Attached files
| file | filename |
|---|---|
| 8-K - 8-K - PIERIS PHARMACEUTICALS, INC. | form8-k.htm |

Jefferies London Healthcare Conference November 2018 (Nasdaq: PIRS)

Forward Looking Statements This presentation contains forward-looking statements as that term is defined in Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934. Statements in this press release that are not purely historical are forward-looking statements. Such forward-looking statements include, among other things, references to novel technologies and methods and our business and product development plans, including the advancement of our proprietary and co-development programs into and through the clinic. Actual results could differ from those projected in any forward- looking statements due to numerous factors. Such factors include, among others, our ability to raise the additional funding we will need to continue to pursue our business and product development plans; the inherent uncertainties associated with developing new products or technologies and operating as a development stage company; our ability to develop, complete clinical trials for, obtain approvals for and commercialize any of our product candidates, including our ability to recruit and enroll patients in our studies; our ability to address the requests of the FDA; competition in the industry in which we operate and market conditions. These forward-looking statements are made as of the date of this press release, and we assume no obligation to update the forward-looking statements, or to update the reasons why actual results could differ from those projected in the forward-looking statements, except as required by law. Investors should consult all of the information set forth herein and should also refer to the risk factor disclosure set forth in the reports and other documents we file with the SEC available at www.sec.gov, including without limitation the Company's Annual Report on Form 10-K for the fiscal year ended December 31, 2017 and the Company's Quarterly Reports on Form 10-Q. 2

Anticalin Proteins – A Novel Therapeutic Class with Favorable Drug Properties • Derived from lipocalins (human extracellular binding proteins) - multifunctional, non-immunogenic polypeptides • Engineerable binding pocket for robust target engagement • Small size (18 kDa vs 150 kDa in the case of antibodies) • Can be formulated for inhalable delivery • Can be formatted into novel bi- and multi-specific constructs Underpinned by a Powerful Drug Discovery Platform • Highly diverse libraries (>1011) of potential drug candidates • Automated high-throughput drug screening technology (phage display) - High hit rates, quick to development candidates, versatile use • Extensive protein engineering know-how 3

Anticalin Protein-based Drug Candidates can be Tailored to Multiple Formats Building Blocks Pure Anticalin Anticalin Multispecific Proteins Protein Fc-Anticalin Proteins Antibody PRS-060 Fc Multispecific Antibody-Anticalin PRS-343 Proteins PRS-344 Potent Multi-target Engagement • Novel Inhaled and Multispecific MoA • Favorable Drug-like Properties 4

Pieris Investment Opportunity • Validation through three anchor partnerships - $120M+ in upfront payments and milestones since January 2017 - Each partnership includes co-development & US-focused commercialization rights Respiratory • Upcoming clinical-based inflection points - Respiratory: co-developed (AstraZeneca) inhaled IL-4Ra antagonist (PRS-060) - IO: wholly owned bispecific 4-1BB agonist (PRS-343) • Strong balance sheet to bridge through clinical inflection points • Partnerships and pipeline supported by IND engine yielding several drug candidates with excellent drug-like properties Immuno-oncology ANCHOR PARTNERSHIPS 5

Recent and Upcoming Milestones Financial(in millions) Update (9/30/18) PRS-060: First-in-human SAD data in 2H18 Cash & Cash Equivalents $137.3 Core Clinical PRS-060: MAD PK/PD biomarker (FeNO) data PRS-343: Initial safety and PD data Debt $0.0 PRS-344 data at SITC 2018 Next-Gen Advance multiple programs in immuno-oncology and Pipeline YTD OPEX (9/30) $35.7 respiratory Non-Core Clinical PRS-080: Phase IIa data in 2H18 (safety, PK, hemoglobin CSO 54.0 change post 5QW dosing) Pipeline Highlights DISCOVERY PRECLINICAL PHASE I PHASE II PRS-080 PRS-343 PRS-060 (AZ) PRS-344 (Servier) Servier PRS-300s AstraZeneca PRS Respiratory Seattle Genetics Two IO INDs planned in 2019 6
Respiratory Franchise Addressing validated targets through inhalation AstraZeneca Collaboration • PRS-060: IL-4 receptor alpha antagonist in clinical development for the treatment of moderate-to-severe uncontrolled asthma • 4 additional committed novel inhaled Anticalin protein programs • Retained co-development and co-commercialization (US) options on PRS-060 and up to two additional programs • Attractive economics • Received $57.5M: $45M upfront & $12.5M Phase I milestone • ~$2.1B in milestone potential, plus up to double-digit royalties • AZ funds all PRS-060 development costs through post-Phase IIa co- development opt-in decision • Access to complementary formulation and device know-how for inhaled delivery • Initiated a discovery program in 2H18 Proprietary Clinical (worldwide rights) • Initiated two proprietary respiratory programs for undisclosed targets in 2H18 7

PRS-060 is an Inhaled Drug Candidate for Uncontrolled Asthma Why did we design this? What We Know Regeneron/Sanofi’s dupilumab (systemically administered anti-IL-4Ra antibody) has demonstrated the following: Reduction in biomarker (FeNO*) Improved lung function Exacerbation Reduction & Steroid Sparing 67% 80% reduction in avg. reduction high-eosinophil in corticosteroid patients use *Fractional exhaled nitric oxide What We Are Testing • Is this a local phenomenon? • First-in-man study underway via inhaled delivery 8

Preclinical In Vivo PoC Supports Clinical Development • First inhaled Anticalin protein to potently engage the highly validated asthma target, IL-4Ra • Localized target engagement in lung tissue supports a rationale for a convenient, low-dose, low-cost alternative to systemically administered antibodies • Preclinical in vivo PoC for pulmonary delivery at doses supportive of daily administration Lung Total and differential cell counts in OVA model Histopathology hIL-13-induced increases in eotaxin Control 48-hr gene expression P ercentage inhibition of lung inflam m atory cells 6 × 10 06 48hr after OVA challenge 1 .5 Percentage inhibition 78% 71% 79% 66% 5 × 10 06 97% 86% 73% 58% 47% 1 .0 ** 4 × 10 06 × 06 3 10 *** 0 .5 expression 2 × 10 06 *** Cell differential ** ** PRS-060 48-hr *** 06 *** 1 × 10 eotaxin Change in Fold ** 0 .0 * 0 hIL-13 T otal M acrophages Eosinophils Lymphocytes Treat: Veh 50µ g 2µ g 48hr timepoint Pre (hr): 0.5 4 8 24 Anticaln control *p<0.05, **p<0.001 compared with control TLPC134, (TLPC134) ** p<0.01, ***p<0.001 compared to anticalin control, unpaired two-tailed t test (n=9-10); data are Mean± SEM PRS-060 unpaired two-tailed t-test (n=5-6); data are Mean ± SEM Inhibits the influx of key inflammatory cells 24-hr duration of action at doses both in lavage fluid and lung tissue feasible for inhalation 9
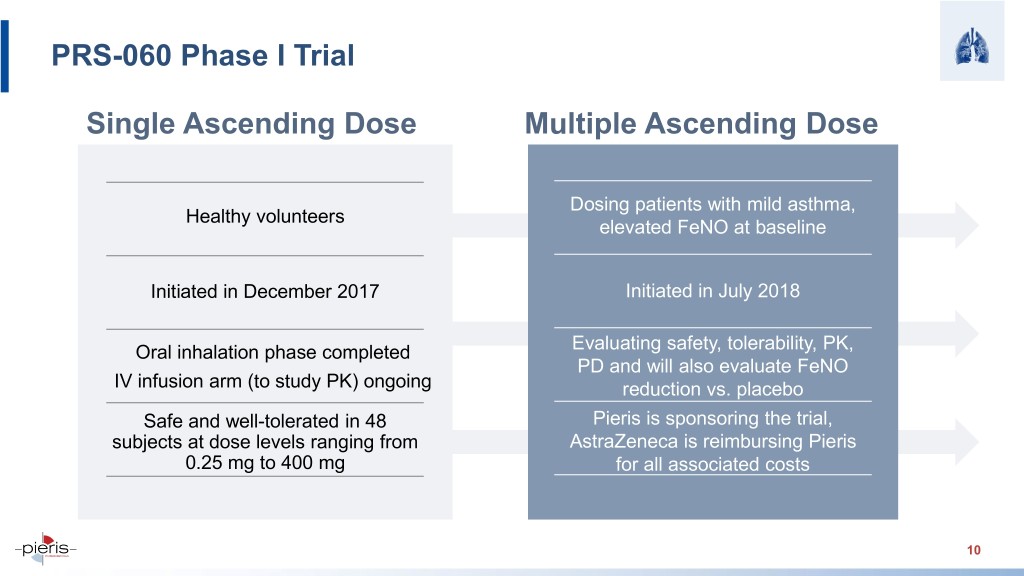
PRS-060 Phase I Trial Single Ascending Dose Multiple Ascending Dose Dosing patients with mild asthma, Healthy volunteers elevated FeNO at baseline Initiated in December 2017 Initiated in July 2018 Oral inhalation phase completed Evaluating safety, tolerability, PK, PD and will also evaluate FeNO IV infusion arm (to study PK) ongoing reduction vs. placebo Safe and well-tolerated in 48 Pieris is sponsoring the trial, subjects at dose levels ranging from AstraZeneca is reimbursing Pieris 0.25 mg to 400 mg for all associated costs 10

Immuno-oncology Franchise Prioritizing PRS-343, “fast-followers” and diversified costim agonism beyond 4-1BB Proprietary Clinical (worldwide rights) • PRS-343: First-in-class bispecific to preferentially activate T cells in the tumor microenvironment (TME) • Committed to advancing several additional tumor-localized costimulatory bispecific fusion proteins Servier Collaboration • PRS-344: PD-L1/4-1BB antibody/anticalin bispecific • 5-program deal (all bispecific fusion proteins) • Pieris retains full U.S. rights for 3 out of 5 programs • $31M upfront payment, $1.8B milestone potential • Up to low double-digit royalties on non-codev products Seattle Genetics Collaboration • 3-program partnership based on tumor-localized costimulatory bispecific fusion proteins • Pieris retains opt-in rights for 50/50 global profit split and U.S. commercialization rights on one of the programs • $30M upfront payment, $1.2B milestone potential • Up to double-digit royalties on non-codev products 11
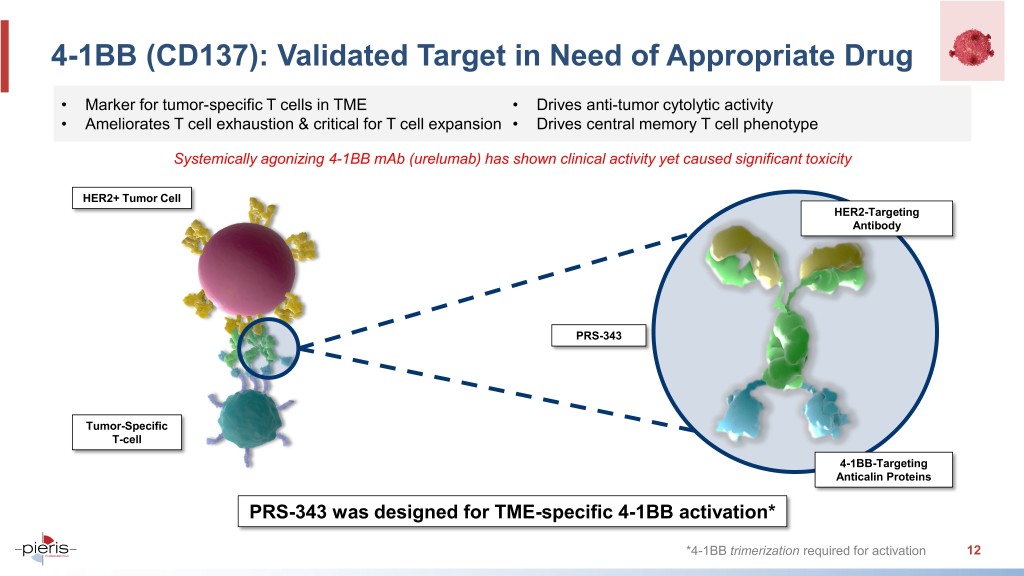
4-1BB (CD137): Validated Target in Need of Appropriate Drug • Marker for tumor-specific T cells in TME • Drives anti-tumor cytolytic activity • Ameliorates T cell exhaustion & critical for T cell expansion • Drives central memory T cell phenotype Systemically agonizing 4-1BB mAb (urelumab) has shown clinical activity yet caused significant toxicity HER2+ Tumor Cell HER2-Targeting Antibody PRS-343 Tumor-Specific T-cell 4-1BB-Targeting Anticalin Proteins PRS-343 was designed for TME-specific 4-1BB activation* *4-1BB trimerization required for activation 12
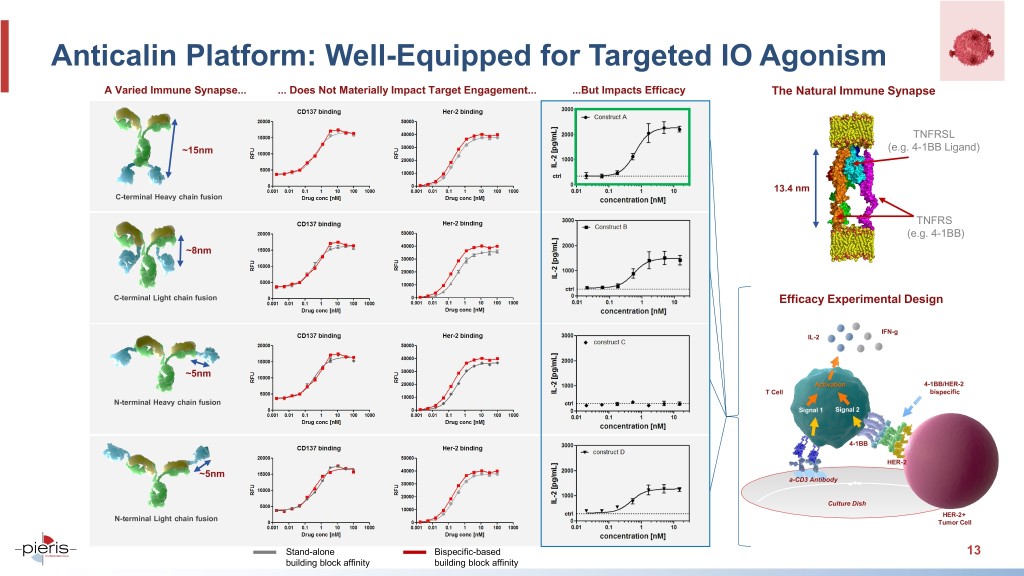
Anticalin Platform: Well-Equipped for Targeted IO Agonism A Varied Immune Synapse... ... Does Not Materially Impact Target Engagement... ...But Impacts Efficacy The Natural Immune Synapse TNFRSL ~15nm (e.g. 4-1BB Ligand) 13.4 nm C-terminal Heavy chain fusion TNFRS (e.g. 4-1BB) ~8nm C-terminal Light chain fusion Efficacy Experimental Design IFN-g IL-2 ~5nm Activation 4-1BB/HER-2 T Cell bispecific N-terminal Heavy chain fusion Signal 1 Signal 2 4-1BB HER-2 ~5nm a-CD3 a-CD3 Antibody antibody Culture Dish HER-2+ N-terminal Light chain fusion Tumor Cell Stand-alone Bispecific-based 13 building block affinity building block affinity
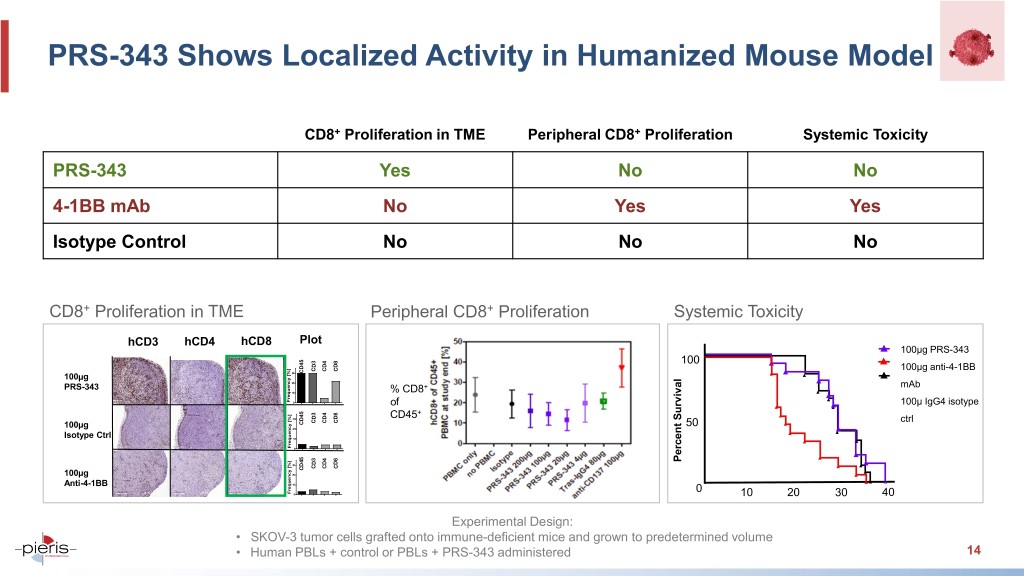
PRS-343 Shows Localized Activity in Humanized Mouse Model CD8+ Proliferation in TME Peripheral CD8+ Proliferation Systemic Toxicity PRS-343 Yes No No 4-1BB mAb No Yes Yes Isotype Control No No No CD8+ Proliferation in TME Peripheral CD8+ Proliferation Systemic Toxicity hCD3 hCD4 hCD8 Plot 100μg PRS-343 100 CD3 CD4 CD8 100μg anti-4-1BB 30 CD45 100µg 20 PRS-343 + mAb 10 % CD8 Frequency [%] 0 of 100μ IgG4 isotype + 30 CD45 CD3 CD4 CD8 ctrl CD45 50 100µg 20 Isotype Ctrl 10 Frequency [%] 0 Percent Survival 30 CD3 CD4 CD8 CD45 100µg 20 Anti-4-1BB 10 Frequency [%] 0 0 10 20 30 40 Experimental Design: • SKOV-3 tumor cells grafted onto immune-deficient mice and grown to predetermined volume • Human PBLs + control or PBLs + PRS-343 administered 14

PRS-343 Phase I Escalation and Expansion Trials HER2+ all-comers to efficiently interrogate therapeutic window during EXPANSION escalation Bladder First patient dosed September 2017 Treating patients with HER2+ solid tumors Gastric Dose-escalation trial Initial PK, safety, tolerability and biomarker data in 1H19 Other(s) First patient dosed in combination with atezolizumab (Tecentriq®) in August 2018 (drug supply agreement with Roche) ESCALATION THE UNIVERSITY OF TEXAS MD Anderson Cancer Center 15
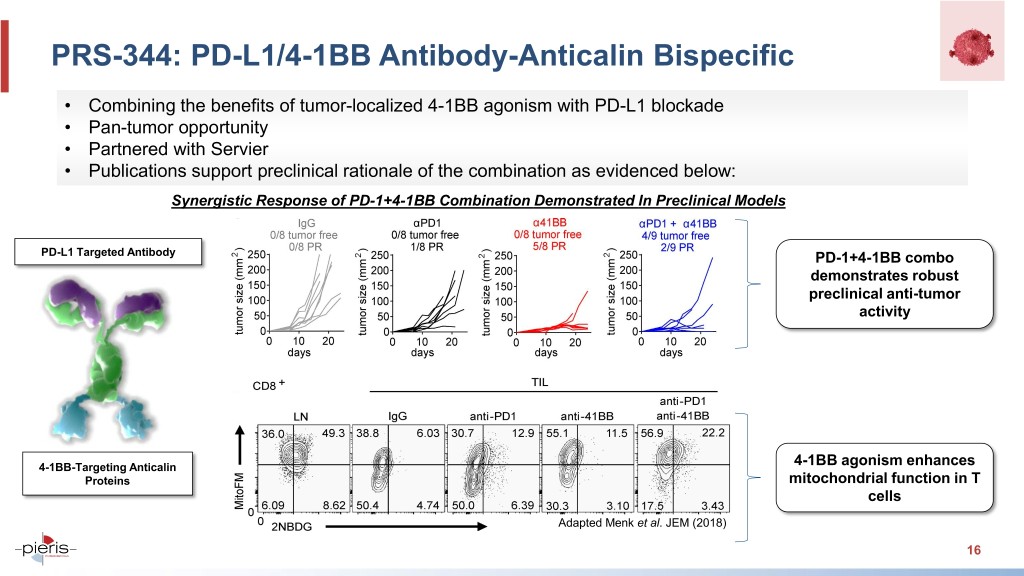
PRS-344: PD-L1/4-1BB Antibody-Anticalin Bispecific • Combining the benefits of tumor-localized 4-1BB agonism with PD-L1 blockade • Pan-tumor opportunity • Partnered with Servier • Publications support preclinical rationale of the combination as evidenced below: Synergistic Response of PD-1+4-1BB Combination Demonstrated In Preclinical Models PD-L1 Targeted Antibody PD-1+4-1BB combo demonstrates robust preclinical anti-tumor activity 4-1BB-Targeting Anticalin 4-1BB agonism enhances Proteins mitochondrial function in T cells Adapted Menk et al. JEM (2018) 16
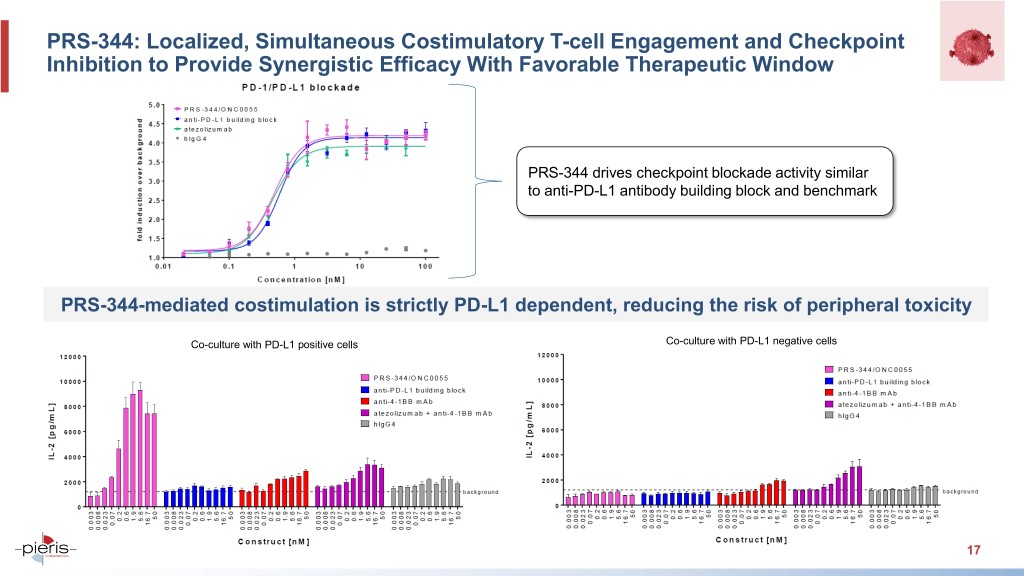
PRS-344: Localized, Simultaneous Costimulatory T-cell Engagement and Checkpoint Inhibition to Provide Synergistic Efficacy With Favorable Therapeutic Window PRS-344 drives checkpoint blockade activity similar to anti-PD-L1 antibody building block and benchmark PRS-344-mediated costimulation is strictly PD-L1 dependent, reducing the risk of peripheral toxicity Co-culture with PD-L1 positive cells Co-culture with PD-L1 negative cells 17

Pieris Investment Opportunity • Validation through three anchor partnerships - $120M+ in upfront payments and milestones since January 2017 - Each partnership includes co-development & US-focused commercialization rights Respiratory • Upcoming clinical-based inflection points - Respiratory: co-developed (AstraZeneca) inhaled IL-4Ra antagonist (PRS-060) - IO: wholly owned bispecific 4-1BB agonist (PRS-343) • Strong balance sheet to bridge through clinical inflection points • Partnerships and pipeline supported by IND engine yielding several drug candidates with excellent drug-like properties Immuno-oncology ANCHOR PARTNERSHIPS 18
Scientific and Clinical Advisory Boards SCIENTIFIC ADVISORY BOARD: SCIENTIFIC ADVISORY BOARD: CLINICAL ADVISORY BOARD: ONCOLOGY RESPIRATORY ONCOLOGY • Michael Curran, PhD • Ian Adcock, PhD • Funda Eric-Bernstam, MD, PhD MD Anderson Cancer Center Imperial College Institute for Personalized Cancer • Vijay Kuchroo DVM, PhD • Gary Anderson, PhD Therapy, MD Anderson Cancer Harvard Medical School University of Melbourne Center • Padmanee Sharma, PhD • Peter Barnes, FRS • Noah Hahnm, MD MD Anderson Cancer Center Imperial College Johns Hopkins University School of Medicine • E. John Werry, PhD • Fan Chung, MD, DSc University of Pennsylvania Imperial College • David Ilson, MD, PhD Memorial Sloan-Kettering Cancer • Dario Vignali, PhD • Oliver Eickelberg, MD Center, Weill Cornell Medical College University of Pittsburgh University of Denver • Sandra Swain, MD • Bruce Levy, MD Georgetown University Cancer Center Harvard University, Brigham and Women’s Hospital • Mario Sznol, MD Yale University • David Schwartz, MD University of Colorado, Denver • Sally Wenzel, MD University of Pittsburgh Medical Center 19

Pieris Pharmaceuticals, Inc. Corporate HQ: 255 State Street, 9th Floor, Boston, MA 02109, USA R&D Hub: Freising, Germany (Munich) info@pieris.com www.pieris.com
