Attached files
| file | filename |
|---|---|
| 8-K - 8-K - Foamix Pharmaceuticals Ltd. | zk1822253.htm |
| EX-99.3 - EXHIBIT 99.3 - Foamix Pharmaceuticals Ltd. | exhibit_99-3.htm |
| EX-99.1 - EXHIBIT 99.1 - Foamix Pharmaceuticals Ltd. | exhibit_99-1.htm |
Exhibit 99.2

Nasdaq: FOMXNovember 2018

Forward-Looking Statements and Where to Find More Information 2 To the extent that statements contained in this presentation are not descriptions of historical facts regarding Foamix, they are forward-looking statements reflecting management’s current beliefs and expectations. Forward-looking statements are subject to known and unknown risks, uncertainties, and other factors that may cause our or our industry’s actual results, levels of activity, performance, or achievements to be materially different from those anticipated by such statements. In some cases, you can identify forward-looking statements by terminology such as “may,” “will,” “should,” “expects,” “plans,” “anticipates,” “believes,” “estimates,” “predicts,” “potential,” “intends,” or “continue,” or the negative of these terms or other comparable terminology. Forward-looking statements contained in this presentation include, but are not limited to, (i) statements regarding the timing of anticipated clinical trials for our product candidates; (ii) the timing of receipt of clinical data for our product candidates; (iii) our expectations regarding the potential safety, efficacy, or clinical utility of our product candidates; (iv) the size of patient populations targeted by our product candidates and market adoption of our product candidates by physicians and patients; (v) the timing or likelihood of regulatory filings and approvals; and (vi) our revenues under our agreements with our licensees, including LEO Pharma and other companies. Although we believe that the expectations reflected in the forward-looking statements are reasonable, various factors may cause differences between our expectations and actual results, including, but not limited to, unexpected safety or efficacy data, unexpected side effects observed during preclinical studies or in clinical trials, lower than expected enrollment rates in clinical trials, changes in expected or existing competition, changes in the regulatory environment for our product candidates and our need for future capital, the inability to protect our intellectual property, and the risk that we become a party to unexpected litigation or other disputes. Except as required by law, we assume no obligation to update these forward-looking statements publicly, or to update the reasons why actual results could differ materially from those anticipated in the forward-looking statements, even if new information becomes available in the future. You should read the documents filed by Foamix with the SEC, including our most recent annual and quarterly reports and prospectuses, including in each case the Risk Factors set forth therein. You may obtain those documents by visiting EDGAR on the SEC website at www.sec.gov or on our website at www.foamix.com under the heading “Investors.” Foamix uses its website (www.foamix.com) as a channel to distribute information about Foamix and its product candidates. Foamix encourages investors to visit our website from time to time, as information is updated and new information is posted, in addition to following our press releases, SEC filings, public conference calls, and webcasts.The trademarks included herein are the property of the owners thereof and are used for reference purposes only. Such use should not be construed as an endorsement of the foam technology or product candidates of Foamix.
FMX103FX2016-11 & FX2016-12Top Line Results Topical Minocycline Foam 1.5%For Moderate-to-Severe Papulopustular Rosacea 3

FMX103 Phase 3 Program Design Studies FX2016-11, FX2016-12 & FX2016-13 4 12-week, randomized, double-blind, vehicle controlled, in subjects with moderate-to-severe papulopustular rosacea; followed by up to an additional 40 weeks open label safety extension Week 12(End of treatment) Week 52 Week 4 Week 8 Double-blind Studies(-11,-12)Randomized (2:1), N=750 (X2) Minocycline Foam 1.5%Foam vehicle Minocycline foam 1.5% – 9 months of treatment Open Label Safety Extension Study (-13) Subjects who complete one of the randomized, Phase 3 studies may enter the open-label study 2 US Double-blinded Studies, 100 sites, 1522 enrolled subjects, >18 years of ageSelf-apply, once daily, for 12 weeksInclusion Criteria15 to 75 inflammatory lesions IGA 5 point scale – Moderate to Severe (Grade 3 or 4)Co-primary Efficacy Endpoints:Mean change from Baseline in inflammatory lesion countProportion of subjects with IGA scores of “Clear” or “Almost Clear”, with improvement of at least 2 grades from BaselineSafety Evaluations: AEs, physical exams, vitals, dermal tolerability, labs BL
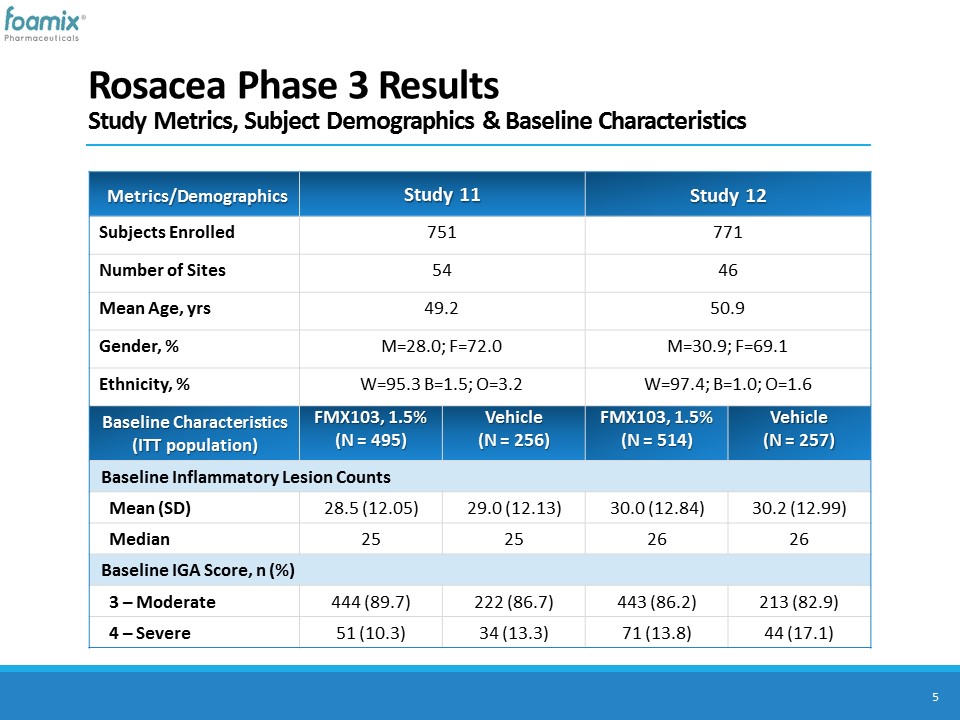
Rosacea Phase 3 ResultsStudy Metrics, Subject Demographics & Baseline Characteristics 5 Metrics/Demographics Study 11 Study 12 Subjects Enrolled 751 771 Number of Sites 54 46 Mean Age, yrs 49.2 50.9 Gender, % M=28.0; F=72.0 M=30.9; F=69.1 Ethnicity, % W=95.3 B=1.5; O=3.2 W=97.4; B=1.0; O=1.6 Baseline Characteristics (ITT population) FMX103, 1.5%(N = 495) Vehicle(N = 256) FMX103, 1.5%(N = 514) Vehicle(N = 257) Baseline Inflammatory Lesion Counts Mean (SD) 28.5 (12.05) 29.0 (12.13) 30.0 (12.84) 30.2 (12.99) Median 25 25 26 26 Baseline IGA Score, n (%) 3 – Moderate 444 (89.7) 222 (86.7) 443 (86.2) 213 (82.9) 4 – Severe 51 (10.3) 34 (13.3) 71 (13.8) 44 (17.1)

Rosacea Phase 3 Results - EfficacyCo-primary endpoint: Absolute Change of Inflammatory Lesion Count at Week 12 6 In Study 11, absolute change in inflammatory lesion count for the FMX103, 1.5% treatment group was –17.57 versus –15.65 in vehicle treatment group (p=0.0031)In Study 12, absolute change in inflammatory lesion count for the FMX103, 1.5% treatment group was –18.54 versus –14.88 in vehicle treatment group (p<0.0001) *ANCOVA, ITT population, multiple imputation P<0.005 P<0.0001 N=XXX N=XXX N=XXX N=XXX N=495 N=256 N=514 N=257

Rosacea Phase 3 Results - EfficacyCo-primary endpoint: IGA Treatment Success at Week 12 [Score Clear (0) or Almost Clear (1)] In Study 11, IGA Treatment Success for FMX103, 1.5% treatment group was 52.1% versus 43.0% in vehicle treatment group (p=0.027)In Study 12, IGA Treatment Success for FMX103, 1.5% treatment group was 49.1% versus 39.0% in vehicle treatment group (p=0.0077) *Cochran–Mantel–Haenszel test stratified by analysis center, ITT population, multiple imputation 7 P<0.05 P<0.01 N=738 N=750 N=333 N=162 N=495 N=256 N=514 N=257

Rosacea Phase 3 Results - EfficacyKey secondary endpoint: Percent Change of Inflammatory Lesion Count at Weeks 4, 8 and 12 8 ^ANCOVA, Intent to Treat (ITT) Population, Observed Cases In Study 11, percent change in inflammatory lesion count for the FMX103, 1.5% treatment group at week 12 was -64.13% versus -56.52% in vehicle (p=0.0039) In Study 12, percent change in inflammatory lesion count for the FMX103, 1.5% treatment group at week 12 was -61.45% versus -50.16% in vehicle (p<0.0001) Statistical significance demonstrated at all timepoints (beginning at Week 4) for both studies ‡P≤.005; †P ≤.001; *P ≤.0001 Study 11 Study 12 * * * † ‡ -64% -61% *
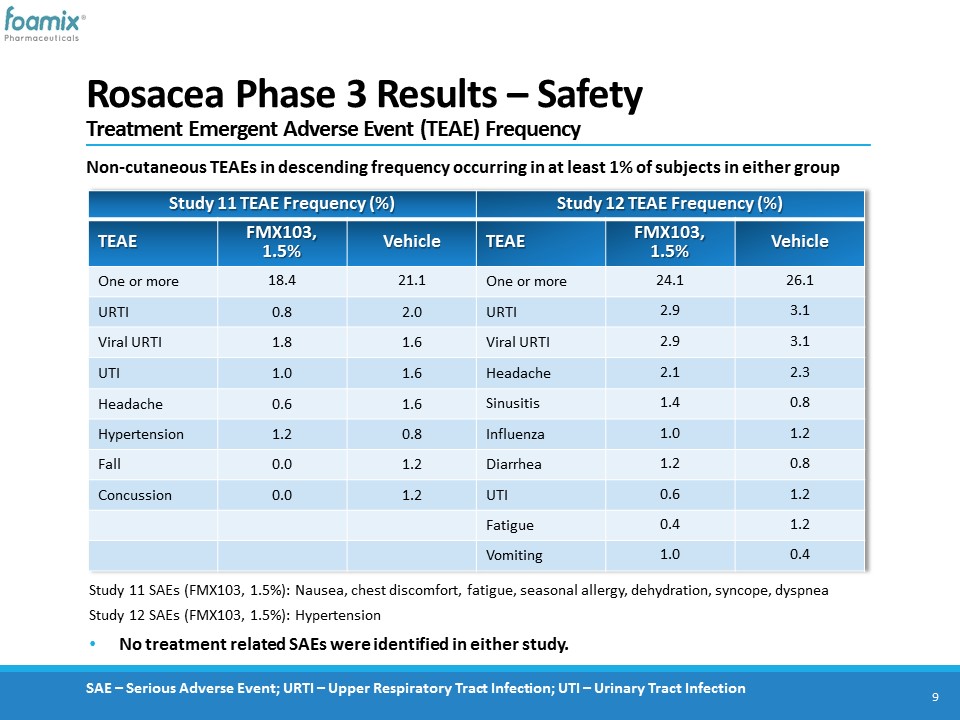
Rosacea Phase 3 Results – SafetyTreatment Emergent Adverse Event (TEAE) Frequency 9 Study 11 TEAE Frequency (%) Study 12 TEAE Frequency (%) TEAE FMX103, 1.5% Vehicle TEAE FMX103, 1.5% Vehicle One or more 18.4 21.1 One or more 24.1 26.1 URTI 0.8 2.0 URTI 2.9 3.1 Viral URTI 1.8 1.6 Viral URTI 2.9 3.1 UTI 1.0 1.6 Headache 2.1 2.3 Headache 0.6 1.6 Sinusitis 1.4 0.8 Hypertension 1.2 0.8 Influenza 1.0 1.2 Fall 0.0 1.2 Diarrhea 1.2 0.8 Concussion 0.0 1.2 UTI 0.6 1.2 Fatigue 0.4 1.2 Vomiting 1.0 0.4 Non-cutaneous TEAEs in descending frequency occurring in at least 1% of subjects in either group Study 11 SAEs (FMX103, 1.5%): Nausea, chest discomfort, fatigue, seasonal allergy, dehydration, syncope, dyspnea Study 12 SAEs (FMX103, 1.5%): Hypertension No treatment related SAEs were identified in either study. SAE – Serious Adverse Event; URTI – Upper Respiratory Tract Infection; UTI – Urinary Tract Infection

Rosacea Phase 3 Results – Safety Summary 10 The most common systemic adverse event was upper respiratory tract infections in both studies with incidence rates <3% for both FMX103 treatment groups.Treatment area cutaneous TEAEs in the FMX103 treatment groups were few; most were mild and included instances of dermatitis, rash, cyst, pain, pruritus, hyperpigmentation and actinic keratosis.No treatment-related serious adverse events were reported in either study.In total, 9 subjects discontinued in Studies 11 and 12 due to a TEAE including 7 in the FMX103 treatment groups and 2 in the vehicle treatment groups.

Rosacea Phase 3 Results – Overall Summary 11 Statistically significant disease improvement of FMX103 vs vehicle was achieved for the co-primary endpoints of absolute reduction of inflammatory lesions and IGA treatment success at Week 12 in both Studies 11 and 12.Treatment emergent adverse events were few in type and frequency; most were mild in severity.No treatment-related serious adverse events were reported.Subject discontinuations due to a TEAE were low in both studies.FMX103 was shown to have a generally favorable safety profile.Study FX2016-13 (open label safety) is currently on-going.

Milestones 12 FMX101 FMX103 Non-clinical Manufacturing scale-up (1-ton) Phase 3 efficacy Phase 3 long-term safety H1 2019 Pre-NDA meeting H1 2019 NDA Submission EOY 2018 H2 2019 PDUFA Q4 2019 H2 2020

Commercial Readiness 13 Well-capitalized to support comprehensive launch for FMX101 into 2020 Scientific message platform executionEfficacy and safety dataVehicle valueAnti-resistanceCongress & convention participationPublications Doryx® Dovonex® Duac®Enstilar®Fabior®Picato®Sorilux®Taclonex® EXPERIENCE AGENCY PARTNERSHIPS MEDICAL COMMUNICATIONS Agency Partners Selected & Outsourced Medical EducationAdvertising & PromotionMarket AccessData Analytics Execution in Progress Payer landscapePricing & Patient assistanceContractingDistribution years combined derm experience Derm product launches: REIMBURSEMENT >80 Market Conditioning& HCP Awareness

Acne Market Product Launch SurrogatesSignificant Rx Adoption Within 12 months of Commercialization 14 Sources: (1) Symphony Health Solutions PHAST; (2) WAC PRICES, MediSpan PriceRx®, Wolters Kluwer, 10/24/18 TRx per month Months since launch Average Exit TRx/Mo. = >25k Average Current WAC = $685 ~45k, $705 ~28k,$594 ~23k,$528 ~16k,$911 Valeant – Philidor Controversy

Rosacea Market Product Launch SurrogatesSignificant Rx Adoption Within 12 months of Commercialization 15 Sources: (1) Symphony Health Solutions PHAST; (2) WAC PRICES, MediSpan PriceRx®, Wolters Kluwer, 10/24/18 TRx per month Months since launch Average Exit TRx/Mo. = >25k Average Current WAC = $465 ~33k, $704 ~27k,$365 ~18k,$327

Nasdaq: FOMXNovember 2018
