Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Jounce Therapeutics, Inc. | jnce110920188-k.htm |

Exhibit 99.1 Jounce Therapeutics A Next Gen Immunotherapy Company

Legal Disclaimer Various statements concerning Jounce’s future expectations, plans and prospects, including without limitation, Jounce’s expectations regarding the timing, progress and results of research and development programs, preclinical studies and clinical trials for Jounce’s product candidates and any future product candidates, the potential benefits of any of these product candidates and the timing or likelihood of regulatory filings may constitute forward-looking statements for the purposes of the safe harbor provisions under The Private Securities Litigation Reform Act of 1995 and other federal securities laws and are subject to substantial risks, uncertainties and assumptions. You should not place reliance on these forward looking statements, which often include words such as “anticipate,” “estimate,” “expect,” “explore,” “goal,” “intend,” “may,” “on track,” “tracking,” “undue,” “plan,” “position,” “predict,” “target,” “potential” or similar terms, variations of such terms or the negative of those terms. Although the Company believes that the expectations reflected in the forward-looking statements are reasonable, the Company cannot guarantee such outcomes. Actual results may differ materially from those indicated by these forward-looking statements as a result of various important factors, including, without limitation, Jounce’s ability to successfully demonstrate the efficacy and safety of its product candidates and future product candidates, the preclinical and clinical results for its product candidates, which may not support further development and marketing approval, the potential advantages of Jounce’s product candidates, the development plans of its product candidates, actions of regulatory agencies, which may affect the initiation, timing and progress of preclinical studies and clinical trials of its product candidates, Jounce’s anticipated milestones, Jounce’s ability to obtain, maintain and protect its intellectual property, Jounce’s ability to enforce its patents against infringers and defend its patent portfolio against challenges from third parties, the timing, cost or other aspects of a potential commercial launch of Jounce’s product candidates and potential future sales of our current product candidates or any other potential products if any are approved for marketing, competition from others developing products for similar uses, Jounce’s ability to manage operating expenses, Jounce’s ability to maintain its collaboration with Celgene and establish or maintain future collaborations, Jounce’s dependence on third parties for development, manufacture, marketing, sales and distribution of product candidates and unexpected expenditures, as well as those risks more fully discussed in the section entitled “Risk Factors” in Jounce’s most recent Annual Report on Form 10-K or Quarterly Report on Form 10-Q filed with the Securities and Exchange Commission as well as discussions of potential risks, uncertainties, and other important factors in Jounce’s subsequent filings with the Securities and Exchange Commission. All such statements speak only as of the date made, and the Company undertakes no obligation to update or revise publicly any forward-looking statements, whether as a result of new information, future events or otherwise. 2 | Jounce Therapeutics © November 2018

DELIVERING VALUE PIPELINE EXECUTION APPROACH & IO OPPORTUNITY 3 | Jounce Therapeutics © November 2018

DELIVERING VALUE PIPELINE EXECUTION APPROACH & IO OPPORTUNITY 4 | Jounce Therapeutics © November 2018

What Makes Jounce Different? Robust Translational Science Platform Diverse, Broad Pipeline • Engine to drive sustained discovery • Three development programs – JTX-2011 (anti-ICOS) Phase 1/2 • Programs targeting multiple immune cell types – JTX-4014 (anti-PD-1) IND active – JTX-8064 (anti-LILRB2) tracking to IND • Additional discovery programs progressing Significant Strategic Collaboration Experienced Founders & Management Team • Strategic collaboration with co- • Founders with fundamental roles in IO biology - development and Coley, Lasker and Nobel awards co-commercialization • Management team with broad R&D and features commercial experience including leadership roles in 5 | Jounce Therapeutics © November 2018
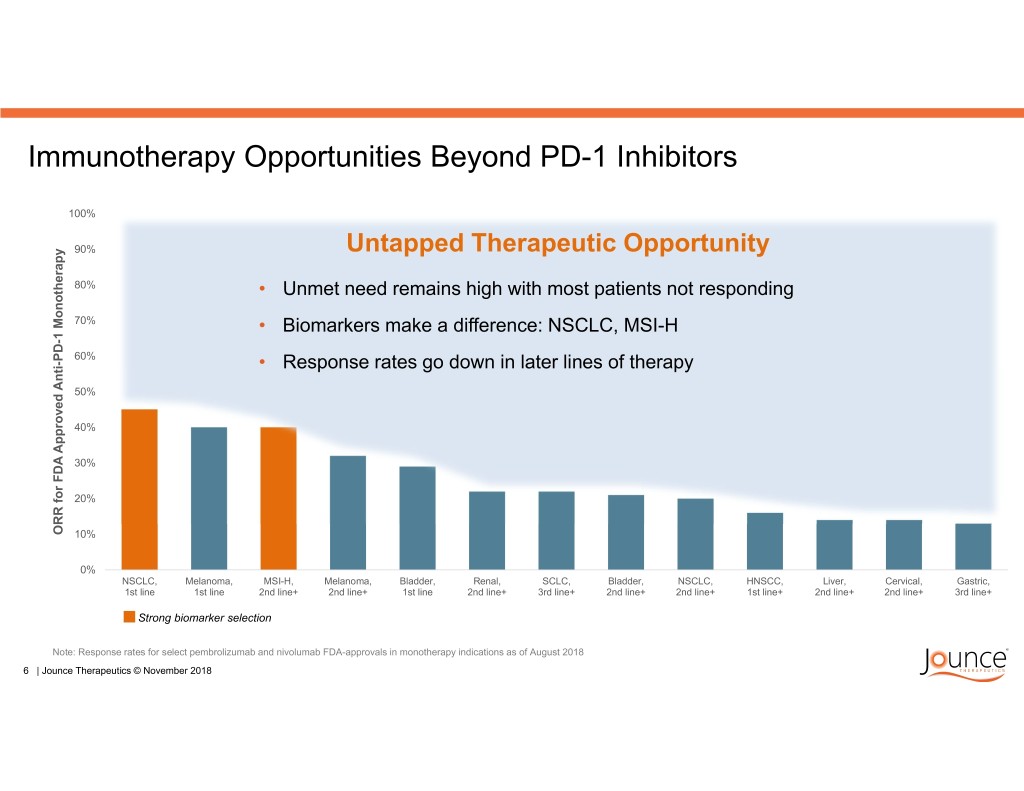
Immunotherapy Opportunities Beyond PD-1 Inhibitors 100% 90% Untapped Therapeutic Opportunity 80% • Unmet need remains high with most patients not responding 70% • Biomarkers make a difference: NSCLC, MSI-H 60% • Response rates go down in later lines of therapy 50% 40% 30% 20% ORR for FDA Approved Anti-PD-1 Monotherapy Anti-PD-1 Approved ORR for FDA 10% 0% NSCLC, Melanoma, MSI-H, Melanoma, Bladder, Renal, SCLC, Bladder, NSCLC, HNSCC, Liver, Cervical, Gastric, 1st line 1st line 2nd line+ 2nd line+ 1st line 2nd line+ 3rd line+ 2nd line+ 2nd line+ 1st line+ 2nd line+ 2nd line+ 3rd line+ Strong biomarker selection Note: Response rates for select pembrolizumab and nivolumab FDA-approvals in monotherapy indications as of August 2018 6 | Jounce Therapeutics © November 2018
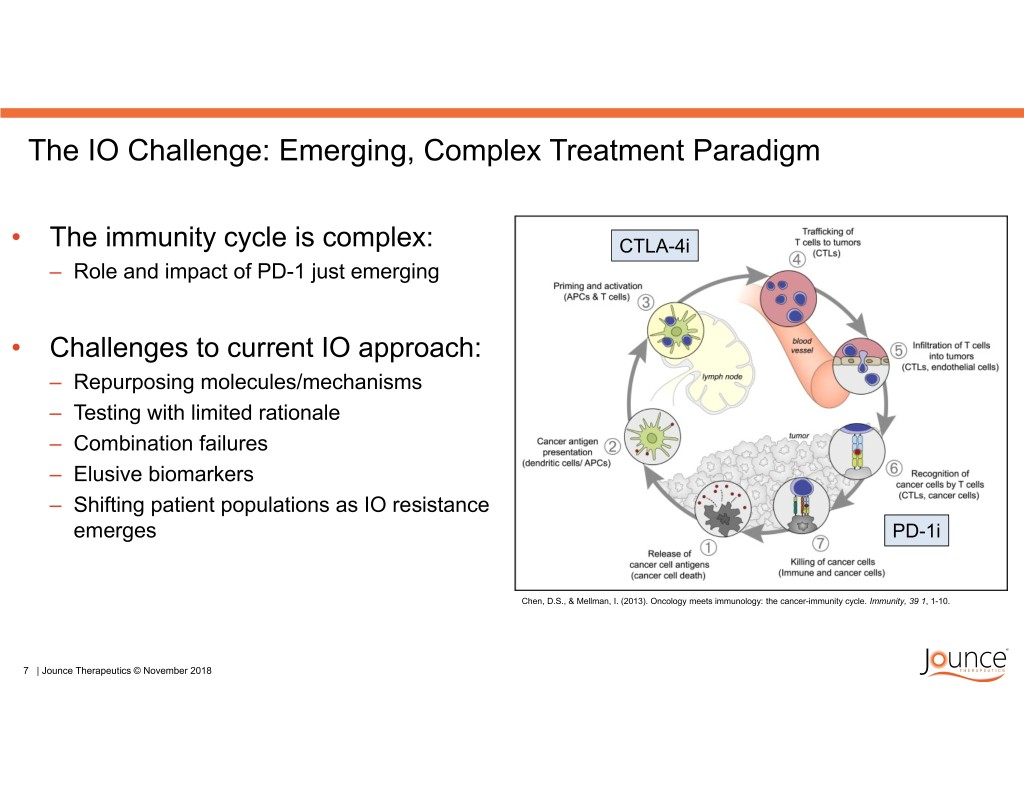
The IO Challenge: Emerging, Complex Treatment Paradigm • The immunity cycle is complex: CTLA-4i – Role and impact of PD-1 just emerging • Challenges to current IO approach: – Repurposing molecules/mechanisms – Testing with limited rationale – Combination failures – Elusive biomarkers – Shifting patient populations as IO resistance emerges PD-1i Chen, D.S., & Mellman, I. (2013). Oncology meets immunology: the cancer-immunity cycle. Immunity, 39 1, 1-10. 7 | Jounce Therapeutics © November 2018

The Jounce Solution: Next Generation IO Tumor Immune Composition • Strong scientific foundation: – Understanding where IO mechanisms act within the immunity cycle – Founders and leadership expertise in checkpoint and IO Discover 1st in Class Therapies biology • Unique approach: – Robust translational science platform • Large scale human tumor data • Human tumor histoculture Mechanistic & Predictive Biomarkers • Multiple integrated technologies and bioinformatics – Targets & biomarkers linked to tumor immune composition – Reverse translational work leading to new targets, methods and paradigms 8 | Jounce Therapeutics © November 2018

DELIVERING VALUE PIPELINE EXECUTION APPROACH & IO OPPORTUNITY 9 | Jounce Therapeutics © November 2018
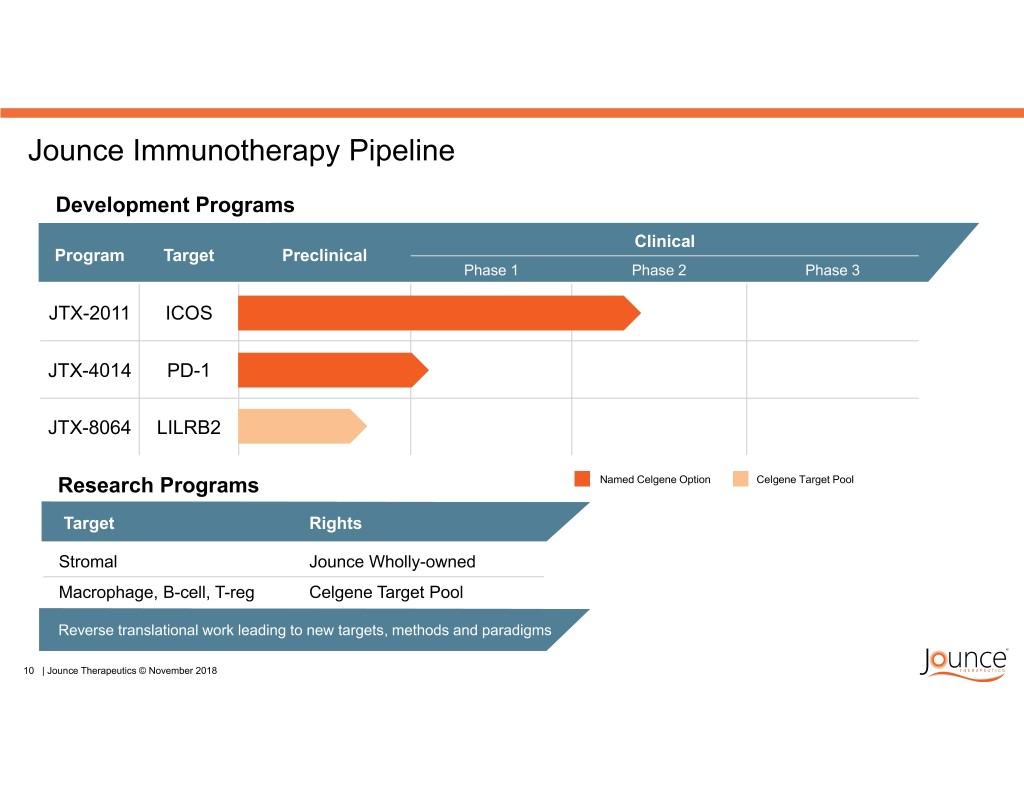
Jounce Immunotherapy Pipeline Development Programs Clinical Program Target Preclinical Phase 1 Phase 2 Phase 3 JTX-2011 ICOS JTX-4014 PD-1 JTX-8064 LILRB2 Research Programs Named Celgene Option Celgene Target Pool Target Rights Stromal Jounce Wholly-owned Macrophage, B-cell, T-reg Celgene Target Pool Reverse translational work leading to new targets, methods and paradigms 10 | Jounce Therapeutics © November 2018
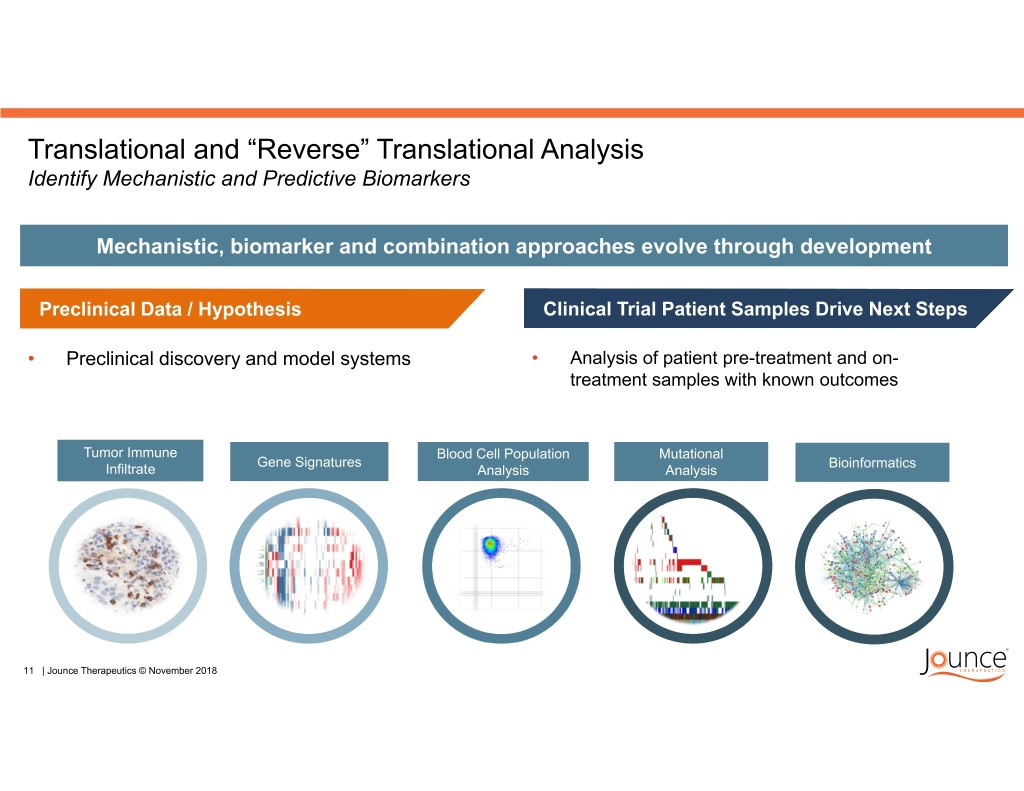
Translational and “Reverse” Translational Analysis Identify Mechanistic and Predictive Biomarkers Mechanistic, biomarker and combination approaches evolve through development Preclinical Data / Hypothesis Clinical Trial Patient Samples Drive Next Steps • Preclinical discovery and model systems • Analysis of patient pre-treatment and on- treatment samples with known outcomes Tumor Immune Blood Cell Population Mutational Gene Signatures Bioinformatics Infiltrate Analysis Analysis 11 | Jounce Therapeutics © November 2018

Development Programs JTX-2011 JTX-4014 JTX-8064 12 | Jounce Therapeutics © November 2018

JTX-2011 Mechanism From Preclinical Studies Activation of T effector cells IFN-Y IFN-Y APC APC IFN-Y IFN-Y 1st1stsignalsignalICOS TCR JTX-2011 TCR TCR ICOS ↑↑IFN-γ ICOS ICOS “Non-Primed” “Primed” T effector cell T effector cell TNK regulatory cell cell T regulatory cell Selective reduction of intratumoral T regulatory cells 13 | Jounce Therapeutics © November 2018

JTX-2011: Key Learnings from Phase 1/2 ICONIC Trial Safety • JTX-2011 safe and well tolerated alone and in combination with nivo Preliminary Efficacy • Responses in both monotherapy and in combination with nivo – Durable (PR ≥ 6 months) RECIST PRs in PD-1 inhibitor naïve patients – Tumor reductions in PD-1 inhibitor failures • Tumor activity associated with mechanism, emergence of ICOS PD biomarker Patient Characteristics • Heavily pre-treated patients; high early discontinuation rate • Late lines of therapy more challenging to treat with IO therapy 14 | Jounce Therapeutics © November 2018

Importance of ICOS hi CD4 T Cells Clinical Trial Patient Samples Drive Preclinical Data / Hypothesis Next Steps Tumor Reverse translational work from responders versus non-responders • ICONIC patient enrichment: • ICOS hi CD4 T cells emerged in the blood in – Patients screened for ICOS(+) immune cells in all ICONIC patients with >30% target lesion the tumors by IHC reduction – ICOS IHC score does not measure levels of • Blood analysis allows discrimination of ICOS ICOS per cell or T cell subsets levels of all individual cells • Analysis of blood ICOS hi cells enables new mechanistic and biomarker plan 15 | Jounce Therapeutics © November 2018

JTX-2011: Key Learnings from ICONIC Reverse Translational Work Mechanism • ICOS hi CD4 T cells emerge upon treatment with JTX- CTLA-4i 2011 JTX-2011 • Emergence of ICOS hi CD4 T cells is correlated to response • JTX-2011 only activates CD4 T cells if they already express high levels of ICOS Combinations • Agents that induce ICOS hi CD4 T cells may be optimal PD-1i combination partners for JTX-2011 • Anti-CTLA-4 can increase ICOS on T cells Chen, D.S., & Mellman, I. (2013). Oncology meets immunology: the cancer-immunity cycle. Immunity, 39 1, 1-10. • CTLA-4 and JTX-2011 may be an ideal combination therapy to create an immune response to cancer 16 | Jounce Therapeutics © November 2018

JTX-2011 Ongoing Studies and Next Steps Phase 1 Phase 2 Safety Preliminary Efficacy Ipilimumab Dose Escalation combo - New combination • Phase 2 combinations anticipated in 2019 - Strong scientific rationale • Multiple cohorts and regimens • Earlier lines of therapy Pembrolizumab Dose Escalation combo • New biomarkers - In line with SOC - Earlier lines of therapy Safety analysis All-comers, no enrichment for ICOS expression 17 | Jounce Therapeutics © November 2018

Development Programs JTX-2011 JTX-4014 JTX-8064 18 | Jounce Therapeutics © November 2018

JTX-4014: Anti-PD-1 Program On Track • IND filed Sept 2018 • Study May Proceed letter received Oct 2018 Well-characterized anti-PD-1 antibody Monotherapy safety and POC • Fully human IgG4 • For use in combinations with potential • Blocks binding to PD-L1 and PD-L2 future product candidates 19 | Jounce Therapeutics © November 2018

Development Programs JTX-2011 JTX-4014 JTX-8064 20 | Jounce Therapeutics © November 2018

LILRB2 Binds HLA-G and MHC I Suppressing Macrophages (M2-like) JTX-8064 Blocks the Interaction with Both Ligands Tumor LILRB2 (aka ILT4) HLA-G JTX-8064 MHC I M1-like Macrophage M2-like Macrophage 21 | Jounce Therapeutics © November 2018

LILRB2 Antibodies Reprogram Tumor Associated Macrophages Pro-Inflammatory Anti-Inflammatory • TNF • CCL2 • IL-1 • IL-10 LILRB2 antibodies promote M1-like activation LILRB2 antibodies attenuate M2-like activation Control mAb JTX-mAb-1 produced JTX-mAb-1 IL-10 produced TNF (relative alone)LPS to (relative to LPS alone) LPS to (relative Control mAb 22 | Jounce Therapeutics © September 2018 Human monocytes in M2-like immunosuppressive state treated with JTX-mAb-1

Deletion of Tumor LILRB2 Results in Reduced Growth of Macrophage- Rich Tumors Mouse expressing normal PirB target (human LILRB2) Mouse lacking PirB target (human LILRB2) MC38 tumor (macrophage staining) WT MC38 PirB MC38 3500 3500 3000 3000 2500 2500 2000 2000 1500 1500 1000 1000 500 500 Tumor Volume (mm3) Volume Tumor Tumor Volume (mm3) 0 0 0102030 0 102030 Day Day Deletion of PirB (human LILRB2) results in anti-tumor activity 23 | Jounce Therapeutics © November 2018

Jounce Immunotherapy Pipeline Development Programs Clinical Program Target Preclinical Phase 1 Phase 2 Phase 3 JTX-2011 ICOS JTX-4014 PD-1 JTX-8064 LILRB2 Research Programs Named Celgene Option Celgene Target Pool Target Rights Stromal Jounce Wholly-owned Macrophage, B-cell, T-reg Celgene Target Pool Reverse translational work leading to new targets, methods and paradigms 24 | Jounce Therapeutics © November 2018

DELIVERING VALUE PIPELINE EXECUTION APPROACH & IO OPPORTUNITY 25 | Jounce Therapeutics © November 2018

Jounce and Celgene Strategic Collaboration Co-develop and Co-commercialize Innovative Immunotherapies Collaboration Highlights • Global strategic collaboration, enabling comprehensive clinical strategy Committed to working together • Option based deal, preset opt-in fees and profit share, cost sharing after opt-in to change the course of • Jounce retains significant development, commercial and economic rights cancer − Upon opt-in, Jounce leads JTX-2011 US co-development/co-commercialization • $225M upfront, $36M equity investment, and up to $2.3B in potential milestones Profit Sharing Program US Jounce US Celgene Ex-US Jounce JTX-2011 Lead 60% 40% Royalty 1st Option post-lead 25% 75% Royalty Options 2-4 50% 50% Royalty JTX-4014 Shared Globally for Combinations with Collaboration Products 26 | Jounce Therapeutics © November 2018

Financial Strength and Flexibility Company History • NASDAQ: JNCE • Strength of balance sheet: Event Date Gross $ Comment – ~$233M cash and investments, Series A 2013 $47M Third Rock as of June 30, 2018 Ventures • Financial guidance for 2018: Series B 2015 $56M Fidelity, Wellington, others – End year with $185M to $195M in cash CELG 2016 $225M US profit sharing – Gross cash burn at the lower end of range, Collaboration upfront on optioned $80M to $100M $36M programs – Revenue of $50M to $60M (non-cash) equity • Common stock outstanding: Initial Public 2017 $117M Upsized deal Offering (gross) – 32.6M shares as of August 3, 2018 27 | Jounce Therapeutics © November 2018

2018 Milestones Report JTX-2011 preliminary clinical efficacy results in Q2 Initiate new JTX-2011 combination (anti-CTLA-4) Advance next development candidate into IND enabling activities Submit IND for JTX-4014 (anti-PD-1) 28 | Jounce Therapeutics © November 2018

Jounce Therapeutics A Next Gen Immunotherapy Company
