Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - NUVASIVE INC | nuva-ex991_8.htm |
| 8-K - 8-K - NUVASIVE INC | nuva-8k_20181030.htm |

Q3 2018 Results Supplemental Presentation to Earnings Press Release October 30, 2018 Exhibit 99.2

NuVasive, Inc. (“NuVasive,” “NUVA” or the “Company”) cautions you that statements included in this presentation that are not a description of historical facts are forward-looking statements that involve risks, uncertainties, assumptions and other factors which, if they do not materialize or prove correct, could cause the Company's results to differ materially from historical results or those expressed or implied by such forward-looking statements. In addition, this presentation contains selected financial results from the third quarter 2018, as well as projections for 2018 financial guidance and longer-term financial and business goals. The Company’s projections for 2018 financial guidance and longer-term financial performance goals represent current estimates, including initial estimates of the potential benefits, synergies and cost savings associated with acquisitions, which are subject to the risk of being inaccurate because of the preliminary nature of the forecasts, the risk of further adjustment, or unanticipated difficulty in selling products or generating expected profitability. The potential risks and uncertainties that could cause actual growth and results to differ materially include, but are not limited to: the risk that NuVasive’s financial guidance and expectations may turn out to be inaccurate because of the preliminary nature of the Company’s forecasts and projections; the risk of further adjustment to financial results or future financial expectations; unanticipated difficulty in selling products, generating revenue or producing expected growth and profitability; the risk that acquisitions will not be integrated successfully or that the benefits and synergies from the acquisition may not be fully realized or may take longer to realize than expected; the loss of key employees; unexpected variations in market growth and demand for the Company’s products and technologies; and those other risks and uncertainties more fully described in the Company’s news releases and periodic filings with the Securities and Exchange Commission. NuVasive’s public filings with the Securities and Exchange Commission are available at www.sec.gov. The forward-looking statements contained herein are based on the current expectations and assumptions of NuVasive and not on historical facts. NuVasive assumes no obligation to update any forward-looking statement to reflect events or circumstances arising after the date on which it was made. Forward-Looking Statements

Management uses certain non-GAAP financial measures such as non-GAAP earnings per share, non-GAAP net income, non-GAAP operating expenses and non-GAAP operating profit margin, which exclude amortization of intangible assets, business transition costs, purchased in-process research and development, one-time restructuring and related items in connection with acquisitions, investments and divestitures, non-recurring consulting fees, certain litigation expenses and settlements, gains and losses from strategic investments, and non-cash interest expense (excluding debt issuance cost). Management also uses certain non-GAAP measures which are intended to exclude the impact of foreign exchange currency fluctuations. The measure constant currency utilizes an exchange rate that eliminates fluctuations when calculating financial performance numbers. The Company also uses measures such as free cash flow, which represents cash flow from operations less cash used in the acquisition and disposition of capital. Additionally, the Company uses an adjusted EBITDA measure which represents earnings before interest, taxes, depreciation and amortization and excludes the impact of stock-based compensation, business transition costs, purchased in-process research and development, one-time restructuring and related items in connection with acquisitions, investments and divestitures, non-recurring consulting fees, certain litigation expenses and settlements, gains and losses on strategic investments, and other significant one-time items. Management calculates the non-GAAP financial measures provided in this presentation excluding these costs and uses these non-GAAP financial measures to enable it to further and more consistently analyze the period-to-period financial performance of its core business operations. Management believes that providing investors with these non-GAAP measures gives them additional information to enable them to assess, in the same way management assesses, the Company’s current and future continuing operations. These non-GAAP measures are not in accordance with, or an alternative for, GAAP, and may be different from non-GAAP measures used by other companies. During the quarter ended June 30, 2018, the Company began excluding from its non-GAAP financial results certain litigation related expenses associated with ongoing litigation with a former Board member and his current employer related to various matters, including infringement of the Company’s intellectual property. For consistency and comparability, the Company has re-casted non-GAAP financial results for each of the quarters ended December 31, 2017 and March 31, 2018 to exclude these litigation expenses in such periods, which were $0.4 million and $0.6 million, respectively. For reconciliations of non-GAAP financial measures to the comparable GAAP financial measure, please refer to the supplemental financial information posted on the Investor Relations section of the Company’s corporate website at www.nuvasive.com. Non-GAAP Financial Measures Accounting Standards Codification 606 The Company adopted Accounting Standards Codification 606 Revenue from Contracts with Customers (“ASC 606”) as of January 1, 2018, electing full retrospective method of adoption, which resulted in a change in its accounting policy for revenue recognition and related adjustments to the Company’s consolidated financial statements. Financial information included in this presentation for prior periods has been re-casted and presented based on the full retrospective method of adoption of ASC 606. For more information regarding the Company’s adoption of ASC 606, please see the Company’s Quarterly Report on Form 10-Q for the quarter ended September 30, 2018.
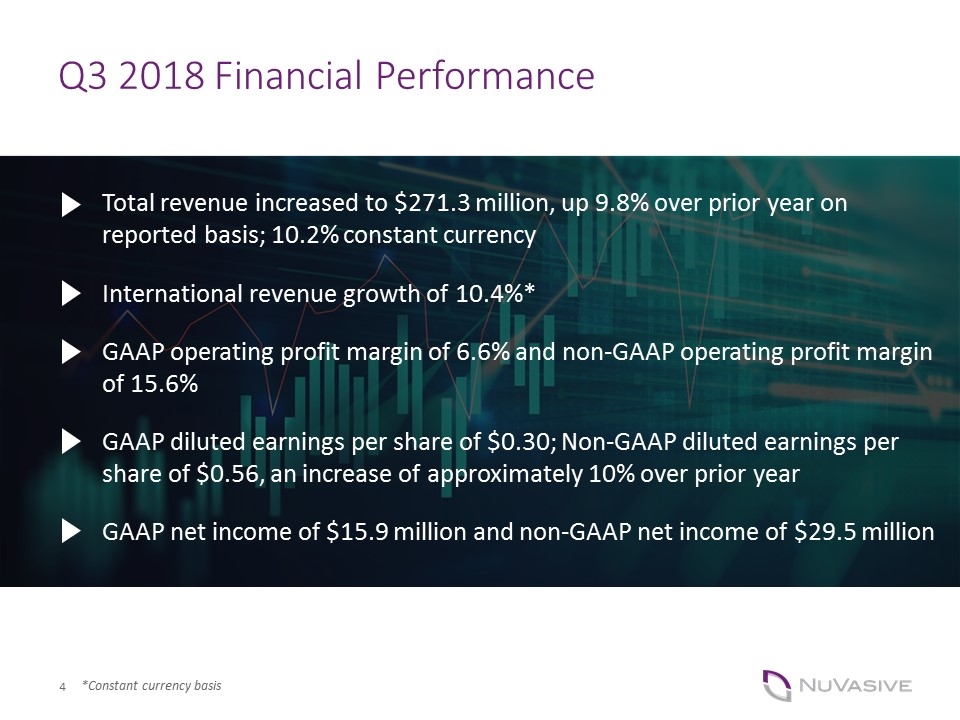
Q3 2018 Financial Performance GAAP diluted earnings per share of $0.30; Non-GAAP diluted earnings per share of $0.56, an increase of approximately 10% over prior year GAAP operating profit margin of 6.6% and non-GAAP operating profit margin of 15.6% Total revenue increased to $271.3 million, up 9.8% over prior year on reported basis; 10.2% constant currency International revenue growth of 10.4%* GAAP net income of $15.9 million and non-GAAP net income of $29.5 million *Constant currency basis
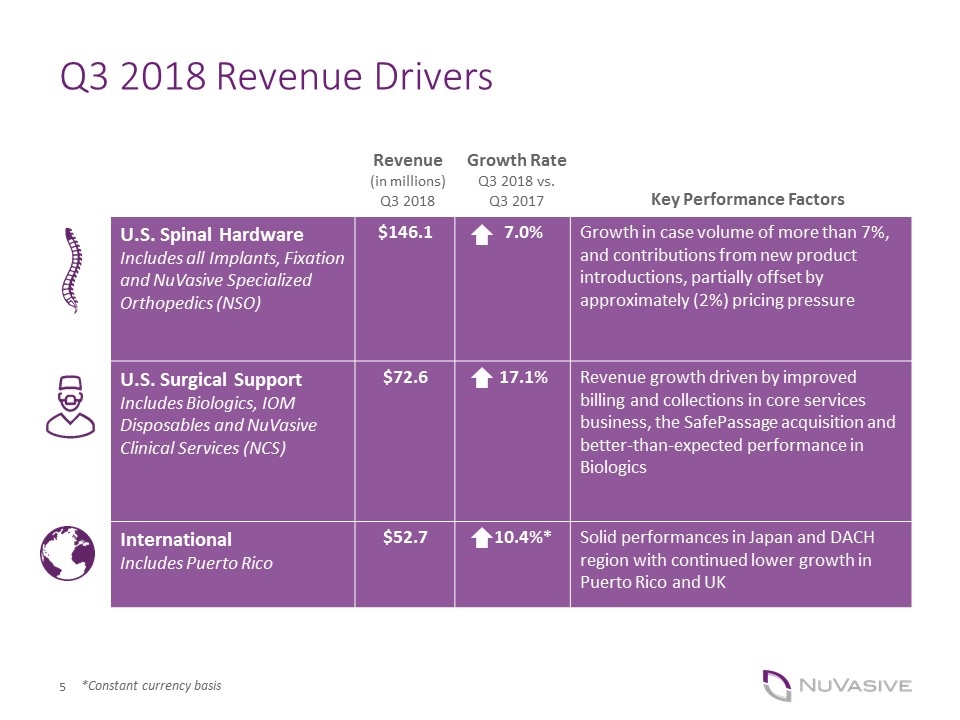
Q3 2018 Revenue Drivers U.S. Spinal Hardware Includes all Implants, Fixation and NuVasive Specialized Orthopedics (NSO) $146.1 7.0% Growth in case volume of more than 7%, and contributions from new product introductions, partially offset by approximately (2%) pricing pressure U.S. Surgical Support Includes Biologics, IOM Disposables and NuVasive Clinical Services (NCS) $72.6 17.1% Revenue growth driven by improved billing and collections in core services business, the SafePassage acquisition and better-than-expected performance in Biologics International Includes Puerto Rico $52.7 10.4%* Solid performances in Japan and DACH region with continued lower growth in Puerto Rico and UK Key Performance Factors Growth Rate Q3 2018 vs. Q3 2017 Revenue (in millions) Q3 2018 *Constant currency basis
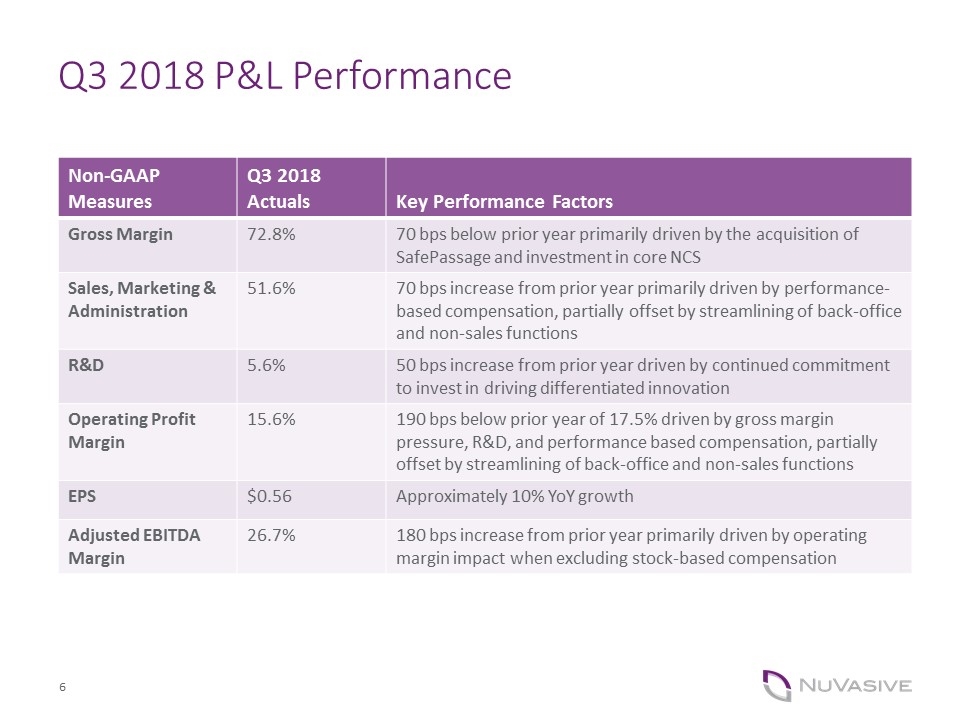
Q3 2018 P&L Performance Non-GAAP Measures Q3 2018 Actuals Key Performance Factors Gross Margin 72.8% 70 bps below prior year primarily driven by the acquisition of SafePassage and investment in core NCS Sales, Marketing & Administration 51.6% 70 bps increase from prior year primarily driven by performance-based compensation, partially offset by streamlining of back-office and non-sales functions R&D 5.6% 50 bps increase from prior year driven by continued commitment to invest in driving differentiated innovation Operating Profit Margin 15.6% 190 bps below prior year of 17.5% driven by gross margin pressure, R&D, and performance based compensation, partially offset by streamlining of back-office and non-sales functions EPS $0.56 Approximately 10% YoY growth Adjusted EBITDA Margin 26.7% 180 bps increase from prior year primarily driven by operating margin impact when excluding stock-based compensation
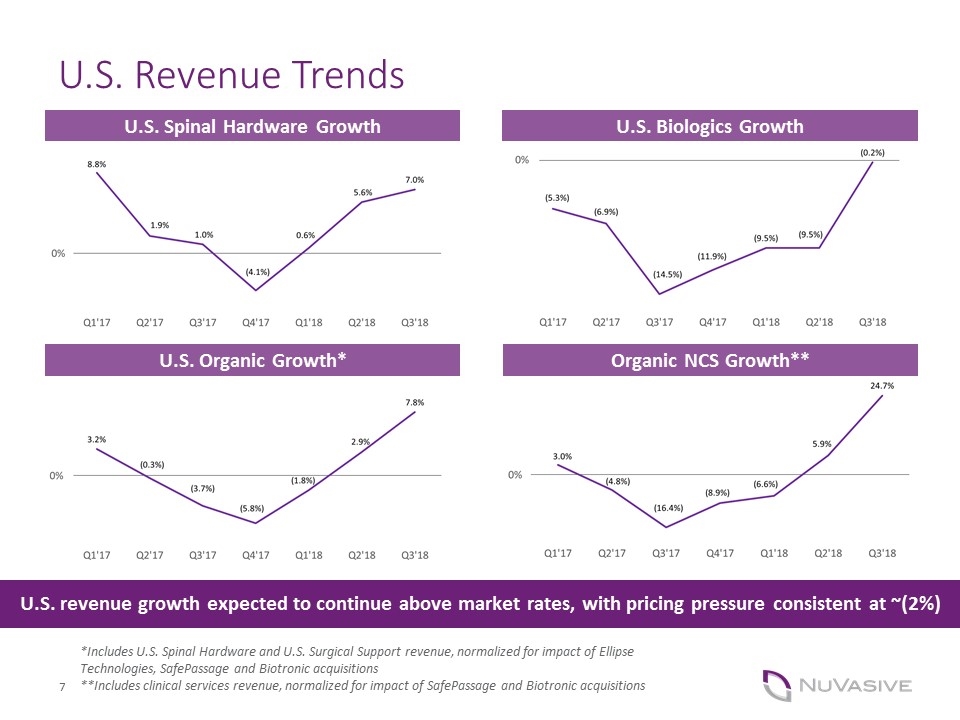
U.S. Revenue Trends U.S. Spinal Hardware Growth U.S. Biologics Growth *Includes U.S. Spinal Hardware and U.S. Surgical Support revenue, normalized for impact of Ellipse Technologies, SafePassage and Biotronic acquisitions **Includes clinical services revenue, normalized for impact of SafePassage and Biotronic acquisitions U.S. Organic Growth* Organic NCS Growth** U.S. revenue growth expected to continue above market rates, with pricing pressure consistent at ~(2%)
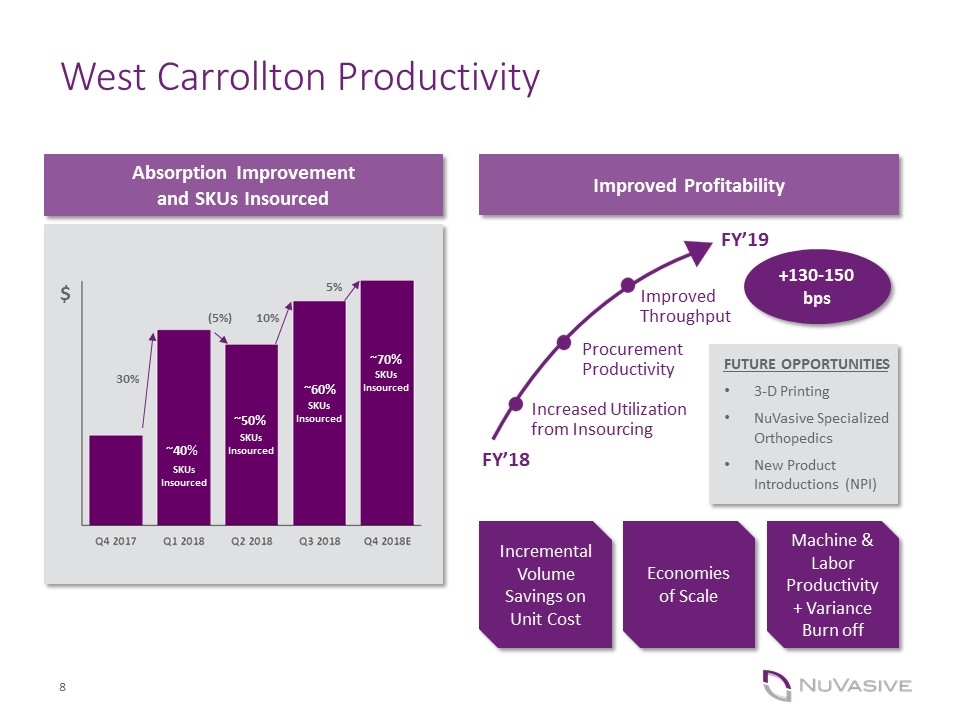
West Carrollton Productivity ~ Absorption Improvement and SKUs Insourced Improved Profitability Increased Utilization from Insourcing Procurement Productivity Improved Throughput FY’19 FY’18 Incremental Volume Savings on Unit Cost +130-150 bps Economies of Scale Machine & Labor Productivity + Variance Burn off FUTURE OPPORTUNITIES 3-D Printing NuVasive Specialized Orthopedics New Product Introductions (NPI) 30% (5%) 10% 5% SKUs Insourced ~50% ~60% ~70% ~40% SKUs Insourced SKUs Insourced SKUs Insourced $

Q3 2018 New Innovations and Partnerships* *Some products not commercially released; NuVasive may not make these products available for commercial sale Surgical Automation Platform with 2D- and 3D-Navigation MAGEC X Early Onset Scoliosis Treatment Biologics Line Extension: Propel DBM, Amniotic Membrane & Traditional Bone Allograft Chips Launched Launched in UK Unveiled at NASS; 510(k) Cleared Strategic partnerships in surgical automation and complex spine deformity TLX 20° Expandable Interbody Launched
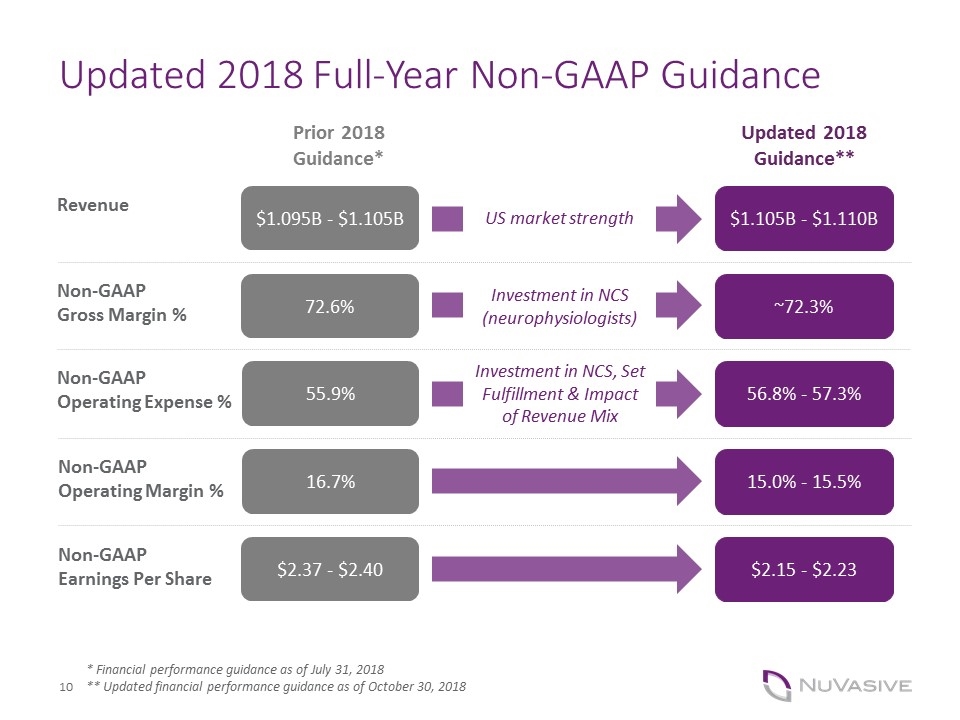
Updated 2018 Full-Year Non-GAAP Guidance * Financial performance guidance as of July 31, 2018 ** Updated financial performance guidance as of October 30, 2018 Prior 2018 Guidance* Updated 2018 Guidance** $1.095B - $1.105B $1.105B - $1.110B 72.6% ~72.3% 55.9% 56.8% - 57.3% 16.7% 15.0% - 15.5% $2.37 - $2.40 $2.15 - $2.23 US market strength Revenue Non-GAAP Gross Margin % Non-GAAP Operating Expense % Non-GAAP Operating Margin % Non-GAAP Earnings Per Share Investment in NCS, Set Fulfillment & Impact of Revenue Mix Investment in NCS (neurophysiologists) Text Here
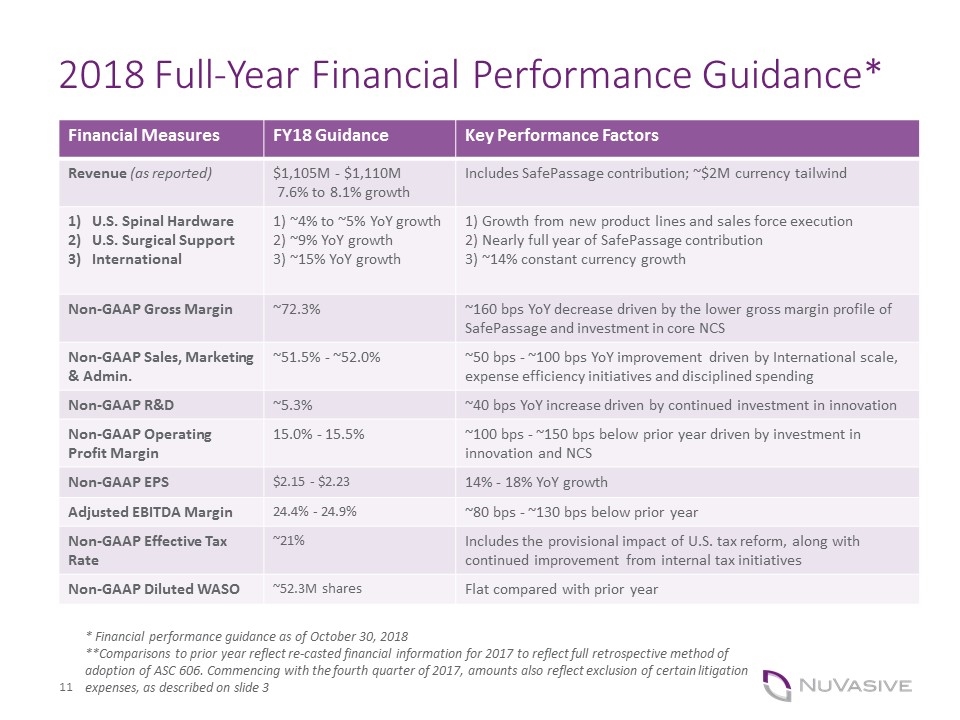
2018 Full-Year Financial Performance Guidance* Financial Measures FY18 Guidance Key Performance Factors Revenue (as reported) $1,105M - $1,110M 7.6% to 8.1% growth Includes SafePassage contribution; ~$2M currency tailwind U.S. Spinal Hardware U.S. Surgical Support International 1) ~4% to ~5% YoY growth 2) ~9% YoY growth 3) ~15% YoY growth 1) Growth from new product lines and sales force execution 2) Nearly full year of SafePassage contribution 3) ~14% constant currency growth Non-GAAP Gross Margin ~72.3% ~160 bps YoY decrease driven by the lower gross margin profile of SafePassage and investment in core NCS Non-GAAP Sales, Marketing & Admin. ~51.5% - ~52.0% ~50 bps - ~100 bps YoY improvement driven by International scale, expense efficiency initiatives and disciplined spending Non-GAAP R&D ~5.3% ~40 bps YoY increase driven by continued investment in innovation Non-GAAP Operating Profit Margin 15.0% - 15.5% ~100 bps - ~150 bps below prior year driven by investment in innovation and NCS Non-GAAP EPS $2.15 - $2.23 14% - 18% YoY growth Adjusted EBITDA Margin 24.4% - 24.9% ~80 bps - ~130 bps below prior year Non-GAAP Effective Tax Rate ~21% Includes the provisional impact of U.S. tax reform, along with continued improvement from internal tax initiatives Non-GAAP Diluted WASO ~52.3M shares Flat compared with prior year * Financial performance guidance as of October 30, 2018 **Comparisons to prior year reflect re-casted financial information for 2017 to reflect full retrospective method of adoption of ASC 606. Commencing with the fourth quarter of 2017, amounts also reflect exclusion of certain litigation expenses, as described on slide 3

For questions, contact investorrelations@nuvasive.com.
