Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Protagonist Therapeutics, Inc | a18-40738_1ex99d1.htm |
| 8-K - 8-K - Protagonist Therapeutics, Inc | a18-40738_18k.htm |
Cautionary Note on Forward-Looking Statements 2 This presentation contains forward - looking statements for purposes of the safe harbor provisions of the Private Securi ties Litigation Reform Act of 1995. Forward - looking statements include statements regarding our intentions or current expectations concerning, among other things, the potential for our programs including PN - 10943, the timing of the initiation and availabil ity of results of our clinical trials and our potential milestone payment receipt and cash runway . In some cases, you can identify these statements by forward - looking words such as "anticipate," "believe," "may," "will," "expect," or the negative or plural of these words or similar expressions. Forward - looking statements are not guarantees of future performance and are subject to risks and uncertainties that could cause actual results and events to differ materially from those anticipated, including, but n ot limited to, our ability to develop and commercialize our product candidates, our ability to earn milestone payments under our collaboration agreement with Janss en, our ability to use and expand our programs to build a pipeline of product candidates, our ability to obtain and maintain regulatory approval of our product candidates, our ability to operate in a competitive industry and compete successfully against competi tors that have greater resources than we do, and our ability to obtain and adequately pr otect intellectual property rights for our product candidates. We discuss many of these risks in greater detail under the heading "Risk Factors" contained in our quarterly rep ort on Form 10 - Q for the three months ended September 30, 2018 , filed with the S ecurities and Exchange Commission. Any forward - looking statements that we make in this presentation speak only as of the date of this presentation . We assume no obligation to update our forward - looking statements, whether as a result of new information, fu ture events or otherwise, after the date of this presentation .

PN-10943: Oral, GI-Restricted a4b7-Integrin Antagonist Standard backup approach to manage risk with the front-runner PN-10943 in comparison to PTG-100 In vitro potency: 5.5 fold more potent in T cell adhesion assay 2.6 fold slower off-rate and therefore longer binding lifetime Target engagement: Similar PD readouts of target engagement (blood %RO and circulating T cells) at 3x lower doses in mice with DSS induced colitis Efficacy: Superior efficacy as measured by colon weight/length and histopathology at similar doses in rats with TNBS induced colitis PK: Similar oral stability and limited blood exposure Different chemical scaffold embedding critical structural features and binding sequences from PTG-100 3 Summary
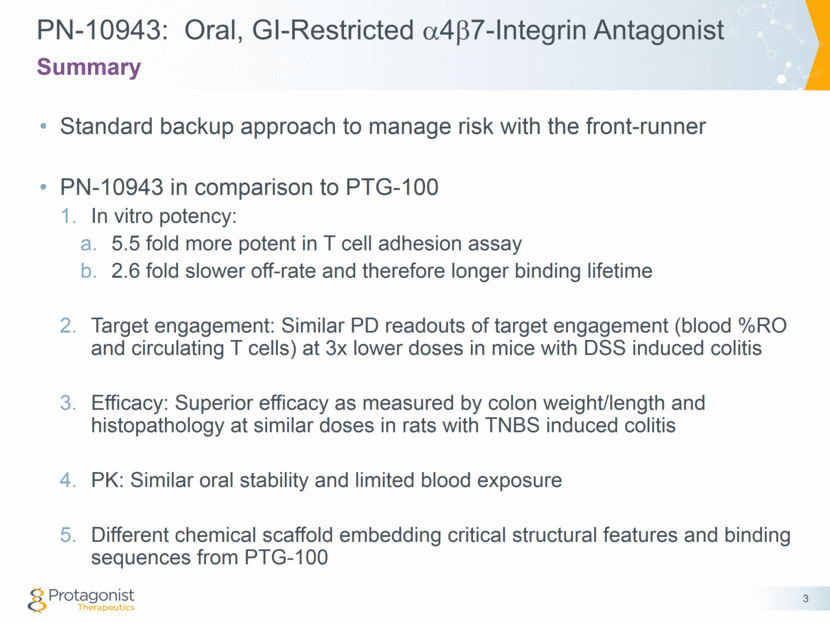
PN-10943: More Potent Than PTG-100 Cell Adhesion or Surface Plasmon Resonance Binding Assays Integrin 47 41 Ligand MAdCAM-1 (IC50 nM) VCAM-1 (IC50 nM) PTG-100 1.5 > 100,000 (> 67,000-fold) PN-10943 0.27 > 12,000 (> 44,000-fold) 5.5-fold more potent in blocking human T cell adhesion assay with similar selectivity 2.6-fold longer binding lifetime (i.e. half-life of dissociation) in SPR assay Ka (on rate) Kd (off rate) Equilibrium constant (KD) Half life of dissociation PTG-100 1060 s 1.2 e-5 s 11.3 nM 16 h PN-10943 1423 s 4.5 e-6 s 3.5 nM 42 h 4
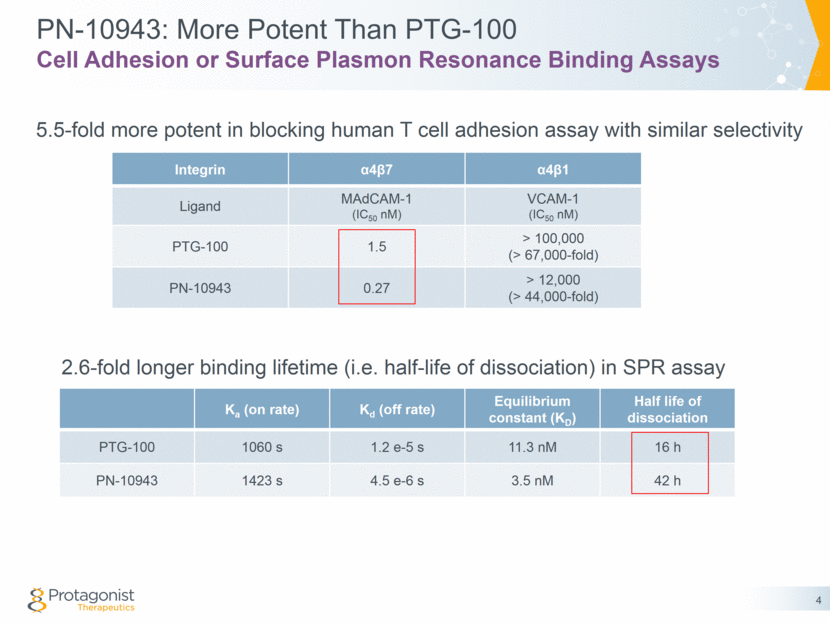
PN-10943: Similar RO% and T Cell Trafficking at Lower Doses 15 Day DSS Colitis Mouse Model Circulating Cell Numbers CD4+ Effector Memory T cells 5 Receptor Occupancy CD4+ Effector Memory T cells V e h i c l e P T G - 1 0 0 , 5 5 m p k / d P N - 9 4 3 A , 5 5 m p k / d P N - 9 4 3 A , 1 8 m p k / d 0 50 100 150 200 250 % m e d i a n v e h i c l e c o n t r o l ** ** *** 43% 59% 52% V e h i c l e P T G - 1 0 0 , 5 5 m p k / d P N - 9 4 3 A , 5 5 m p k / d P N - 9 4 3 A , 1 8 m p k / d 0 50 100 150 % r e c e p t o r o c c u p a n c y **** **** **** 71% 92% 89%
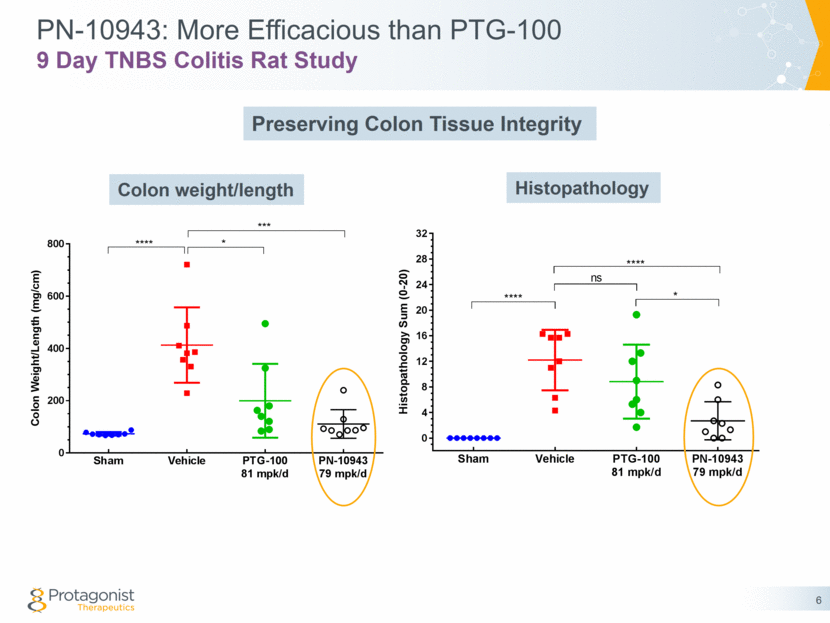
PN-10943: More Efficacious than PTG-100 9 Day TNBS Colitis Rat Study Colon weight/length 6 Histopathology Preserving Colon Tissue Integrity Sham Vehicle PTG-100 81 mpk/d PN-10943 79 mpk/d 0 200 400 600 800 C o l o n W e i g h t / L e n g t h ( m g / c m ) **** * *** Sham Vehicle PTG-100 81 mpk/d PN-10943 79 mpk/d 0 4 8 12 16 20 24 28 32 H i s t o p a t h o l o g y S u m ( 0 - 2 0 ) **** ns **** *
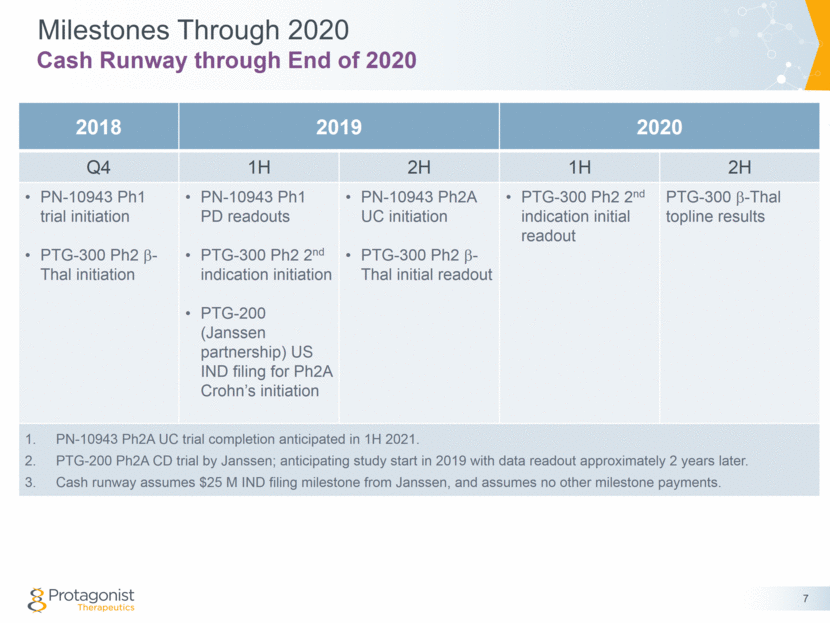
2018 2019 2020 Q4 1H 2H 1H 2H PN-10943 Ph1 trial initiation PTG-300 Ph2 b-Thal initiation PN-10943 Ph1 PD readouts PTG-300 Ph2 2nd indication initiation PTG-200 (Janssen partnership) US IND filing for Ph2A Crohn’s initiation PN-10943 Ph2A UC initiation PTG-300 Ph2 b-Thal initial readout PTG-300 Ph2 2nd indication initial readout PTG-300 b-Thal topline results PN-10943 Ph2A UC trial completion anticipated in 1H 2021. PTG-200 Ph2A CD trial by Janssen; anticipating study start in 2019 with data readout approximately 2 years later. Cash runway assumes $25 M IND filing milestone from Janssen, and assumes no other milestone payments. Milestones Through 2020 7 Cash Runway through End of 2020