Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Theravance Biopharma, Inc. | a18-39648_1ex99d1.htm |
| 8-K - 8-K - Theravance Biopharma, Inc. | a18-39648_18k.htm |
3Q 2018 Financial Results and Business Update November 6, 2018 Theravance Biopharma, Inc. (NASDAQ: TBPH)

Cautionary Statement Regarding Forward-Looking Statements Under the safe harbor provisions of the U.S. Private Securities Litigation Reform Act of 1995, the company cautions investors that any forward-looking statements or projections made by the company are subject to risks and uncertainties that may cause actual results to differ materially from the forward-looking statements or projections. Examples of forward-looking statements in this presentation include statements relating to the expected closing date for the sale of VIBATIV®, the company’s business plans and objectives, including financial and operating results, potential partnering transactions and sales targets, the company’s regulatory strategies and timing and results of clinical studies, the potential benefits and mechanisms of action of the company’s product and product candidates (including their potential as components of combination therapies). The company’s forward-looking statements are based on the estimates and assumptions of management as of the date of this presentation and are subject to risks and uncertainties that may cause the actual results to be materially different than those projected, such as risks related to delays or difficulties in commencing or completing clinical studies, the potential that results from clinical or non-clinical studies indicate product candidates are unsafe or ineffective (including when our product candidates are studied in combination with other compounds), delays or failure to achieve and maintain regulatory approvals for product candidates, risks of collaborating with third parties to discover, develop and commercialize products, risks associated with establishing and maintaining sales, marketing and distribution capabilities. Other risks affecting the company are described under the heading “Risk Factors” and elsewhere in the company’s Form 10-Q filed with the Securities and Exchange Commission (SEC) on August 2, 2018, and other periodic reports filed with the SEC.
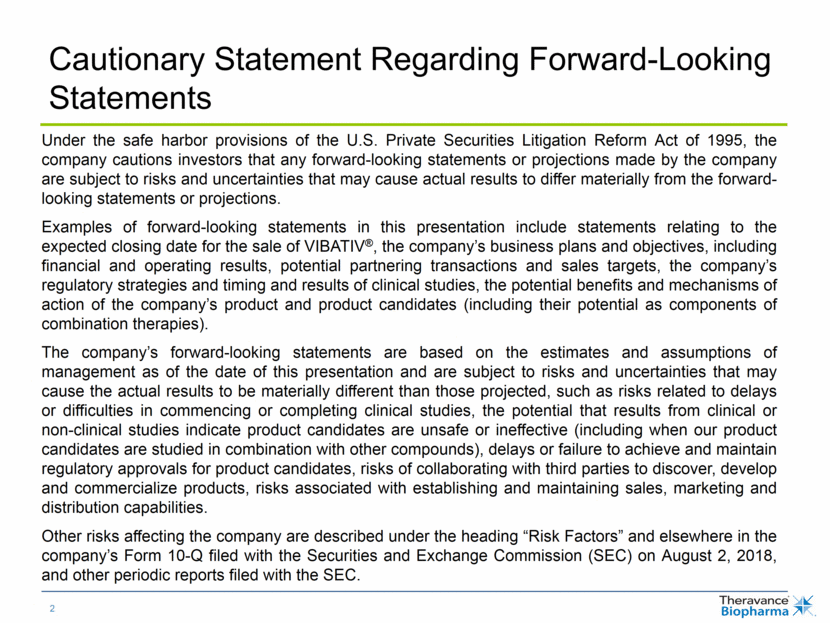
Strategic Focus in 2018 JAK = Janus kinase. NSRI = noreprenephrine serotonin reuptake inhibitor. nOH = neurogenic orthostatic hypotension. SBP = systolic blood pressure. QD = once-daily. LAMA = long-acting muscarinic antagonist. PDUFA = Prescription Drug User Fee Act. YUPELRI is the proposed brand name for revefenacin inhalation solution. 1 YUPELRITM (revefenacin) inhalation solution. 2 TBPH holds 85% economic interest in upward-tiering royalty stream of 6.5% – 10% payable by GSK (net of TRC LLC expenses paid and the amount of cash, if any, expected to be used by TRC pursuant to the TRC LLC Agreement over the next four fiscal quarters). NDA accepted by FDA and under review Assigned PDUFA date of November 13, 2018; proposed as first QD nebulized LAMA for treatment of COPD Positive top-line four-week results in nOH Initiating pivotal Phase 3 program in symptomatic nOH TD-1473 (JAKi) TD-9855 (NSRI) YUPELRITM (LAMA)1 Partnership with global leader in Immunology Initiating Phase 2 study in Crohn’s disease and pivotal Phase 2b/3 study in ulcerative colitis Commercial organization to concentrate exclusively on YUPELRI if approved Economic interest in Trelegy Ellipta serves as an important strategic asset2 Promising initial launch following approvals in US and EU in late 2017 TD-1473, TD-9855, and YUPELRI each internally discovered and developed by R&D engine which serves as important driver of long term value
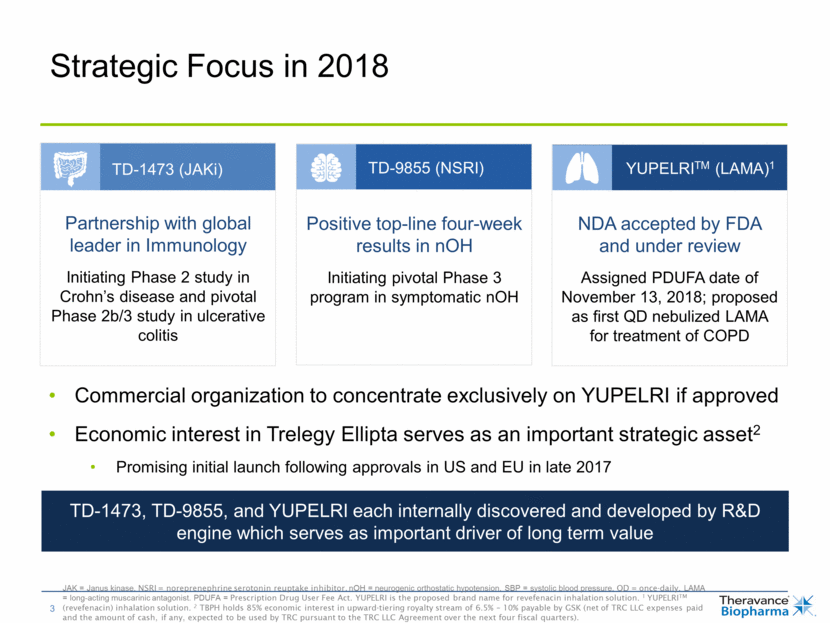
TD-9855: Phase 2 Results Support Progression Three-part proof of concept study in nOH 1 Symptomatic defined as OSHA #1 > 4. OHSA = Orthostatic Hypotension Symptom Assessment. OHSA #1 measures dizziness (cardinal symptom of nOH), lightheadedness, feeling faint, or feeling of impending black out Key Findings A SAD portion Responses reported in majority of patients treated with TD-9855 27 of 34 patients enrolled in Part A showed improvements in SBP and/or standing time B Single dose, placebo-controlled TD-9855 increased SBP from a low baseline SBP dropped on placebo during the day as expected, in response to postural changes and eating No evidence of supine hypertension with TD-9855 overnight C Repeat dose extension phase Durability of effect observed through four weeks Reductions in symptom severity, most pronounced benefit in patients with symptomatic nOH1 Consistent increases in SBP through four weeks Generally well tolerated; no serious adverse events assessed as drug-related Patients started on Part A, and responders moved to Part B and/or Part C (extension phase) Registrational Phase 3 program in symptomatic nOH in advanced planning
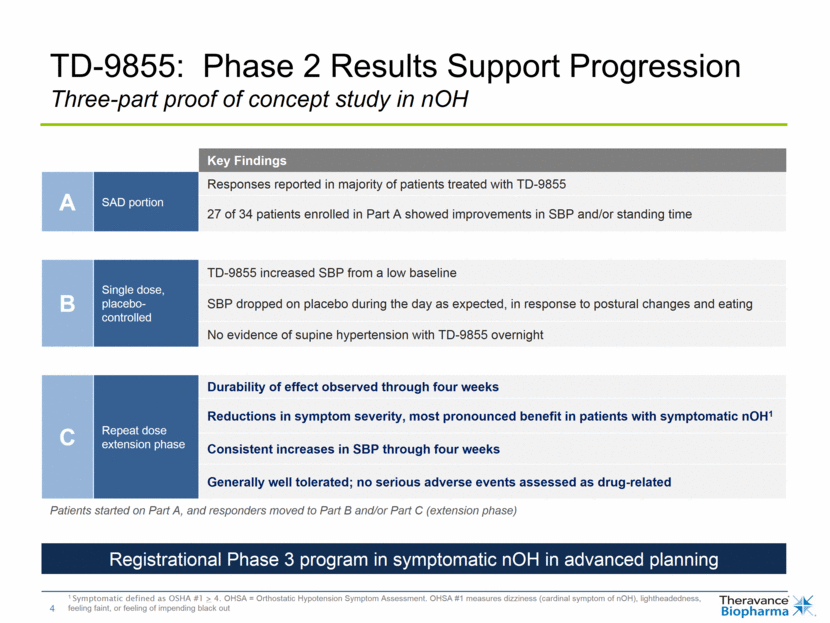
TD-1473: Encouraging Findings in Phase 1b Study 4-week treatment in 40 patients with active ulcerative colitis 1 Clinical response as measured by both partial and full Mayo 2 Surrogate biomarkers include C-reactive protein (CRP) and fecal calprotectin Key Findings Favorable overall safety and tolerability No systemic or opportunistic infections (including herpes zoster) No evidence of reduce white cell counts Minimal systemic exposure Plasma levels of TD-1473 very low Consistent in all cohorts to levels observed in healthy volunteers Biologic activity in GI tract Endoscopic improvements and mucosal healing reported in all active arms; none reported in placebo arm Rectal bleeding scores improved above placebo at highest two doses Rates of clinical response higher for all active doses compared to placebo1 Clinical responses matched by dose-dependent reductions in surrogate biomarkers2 Dose-related increases in local GI tissue drug concentrations; higher two doses produced mean concentrations above the JAK IC50 Detailed results presented in oral late-breaker at UEGW 2018; progressing into Phase 2 in Crohn’s disease and Phase 2b/3 in UC
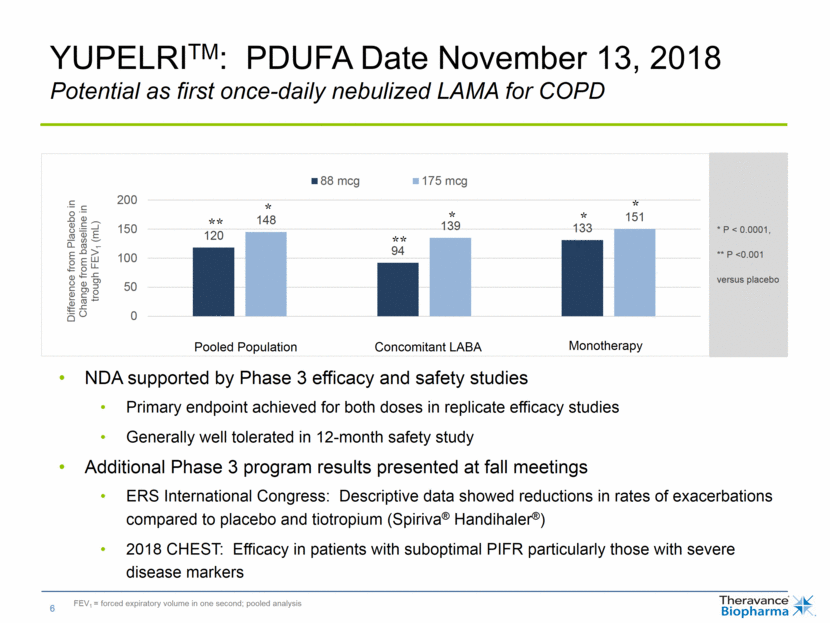
NDA supported by Phase 3 efficacy and safety studies Primary endpoint achieved for both doses in replicate efficacy studies Generally well tolerated in 12-month safety study Additional Phase 3 program results presented at fall meetings ERS International Congress: Descriptive data showed reductions in rates of exacerbations compared to placebo and tiotropium (Spiriva® Handihaler®) 2018 CHEST: Efficacy in patients with suboptimal PIFR particularly those with severe disease markers YUPELRITM: PDUFA Date November 13, 2018 Potential as first once-daily nebulized LAMA for COPD FEV1 = forced expiratory volume in one second; pooled analysis * ** * * * ** Monotherapy Concomitant LABA Pooled Population * P < 0.0001, ** P <0.001 versus placebo
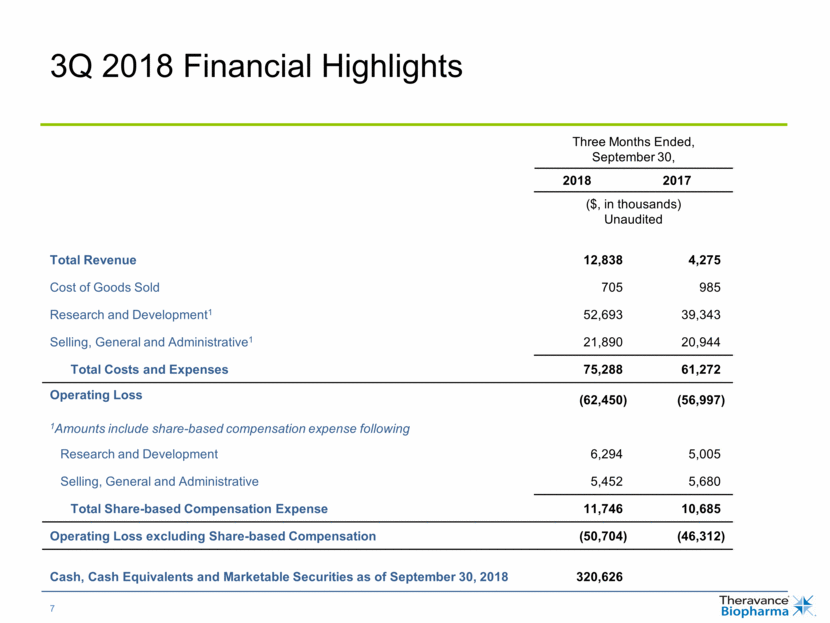
3Q 2018 Financial Highlights Three Months Ended, September 30, 2018 2017 ($, in thousands) Unaudited Total Revenue 12,838 4,275 Cost of Goods Sold 705 985 Research and Development1 52,693 39,343 Selling, General and Administrative1 21,890 20,944 Total Costs and Expenses 75,288 61,272 Operating Loss (62,450) (56,997) 1Amounts include share-based compensation expense following Research and Development 6,294 5,005 Selling, General and Administrative 5,452 5,680 Total Share-based Compensation Expense 11,746 10,685 Operating Loss excluding Share-based Compensation (50,704) (46,312) Cash, Cash Equivalents and Marketable Securities as of September 30, 2018 320,626
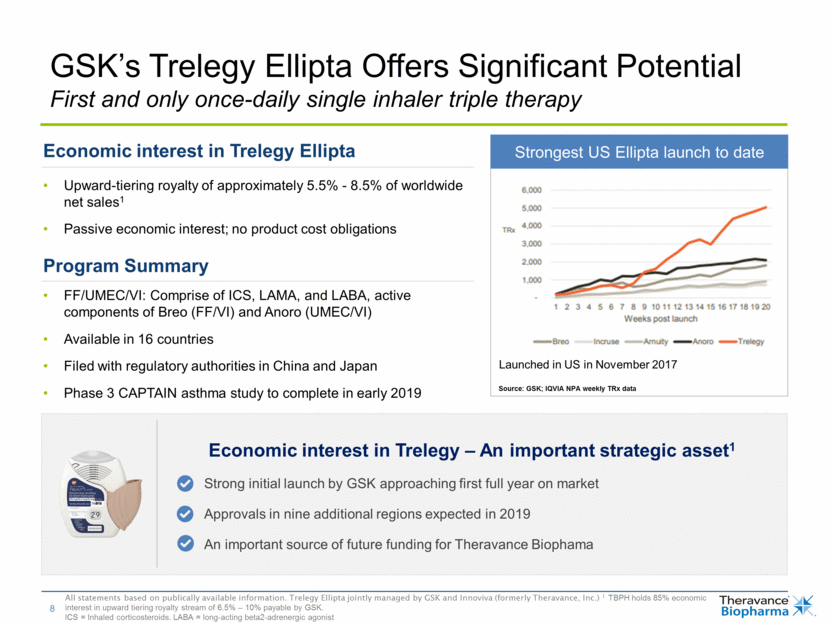
Economic interest in Trelegy Ellipta Upward-tiering royalty of approximately 5.5% - 8.5% of worldwide net sales1 Passive economic interest; no product cost obligations Program Summary FF/UMEC/VI: Comprise of ICS, LAMA, and LABA, active components of Breo (FF/VI) and Anoro (UMEC/VI) Available in 16 countries Filed with regulatory authorities in China and Japan Phase 3 CAPTAIN asthma study to complete in early 2019 GSK’s Trelegy Ellipta Offers Significant Potential First and only once-daily single inhaler triple therapy All statements based on publically available information. Trelegy Ellipta jointly managed by GSK and Innoviva (formerly Theravance, Inc.) 1 TBPH holds 85% economic interest in upward tiering royalty stream of 6.5% – 10% payable by GSK. ICS = Inhaled corticosteroids. LABA = long-acting beta2-adrenergic agonist So Source: GSK; IQVIA NPA weekly TRx data Strongest US Ellipta launch to date Launched in US in November 2017 Economic interest in Trelegy – An important strategic asset1 Strong initial launch by GSK approaching first full year on market Approvals in nine additional regions expected in 2019 An important source of future funding for Theravance Biophama
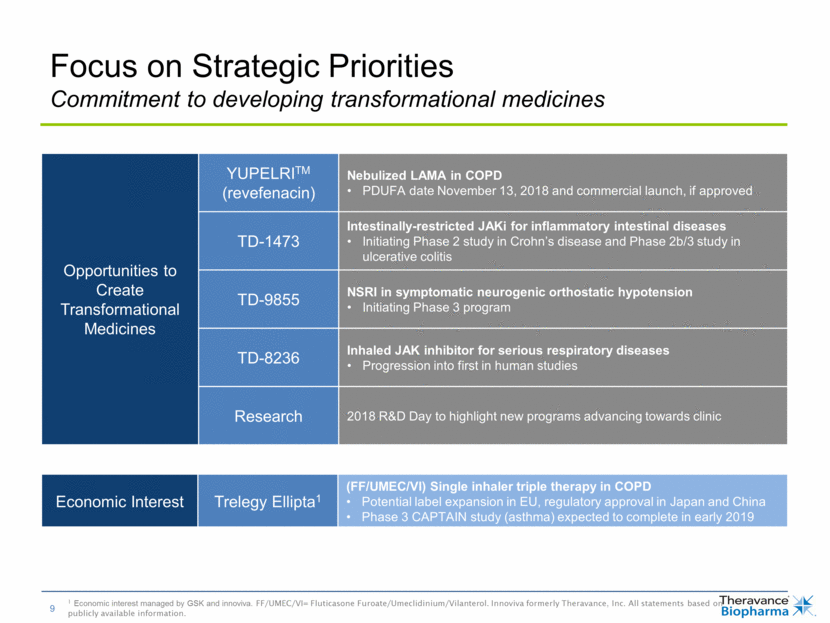
Opportunities to Create Transformational Medicines YUPELRITM (revefenacin) Nebulized LAMA in COPD PDUFA date November 13, 2018 and commercial launch, if approved TD-1473 Intestinally-restricted JAKi for inflammatory intestinal diseases Initiating Phase 2 study in Crohn’s disease and Phase 2b/3 study in ulcerative colitis TD-9855 NSRI in symptomatic neurogenic orthostatic hypotension Initiating Phase 3 program TD-8236 Inhaled JAK inhibitor for serious respiratory diseases Progression into first in human studies Research 2018 R&D Day to highlight new programs advancing towards clinic Focus on Strategic Priorities Commitment to developing transformational medicines 1 Economic interest managed by GSK and innoviva. FF/UMEC/VI= Fluticasone Furoate/Umeclidinium/Vilanterol. Innoviva formerly Theravance, Inc. All statements based on publicly available information. Managed by GSK and Innoviva1 Economic Interest Trelegy Ellipta1 (FF/UMEC/VI) Single inhaler triple therapy in COPD Potential label expansion in EU, regulatory approval in Japan and China Phase 3 CAPTAIN study (asthma) expected to complete in early 2019
Q&A Thank you

