Attached files
| file | filename |
|---|---|
| EX-99.2 - EX-99.2 - Syros Pharmaceuticals, Inc. | a18-40247_1ex99d2.htm |
| 8-K - 8-K - Syros Pharmaceuticals, Inc. | a18-40247_18k.htm |
Forward-looking statements This presentation contains forward-looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 that involve substantial risks and uncertainties. All statements, other than statements of historical facts, contained in this presentation, including statements regarding the potential benefits of CDK7 inhibition and of SY-1365 and statements regarding our strategy, research and clinical development plans, collaborations, future operations, future financial position, future revenues, projected costs, prospects, plans and objectives of management, are forward-looking statements. The words “anticipate,” “believe,” “estimate,” “expect,” “intend,” “may,” “plan,” “predict,” “project,” “target,” “potential,” “will,” “would,” “could,” “should,” “continue,” and similar expressions are intended to identify forward-looking statements, although not all forward-looking statements contain these identifying words. We may not actually achieve the plans, intentions or expectations disclosed in our forward-looking statements, and you should not place undue reliance on our forward-looking statements. Actual results or events could differ materially from the plans, intentions and expectations disclosed in these forward-looking statements as a result of various important factors, including Syros’ ability to: advance the development of its programs, including SY-1365, under the timelines it projects in current and future clinical trials; demonstrate in any current and future clinical trials the requisite safety, efficacy and combinability of its drug candidates; replicate scientific and non-clinical data in clinical trials; obtain and maintain patent protection for its drug candidates and the freedom to operate under third party intellectual property; obtain and maintain necessary regulatory approvals; identify, enter into and maintain collaboration agreements with third parties; manage competition; manage expenses; raise the substantial additional capital needed to achieve its business objectives; attract and retain qualified personnel; and successfully execute on its business strategies; risks described under the caption “Risk Factors” in Syros’ Annual Report on Form 10-K for the year ended December 31, 2017, as updated in its Quarterly Reports on Form 10-Q for the quarters ended March 31, June 30 and September 30, 2018, each of which is on file with the Securities and Exchange Commission; and risks described in other filings that Syros makes with the Securities and Exchange Commission in the future. Any forward-looking statements contained in this presentation speak only as of the date this presentation is made, and we expressly disclaim any obligation to update any forward-looking statements, whether because of new information, future events or otherwise. 2

SY-1365 (CDK7 inhibitor): Controlling expression of tumor-driving genes First-in-class selective inhibitor of CDK7 CDK7 inhibition induces apoptosis and preferentially kills cancer cells over non-cancerous cells Currently in Phase 1 clinical trial as single and combination agent in ovarian and breast cancers Opened expansion cohorts in September 2018 Data from dose escalation phase presented today in oral presentation at EORTC-NCI-AACR 2018 meeting Broad potential to expand into additional solid tumors and blood cancers Difficult-to-treat solid tumors and blood cancers 3
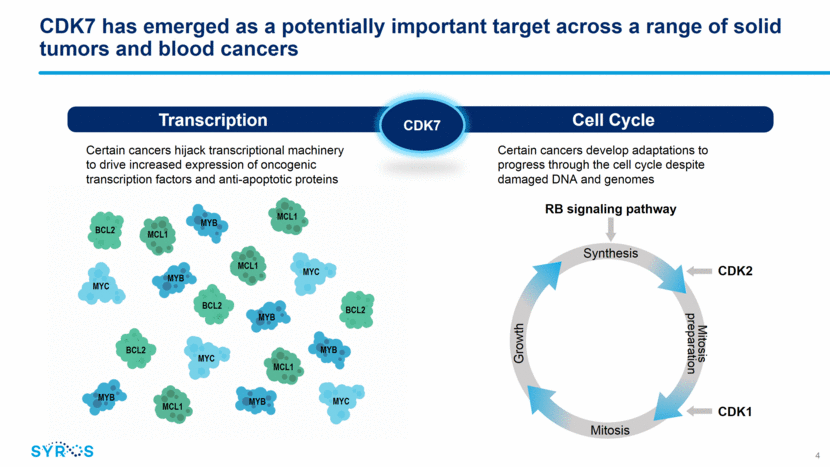
CDK7 has emerged as a potentially important target across a range of solid tumors and blood cancers Cell Cycle Transcription Certain cancers hijack transcriptional machinery to drive increased expression of oncogenic transcription factors and anti-apoptotic proteins Certain cancers develop adaptations to progress through the cell cycle despite damaged DNA and genomes MYC MYB MCL1 BCL2 MYC MYB MCL1 BCL2 BCL2 BCL2 MYB MYB MYB MYC MCL1 MCL1 MCL1 MYB MYC CDK7 4 CDK2 CDK1 Synthesis Growth Mitosis preparation Mitosis RB signaling pathway
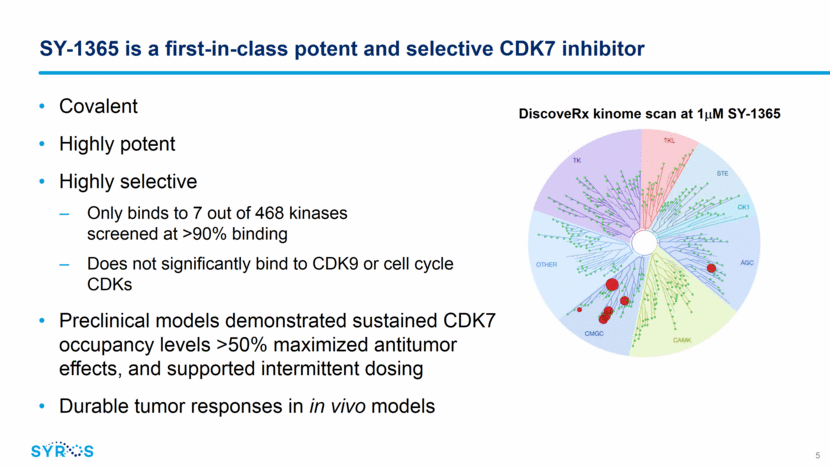
SY-1365 is a first-in-class potent and selective CDK7 inhibitor DiscoveRx kinome scan at 1mM SY-1365 Covalent Highly potent Highly selective Only binds to 7 out of 468 kinases screened at >90% binding Does not significantly bind to CDK9 or cell cycle CDKs Preclinical models demonstrated sustained CDK7 occupancy levels >50% maximized antitumor effects, and supported intermittent dosing Durable tumor responses in in vivo models 5
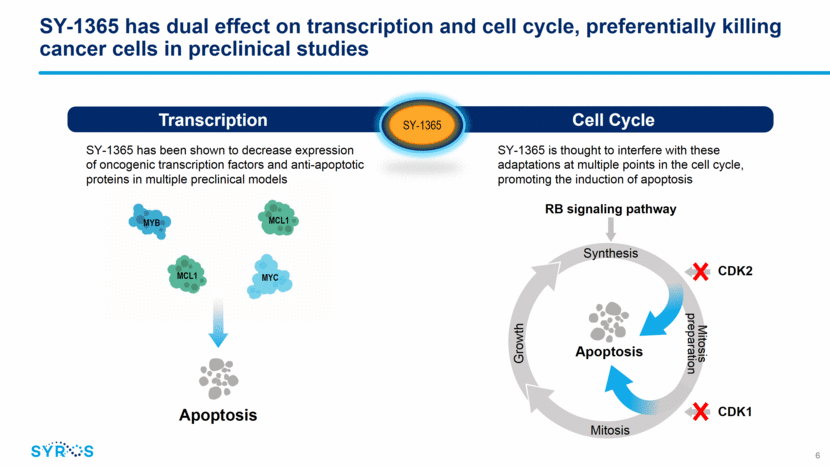
SY-1365 has dual effect on transcription and cell cycle, preferentially killing cancer cells in preclinical studies Apoptosis SY-1365 has been shown to decrease expression of oncogenic transcription factors and anti-apoptotic proteins in multiple preclinical models SY-1365 is thought to interfere with these adaptations at multiple points in the cell cycle, promoting the induction of apoptosis MYC MYB MCL1 MCL1 Cell Cycle Transcription CDK7 SY-1365 6 Apoptosis RB signaling pathway Synthesis Growth Mitosis preparation Mitosis CDK2 CDK1
Expansion cohorts in Phase 1 trial exploring SY-1365 as single agent and in combination in multiple ovarian and breast cancer patient populations Phase 1 clinical trial design Relapsed ovarian cancer, 3+ prior lines Single agent (N=24) Relapsed ovarian cancer, 1+ prior lines (platinum sensitive) Combination with carboplatin (N=24) Primary platinum refractory ovarian cancer Single agent pilot (N=12) Solid tumors accessible for biopsy Single agent (N=10) HR+ metastatic breast cancer, CDK4/6 inhibitor resistant Combination with fulvestrant (N=12) Enrolled patients with advanced solid tumors of any histology Explored once- and twice-a-week dosing Primary objective to establish MTD and optimal dose and regimen Assessed safety, PK/PD, proof-of-mechanism Dose escalation Status: Completed, data at EORTC-NCI-AACR Expansion Status: Ongoing 7
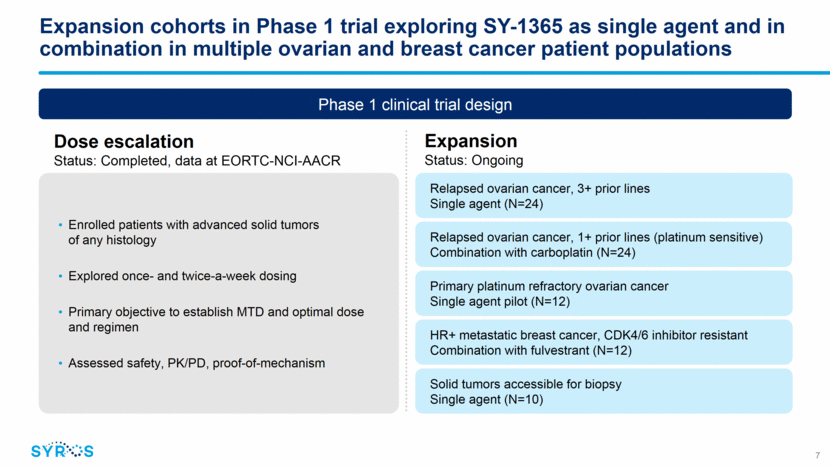
Dose escalation portion of SY-1365 Phase 1 trial Dosing IV dosing over one hour 3 weeks every 4 weeks Trial Endpoints Primary: DLTs and safety Secondary: PK and PD Exploratory: Anti-tumor activity Data snapshot: Oct. 15, 2018 Part 1 Dose Escalation (N=32) Weekly (mg/m2) Twice Weekly (mg/m2) Single patient cohort 3+3 escalation All Solid Tumors 8
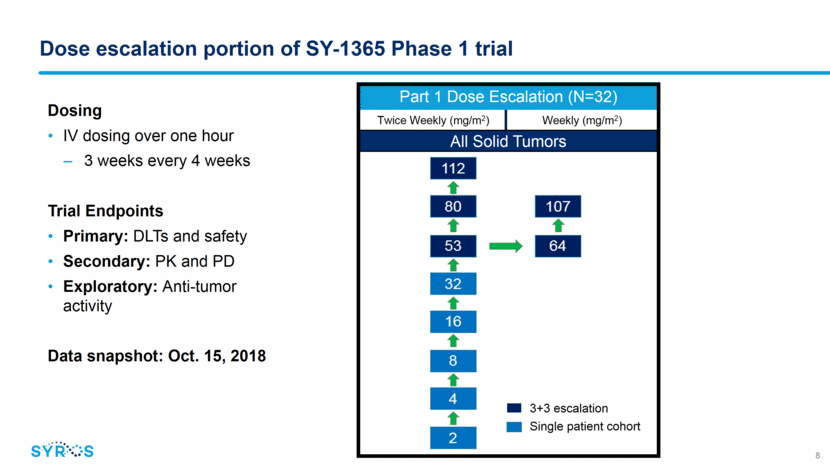
SY-1365 dose escalation: patient baseline characteristics 9 Characteristics N(%) N=32 Median Age, years (range) 63 (25-87) Female sex, n (%) 25 (78.1) 4 Prior Lines of Therapy 28 (87.5) Median Number Prior Lines (range) 5 (1-13) Cancer Type Breast 8 (25) Ovarian 8 (25) Endometrial 5 (16) Pancreatic 2 (6) Other 9 (28)
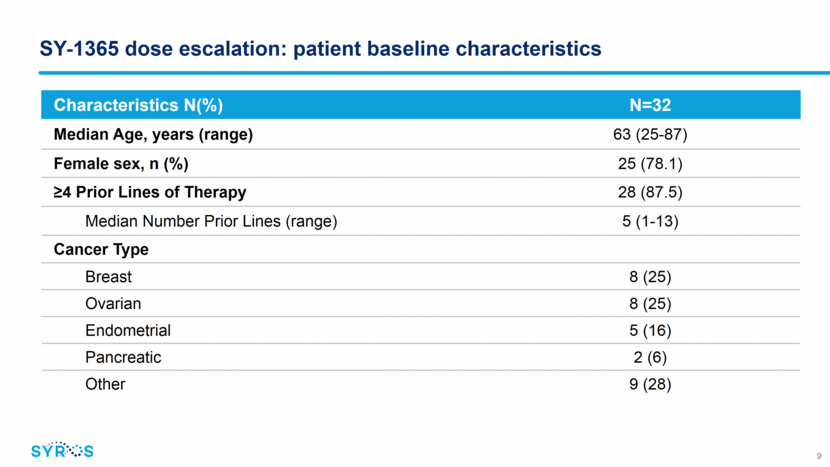
SY-1365 dose escalation: patient disposition 10 *Due to progression of disease Number of Patients Enrolled N (%) Duration of Treatment: Median days (range) 46.5 (2 – 147) Patients withdrawn from treatment 28 (87.5) Progressive Disease per RECIST 1.1 16 (50.0) Clinical Progression 7 (21.9) Withdrawal of Consent 4 (12.5) Death* 1 (3.1) Number of Patients Enrolled By Dose Level N Dose (mg/m2) 2 4 8 16 32 53 64 80 107 112 Total Safety Population 1 2 1 1 1 6 7 6 6 1 32 Response Evaluable 1 1 1 1 1 3 5 3 3 0 19
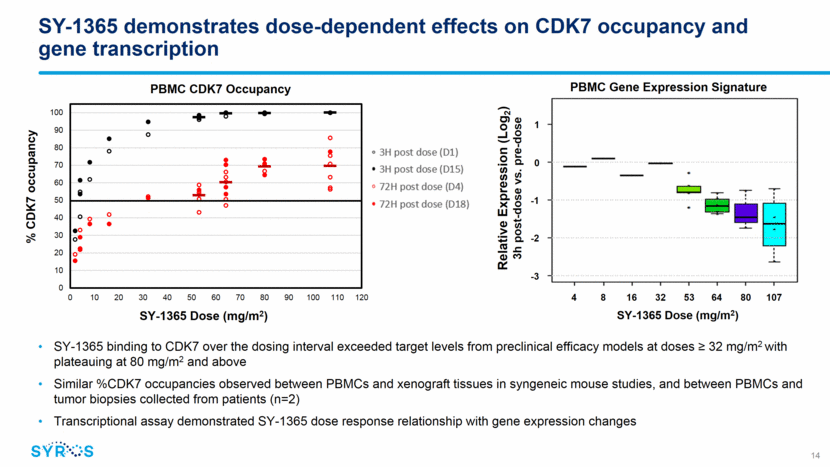
SY-1365 safety overview: dose escalation (N=32) 11 Predominantly low grade, reversible, and generally manageable Most frequent related AEs include headache, nausea, vomiting, and fatigue No reports of neutropenia DLTs: headache (64 mg/m2), coronary vasospasm (80 mg/m2), and fatigue (112 mg/m2) MTD not defined 9 13 3 13 19 16 28 31 3 6 3 13 22 9 16 3 6 6 6 0 10 20 30 40 50 60 Anaemia Dysgeusia Thrombocytopenia Diarrhoea Fatigue Nausea Vomiting Headache Patients (%) Related Adverse Events (?10%) Grade 1 Grade 2 Grade 3 16 13 3 6 16 16 16 19 13 31 31 3 13 9 3 3 13 25 16 19 3 6 6 9 6 0 10 20 30 40 50 60 ALT increased Dizziness Hypoalbuminaemia Anaemia Decreased appetite Diarrhoea Hyponatraemia Fatigue Nausea Vomiting Headache Patients (%) Adverse Events (?15%), All Causality Grade 1 Grade 2 Grade 3
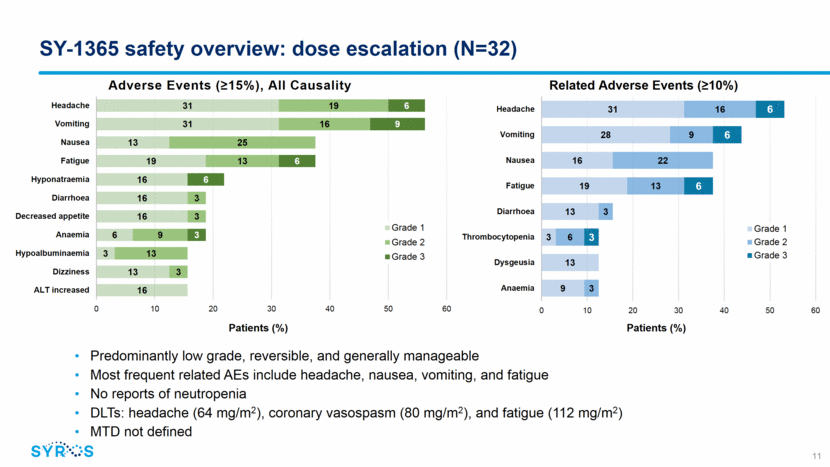
SY-1365 plasma pharmacokinetics Plasma PK exposures (Cmax, AUC) are linear from doses of 2 to 107 mg/m2 No SY-1365 accumulation with repeat dosing SY-1365 day 1 PK parameters at 80 mg/m2 Cmax: 7,498 ± 1,116 ng/mL AUC: 11,696 ± 2,848 ng/mL•h Half-life: 17.9 ± 4.2 h 12
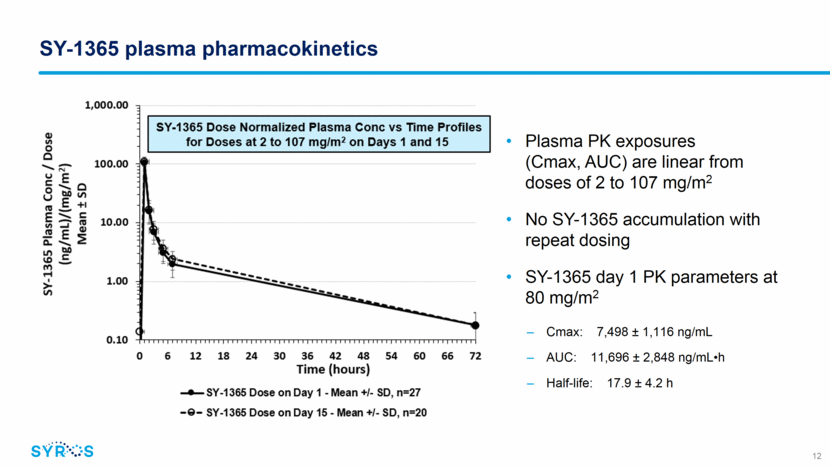
SY-1365 PD effects evaluated by CDK7 occupancy and transcriptional assays Sample containing bound and free CDK7 Lyse and incubate with biotinylated probe Bound CDK7 Bound CDK7 Pull-down with Streptavidin beads Use whole lysate total CDK7 free CDK7 SY-1365 treatment (nM) SY-1365-biotin probe molecule to capture unbound/free CDK7 MSD format for high-throughput assessment CDK7 Occupancy: relative measure of free CDK7 to total CDK7 Transcriptional assay: gene expression signature SY-1365 dose-response gene signature developed in PBMCs in vitro ~25 early response genes (3-5 hrs post treatment) Custom Nanostring codeset to evaluate a subset of response and control genes in patient PBMCs 13 Free CDK7 Bound CDK7 Free CDK7 Bound CDK7 Free CDK7 Free CDK7 Free CDK7 Free CDK7 Free CDK7 Free CDK7 Bound CDK7 Bound CDK7
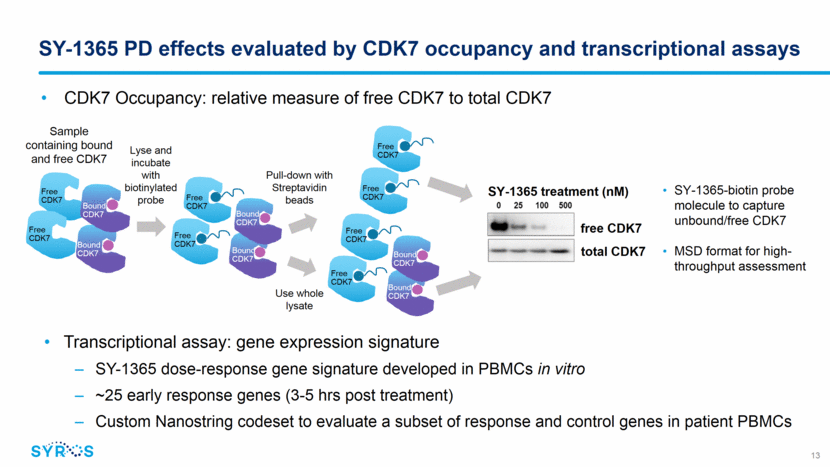
SY-1365 demonstrates dose-dependent effects on CDK7 occupancy and gene transcription Relative Expression (Log2) 3h post-dose vs. pre-dose SY-1365 Dose (mg/m2) 0 -1 -2 -3 1 4 8 16 32 53 64 80 107 PBMC Gene Expression Signature PBMC CDK7 Occupancy SY-1365 binding to CDK7 over the dosing interval exceeded target levels from preclinical efficacy models at doses 32 mg/m2 with plateauing at 80 mg/m2 and above Similar %CDK7 occupancies observed between PBMCs and xenograft tissues in syngeneic mouse studies, and between PBMCs and tumor biopsies collected from patients (n=2) Transcriptional assay demonstrated SY-1365 dose response relationship with gene expression changes 14
Early evidence of SY-1365 clinical activity Clinical activity per RECIST 1.1 criteria was observed in 7 out of 19 evaluable patients Disease Control Rate (CR+PR+SD) of 37% One confirmed PR (in clear cell ovarian cancer patient) observed at 80 mg/m2 BIW 6 additional patients with stable disease, mostly at higher doses ( 32 mg/m2 BIW) Consists of 2 ovarian, 2 breast and 2 endometrial cancer patients Duration of treatment for these patients ranged from 50 - 127 days 15
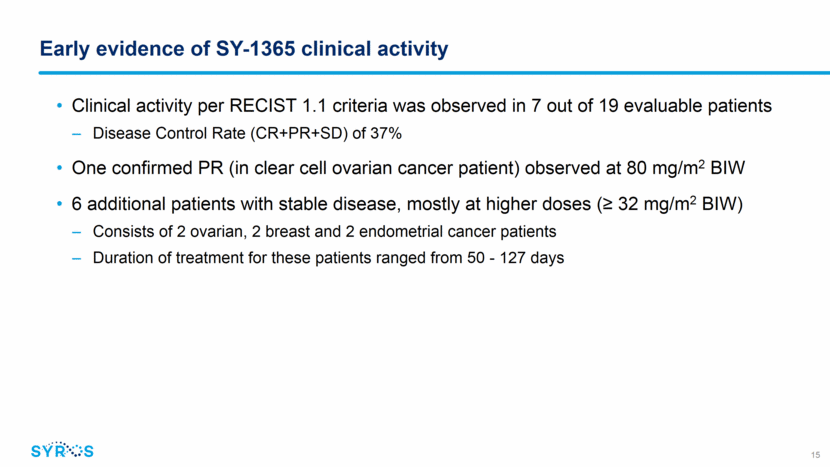
Early evidence of SY-1365 clinical activity CT images of 52 year old woman with relapsed ovarian cancer on SY-1365 80 mg/m2 BIW 16 Stage IV clear cell in 4th relapse ARID1A, PIK3CA, NF1 mutations Best response to prior lines of therapy: SD Confirmed PR after 2 cycles 31.8% reduction (C3D1) Remains on study in PR in 7th month of SY-1365 treatment 49% decrease at C7D1 Before treatment After 2 months After 6 months
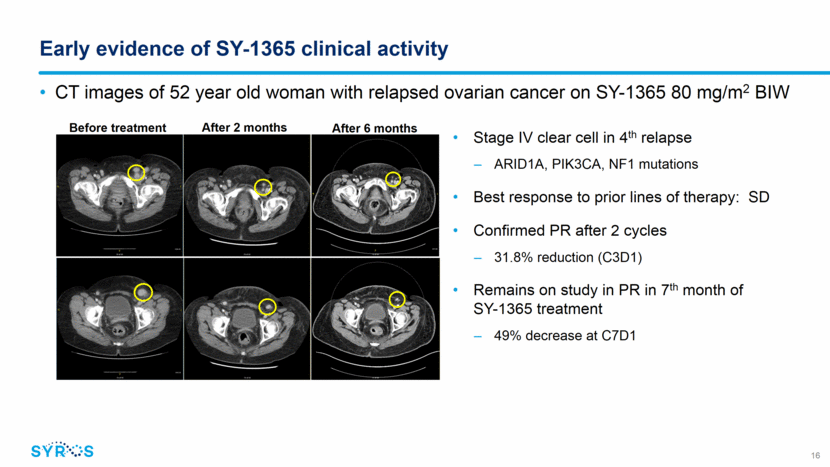
Dose and schedule selected for ongoing expansion cohorts Evaluating SY-1365 as single agent and in combination in multiple ovarian and breast cancer patient populations Dose selection supported by PK/PD analyses of drug exposure and target occupancy, tolerability profile and early signs of clinical activity Single agent: 80 mg/m2 twice weekly Combination: 80 mg/m2 once weekly 17 Relapsed ovarian cancer, 3+ prior lines Single agent (N=24) Relapsed ovarian cancer, 1+ prior lines (platinum sensitive) Combination with carboplatin (N=24) Primary platinum refractory ovarian cancer Single agent pilot (N=12) Solid tumors accessible for biopsy Single agent (N=10) HR+ metastatic breast cancer, CDK4/6 inhibitor resistant Combination with fulvestrant (N=12) SY-1365 Phase 1 Expansion Cohorts
Breast Cancer : ~266,0002 Ovarian Cancer : ~59,0001 Significant need for new therapies in advanced high-grade serous ovarian and HR+ metastatic breast cancer 70% have high-grade serous ovarian cancer and most present with advanced disease at initial diagnosis Standard-of-care includes platinum-based chemotherapy as foundation Majority of patients, even those who initially respond to platinum-based chemotherapy, relapse within a year Significant unmet need and/or limited treatment options in platinum sensitive, resistant and refractory patients ~80% are HR+ breast cancer Standard-of-care for metastatic HR+ breast cancer includes CDK4/6 inhibitor plus an aromatase inhibitor ~50% of patients progress within ~2 years Second-line hormone-based therapies have limited efficacy, creating a need for new therapies 1Annual ovarian cancer diagnoses in the U.S., Canada, Japan and the EU 5 (UK, Germany, France, Spain and Italy). Health Advances analysis. 2American Cancer Society estimate of new cases diagnosed in U.S. in 2018 Sources: Hanker et al. Ann Oncol. 2012 Oct;23(10):2605-12. SEER, Cancer Research UK 2013. NCCN Guidelines Nov. 2017. McCluggage WG. Pathology 2011; 43: 420–432. Gabra H. EJC Suppl. 2014 Dec;12(2):2-6. and Herzog TJ and Monk BJ. Onitilo AA et al., Clin Med Res 2009; 7(1-2):4-13. Rugo HS et al., JCO 2016; 34: 3069-3103. Finn RS et al, N Engl J Med 2016; 375(20): 1925-1936. Faslodex USPI 18
Rapidly advancing toward our vision 19 Progressing to pivotal development Advancing multiple programs in clinic Preparing for commercial launch Ongoing investment in discovery Fully integrated company with medicines that provide a profound benefit for patients Driving SY-1425 and SY-1365 to key milestones Advancing SY-5609 into IND-enabling studies Investing in discovery to support goal of one IND every other year Cash to fund planned operations into 2020 Now Next Future
[LOGO]


