Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Zyla Life Sciences | a18-40120_18k.htm |
2 Forward Looking Statements Statements included in this presentation that are not historical in nature are "forward-looking statements" within the meaning of the Private Securities Litigation Reform Act of 1995. These forward-looking statements are based on management's current expectations, and are subject to known and unknown uncertainties, risks and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. You can identify forward-looking statements by terminology such as “may,” “could,” “plans,” “future,” “expects,” “goal,” “intends,” “assess,” “continue to,” “potential,” “anticipates,” “believes,” “estimates,” “predicts,” or “focus” or the negative of these terms or other comparable terminology. Forward-looking statements contained in this presentation include, but are not limited to statements regarding: (i) our proposed acquisition of assets from Iroko Pharmaceuticals Inc. (the “Iroko Acquisition”), our proposed plan of reorganization under Chapter 11 of the Bankruptcy Code (the “Restructuring”) and the risks related to described below; (ii) the potential market size for our products; (iii) the timing or likelihood of regulatory filings, decisions and approvals for our products and product candidates; (iv) our expectations regarding the potential safety, efficacy, or clinical utility of our product candidates; (v) the impact of our existing commercial presence on the commercialization of our new products; (vi) the impact of the addition of our new products on our market presence; (vii) statements regarding the expansion of new prescribers and prescriptions for our products; (viii) the timing of the expansion of dosage levels for our products; (viii) the implications for the success of our products based on our current demand experience; (ix) our expectations regarding our path to sustainability and growth, including our business development plans; (x) the strategic imperatives with regard to our products, including our goals with regard to market access; and (xi) our expectations regarding our finances, including our expenses, and our funding sources, our use of funds and potential payments under our notes and our royalty rights agreements. In addition, we, through our senior management, from time to time make forward-looking public statements concerning our expected future operations and performance and other developments. Actual results could differ materially from those discussed due to a number of factors, including, but not limited to: the costs of the restructuring and the ability to emerge expeditiously, including there being no substantial objection to or litigation with respect to the restructuring; the Company’s ability to satisfy the requirements of the Company’s restructuring support agreement with certain of its creditors, including consummation of the proposed plan of reorganization; the Company’s expected motions to be filed in the Chapter 11 proceeding and the dispositions of such motions; the Company’s continued operations and customer and supplier relationships while in a Chapter 11 proceeding; the resources needed to support the Company’s operations while in a Chapter 11 proceeding; the Company’s ability to lower debt and interest payments, operate its business and satisfy its obligations while in a Chapter 11 proceeding; the public disclosure of sensitive business information, including projections, as part of the Chapter 11 proceedings; the anticipated benefits of the Iroko Acquisition and the impact of the Iroko Acquisition on the Company’s earnings, capital structure, strategic plan and results of operations; the occurrence of any event, change or other circumstance that could give rise to the termination of the purchase agreement related to the Iroko Acquisition, the failure of the closing conditions to the Iroko Acquisition to be satisfied (or any material delay in satisfying such conditions); the failure to consummate the Iroko Acquisition; the costs, fees, expenses and charges (if any) related to the Iroko Acquisition and the Company’s Chapter 11 proceedings; the Company’s ability to continue as a going concern; the trading price of the Company’s common stock and the liquidity of the trading market with respect thereto, including the fact that the plan or reorganization contemplated by the Company’s restructuring support agreement with certain of its creditors provides for all existing equity interests of our common stockholders to be cancelled and for our common stockholders to lose the full amount of their investment; the Company’s ability to recruit or retain key scientific or management personnel or to retain our executive officers;

3 Forward Looking Statements Continued our ability to obtain regulatory approval of our product candidates and supplemental applications relating to our products; our ability to successfully commercialize our products and gain broader acceptance and use of our products; our ability to execute on our sales and marketing strategy, including developing relationships with customers, physicians, payers and other constituencies and other commercial capabilities; the accuracy of our estimates of the size and characteristics of the potential markets for our product candidates and our ability to serve those markets; unexpected safety or efficacy data; competitive factors; changes in the regulatory environment for our products; general market conditions; our need for and ability to obtain future capital; our ability to service our current and future indebtedness; our estimates regarding expenses, future revenues, capital requirements and needs for additional financing; our ability to execute on our business development strategy; and other risk factors described in our filings with the United States Securities and Exchange Commission. Egalet assumes no obligation to update or revise any forward-looking statements contained in this presentation whether as a result of new information or future events, except as may be required by law. The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be constitute as an endorsement of such products. This presentation shall not constitute an offer to sell or the solicitation of an offer to buy, nor shall there be any sale of these securities in any state or other jurisdiction in which such offer, solicitation or sale would be unlawful prior to registration or qualification under the securities laws of any such state or other jurisdiction. See Sprix.com and Oxaydo.com for full prescribing information including boxed warning and medication guide.

4 Q3 SPRIX Nasal Spray & OXAYDO Growth Q3 net revenues of $6.1 MM an increase of 13% over previous quarter Highest quarterly revenue since acquisition of SPRIX in January 2015 Indication: Use in adult patients for the short-term (up to five days) management of moderate to moderately severe pain that requires analgesia at the opioid level. Q3 net revenues of $1.9 MM an increase of 11% over previous quarter Highest quarterly revenue since OXAYDO was licensed in January 2015 Indication: Management of acute and chronic moderate to severe pain where the use of an opioid analgesic is appropriate

Purchase Agreement Signed to Acquire Iroko Assets* 5 5 + *Transaction expected to close in Q1:19.

PHASE 1 PHASE 2 PHASE 3 NDA MARKETED Acute pain and Osteoarthritis pain2,3 Acute pain4 Osteoarthritis pain5 6 Portfolio to Address Needs of Broader Patient Population PROGRAM 1. SPRIX® (ketorolac tromethamine) Nasal Spray is approved for use in adult patients for the short-term (up to five days) management of moderate to moderately severe pain that requires analgesia at the opioid level. 2. ZORVOLEX® is approved for the treatment of mild to moderate acute pain in adults. 3. ZORVOLEX® is approved for the management of osteoarthritis pain. 4. TIVORBEX ® is approved for the treatment of mild to moderate acute pain in adults. 5.VIVLODEX™ is approved for the management of osteoarthritis. 6. INDOCIN ORAL SUSPENSION is approved for moderate to severe rheumatoid arthritis including acute flares of chronic disease, moderate to severe ankylosing spondylitis, moderate to severe osteoarthritis, acute painful shoulder bursitis and/or tendinitis) and acute gouty arthritis and INDOCIN SUPPOSITORIES are approved to treat arthritis, to prevent gouty arthritis and ankylosing spondylitis. 7. OXAYDO is Indicated for the management of acute and chronic moderate to severe pain where the use of an opioid analgesic is appropriate. Acute pain1 (oxycodone HCL, USP) tablets CII Acute and chronic pain7 Joint pain6 Osteoarthritis pain Pipeline SOLUMATRIX® naproxen Approved *Acquisition expected to close in Q1:19. * * * * *

7 Restructured Egalet: More Potential Revenue and Less Debt1 Pre-Restructuring/Pre-Acquisition Egalet Post-Restructuring/Post-Acquisition Egalet2 Marketed Products Two Six Primary Focus Abuse-deterrent opioids and NSAIDs NSAIDs Senior Debt $129 Million $95 Million Projected Annual Revenue $43 Million $80 - $90 Million 1.Acquisition expected to close in Q1:19. 2. Assumes Iroko Acquisition and Restructuring are consummated on terms substantially similar to those currently proposed in the Plan of Reorganization filed October 30, 2018.

Expanding and Focusing Commercial Business 8

9 Current Environment Supports NSAID Focus U.S. Annual NSAID Prescription Volume (MM) Source: IMS NPA as of Jan 2016. 1. Assumes all 127 MM prescriptions in 2015 were sold at pricing levels comparable to ZORVOLEX® . Decline in opioid use, while NSAID market continues to grow NSAIDs are one of the largest therapeutic classes in the U.S. NSAID Market represents ~$20 billion1 in U.S. domestic annual market opportunity at branded prices 104 127 33 42 18 20 17 29 10 8 12 9 11 16 3 3 2010 2015 Ibuprofen Naproxen Meloxicam Celecoxib Others Diclofenac Indomethacin

10 Leverage Current Egalet Salesforce, Expand Number of Targets and Call on Large Iroko Product Prescribers [World Map] 82 territory managers Plan to expand targets to Iroko high prescriber targets Targeting: Pain Physical Medicine Rehabilitation Primary care Orthopedic Surgeons Neurology Nurse Practitioners Focusing on commercially insured Partnered with Ascend and OraPharma to promote SPRIX

11 SoluMatrix Fine Particle Technology™ Makes Low-Dose NSAIDs Possible SoluMatrix Fine Particle Technology™ is a proprietary technology that is used to create NSAID drug particles that are approximately 10 to 20 times smaller than their original size1 Reduction in particle size increases surface area, which leads to the product being quickly dissolved and rapidly absorbed With SoluMatrix Fine Particle Technology, NSAIDs are delivered at low-dosage strengths with rapid absorption and low systemic exposure2-4 1. Iroko Pharmaceuticals, LLC. Website. 2. Hussaini A, Solorio D, Young C. Pharmacokinetic properties of low-dose SoluMatrix meloxicam in healthy adults. Clin Rheumatol. [epub ahead of print] 3. Desjardins PJ, Olugemo K, Solorio D, Young CL. Pharmacokinetic properties and tolerability of low-dose SoluMatrix diclofenac. Clin Ther. 2015;37(2):448-461. 4. Olugemo K, Solorio D, Sheridan C, Young CL. Pharmacokinetics and safety of low-dose submicron indomethacin 20 and 40 mg compared with indomethacin 50 mg. Postgrad Med. 2015;127(2):223-231.

12 VIVLODEX® (meloxicam) Once daily dosing Meloxicam is the second highest prescribed NSAID in market with >500,000 prescriptions/week in US Multiple international partnerships in place INDICATION: FDA-approved for management of osteoarthritis pain in October 2015

13 ZORVOLEX® (diclofenac) 20% lower dose and lower systemic exposure than other oral diclofenac products2 Multiple international partnerships in place 1. Full prescribing information accessible at ZORVOLEX.com 2.. 35mg ZORVOLEX® contains 20% less diclofenac than other commercially available 50mg diclofenac potassium drug products; 18mg ZORVOLEX® contains 20% less diclofenac than other 25mg diclofenac products 1. ZORVOLEX ® package insert. 2. Data on File INDICATION: FDA-approved for mild to moderate acute pain in January 2014 and osteoarthritis pain in September 20141

. Lower dose and lower systemic exposure compared to other immediate release indomethacin products1 Opioid-sparing data published2 14 TIVORBEX® (indomethacin) 40mg Tivorbex® contains 20% less indomethacin than other commercially available 50mg indomethacin immediate release drug products; 20mg Tivorbex® contains 20% less indomethacin than other 25mg indomethacin products Altman, Physician and Sports Medicine, 2013 INDICATION: FDA-approved for treatment of mild to moderate acute pain in adults in February 2014

15 INDOCIN® (indomethacin) INDICATION: FDA-approved for treatment of moderate to severe rheumatoid arthritis including acute flares of chronic disease, moderate to severe ankylosing spondylitis, moderate to severe osteoarthritis, acute painful shoulder (bursitis and/or tendinitis) and acute gouty arthritis Two forms of INDOCIN: oral suspension and suppositories No marketing activity for INDOCIN in the U.S. since 2014

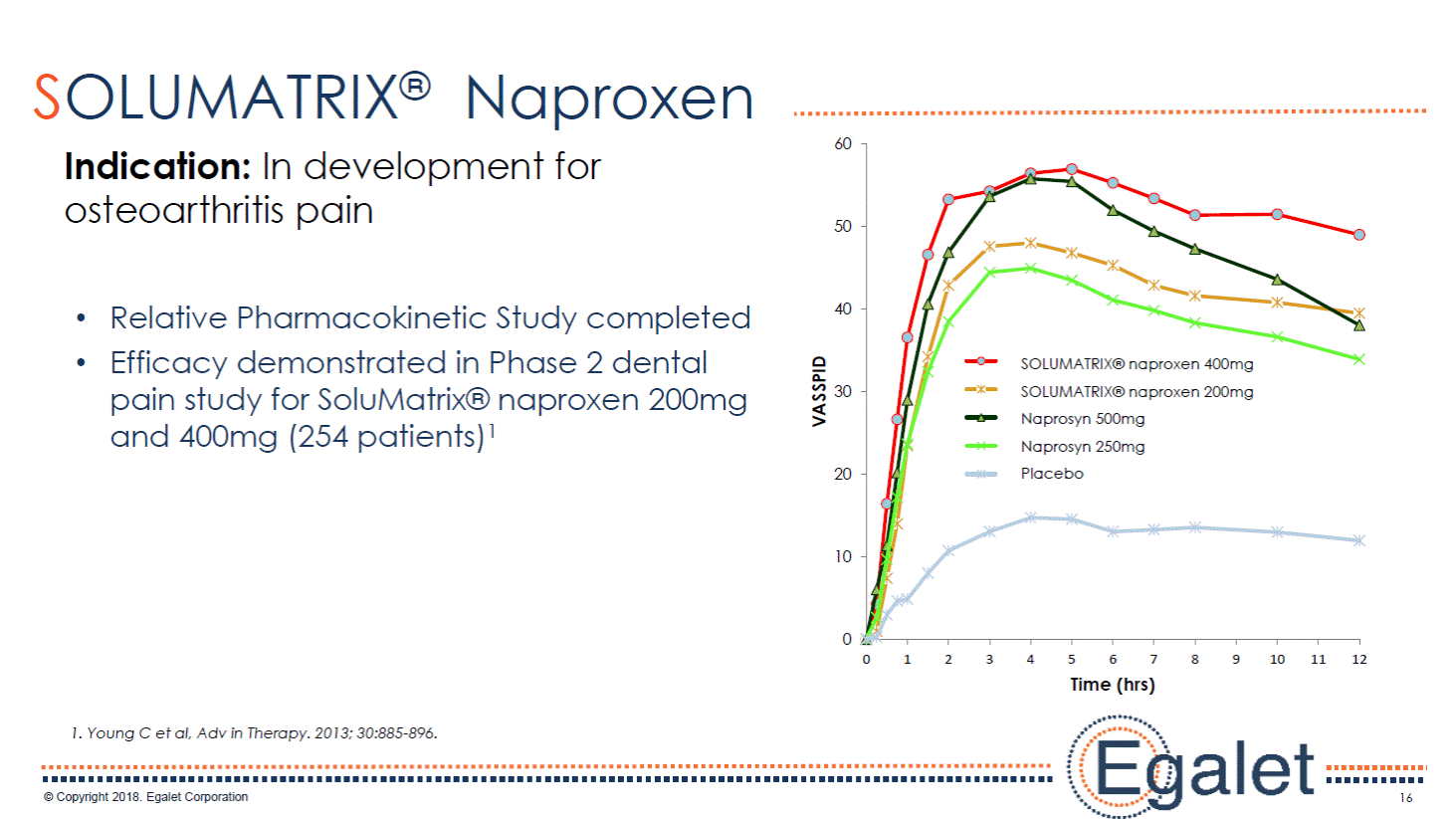
16 SOLUMATRIX® Naproxen Relative Pharmacokinetic Study completed Efficacy demonstrated in Phase 2 dental pain study for SoluMatrix® naproxen 200mg and 400mg (254 patients)1 1. Young C et al, Adv in Therapy. 2013; 30:885-896. Indication: In development for osteoarthritis pain 0 10 20 30 40 50 60 0 1 2 3 4 5 6 7 8 9 10 11 12 VASSPID Time (hrs) Nano Test 400 mg Nano Test 200 mg Naprosyn 500 mg Naprosyn 250 mg Placebo SOLUMATRIX® naproxen 400mg SOLUMATRIX® naproxen 200mg Naprosyn 500mg Naprosyn 250mg Placebo
Proposed Restructuring and Financials 17

Certain Terms of the Iroko Acquisition1 18 No upfront cash payment to purchase assets If transaction is approved, Iroko would receive: $45 million in new senior secured debt 49% of post-restructuring Egalet equity2 Royalty on Indocin annual net sales of more than $20 million Please refer to the Company’s Form 8-k filed with the Securities and Exchange Commission on November 1, 2018 for additional information. Pursuant to the proposed Plan of Reorganization, all existing equity interest in Egalet Corporation will be extinguished.

Asset purchase agreement is tied to proposed plan of financial reorganization of Egalet In advance of bankruptcy filing, Egalet signed a restructuring support agreement with over 2/3 (in dollar amount) of senior debt holders The plan calls for Egalet to issue new Egalet common stock to satisfy all claims related to the 5.5% and 6.5% convertible debt and a portion of the senior secured debt Egalet plans to reduce its senior debt by $34 million to a total of $95 million of senior secured debt (including the $45 million to be issued as part of the Iroko Acquisition purchase price) The pre-arranged reorganization will require Court approval, and such approval is a condition to closing the Iroko Acquisition Egalet management expected to remain with changes to Board composition 19 Voluntary Plan of Reorganization1 Anticipate closing in the first quarter of 2019 Please refer to the Company’s Form 8-k filed with the Securities and Exchange Commission on November 1, 2018 for additional information.

Acquisition would add four approved, NSAIDs to commercial portfolio Goal is to adapt to market pressures and increase company focus on non-narcotics Would leverage current commercial infrastructure Acquisition would require no upfront cash, only debt and equity Reorganization to reduce total debt Potential annual revenue of between $80 and $90 million 20 Transaction Expected to Increase Egalet Potential Revenue While Reducing Debt

Thank You 21 @EgaletCorp Egalet.com


