effect Size 0.54 0.57 p-value 0.0004 <.0001 11 MMRM = Mixed Model for Repeated Measure ITT = Intent to Treat EOS = End of study Effect size in Phase IIb study ranged from 0.46 to 0.63 Primary Analysis of ADHD-RS-5 based on MMRM (ITT Population)
SPN-812 P301: Met Primary Endpoint Efficacy Starting in Week 1 Visit Statistics Placebo (N=155) 100 mg (N=147) 200 mg (N=158) Baseline Mean 43.6 45.0 44.0 Week 1 LS Mean -5.8 -9.5 -8.1 p-value 0.0004 0.0244 Week 2 LS Mean -7.9 -12.6 -12.3 p-value <.0001 0.0001 Week 3 LS Mean -9.9 -15.4 -15.7 p-value <.0001 <.0001 Week 4 LS Mean -11.2 -17.0 -17.7 p-value <.0001 <.0001 Week 5 LS Mean -11.9 -17.3 -18.4 p-value 0.0006 <.0001 Week 6 (EOS) LS Mean -10.9 -16.6 -17.7 p-value 0.0004 <.0001 12 MMRM = Mixed Model for Repeated Measure ITT = Intent to Treat EOS = End of study
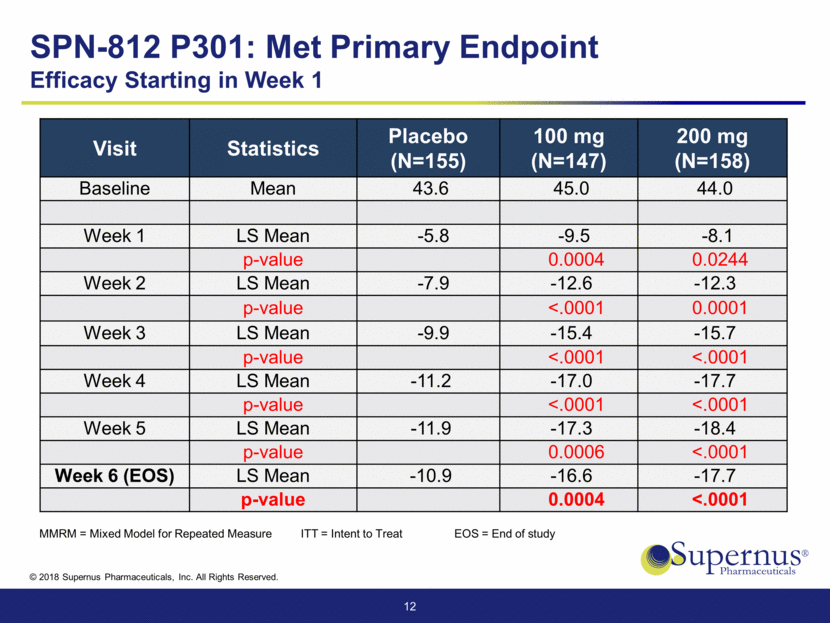
SPN-812 P301 – Efficacy Starting in Week 1 LS Mean Change from Baseline over Time – MMRM (ITT Population) 13 ** ** ** * ** ** ** ** ** ** ** ** * p < 0.05 ** p< 0.01 MMRM = Mixed Model for Repeated Measure ITT = Intent to Treat -20 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 Change from Baseline on ADHD - RS - 5 Placebo SPN-812 100 mg SPN-812 200 mg
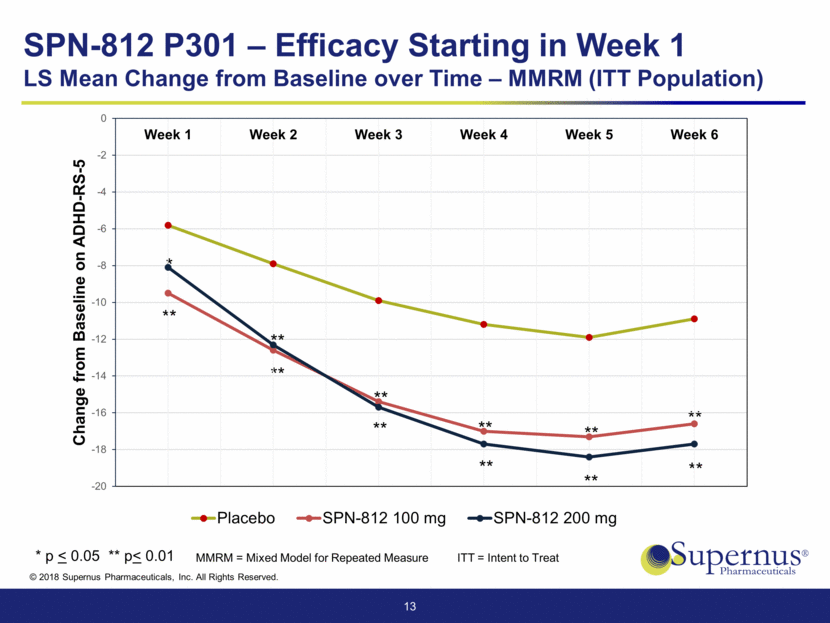
SPN-812 P301: Met Primary Endpoint Visit Statistics Placebo (N=155) 100 mg (N=147) 200 mg (N=158) Baseline Mean 43.6 45.0 44.0 Week 6 (EOS) LS Mean -11.1 -16.5 -17.8 p-value 0.0008 <.0001 14 ANCOVA = Analysis of Covariance ITT = Intent to Treat EOS = End of study Sensitivity Analysis of ADHD-RS-5 based on ANCOVA at Week 6 (EOS) Confirms Primary Analysis (ITT Population)
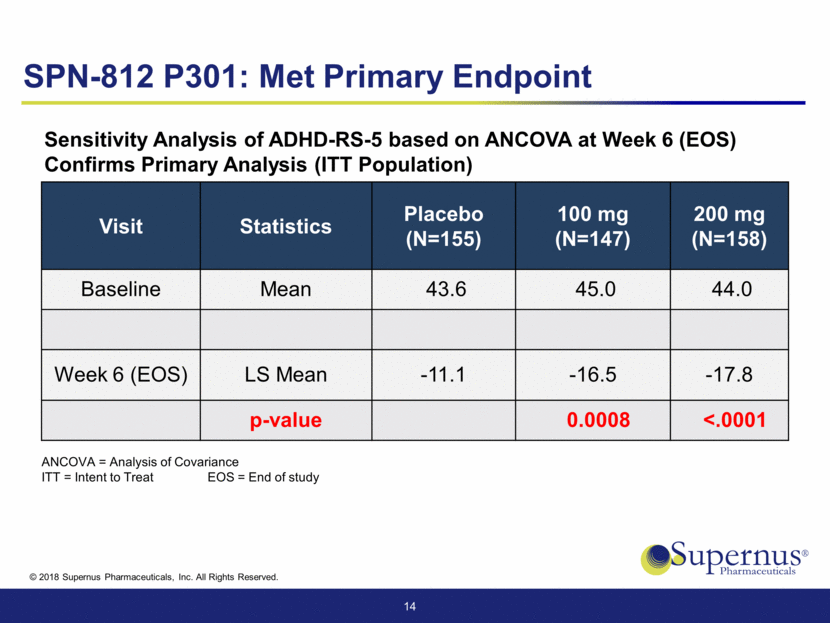
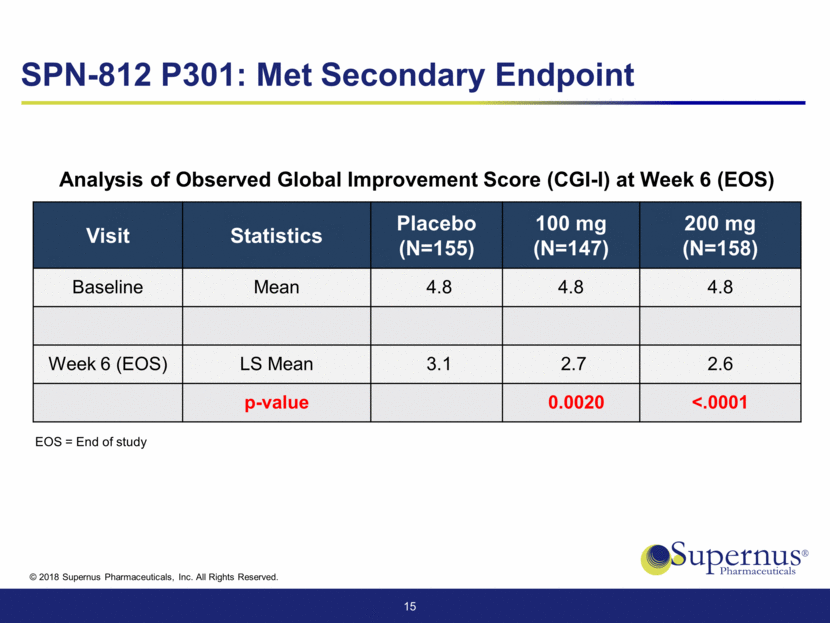
Visit Statistics Placebo (N=155) 100 mg (N=147) 200 mg (N=158) Baseline Mean 4.8 4.8 4.8 Week 6 (EOS) LS Mean 3.1 2.7 2.6 p-value 0.0020 <.0001 15 SPN-812 P301: Met Secondary Endpoint EOS = End of study Analysis of Observed Global Improvement Score (CGI-I) at Week 6 (EOS)
Visit Statistics Placebo (N=155) 100 mg (N=147) 200 mg (N=158) Baseline Mean 75.9 77.4 75.5 Week 6 (EOS) LS Mean -4.8 -9.1 -9.5 p-value 0.0004 <.0001 16 EOS = End of study Analysis of Change from Baseline at Week 6 (EOS) in Composite T-score for Conners 3 - Parent Reported Scores SPN-812 P301: Met Secondary Endpoint
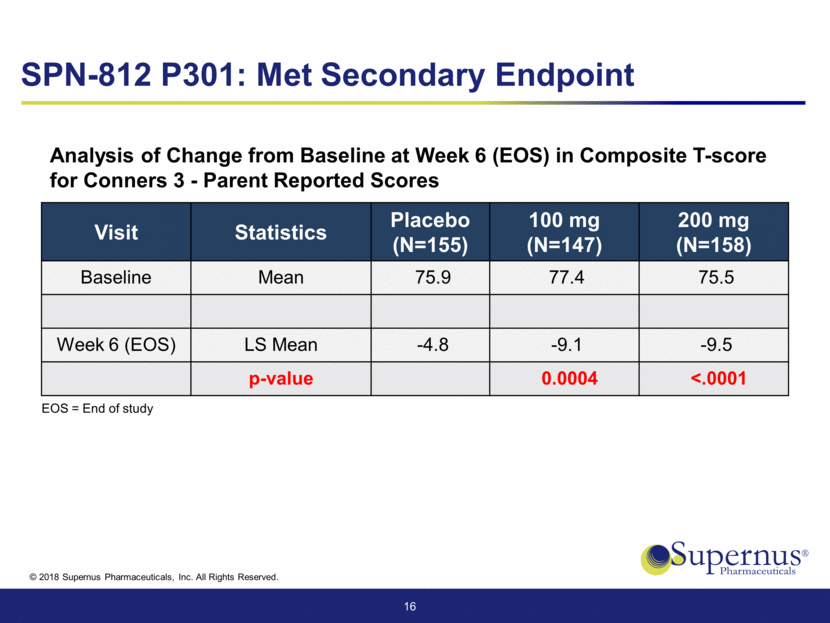
Visit Statistics Placebo (N=155) 100 mg (N=147) 200 mg (N=158) Baseline Mean 1.08 1.15 1.10 Week 6 (EOS) LS Mean -0.22 -0.36 -0.39 p-value 0.0019 0.0002 17 EOS = End of study WFIRS-P = Weiss Functional Impairment Rating Scale - Parent Report Analysis of Change from Baseline at Week 6 (EOS) in WFIRS-P SPN-812 P301: Met Secondary Endpoint
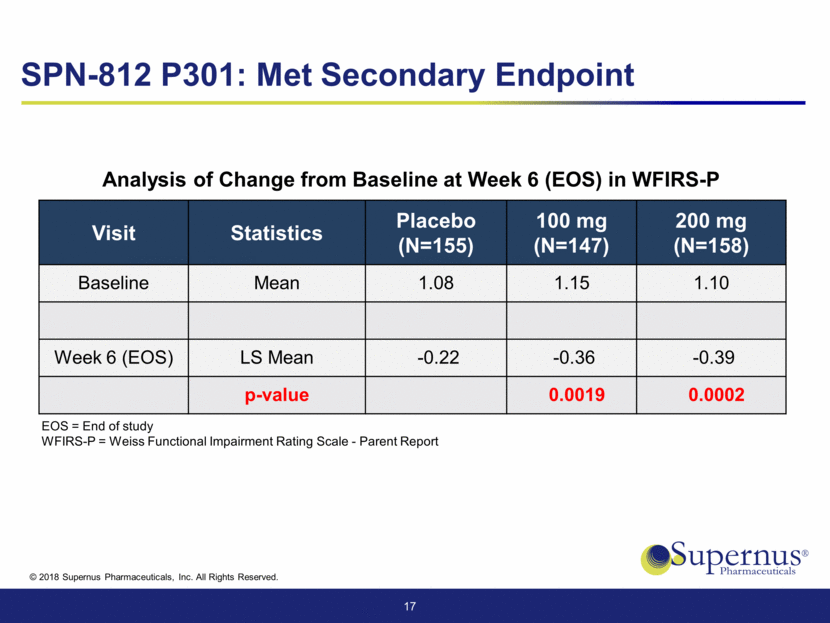
Visit Statistics Placebo (N=155) 100 mg (N=147) 200 mg (N=158) ADHD-RS-5 Hyperactivity/Impulsivity Baseline Mean 21.1 22.2 21.1 Week 6 (EOS) LS Mean -5.5 -8.0 -8.7 p-value 0.0026 <.0001 ADHD-RS-5 Inattention Baseline Mean 22.5 22.8 22.9 Week 6 (EOS) LS Mean -5.7 -8.6 -9.2 p-value 0.0006 <.0001 18 Analysis in ADHD-RS-5 Inattention and Hyperactivity/Impulsivity Subscales SPN-812 P301 Significant Reduction in Hyperactivity and Inattention EOS = End of study
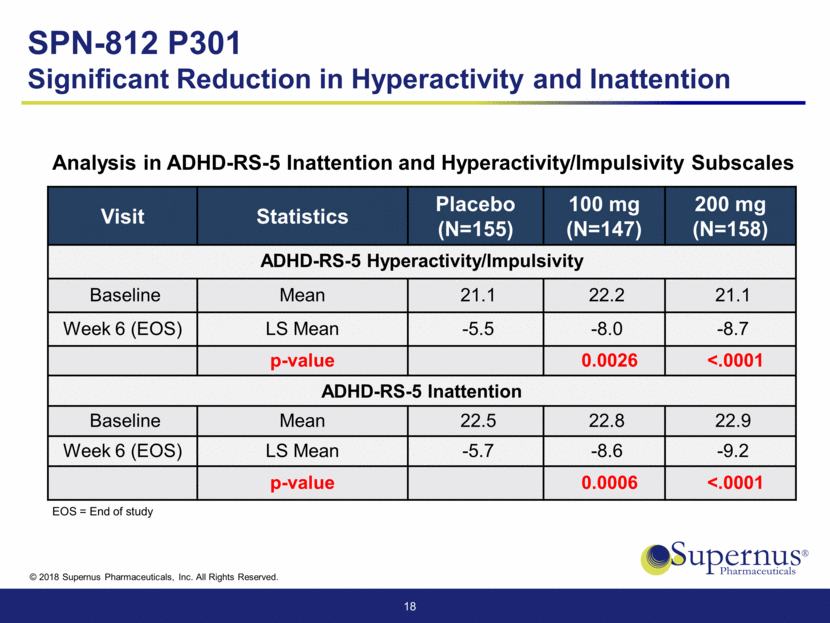
Placebo (N=159) 100 mg (N=154) 200 mg (N=161) Overall SPN-812 (N=315) Somnolence 3 ( 1.9) 16 (10.4) 15 ( 9.3) 31 ( 9.8) Headache 6 ( 3.8) 15 ( 9.7) 16 ( 9.9) 31 ( 9.8) Decreased appetite 0 8 ( 5.2) 14 ( 8.7) 22 ( 7.0) Sedation 0 3 ( 1.9) 9 ( 5.6) 12 ( 3.8) Vomiting 3 ( 1.9) 7 ( 4.5) 8 ( 5.0) 15 ( 4.8) Discontinuation Due to AE’s 2 ( 1.3) 5 ( 3.2) 2 ( 1.2) 7 ( 2.2) 19 Number (%) of Patients Reporting Common AEs (> 5% and greater than placebo) SPN-812 P301 Well Tolerated
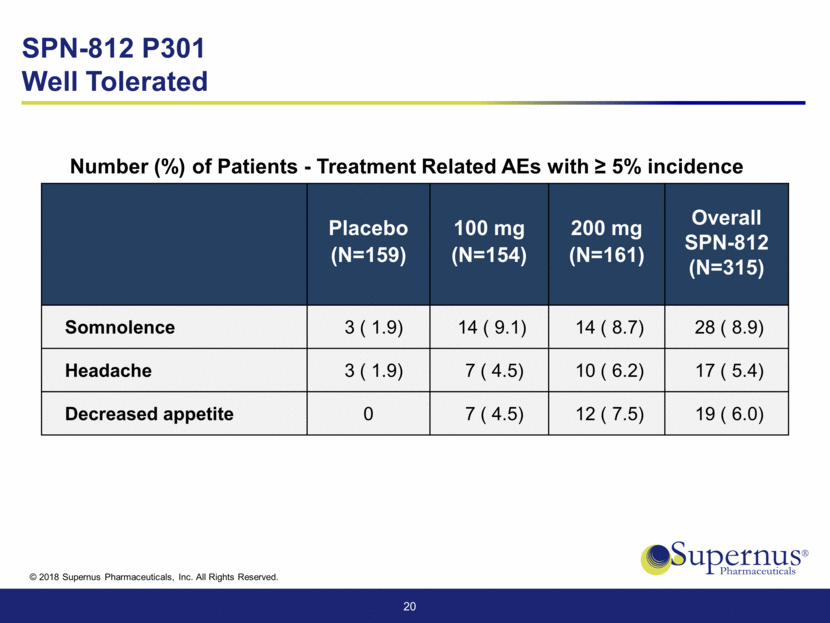
Placebo (N=159) 100 mg (N=154) 200 mg (N=161) Overall SPN-812 (N=315) Somnolence 3 ( 1.9) 14 ( 9.1) 14 ( 8.7) 28 ( 8.9) Headache 3 ( 1.9) 7 ( 4.5) 10 ( 6.2) 17 ( 5.4) Decreased appetite 0 7 ( 4.5) 12 ( 7.5) 19 ( 6.0) 20 Number (%) of Patients - Treatment Related AEs with > 5% incidence SPN-812 P301 Well Tolerated
SPN-812 P303 Study Topline Results

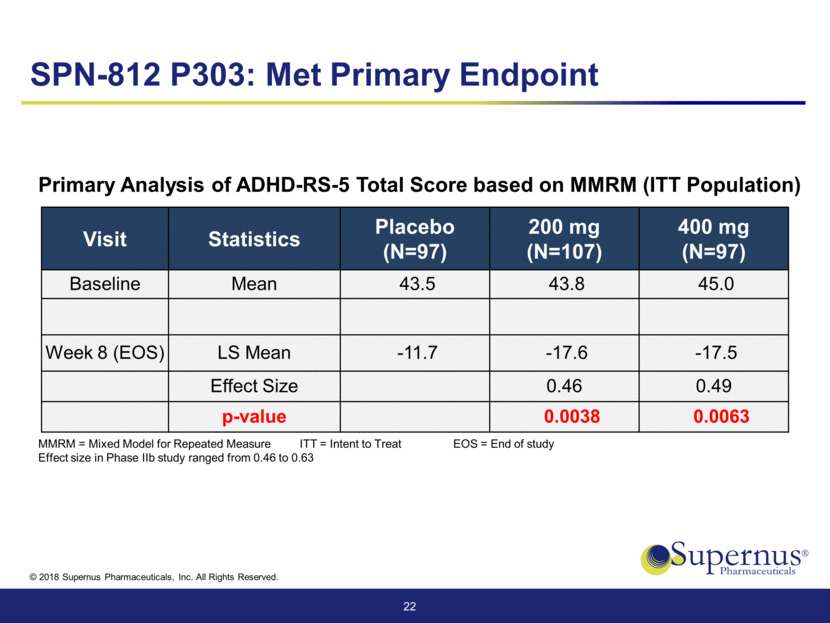
Visit Statistics Placebo (N=97) 200 mg (N=107) 400 mg (N=97) Baseline Mean 43.5 43.8 45.0 Week 8 (EOS) LS Mean -11.7 -17.6 -17.5 Effect Size 0.46 0.49 p-value 0.0038 0.0063 22 Primary Analysis of ADHD-RS-5 Total Score based on MMRM (ITT Population) SPN-812 P303: Met Primary Endpoint MMRM = Mixed Model for Repeated Measure ITT = Intent to Treat EOS = End of study Effect size in Phase IIb study ranged from 0.46 to 0.63
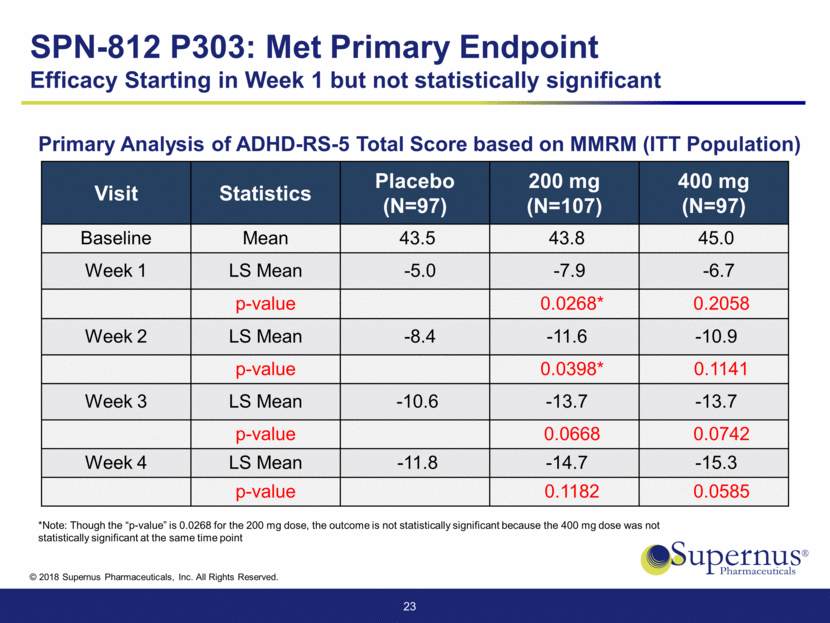
Visit Statistics Placebo (N=97) 200 mg (N=107) 400 mg (N=97) Baseline Mean 43.5 43.8 45.0 Week 1 LS Mean -5.0 -7.9 -6.7 p-value 0.0268* 0.2058 Week 2 LS Mean -8.4 -11.6 -10.9 p-value 0.0398* 0.1141 Week 3 LS Mean -10.6 -13.7 -13.7 p-value 0.0668 0.0742 Week 4 LS Mean -11.8 -14.7 -15.3 p-value 0.1182 0.0585 23 *Note: Though the “p-value” is 0.0268 for the 200 mg dose, the outcome is not statistically significant because the 400 mg dose was not statistically significant at the same time point SPN-812 P303: Met Primary Endpoint Efficacy Starting in Week 1 but not statistically significant Primary Analysis of ADHD-RS-5 Total Score based on MMRM (ITT Population)
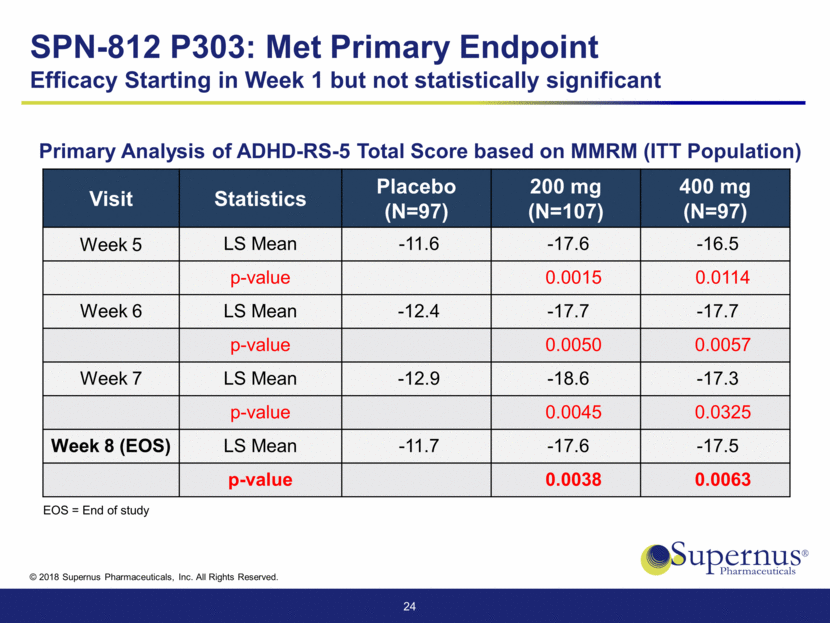
Primary Analysis of ADHD-RS-5 Total Score based on MMRM (ITT Population) Visit Statistics Placebo (N=97) 200 mg (N=107) 400 mg (N=97) Week 5 LS Mean -11.6 -17.6 -16.5 p-value 0.0015 0.0114 Week 6 LS Mean -12.4 -17.7 -17.7 p-value 0.0050 0.0057 Week 7 LS Mean -12.9 -18.6 -17.3 p-value 0.0045 0.0325 Week 8 (EOS) LS Mean -11.7 -17.6 -17.5 p-value 0.0038 0.0063 24 SPN-812 P303: Met Primary Endpoint Efficacy Starting in Week 1 but not statistically significant EOS = End of study
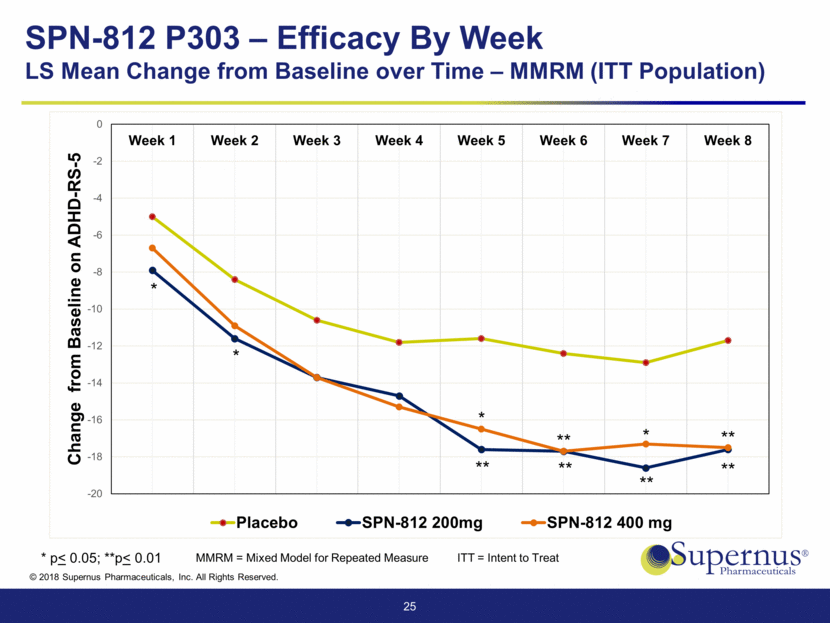
25 ** ** ** ** * ** ** * * * * p< 0.05; **p< 0.01 SPN-812 P303 – Efficacy By Week LS Mean Change from Baseline over Time – MMRM (ITT Population) MMRM = Mixed Model for Repeated Measure ITT = Intent to Treat -20 -18 -16 -14 -12 -10 -8 -6 -4 -2 0 Week 1 Week 2 Week 3 Week 4 Week 5 Week 6 Week 7 Week 8 Change from Baseline on ADHD - RS - 5 Placebo SPN-812 200mg SPN-812 400 mg
Visit Statistics Placebo (N=97) 200 mg (N=107) 400 mg (N=97) Baseline Mean 43.5 43.8 45.0 Week 8 (EOS) LS Mean -11.3 -17.0 -16.6 p-value 0.0058 0.0121 26 Sensitivity Analysis of ADHD-RS-5 based on ANCOVA at Week 8 (EOS) (ITT Population) SPN-812 P303: Met Primary Endpoint ANCOVA = Analysis of Covariance ITT = Intent to Treat EOS = End of study

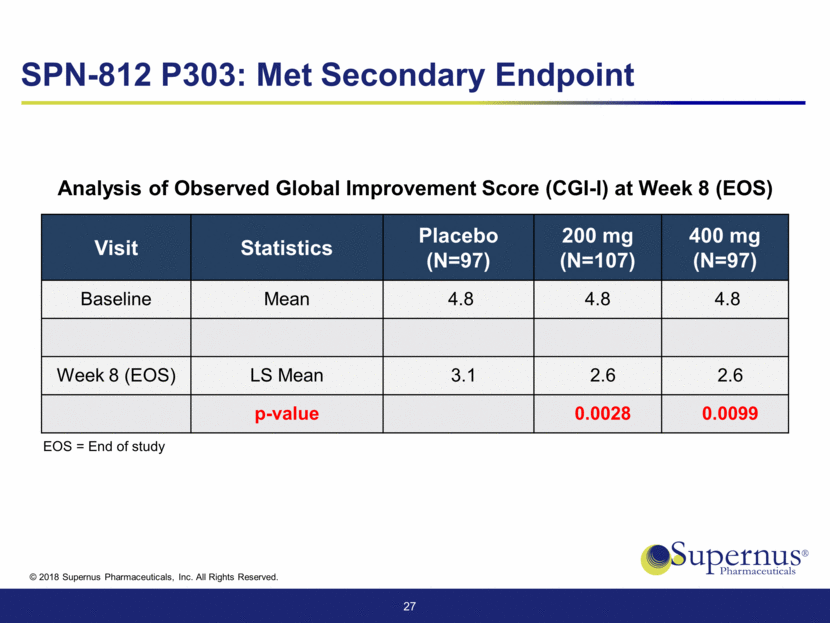
Visit Statistics Placebo (N=97) 200 mg (N=107) 400 mg (N=97) Baseline Mean 4.8 4.8 4.8 Week 8 (EOS) LS Mean 3.1 2.6 2.6 p-value 0.0028 0.0099 27 Analysis of Observed Global Improvement Score (CGI-I) at Week 8 (EOS) SPN-812 P303: Met Secondary Endpoint EOS = End of study
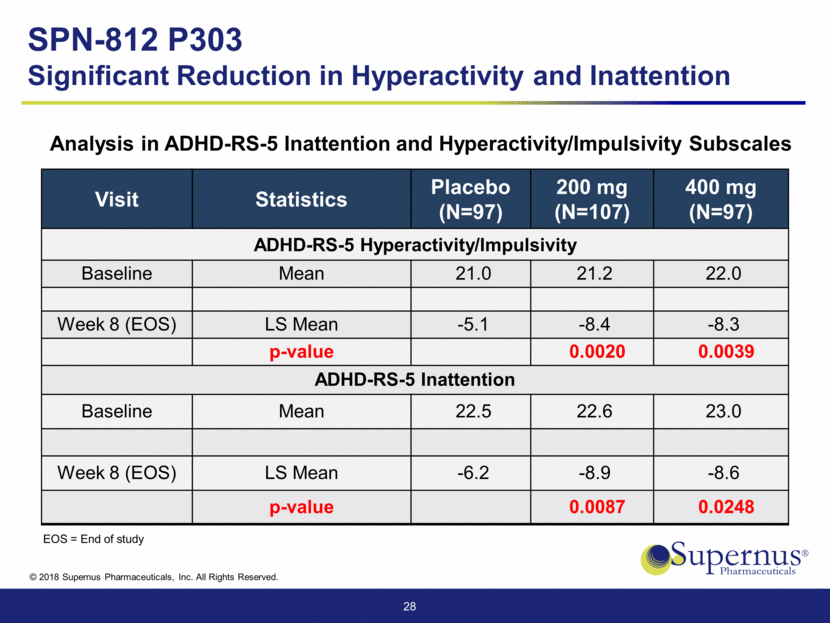
Visit Statistics Placebo (N=97) 200 mg (N=107) 400 mg (N=97) ADHD-RS-5 Hyperactivity/Impulsivity Baseline Mean 21.0 21.2 22.0 Week 8 (EOS) LS Mean -5.1 -8.4 -8.3 p-value 0.0020 0.0039 ADHD-RS-5 Inattention Baseline Mean 22.5 22.6 23.0 Week 8 (EOS) LS Mean -6.2 -8.9 -8.6 p-value 0.0087 0.0248 28 SPN-812 P303 Significant Reduction in Hyperactivity and Inattention Analysis in ADHD-RS-5 Inattention and Hyperactivity/Impulsivity Subscales EOS = End of study
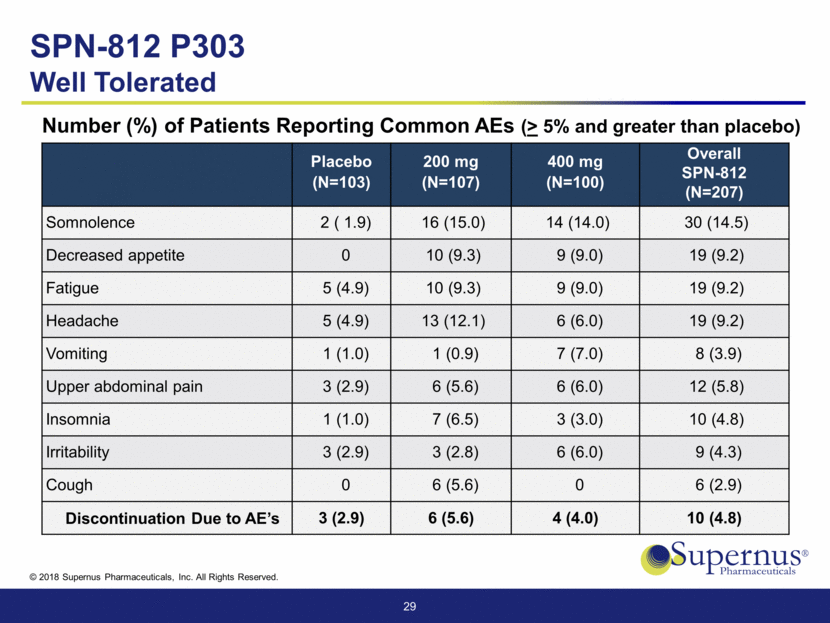
Placebo (N=103) 200 mg (N=107) 400 mg (N=100) Overall SPN-812 (N=207) Somnolence 2 ( 1.9) 16 (15.0) 14 (14.0) 30 (14.5) Decreased appetite 0 10 (9.3) 9 (9.0) 19 (9.2) Fatigue 5 (4.9) 10 (9.3) 9 (9.0) 19 (9.2) Headache 5 (4.9) 13 (12.1) 6 (6.0) 19 (9.2) Vomiting 1 (1.0) 1 (0.9) 7 (7.0) 8 (3.9) Upper abdominal pain 3 (2.9) 6 (5.6) 6 (6.0) 12 (5.8) Insomnia 1 (1.0) 7 (6.5) 3 (3.0) 10 (4.8) Irritability 3 (2.9) 3 (2.8) 6 (6.0) 9 (4.3) Cough 0 6 (5.6) 0 6 (2.9) Discontinuation Due to AE’s 3 (2.9) 6 (5.6) 4 (4.0) 10 (4.8) 29 Number (%) of Patients Reporting Common AEs (> 5% and greater than placebo) SPN-812 P303 Well Tolerated
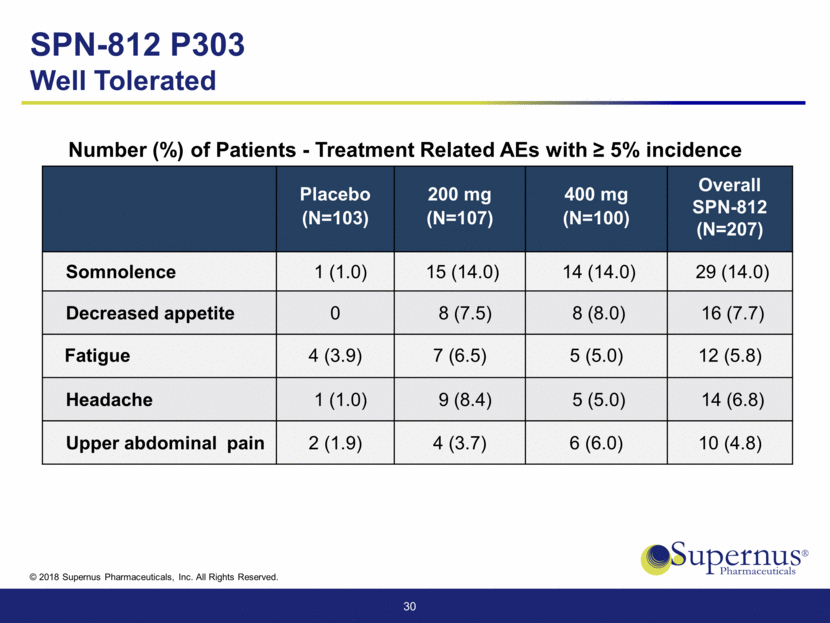
30 Placebo (N=103) 200 mg (N=107) 400 mg (N=100) Overall SPN-812 (N=207) Somnolence 1 (1.0) 15 (14.0) 14 (14.0) 29 (14.0) Decreased appetite 0 8 (7.5) 8 (8.0) 16 (7.7) Fatigue 4 (3.9) 7 (6.5) 5 (5.0) 12 (5.8) Headache 1 (1.0) 9 (8.4) 5 (5.0) 14 (6.8) Upper abdominal pain 2 (1.9) 4 (3.7) 6 (6.0) 10 (4.8) SPN-812 P303 Well Tolerated Number (%) of Patients - Treatment Related AEs with > 5% incidence
SPN-812 P301/303 Topline Results Executive Summary Two positive phase III trials in children 6-11 years old Clinical data point to a well-differentiated ADHD product Strong efficacy with robust statistical significance Efficacy on both Hyperactivity and Inattention Fast onset of action Works as early as the first week Very well tolerated Data on the P302 adolescent trial before year-end Data on the P304 adolescent trial in 1Q 2019 Targeted NDA filing 2H 2019, and if approved, launch 2H 2020 31
Growth Potential for Existing Products Potential Peak Sales for Oxtellar XR® and Trokendi XR® >$500M Innovative Late Stage Portfolio in Psychiatry SPN-812 Well Differentiated Novel Non-Stimulant SPN-810 First Product to be Developed for Impulsive Aggression SPN-604 Novel Product for Bipolar Disorder Positioned For Continued Strong Growth 32

