Attached files
| file | filename |
|---|---|
| EX-99.1 - EXHIBIT 99.1 - AMAG PHARMACEUTICALS, INC. | a3q2018ex991.htm |
| 8-K - 8-K - AMAG PHARMACEUTICALS, INC. | amag3q20188-k.htm |

Q3-2018 Financial Results November 1, 2018 © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 1

Forward-Looking Statements This presentation contains forward‐looking statements within the meaning of the Private Securities Litigation Reform Act of 1995 (PSLRA) and other federal securities laws. Any statements contained herein which do not describe historical facts, including, among others, expectations for Feraheme pricing; beliefs about Feraheme’s resiliency to INFeD®; beliefs about the conversion from the Makena IM to SC auto-injector; plans to protect the Makena brand by continued conversion to the SC auto-injector and patient access; expectations about supply of Makena, including when additional supply will be available; beliefs about generic economics; beliefs about Intrarosa market share; beliefs about Intrarosa media coverage and marketing initiatives; expectations for 2018 financial guidance, including revenues, operating loss and adjusted EBITDA; expectations that the fourth quarter will be a period of continued investment to drive future value; AMAG’s key themes for 2019, including product revenue growth, a decline in Makena IM revenues, the planned launch of Vylessi (if approved), increased investments in AMAG-423, Vyleesi and Intrarosa and capital allocations from AMAG’s balance sheet to fuel its investments; beliefs that AMAG’s balance sheet is strengthening; beliefs about shareholder value and AMAG’s portfolio; beliefs about annual peak revenue opportunities for AMAG-423, Vyleesi and Intrarosa; beliefs about preeclampsia, the market for, and the anticipated timeline for the FDA’s Advisory Committee meeting, launch of, AMAG-423 (if approved); beliefs about the Vyleesi Phase 3 studies, including favorable safety profile; beliefs about the market for, and the anticipated timeline for launch of, Vyleesi (if approved); beliefs about the market for Intrarosa; beliefs that the AMAG portfolio is innovative and plans to deliver multiple value drivers; the anticipated regulatory timeline for AMAG’s products and product candidates; and plans to undertake additional licensing and acquisition transactions and the expected areas of such expansion are based on management’s current expectations and beliefs and are forward‐looking statements which involve risks and uncertainties that could cause actual results to differ materially from those discussed in such forward‐looking statements. Such risks and uncertainties include, among others, the risk that sales of Makena will continue to be negatively impacted by the supply disruption and recent and future generic entries in the market; the risk that AMAG may be unable to gain approval of its product candidates, including Vyleesi and AMAG-423, on a timely basis, or at all; the potential for such approvals, if obtained, to include unanticipated restrictions or warnings and the risk that the costs and time investments for AMAG’s development efforts will be higher than anticipated, or that AMAG has over-estimated the market and potential revenues for its products and product candidates, if approved, including AMAG’s beliefs about annual peak sales for AMAG-423, Vyleesi and Intrarosa, as well as those risks identified in AMAG’s filings with the U.S. Securities and Exchange Commission (the “SEC”), including its Annual Report on Form 10‐K for the year ended December 31, 2017 and subsequent filings with the SEC, which are available at the SEC’s website at www.sec.gov. Any such risks and uncertainties could materially and adversely affect AMAG’s results of operations, its profitability and its cash flows, which would, in turn, have a significant and adverse impact on AMAG’s stock price. AMAG cautions you not to place undue reliance on any forward‐looking statements, which speak only as of the date they are made AMAG disclaims any obligation to publicly update or revise any such statements to reflect any change in expectations or in events, conditions or circumstances on which any such statements may be based, or that may affect the likelihood that actual results will differ from those set forth in the forward‐looking statements. AMAG Pharmaceuticals® and Feraheme® are registered trademark of AMAG Pharmaceuticals, Inc. VyleesiTM is a trademark of AMAG Pharmaceuticals, Inc. MuGard® is a registered trademark of Abeona Therapeutics, Inc. Makena® is a registered trademark of AMAG Pharma USA, Inc. Intrarosa® is a registered trademark of Endoceutics, Inc. Other trademarks referred in this report are the property of their respective owners. Further, the endnotes included at the end of this presentation include information that is critical to an investor’s understanding of the materials presented and we strongly encourage investors to read such information carefully and in its entirety. © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 2

Agenda 1 Q3-2018 Highlights 2 Commercial Product Portfolio Update 3 Q3-2018 Financial Overview 4 Value Drivers 5 Q&A © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 3

Third Quarter 2018 Highlights and Recent Developments 42% Feraheme revenue growth year over year Completed the sale of CBR • Underscores focus on development and commercialization of pharmaceutical products Makena brand revenue from subcutaneous (SC) $475M high yield notes paid-off 57% auto-injector • Eliminated ~$40M/year cash interest expense • Significant de-levering event ~$430M cash and $21M short-term debt Exceeded campaign benchmark metrics with condition awareness campaign featuring actress Cheryl Hines and branded Intrarosa campaign AMAG-423 © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 4

Feraheme Makena Intrarosa 5

Feraheme Continues to Grow with Expanded Label Feraheme Revenue1 ($M) Feraheme Growth $37.0 • Q3-2018 vs. Q3-2017 – Volume increased by 31% – Market share rose from 12.3% to 15.8%2 42% $26.1 – Performance based contracting supports increased net price by 11% • Launched OB/GYN IV iron pilot program utilizing Maternal Health sales team • Continued account conversion demonstrates resiliency to INFeD® resupply (especially in hem/onc segment) Q3-2017 Q3-2018 IV IRON DEFICIENCY ANEMIA: FERAHEME © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 6

Feraheme Continues to Grow with Expanded Label Feraheme volume demand grew versus Q2-2018, despite INFeD return IV Iron Deficiency Anemia (IDA) Quarterly Market Share by Product3 Q3 INFeD Return to Market Impact4 Ferrlecit / Q3-2018 Q3 vs. Q2 INFeD Feraheme Injectafer Venofer Total Nulecit 5.9% Market Share Change +5 pp 0 pp -1 pp -3 pp -1 pp -- All 10.7% Channels +10,860 Volume Change +18,129 +318 -823 -4,686 -2,078 +3.3% 34.2% 15.8% Market Share Change +5 pp 0 pp -4 pp -1 pp 0 pp -- Hem/Onc +1,432 Volume Change +4,658 +500 -3,123 -400 -203 33.4% +1.6% Feraheme Injectafer® Venofer® INFeD® Ferrlecit®/Nulecit IV IRON DEFICIENCY ANEMIA: FERAHEME © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 7

Feraheme Makena Intrarosa 8

Continued Strong Conversion from Makena IM to SC Auto-injector 57% of Q3-2018 Makena brand revenue from subcutaneous auto-injector Makena Revenue ($M) Q3-2018 Results $105.2 • Continued strong conversion from IM to SC auto-injector $97.6 – 57% of brand revenue from SC auto-injector $80.2 • First generic to Makena single-dose IM entered market in July 2018 • Authorized generic (AGx) partner (Prasco) launched immediately following availability of first generic – Launched single- and multi-dose IM – Immediately gained >75% of generic market share5 • Managing supply issues with branded IM products – AGx IM available – Branded single-dose IM vials currently out of stock Q3-2017 Q2-2018 Q3-2018 Makena SC Makena IM AGx IM MATERNAL HEALTH: MAKENA © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 9

Optimize Makena Franchise Performance & Sustainability Protect the Brand Participate in Generic Market • Continue to convert usage to Makena SC auto-injector • Partnered with Prasco, an experienced – Promote the benefits of the SC auto-injector authorized generic company – Brand loyalty: 56% of Q3 scripts through Makena Care • Captured significant majority of generic Connection® state “dispense as written” vs. 40% in Q2 market share in Q3 • Protect patient access to Makena SC auto-injector • Attractive economics to AMAG – Reinforce value of Makena Care Connection – Proactive, targeted payer strategy MATERNAL HEALTH: MAKENA © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 10

Feraheme Makena Intrarosa 11

Intrarosa HCP Prescribing and Market Share Continue to Increase 6 Intrarosa Rx Volume As of September 30, 2018 (data reported as of last week of each month) 5,000 • >146,000 TRx’s written since launch7 4,500 – Increase of 53,000 since June 30 4,000 • 11,300 HCP prescribers since launch7 3,500 – Increase of 2,200 prescribers since June 30 3,000 2,500 • 83% commercial lives covered8 2,000 7 Number Number of Scripts • Continued growth in market share 1,500 – 4.5% TRX weekly share (overall) 1,000 – 6.3% TRx weekly share (commercial only) 500 0 Jul-17 Jul-18 Jan-18 Jun-18 Oct-17 Apr-18 Sep-17 Feb-18 Sep-18 Dec-17 Aug-18 Aug-17 Nov-17 Mar-18 May-18 TRx NRx WOMEN’S HEALTH: INTRAROSA © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 12

September Campaigns Kick Started Integrated Direct-to-Consumer Program Condition Awareness Campaign Branded Campaign Patient Consults MD Cheryl Hines, Emmy®-nominated actress Office visit or on-line 360M+ impressions 650K unique visitors to site 10M+ women reached in Sept 40% ahead of benchmark 2x benchmark 30% ahead of benchmark Garnered coverage in top Robust digital campaign generating Learnings from condition awareness consumer media - The View, engagement significantly above campaign leveraged for strong start to People.com, Parade, US industry average branded campaign Weekly, Healthline and more 5x increase in non-paid site traffic 30% increase in Intrarosa site traffic © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 13

Financial Overview 14

Revenue, Operating Loss and Adjusted EBITDA ($M)* Q3-2017 Q3-2018 $124.3 $0.4 $122.2 $4.9 • Makena SC auto-injector revenue of $40M $97.6 $80.2 • Feraheme revenue growth of 42% $45.1 • Q3-2017 operating loss includes $319M 45.1 $30.0 impairment charge and $50M expense reversal 30 $26.3 $37.1 • Q3-2018 operating loss includes IPR&D of $12.5M for acquisition of AMAG-423 ($19.3) • $30M of adjusted EBITDA with ($254.2) significant investments in SG&A Feraheme/MuGard Revenue Makena Revenue Intrarosa Revenue GAAP Operating Loss Non-GAAP Adjusted EBITDA * See slide 26 for a reconciliation of GAAP to non-GAAP financial results. FINANCIAL OVERVIEW © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 15
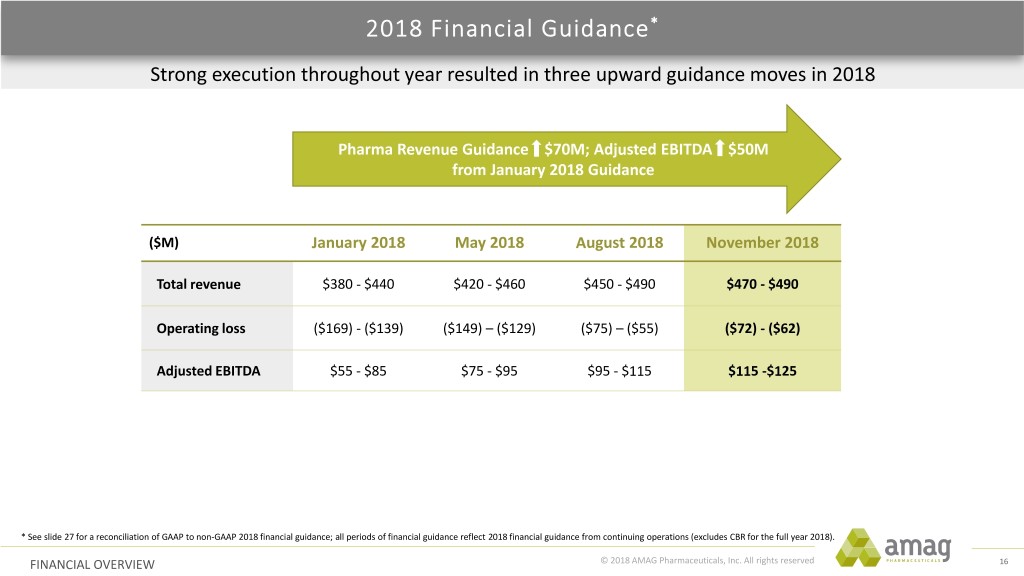
2018 Financial Guidance* Strong execution throughout year resulted in three upward guidance moves in 2018 Pharma Revenue Guidance $70M; Adjusted EBITDA $50M from January 2018 Guidance ($M) January 2018 May 2018 August 2018 November 2018 Total revenue $380 - $440 $420 - $460 $450 - $490 $470 - $490 Operating loss ($169) - ($139) ($149) – ($129) ($75) – ($55) ($72) - ($62) Adjusted EBITDA $55 - $85 $75 - $95 $95 - $115 $115 -$125 * See slide 27 for a reconciliation of GAAP to non-GAAP 2018 financial guidance; all periods of financial guidance reflect 2018 financial guidance from continuing operations (excludes CBR for the full year 2018). FINANCIAL OVERVIEW © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 16
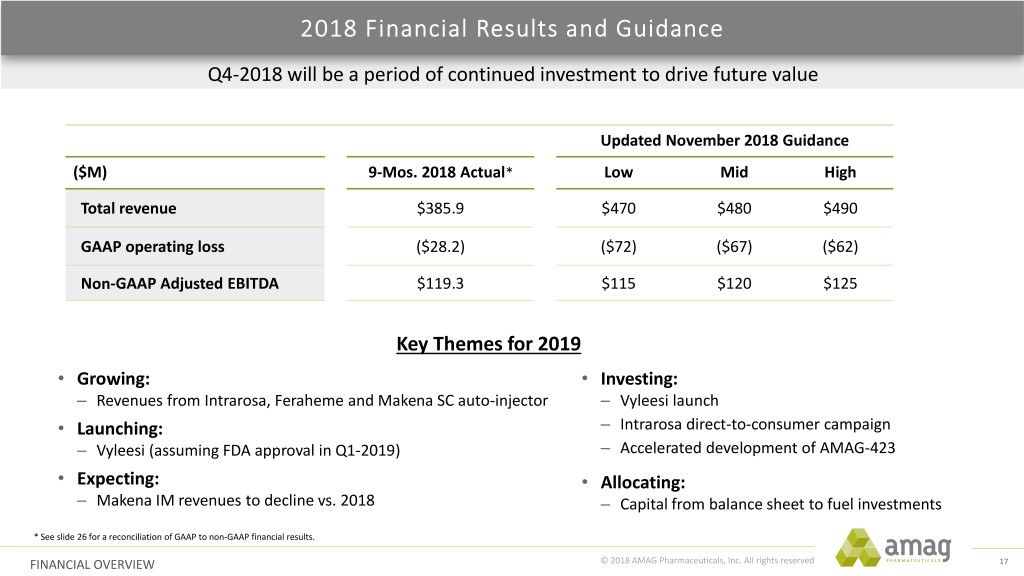
2018 Financial Results and Guidance Q4-2018 will be a period of continued investment to drive future value Updated November 2018 Guidance ($M) 9-Mos. 2018 Actual* Low Mid High Total revenue $385.9 $470 $480 $490 GAAP operating loss ($28.2) ($72) ($67) ($62) Non-GAAP Adjusted EBITDA $119.3 $115 $120 $125 Key Themes for 2019 • Growing: • Investing: – Revenues from Intrarosa, Feraheme and Makena SC auto-injector – Vyleesi launch • Launching: – Intrarosa direct-to-consumer campaign – Vyleesi (assuming FDA approval in Q1-2019) – Accelerated development of AMAG-423 • Expecting: • Allocating: – Makena IM revenues to decline vs. 2018 – Capital from balance sheet to fuel investments * See slide 26 for a reconciliation of GAAP to non-GAAP financial results. FINANCIAL OVERVIEW © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 17

Strengthening Balance Sheet ($M) 9/30/18 12/31/17 Cash, cash equivalents and investments $428 $3299 Short-term debt: Convertible senior notes (2.5%) due 2019 $ 21 $ 21 Long-term debt: Convertible senior notes (3.25%) due 2022 $320 $320 Senior notes (7.875%) due 2023 $ 0 $475 FINANCIAL OVERVIEW © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 18

Significant Shareholder Value at AMAG Historical Value Drivers Fund Future Value Drivers AMAG-423 AMAG-423 ™ Vyleesi™ Vyleesi HISTORICAL Value at AMAG FUTURE (≥2020) Value at AMAG © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 19

Significant Unmet Medical Need with Global Commercial Opportunity AMAG-423 in development for the treatment of severe preeclampsia • Preeclampsia is the leading cause of:10 – Maternal morbidity and mortality – Adverse neonatal outcomes • No effective treatments for preeclampsia Annual U.S. incidence of preeclampsia: – Only “treatment” is delivery of the baby, often times very ~140,000 pregnant women15 preterm11 • FDA granted AMAG-423 orphan status (7-years Annual U.S. incidence of severe preeclampsia: exclusivity expected at approval) and fast track review ~50,000 pregnant women15,16 • Significant $2.2 billion annual burden to U.S. healthcare system12 Annual U.S. peak • Ex-U.S. estimated incidence of severe preeclampsia: revenue opportunity ~1.6M pregnant women13 – Developing countries incidence of preeclampsia is 7x 17 higher than in developed countries14 >$1B + • 2021 commercial launch, if approved MATERNAL HEALTH: AMAG-423 © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 20

Significant Unmet Medical Need for Millions of Women Vyleesi: investigational product for premenopausal women with HSDD* • Medical condition of low sexual desire/libido associated with distress • Novel mechanism of action – Melanocortin receptor agonist (MCR4) 12 million U.S. women with HSDD18 • Used in anticipation of sexual activity – Self-administered auto-injector pen 5.8 million U.S. premenopausal women with HSDD19 • Two Large Phase 3 studies (1 in 10 premenopausal women)20,21 – Met co-primary, pre-specified endpoints • Improvement in desire 99% (5.7M) of premenopausal • Reduction in distress women not on therapy19 – Favorable safety profile • Regulatory/Launch timeline Annual U.S. peak revenue opportunity – January 2019 FDA Advisory Committee – March 23, 2019 PDUFA Action date 17 – Q2-2019 commercial launch, if approved >$700M * HSDD: Hypoactive Sexual Desire Disorder WOMEN’S HEALTH: VYLEESI © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 21

Significant Unmet Medical Need in Large Underserved Market Intrarosa: First-in-class therapy to treat moderate to severe dyspareunia • Dyspareunia – Common symptom of VVA* in post- menopausal women • Only FDA approved locally administered non-estrogen therapy22 Annual U.S. incidence of dyspareunia, a symptom of VVA • Differentiated mechanism of action22 20 million women23 – Intrarosa converts locally into active androgens and estrogens to help restore U.S. women with dyspareunia not on Rx therapy vaginal tissue 18 million women (90%)23 • Unique safety profile – No boxed warning and no limitation on duration of use #1 reason affected patients not on Rx therapy: – Estrogen therapies contain a boxed warning Don’t want estrogen24 about: • Increased risk of cancer Annual U.S. peak • Increased risk of cardiovascular disease revenue opportunity • Probable dementia • Launched July 2017 >$500M 17 * VVA: vulvar vaginal atrophy WOMEN’S HEALTH: INTRAROSA © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 22

Innovative Pipeline Delivering Multiple Value Drivers Approved/ PHASE 1 PHASE 2 PHASE 3 Regulatory Review Marketed AMAG-423 Digoxin Immune Treatment of severe preeclampsia 2020 2021 Fab (ovine) Treatment of low desire or libido with PDUFA TM AdCom: January 2019 Vyleesi associated distress in premenopausal 03/23/19 women Treatment for moderate to severe dyspareunia (pain during sex) in postmenopausal women Data 2020 2021 HSDD* Indication 1H-2019 Treatment of iron deficiency anemia Treatment to reduce recurrent preterm birth in certain at-risk women Additional Women’s healthcare, hematology and licensing & adjacencies acquisitions * HSDD: Hypoactive Sexual Desire Disorder © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 23

Q&A © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 24

Appendix 25

Reconciliation of GAAP to Non-GAAP Financial Results ($M) Q3-2017 Q3-2018 YTD-2018 GAAP operating loss ($254.2) ($19.3) ($28.2) Depreciation and intangible asset amortization 24.0 31.4 146.0 Non-cash inventory step-up adjustments 0.4 0.4 3.6 Stock-based compensation 5.6 5.0 14.6 Adjustments to contingent consideration (49.9) -- (49.2) Impairment charges of intangible assets 319.2 -- -- Acquired IPR&D -- 12.5 32.5 Non-GAAP adjusted EBITDA $45.1 $30.0 $119.3 APPENDIX © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 26

Reconciliation of GAAP to Non-GAAP 2018 Financial Guidance Financial guidance issued throughout 201825 Updated ($M) January 2018 May 2018 August 2018 November 2018 GAAP operating loss ($169) – ($139) ($149) – ($129) ($75) - ($55) ($72) - ($62) Depreciation & intangible asset amortization 179 179 174 177 Stock-based compensation 21 21 21 22 Non-cash inventory step up and 4 4 (45) (45) adjustments to contingent consideration Acquired IPR&D 20 20 20 33 Non-GAAP adjusted EBITDA $55 - $85 $75 - $95 $95 - $115 $115 - $125 APPENDIX © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 27

Endnotes 1. Represents Feraheme revenue only. Excludes MuGard revenue as reported on financial statements. 2. Feraheme market share based on IQVIA data and internal analytics. 3. Average quarterly market share of IV iron products based on IQVIA data. 4. Market share and volume data of IV iron products based on IQVIA data. 5. Prasco market share based on Symphony data. 6. Intrarosa Rx volume based on weekly IQVIA data as reported in the last week of each month. 7. Intrarosa TRx’s, HCP prescribers and market share based on IQVIA data. 8. MMIT data for commercial lives, unrestricted and adjusted for non-disadvantaged plans under contract. 9. Includes cash of $29.3 million previously held in a CBR account, which was reported in ‘assets held for sale’ at December 31, 2017. These funds were returned to AMAG upon close of the transaction in August 2018. 10. Task Force Report “Hypertension in Pregnancy,” issued by ACOG (November 2013). 11. Agunanne E et al, Marinobufagenin Levels in Preeclamptic Patients: A Preliminary Report. American Journal of Perinatology/Volume 28, Number 7, 2011, p 509. 12. Stevens W et al, Short-term costs of preeclampsia to the U.S. health care system. American Journal of Obstetrics & Gynecology. September 2017, Volume 217, Issue 3, pp237-248.e16. 13. Society for Maternal Fetal Medicine Clinical Opinion: Evaluation and management of severe preeclampsia before 34 weeks’ gestation. SMFS Publications Committee, with the assistance of Baha M. Sibai. AJOG 2011; AMAG internal analytics. 14. Preeclampsia Foundation, “Preeclampsia and Maternal Mortality: a Global Burden,” www.preeclampsia.org, accessed 10/31/18. 15. Ananth, C. V., Keyes, K. M., & Wapner, R. J. (2013). Pre-eclampsia rates in the United States, 1980-2010: age-period-cohort analysis. The BMJ, 347, f6564. http://doi.org/10.1136/bmj.f6564. 16. AMAG Phase 2b/3a clinical trial population is a subset of the severe preeclampsia population. 17. Annual U.S. peak revenue opportunity is not guidance, but instead represents what the company believes to be AMAG's peak revenue opportunity based on internal estimates, including early market research conducted for each product. 18. Shifren et al, Sexual Problems and Distress in United States Women; Obstetrics & Gynecology, Vol. 112, No. 5, November 2008; 2014 U.S. Census data. 19. Patient & Economic Flow Study sponsored by Palatin Technologies, Inc. and conducted by Burke Inc., April 2016. 20. Shifren JL, Monz BU, Russo PA, Segreti A, Johannes CB. Sexual problems and distress in United States women: prevalence and correlates. Obstet Gynecol. 2008;112(5):970–978. 21. Goldstein I, Kim NN, Clayton AH, et al. Hypoactive sexual desire disorder: International Society for the Study of Women’s Sexual Health (ISSWSH) expert consensus panel review. Mayo Clin Proc. 2017;92(1):114‐128. 22. Intrarosa is converted by enzymes in the body into androgens and estrogens, though the mechanism of action is not fully established. 23. AMAG estimate based on Wysocki et al. Management of Vaginal Atrophy: Implications from the REVIVE Survey. Clinical Medicine Insights: Reproductive Health 2014:8 23–30; Kingsberg et al. Vulvar and Vaginal Atrophy in Postmenopausal Women: Findings from the REVIVE Survey. J Sex Med 2013;101790-1799; and F. Palma et al: Vaginal atrophy of women in postmenopause. Results from a multicentric observational study: The AGATA study; Intrarosa is a steroid indicated for the treatment of moderate to severe dyspareunia, a symptom of vulvar and vaginal atrophy, due to menopause. 24. Market research sponsored by AMAG and conducted by Hall and Partners. June 2018. 25. All periods of financial guidance reflect 2018 financial guidance from continuing operations (excludes CBR for the full year 2018). © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 28

Q3-2018 Financial Results November 1, 2018 © 2018 AMAG Pharmaceuticals, Inc. All rights reserved 29
