Attached files
| file | filename |
|---|---|
| 8-K - CURRENT REPORT - ReWalk Robotics Ltd. | f8k111318_rewalkrobotics.htm |
Exhibit 99.1

Human and Robotic Intersection Take the Next Step 1 November 2018 ReWalk Confidential. © 2018 ReWalk Robotics, Inc.

Disclaimer This presentation contains statistical data that were obtained from industry publications and reports generated by third parties. Although ReWalk believes that the publications and reports are reliable, it has not independently verified this statistical d ata . The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use shou ld not be construed as an endorsement of the products or services of ReWalk. This document may not be retained, reproduced or distributed, in whole or in part, by any means (including electronic) withou t t he prior written consent of ReWalk. This document will not be left behind after this presentation, and by accepting this document and attending the presentation, yo u agree to be bound by the foregoing limitations. www.rewalk.com 2

Forward Looking Statements In addition to historical information, this presentation contains forward - looking statements within the meaning of Section 27 A of the Securities Act, Section 21 E of the Exchange Act, and the safe harbor provisions of the U . S . Private Securities Litigation Reform Act of 1995 , that are based on our management’s beliefs and assumptions and on information currently available to our management . Forward - looking statements include information concerning our possible or assumed future results of operations, business strategies, financing plans, competitive position, industry environment, potential growth opportunities, potential market opportunities and the effects of competition . Forward - looking statements may include projections regarding our future performance and, in some cases, can be identified by words like “anticipate,” “assume,” “believe,” “could,” “seek,” “estimate,” “expect,” “intend,” “may,” “plan,” “potential,” “predict,” “project,” “future,” “should,” “will,” “would” or similar expressions that convey uncertainty of future events or outcomes and the negatives of those terms . These forward - looking statements are based on our management’s current expectations, which are subject to uncertainty, risks and changes in circumstances that are difficult to predict, and many of which are outside of our control . Important factors that could cause our actual results, levels of activity or performance to differ materially from those indicated in the forward - looking statements include, among others : our expectations regarding future growth, including our ability to increase sales in our existing geographic markets, expand to new markets and achieve our planned expense reductions ; our management’s conclusion, and our independent registered public accounting firm’s statement in its opinion relating to our accompanying consolidated financial statements, that there is a substantial doubt as to our ability to continue as a going concern ; our ability to regain compliance with the continued listing requirements of the Nasdaq Capital Market and the risk that our ordinary shares will be delisted if we cannot do so ; our ability to maintain and grow our reputation and the market acceptance of our products ; our ability to achieve reimbursement from third - party payors for our products ; our expectations as to our clinical research program and clinical results ; our expectations as to the results of the FDA’s regulatory developments with respect to our mandatory 522 postmarket surveillance study ; the outcome of ongoing shareholder class action litigation relating to our IPO ; our ability to repay our secured indebtedness ; our ability to improve our products and develop new products ; our ability to close periodic issuances of our ordinary shares to, and to form a joint venture in China with, Timwell and the resulting effect on our liquidity and financial condition ; the risk of substantial dilution resulting from additional issuances, if any, of our ordinary shares to Timwell ; the significant voting power and de facto voting control Timwell may acquire upon additional issuances, if any, of our ordinary shares to Timwell ; our ability to maintain adequate protection of our intellectual property and to avoid violation of the intellectual property rights of others ; our ability to gain and maintain regulatory approvals ; our ability to secure capital from equity and debt financings in light of limitations under our effective registration statement on Form S - 3 , the price range of our ordinary shares and conditions in the financial markets, and the risk that such financings may dilute our shareholders or restrict our business ; our ability to use effectively the proceeds of offerings of our securities ; the impact of the market price of our ordinary shares on the determination of whether we are a passive foreign investment company ; our ability to maintain relationships with existing customers and develop relationships with new customers ; and our compliance with medical device reporting regulations to report adverse events involving our products and the potential impact of such adverse events on ReWalk’s ability to market and sell its products . Any forward - looking statement made in this presentation speaks only as of the date hereof . Factors or events that could cause our actual results to differ from the statements contained herein may emerge from time to time, and it is not possible for us to predict all of them . Except as required by law, we undertake no obligation to update publicly any forward - looking statements for any reason after the date of this presentation to conform these statements to actual results or to changes in our expectations, whether as a result of new information, future developments or otherwise . www.rewalk.com 3

Fundamentally change the Quality of Life for individuals with lower limb disability through the creation and development of market leading robotic technologies Our Mission www.rewalk.com 4

ReWalk rigid exoskeleton • Assists individuals with spinal cord injury to stand and walk • FDA & CE mark clearance • Sixth generation device • Commercialized with 450+ systems worldwide • First mover advantage ReStore soft exo - suit • In development for stroke patients and other lower limb disabilities • Clinical testing underway • Potential for additional indications including multiple sclerosis, cerebral palsy, Parkinson’s disease and elderly assistance ReWalk Overview Market leading global developer of robotic therapy and mobility assistance solutions www.rewalk.com 5

Multiple Markets for Growth Significant Mobility Impairment TODAY • 282,000 people in the U.S. living with SCI 1 • 17,000 new cases annually FUTURE POTENTIAL • 1,000+ US primary stroke centers 2 , ~100,000 general rehab clinics 3 • EU market similar to US • 6,000+ rehab hospitals in China 4 • 200,000+ Licensed Physical Therapists in US 5 • 1 million MS diagnosis in US, 2.3 million worldwide 6 • 75% will experience clinically significant walking disturbance 7 • Potential additional markets • 10M WW Parkinson’s 8 • Cerebral Palsy • Elderly assistance ___________________________ 1. Source: https://www.nscisc.uab.edu/Public/Facts%202016.pdf 2. US prevalence 2014, American Heart Association 3. https://www.ibisworld.com/industry - trends/market - research - reports/healthcare - social - assistance/ambulatory - health - care - services/p hysical - therapists.html 4. http:// www.chyxx.com/industry/201609/450634.html 5. http://www.apta.org/WorkforceData/ModelDescriptionFigures/ 6. https:// www.healthline.com/health/multiple - sclerosis/facts - statistics - infographic 7. Evaluating Walking in Patients with Multiple Sclerosis Which Assessment Tools Are Useful in Clinical Practice? Francois Bethoux , MD; Susan Bennett, PT, DPT, EdD , NCS, MSCS 8. Parkinson’s Disease Foundation Institutional Use Mild Mobility Impairment In market Pipeline – soft suit SCI ReWalk Rehab SCI ReWalk Personal Stroke - Rehab Multiple Sclerosis Elderly Assist Parkinson’s Cerebral Palsy Home Use Targeting 2019 Launch Stroke - H ome

www.rewalk.com Breakthrough Technology Addresses the SCI Need ___________________________ 1. Source: https:// www.nscisc.uab.edu/Public/Facts%202016.pdf 2. Source : https://www.nscisc.uab.edu/public/2016%20Annual%20Report%20 - % 20Complete%20Public%20Version.pdf Wheelchair confinement can cause severe physical and psychological deterioration resulting in significant costs to the healthcare system Difficulty with bowel and urinary tract function Osteoporosis Loss of lean mass / gain in fat mass Insulin resistance Diabetes Heart disease 87% of spinal cord injury patients discharged to private, non - institutional residences 2 Secondary Medical Consequences of Paralysis: $ 520K Avg. Cost of Healthcare First Year of Injury for Paraplegia 1 $69K Avg. Annual Cost of Healthcare for Paraplegia 1 $2.3M Est. Lifetime Cost of Healthcare for Paraplegia Injury at age 25 1 $1.5M Est. Lifetime Cost of Healthcare for Paraplegia Injury at age 50 1

ReWalk’s Breakthrough Technology Patented tilt - sensor technology mimics natural gait 8 https://www.youtube.com/watch?v=d5zl7fglMgo


• First national coverage policy updated in June 2018 • For evaluation, training and issuance of ReWalk exoskeletons • Exclusive to ReWalk exoskeletons • Large multi - center community based exoskeleton study • Evaluating quality of life and health benefits of walking Scope • ~44,000 Paralyzed Individuals are eligible for VA Benefits (2 ) • 160 SCI veterans • Duration: 4 years Facilities • Evaluation - 24 SCI “Hub” Centers (3 ) • Training with the VA “Choice” program through - • Trained VA “Spoke” sites , or • 113 certified ReWalk training centers • Currently 12 centers active over 18 months VA Department • Clinical group – prosthetics (same area that provides legs and arms) • Research department only Units • 19 • Over 60% conversion rate • 88 National Coverage Policy Research Study ( 1) (1) ExoskCSP #2003 exoskeleton Assisted - Walking in Persons With SCI: Impact on Quality of Life (2) http://imperial.networkofcare.org/veterans/library/article.aspx?id=1687 (3) https://www.sci.va.gov/VAs_SCID_System_of_Care.asp ___________________________ Strong Partnership with the VA

Medical Aid Code : 23.29.01.2001 ReWalk Personal 6.0: first and only exoskeleton officially recognized as a medical aid throughout Germany. German SCI market has 80,000 1 paralyzed individuals Official publication in Federal Gazette on June 11, 2018 The listing enables any medically qualified individual to obtain reimbursement for ReWalk Personal 6.0 exoskeleton through German Statutory Health Insurance Funds (GKV) - General product safety - Safe use in home environment confirmed - Individual supply requirements - Trial/Rental period - Companion training Medical Aid Code confirms Since medical aid confirmation – 7 more Statutory Health Insurers (SHI) have approved ReWalk Personal 6.0 • Contract the different payors with a formal training and supply agreement • There are 134 claims in the pipeline that we are working to process 1. Source: https:// www.dgn.org/images/red_leitlinien/LL_2012/pdf/ll_71_2012_querschnittlhmung.pdf . 10 - 30 SCI paralyzed individuals per 1 million population ___________________________

ReStore - Shaping the Future • Initially targeting rehabilitation related to stroke • Technology licensed from Harvard • Provides powered plantarflexion • Clinical trial enrollment underway • CE mark submission at the beginning of Q4 2018; FDA submission is planned by Q1 2019; commercialization in EU and US planned for Q3 2019 12 www.rewalk.com Leveraging ReWalk’s technology and commercial expertise to develop a lightweight, wearable soft exo - suit that facilitates a natural gait

ReStore in Action • Light, soft components and powered dorsi / plantar flexion facilitate natural gait pattern • Provides therapist real - time analytics and enhanced session control for optimized results • Multiple modes of function and rapid donning/ doffing and adjustment for efficient therapy sessions • Session data capture with reporting and comparison across sessions Key Differentiators https://www.youtube.com/watch?v=kreK8VDh0ZI

Real Time Analytics and Control 14 www.rewalk.com

Near - term ReStore Market: Stroke US: 7 million stroke survivors 1 EU: 9.6 million stroke survivors 2 China: 11 million stroke survivors ⁸ US: 2.5 million 5,6 potentially eligible for ReStore system EU: 3.4 million 7 potentially eligible for ReStore system China: 3.9 million 7 US: ~ 795K 3 EU / Western Europe: ~ 1.1 million 4 China: ~ 2.4 million⁸ US: ~ 370K 5, 6 EU: ~500K 7 China: ~1.1M 7 Annual Incidence Annual Addressable Market - Incidence 1. https:// www.ncbi.nlm.nih.gov/pmc/articles/PMC3250269/pdf/13311_2011_Article_53.pdf 2. Extrapolated from the incidence numbers based on the rate for US. 3. American Heart Association 2017 Heart Disease and Stroke Statistics 2017 4. European Journal of Neurology 6JUN2016, Vol 13, Issue 6 “Stroke Incidence and Prevalence in Europe: a review of available dat a”; as of 2000. 5. Assumption, 60% lower limb disability rate after stroke - Source : Rehabilitation after Stroke Bruce H. Dobkin , M.D. N Engl J Med 2005; 352:1677 - 1684 April 21, 2005 DOI: 10.1056/NEJMcp043511 . 6. Assumptions: For the incidence pool, 60% have lower limb disability per (6), and estimate 22% fall our rate; for prevalence pool, estimate 40% fall our rate of the 60 % w ith lower limb disability 7. Assuming similar rates as the US market in 5 and 6 above. 8. Prevalence, Incidence, and Mortality of Stroke in China - Results from a Nationwide Population - Based Survey of 480 687 Adults https :// pdfs.semanticscholar.org/f59d/209fe597e6dabdf966628b99b44762273497.pdf 9. US prevalence 2014, American Heart Association 10. Estimate similar to US 11. http://www.chyxx.com/industry/201609/450634.html Prevalence Addressable Market - Prevalence Eligible population adjusted by physical qualifications PHASE I: Top Tier Stroke Rehab Centers PHASE II: Thousands of Hospitals & Physical Therapy Clinics US 1,000 primary stroke centers 9 EU 1,000 clinics 10 China 6,000 Rehab Hospitals 11 Penetration strategy - ___________________________
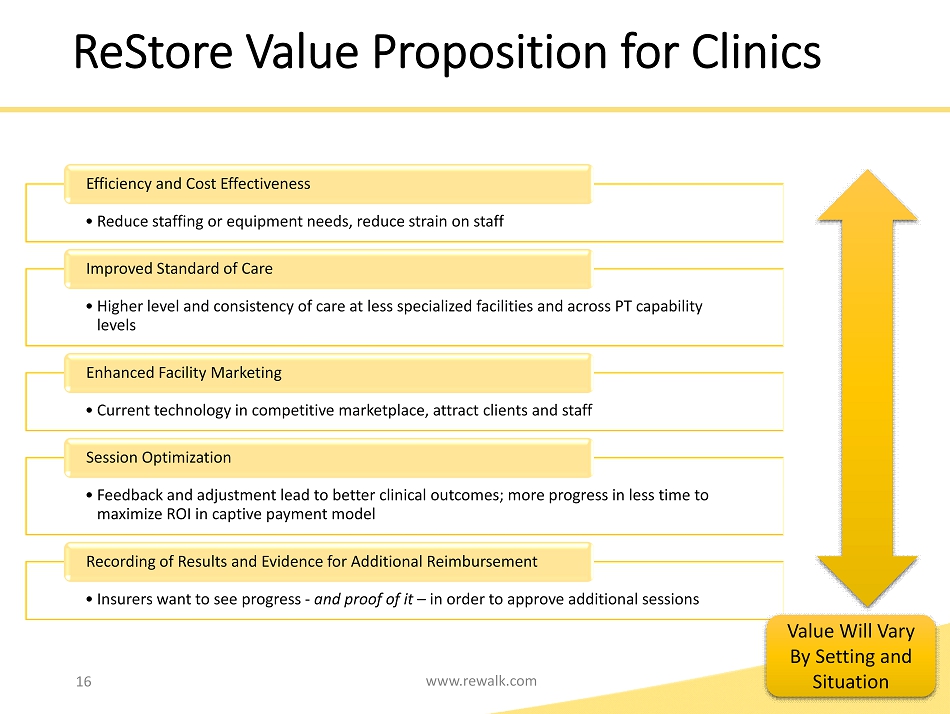
16 www.rewalk.com • Reduce staffing or equipment needs, reduce strain on staff Efficiency and Cost Effectiveness • Higher level and consistency of care at less specialized facilities and across PT capability levels Improved Standard of Care • Current technology in competitive marketplace, attract clients and staff Enhanced Facility Marketing • Feedback and adjustment lead to better clinical outcomes; more progress in less time to maximize ROI in captive payment model Session Optimization • Insurers want to see progress - and proof of it – in order to approve additional sessions Recording of Results and Evidence for Additional Reimbursement Value Will Vary By Setting and Situation ReStore Value Proposition for Clinics
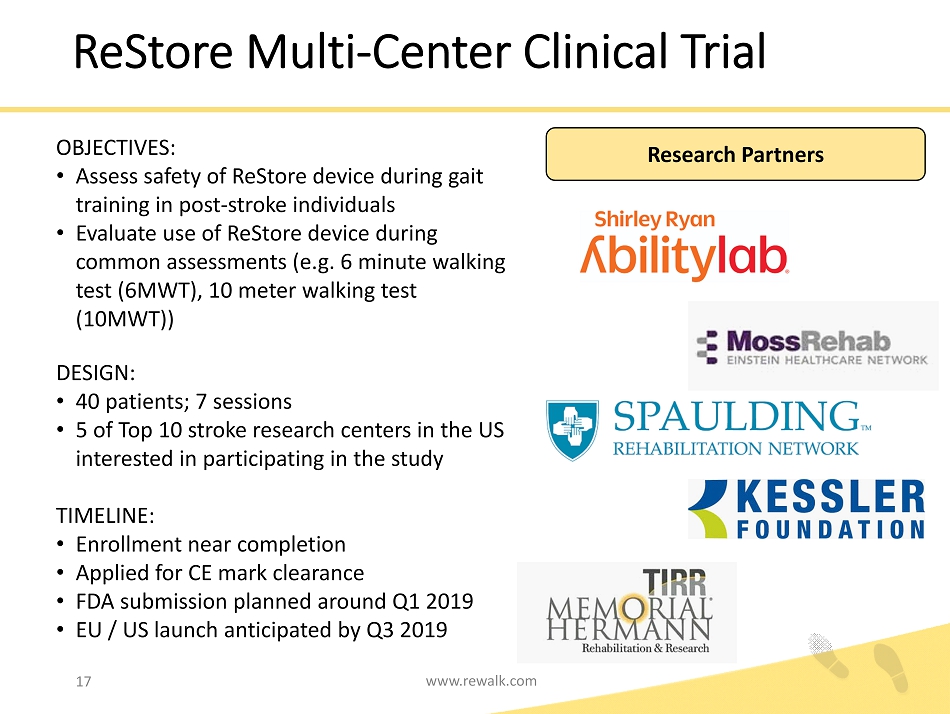
ReStore Multi - Center Clinical Trial OBJECTIVES: • Assess safety of ReStore device during gait training in post - stroke individuals • Evaluate use of ReStore device during common assessments (e.g. 6 minute walking test (6MWT), 10 meter walking test (10MWT)) DESIGN: • 40 patients; 7 sessions • 5 of Top 10 stroke research centers in the US interested in participating in the study TIMELINE: • Enrollment near completion • Applied for CE mark clearance • FDA submission planned around Q1 2019 • EU / US launch anticipated by Q3 2019 Research Partners www.rewalk.com 17


Strategic Approach – China • Large market opportunity in China • 11 million stroke survivors 1 • 2.4 million people suffer a stroke each year 2 • Number of stroke rehabilitation centers in China expected to exceed those in the US and EU combined by 2021 • March 2018: ReWalk signed a $20 million strategic investment which included establishment of JV, licensing, and supply agreements with Timwell Corporation ( Realcan pharma affiliate) • First tranche of $5 million completed May 2018; later tranches of $10 million with JV and $5 million with manufacturing have not occurred due to complexity of structure • Currently considering simplified investment alternatives, one of which is for an investment in parallel with an exclusive distribution contract • A lso evaluating alternative paths with different groups to penetrate the Chinese market Developing a platform for accelerated growth in largest single stroke market www.rewalk.com 19 1. Prevalence , Incidence, and Mortality of Stroke in China - Results from a Nationwide Population - Based Survey of 480 687 Adults https :// pdfs.semanticscholar.org/f59d/209fe597e6dabdf966628b99b44762273497.pdf 2. Id . ___________________________

Financial Snapshot 128 149 218 226 Q3 '15 Q3 '16 Q3 '17 Q3 '18 Pending Insurance Claims $4.0 $3.7 $5.9 $7.8 $5.0 Revenue ($M) www.rewalk.com 20 74 73 119 107 66 Units Placed

Company Highlights • Market leading global exoskeleton developer with two breakthrough device platforms • Rigid ReWalk exoskeleton for spinal cord injury market • FDA and CE mark clearance for both Personal and Rehabilitation use; sixth generation device • 500 systems placed, with majority for home and community use; other systems used in rehab centers • Global commercial infrastructure with market leading sales and reimbursement track record • ReStore soft - suit exoskeleton for stroke rehabilitation • First study underway in collaboration with Harvard University’s Wyss Institute • CE submission completed Q4 2018; FDA submission planned for early 2019 ; EU and US launch anticipated Q3 2019 • Potential for other indications including multiple sclerosis and Parkinson’s disease • Innovation through research collaboration agreement with Wyss Institute at Harvard • Proven insurance reimbursement success. Currently working with other groups in the US and Germany to secure broader coverage • Extensive relationship with Department of Veterans Affairs, the single largest network of care for spinal cord injury (SCI) patients in the United States www.rewalk.com 21

Take the Next Step 22 www.rewalk.com
