UNITED STATES
SECURITIES AND EXCHANGE COMMISSION
Washington, D.C. 20549
FORM 8-K
CURRENT REPORT
Pursuant to Section 13 or 15(d) of the Securities Exchange Act of 1934
October 30, 2018
Date of Report
MARKER THERAPEUTICS, INC.
(Exact name of registrant as specified in its charter)
| Delaware | 001-37939 | 45-4497941 |
| (State or other jurisdiction of incorporation) |
(Commission File Number) | (IRS Employer Identification No.) |
|
5 West Forsyth Street Suite 200 Jacksonville, FL |
32202 | |
| (Address of principal executive offices) | (Zip Code) |
(904) 516-5436
Registrant’s telephone number, including area code
N/A
(Former name or former address, if changed since last report)
Check the appropriate box below if the Form 8-K is intended to simultaneously satisfy the filing obligation of the registrant under any of the following provisions:
| ¨ | Written communications pursuant to Rule 425 under the Securities Act (17 CFR 230.425) |
| ¨ | Soliciting material pursuant to Rule 14a-12 under the Exchange Act (17 CFR 240.14a-12) |
| ¨ | Pre-commencement communications pursuant to Rule 14d-2(b) under the Exchange Act (17 CFR 240.14d-2(b)) |
| ¨ | Pre-commencement communications pursuant to Rule 13e-4(c) under the Exchange Act (17 CFR 240.13e-4(c)) |
Indicate by check mark whether the registrant is an emerging growth company as defined in Rule 405 of the Securities Act of 1933 (§230.405 of this chapter) or Rule 12b-2 of the Securities Exchange Act of 1934 (§240.12b-2 of this chapter). Emerging growth company ¨
If an emerging growth company, indicate by check mark if the registrant has elected not to use the extended transition period for complying with any new or revised financial accounting standards provided pursuant to Section 13(a) of the Exchange Act. ¨
Introductory Note
On October 17, 2018, Marker Therapeutics, Inc., formerly known as TapImmune Inc. (“Marker” or the “Company”, and prior to the merger described below, “TapImmune”), completed its business combination with Marker Cell Therapy, Inc. (formerly Marker Therapeutics, Inc.), a privately-held Delaware corporation (“Marker Cell”) dedicated to the development of non-gene modified T cell therapies for the treatment of hematologic malignancies and solid tumors, in accordance with the terms of an Agreement and Plan of Merger and Reorganization, dated as of May 15, 2018 (the “Merger Agreement”), by and among the Company, Timberwolf Merger Sub, Inc., a Delaware corporation and wholly-owned subsidiary of the Company (“Merger Sub”), and Marker Cell. On October 17, 2018, pursuant to the Merger Agreement, Merger Sub was merged with and into Marker Cell (the “Merger”), with Marker Cell being the surviving corporation and becoming a wholly-owned subsidiary of the Company. In connection with the Merger, the Company reincorporated to a Delaware corporation and changed its name to Marker Therapeutics, Inc., and Marker Cell changed its name to Marker Cell Therapy, Inc. The Company is filing this Current Report on Form 8-K to update the description of the Company’s business and risk factors after giving effect to the Merger.
Item 8.01. Other Events.
Cautionary Note Regarding Forward-Looking Statements
This Current Report on Form 8-K includes forward-looking statements within the meaning of Section 21E of the Securities Exchange Act of 1934, as amended (the “Exchange Act”) and Section 27A of the Securities Act of 1933, as amended (the “Securities Act”). For this purpose, any statements contained herein, other than statements of historical fact, including future financial and operating results, targeted product milestones and potential revenues; future opportunities of the Company; the progress and timing of product development programs and related trials; the potential efficacy of products and product candidates; and the strategy, projected costs, prospects, plans and objectives of management of the Company, may be forward-looking statements under the provisions of The Private Securities Litigation Reform Act of 1995. Words such as “anticipate,” “believe,” “could,” “estimate,” “expect,” “intend,” “may,” “plan,” “project,” “should,” “target,” “will,” “would” or other words that convey uncertainty of future events or outcomes are used to identify these forward-looking statements. Actual results may differ materially from those indicated by such forward-looking statements as a result of various important factors, including “critical accounting estimates” and risks relating to: the ability to maintain compliance with NASDAQ listing standards; the liquidity and trading market for the Company’s common stock; clinical trials, including difficulties or delays in the completion of patient enrollment, data collection or data analysis; uncertainties in obtaining successful pre-clinical and clinical results for product candidates and unexpected costs that may result therefrom; ability to manufacture sufficient product to conduct clinical trials; ability to manage potential conflicts of interest concerning manufacturing and licensing matters; ability to obtain required regulatory approvals for product candidates; costs, timing and regulatory review of the Company’s studies and clinical trials; failure to realize any value of certain product candidates being developed, in light of inherent risks and difficulties involved in successfully bringing product candidates to market; the ability to develop new product candidates; the ability to commercialize and launch any product candidate that receives regulatory approval; the ability to attain market acceptance among physicians, patients, patient advocacy groups, health care payors and the medical community for future products of the Company; the ability to market any approved drug successfully or at all once it is on the market in light of challenges relating to regulatory compliance, pricing, market acceptance and competition; the ability to obtain the substantial additional funding required to conduct development and commercialization activities; and the ability to obtain, maintain and enforce patent and other intellectual property protection for currently marketed products and product candidates. Many of these factors that will determine actual results are beyond the Company’s ability to control or predict. If one or more of these factors materialize, or if any underlying assumptions prove incorrect, actual results, performance or achievements may vary materially from any future results, performance or achievements expressed or implied by these forward-looking statements. In addition, any forward-looking statements in this Current Report on Form 8-K represent the Company’s views only as of the date of this Current Report on Form 8-K and should not be relied upon as representing the Company’s views as of any subsequent date. The Company anticipates that subsequent events and developments will cause its views to change. However, while the Company may elect to update these forward-looking statements publicly at some point in the future, the Company specifically disclaims any obligation to do so, except as may be required by law, whether as a result of new information, future events or otherwise. The Company’s forward-looking statements generally do not reflect the potential impact of any future acquisitions, mergers, dispositions, joint ventures or investments it may make.
DESCRIPTION OF THE COMPANY’S BUSINESS
See the Glossary at the end of this Form 8-K for definitions of certain technical terms frequently used herein.
Overview
The Company is a clinical-stage immuno-oncology company specializing in the development and commercialization of innovative cell-based immunotherapies for the treatment of hematological malignancies and solid tumor indications and novel peptide-based vaccines for the treatment of breast and ovarian cancers. The Company’s cell-based immunotherapy technology is based on the selective expansion of non-engineered, tumor-specific T cells that recognize tumor associated antigens (i.e. tumor targets) and kill tumor cells expressing those targets. Once infused into patients, this population of T cells recognizes multiple tumor targets to produce broad spectrum anti-tumor activity. Because the Company does not genetically engineer its T cells, when compared to current engineered CAR-T and TCR-based approaches, its products (i) are significantly less expensive and easier to manufacture, (ii) appear to be markedly less toxic, and (iii) are associated with meaningful clinical benefit. As a result, the Company believes its portfolio of T cell therapies has a compelling therapeutic product profile, as compared to current gene-modified CAR-T and TCR-based therapies. In addition, the Company’s legacy TapImmune Folate Receptor Alpha program (TPIV200) for breast and ovarian cancers and its HER2/neu program (TPIV100/110) are in Phase II clinical trials. In parallel, the Company has been working on a proprietary nucleic acid-based antigen expression technology named PolyStart™ to improve the ability of the immune system to recognize and destroy diseased cells.
Immuno-oncology, which utilizes a patient’s own immune system to combat cancer, is one of the most actively pursued areas of research by biotechnology and pharmaceutical companies today. Interest and excitement about immunotherapy is driven by compelling efficacy data in cancers with historically bleak outcomes, and the potential to achieve a cure or functional cure for some patients. Harnessing the power of the immune system is an important component of fighting cancerous cells in the body. The Company’s MultiTAA T cell therapy platform identifies and selects for effectively all T cells that are specific for any peptide from the antigens that the Company targets (e.g., WT1, MAGE-A4, PRAME, Survivin, NY-ESO-1, and SSX2). The Company’s in-vitro manufacturing process promotes proliferation of very rare cancer-killing T cells to augment their anti-tumor properties and provide benefit to patients following their infusion. By using the multi-antigen targeted approach, the Company’s proprietary technology can kill heterogeneous tumor cell populations more effectively than single-antigen targeted approaches, thereby reducing the likelihood of tumor escape and potentially increasing the durability of a patient’s response to therapy.
The Company believes that its therapy presents a promising innovation in immuno-oncology. The Company’s therapy has been developed through its collaboration with the Cell and Gene Therapy Center at the Baylor College of Medicine (“BCM”) founded by Malcolm K. Brenner, M.D., Ph.D., a recognized pioneer in immuno-oncology. Marker Cell’s founders include Drs. Malcolm Brenner M.D., PhD, Ann Leen, PhD., Juan Vera, M.D., Helen Heslop, M.D., DSc (Hon) and Cliona Rooney, PhD, who have significant experience in this field.
The Company’s Strategy
The goal of the Company is to be the leader in the development and commercialization of transformative and best-in-class immunotherapies for the treatment of hematological malignancies and solid tumors. The Company will be developing a portfolio of highly-differentiated T cell therapies utilizing its MultiTAA platform that has the potential to significantly disrupt the current cell therapy landscape, while substantially improving survival and quality of life for patients with cancers.
| 2 |
Key elements of the Company’s strategy include:
• Expedite clinical development, regulatory approval, and commercialization of the Company’s lead product candidates.
Based on results in the Phase I clinical trials conducted at BCM, the Company plans to advance its lead product candidates into Phase II clinical trials and facilitate the initiation of Company-sponsored clinical trials in post-transplant AML and in lymphoma. The Company expects to finalize its clinical trial protocols by the end of 2018.
The Company plans to initiate a Phase II clinical trial in post-transplant AML in the second quarter of 2019 and a Phase II clinical trial in relapsed/refractory (“r/r”) Non-Hodgkin’s Lymphoma (“NHL”) in 2020. The Company anticipates that product manufacturing in support of those clinical trials will be conducted at BCM within its GMP cell manufacturing facility.
In 2019, the Company expects to begin the technology transfer process and begin the planning and implementation of additional GMP manufacturing capacity that would be capable of supporting the Company’s manufacturing needs with respect to pivotal trials. If the results of its Phase II studies are positive, the Company will explore potential avenues to achieve regulatory approval for the use of its products in these indications, including any potential avenues for accelerated approval. The U.S. Food and Drug Administration (“FDA”) may grant accelerated approval for product candidates for serious conditions that fill an unmet medical need based on a surrogate or intermediate endpoint. The Company believes that an accelerated approval strategy may be warranted given the limited options available for patients with post-transplant AML. However, if the FDA grants accelerated approval, confirmatory trials will be required by the FDA.
• Continue collaboration with the Company’s partners, and increase the Company’s internal research and development activities, to improve and develop adoptive cell therapy technologies.
The Company intends to finalize a strategic alliance with BCM, in which the Company would sponsor selected research at the institution in support of the Company’s technology. In conjunction with this strategic alliance, BCM will conduct selected Phase I/II clinical trials using the Company’s technology. If data from these early clinical trials appear positive, the Company will consider the therapeutic and commercial potential for such therapies to be advanced as new products for the Company.
In addition, the Company plans to use BCM facilities to enable the process development and manufacturing required to support the Phase II clinical trials of the Company’s product candidates. Outside of its relationship with BCM, the Company will invest in its research and development and CMC capabilities to enhance its ability to conduct process development to optimize its manufacturing process, product quality and commercial scalability.
The Company believes that its G-Rex® based manufacturing process is highly robust and scalable, and it will continue to invest resources in further refining the manufacturing process to create a product with highly attractive commercial attributes. The Company plans to engage Wilson Wolf Manufacturing Corporation (a company controlled by John Wilson, who is a director of the Company) in discussions to further customize the G-Rex® to optimally match the Company’s manufacturing requirements, as well as to develop a scalability plan to drive efficiencies for a commercial product.
• Invest in the Company’s platform to maximize the beneficial outcomes for cancer patients.
The Company plans to explore new product opportunities by expanding and/or customizing the antigens the Company targets, in order to expand the indications in which the Company’s products may be used, including solid tumors or other hematologic malignancies. Additionally, the Company’s research and development efforts may include the exploration of dosing and/or frequency of product administration and the relationship of these factors with potential therapeutic benefit.
| 3 |
• Leverage the Company’s relationships with its founding institutions, scientific founders and other scientific advisors.
The Company’s world renowned scientific founders and scientific advisors have made seminal contributions to major discoveries in the field of immuno-oncology, and have significant experience in oncology, immunology and cell therapy. The Company intends to be a science-driven company in its strategic decision-making and thus it intends to significantly leverage the knowledge, experience and advice of its scientific founders and advisors, as well as the institutional expertise of BCM, the Mayo Clinic and our other major institutional partners, to advance its therapies through the clinic and into commercialization.
The Company is in the process of evaluating the legacy therapeutic products and programs of the Company to determine the future strategy and the proper allocation of resources to best maximize stockholder value in the Company. In conjunction with this strategic review, the reconstituted board of directors and management of the Company may de-emphasize or terminate therapeutic products or programs, as appropriate. The Company’s board and management plan to make this strategic review a high priority now that the Merger has been completed, and expect to continue this strategic review on an ongoing basis.
Legacy Marker Cell Products
Multi Tumor-Associated Antigen (MultiTAA) Approach
By their nature, cancers are heterogeneous in their expression of antigens, meaning that a tumor generally consists of individual cancer cells that express different antigens, and each of those antigens can be present at a different level that can change over time. If a therapy targets only a single antigen, it is vulnerable to evolutionary escape mechanisms.
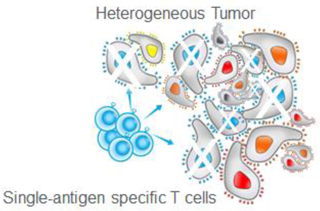
Even if the single-antigen specific therapy can eliminate all the tumor cells expressing the targeted antigen, the residual tumor cells that do not express that antigen may survive and expand. In addition, tumor cells may also downregulate or mutate the targeted antigen, thus becoming invisible to the T cell therapy. Both phenomena create a transformed tumor that is impervious to that therapy. This process is referred to as antigen-negative tumor immune escape. The Company’s solution to the problem of tumor heterogeneity was to develop T cell products that simultaneously attack multiple tumor-expressed antigens and thereby enable more complete initial tumor targeting, thus minimizing the subsequent opportunity for the cancer to engage escape mechanisms. Of note, data suggest this strategy may be responsible for recruitment and activation of unique cancer-killing cells from the patient’s own immune repertoire to participate in cancer eradication, further minimizing the possibility for tumor cell escape.
| 4 |
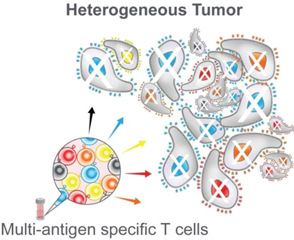
The Company’s proprietary MultiTAA T cell platform may have meaningful advantages over current CAR-T and TCR cell therapy approaches. Compared to current gene-modified T cell therapies, the Company’s programs are characterized by the following:
•Demonstrated clinical benefit, without the need for lymphodepletion before infusion: In its Phase I lymphoma study, the Company saw complete responses (“CRs”) in 50 – 60% of its evaluable patients. The Company believes it is significant that no patient with a CR has subsequently relapsed with disease, whereas typically 30% or more of patients with CR in reported CAR-T studies relapse within one year. In patient results to date, observed therapeutic responses appear to be highly durable, with some patients being relapse-free beyond two years.
•Non-gene-modified: Unlike CAR-T and TCR approaches, the Company’s therapy requires no genetic modification of T cells, a costly and complex process that significantly complicates the manufacturing of a patient product. The Company believes its therapy can be manufactured at a fraction of the cost of a gene-modified T cell product, with substantially reduced complexity of manufacturing.
•Low incidence rate of adverse events: In approximately 60 patients treated to date, the Company has seen only one grade III adverse reaction possibly related to its therapy. This appears to compare favorably with published CD19 CAR-T studies, wherein up to 95% of patients had associated grade III or higher adverse events during treatment. The Company believes that it is notable that there have been no cases of cytokine-release syndrome (“CRS”), or related serious adverse events (“SAEs”) in patients treated with its therapy to date.
•Capable of addressing a broad repertoire of cancer cells: While CAR-T and TCR therapies generally target a single epitope, the Company’s manufacturing process selects for T cells that are specific for multiple peptides derived from several targeted antigens. Deep gene sequencing of the Company products shows that a typical patient dose usually consists of approximately 4,000 unique T cell clonotypes targeting up to five different tumor-associated antigens. In layman’s terms, the five antigen targets can be recognized by a very wide range of T cells, facilitating robust killing of targeted cancer cells.
•Appears to drive endogenous immune responses: The Company sees evidence of “epitope spreading” in its patients, meaning that the Company’s therapy is potentially inducing an enhanced response by the patient’s own T cells (specific for an expanded set of tumor-associated antigens beyond those targeted by the Company’s infused product). The Company’s correlative analyses show expansion of endogenous T cells, other than those present in the Company product, in the months following the infusion of the Company’s product. This phenomenon, also known as “antigen spreading,” is potentially important in generating a durable response for a patient, because it enables the killing of tumors that do not express any of the antigens initially targeted by the Company’s product.
| 5 |
Legacy TapImmune Products
In contrast to standard therapies for cancer treatment including surgery, radiation therapy and chemotherapy that target both cancer cells and normal cells, the Company has been developing vaccines that precisely target breast, colorectal, ovarian and non-small cell lung cancers. The Company is currently developing three core technology platforms:
1) an exclusively licensed peptide-based vaccine (composition and methods of use) for the treatment of HER2/neu+ breast cancer that overexpresses Human Epidermal Growth Factor Receptor 2 (HER2/neu+) (TPIV100/110),
(2) an exclusively licensed peptide-based vaccine (composition and methods of use) for treating breast and ovarian cancers that overexpress Folate Receptor Alpha (TPIV200), and
(3) a wholly-owned nucleic acid-based vaccine (composition and methods of use) technology (PolyStart™) for treatment of various cancers or infectious disease.
The Company’s peptide vaccines derived from naturally processed T cell antigens discovered using cancer patient samples and the Company’s PolyStart™ expression technology, which improves antigen presentation to T cells, are not just effective therapies on their own, but can also to enhance the efficacy of other immunotherapy approaches such as CAR-T and PD-1 inhibitors, for example.
Products and Technology in Development
| Product/Candidate | Description | Application | Status | |||||||
| TPIV100/110 HER2/neu Breast Cancer Vaccine | Peptide Vaccine | Treatment of HER2/neu+ Breast Cancer | Phase I trial completed Phase I(b) trial to start in 2018 Phase I/II to start in 2018 (TPIV110) |
|||||||
| TPIV200 Folate Receptor Alpha Vaccine | Peptide Vaccine | Treatment of Folate Receptor Alpha+/Triple-Negative Breast and Ovarian Cancer | Phase I trial completed Multiple Phase II trials started in 2016 and 2017 | |||||||
| PolyStart™ | Nucleic acid expression technology | Broad Application to “Prime”- and- “Boost” | Preclinical |
Background and History of Cancer Immunotherapies
Despite advances in options for treatment, cancer continues to be one of the main causes of death in developed countries. Historically, cancer therapy has been constrained to surgery, radiation, and chemotherapy. More recently, advances in the understanding of the immune system’s role in cancer immune surveillance have led to immunotherapy becoming an important treatment approach. Cancer immunotherapy began with treatments that nonspecifically activated the immune system and had limited efficacy and/or significant toxicity. In contrast, newer immunotherapy treatments can activate specific, potent immune cells, leading to improved efficacy and safety. Within the immunotherapy category, treatments have included vaccines, cytokine therapies, antibody therapies, and adoptive cell therapies.
In 1996, Leach, Krummel and Allison reported that monoclonal antibodies (“mAbs”) blocking CTLA-4 could treat tumors in animal models. Subsequently, mAbs that targeted CTLA-4 and PD-1 became known as “immune checkpoint inhibitors” (“ICIs”). Immune checkpoints are a means by which cancer cells are able to inhibit or turn down the body’s immune response to cancer. By interfering with these cloaking mechanisms, ICIs have shown an ability to activate T cells, shrink tumors, and improve patient survival. Recent clinical data from checkpoint inhibitors such as ipilimumab, nivolumab and pembrolizumab have confirmed both the validity of this approach and the importance of T cells as promising tools for the treatment of cancer.
| 6 |
Despite these many advances, there persists a significant unmet need in cancer therapeutics. We believe that the use of human cells as a therapeutic modality to re-engage the immune system will be the next significant advancement in the treatment of cancer. These cellular therapies may avoid the long-term side effects associated with current treatments and have the potential to be effective regardless of the type of previous treatments patients have experienced.
T Cell Therapy Overview
The field of adoptive cell transfer (“ACT”) is currently comprised primarily of CAR and TCR engineered T cells and has emerged from principles of basic immunology to become a paradigm-shifting clinical immunotherapy. T cell therapy has evolved as one of the most promising branches of immunotherapy. T cell immunotherapy involves the infusion of immune cells into a patient. Immune cells used for immunotherapy treatments can either be collected from the patient (autologous) or harvested from a donor (allogenic). The cells are retrieved and mixed with specific antigens, then cultured to proliferate to reach a sufficient number before infusion into the patient. Upon infusion, the cells are capable of targeting and eliminating cancerous cells. Unlike chemotherapy, which is unable to distinguish between healthy and malignant cells, T cells produced for immunotherapy are able to selectively attack cancer cells that express the target antigen(s). This leads to a more effective treatment platform with fewer side effects. In addition, because of immunological memory, these infused T cells remain in the body for long periods of time, thus leading to longer and more durable responses.
TCRs and CARs have distinct signaling properties and antigen sensitivities. TCRs recognize peptide fragments from proteins expressed either inside the cell or on the cell surface. CARs are programmed to recognize a specific cell surface protein. Because CARs are specific for a single antigen, or more precisely a single epitope within the single antigen, they are very narrowly focused and come with limitations. While it is true that they may eliminate the tumor cells that express the target antigen, when applying a CAR-T cell product to a specific antigen of a heterogeneous disease, CAR-T cells may leave behind tumor cells that do not express the target antigen, which can lead to tumor relapse due to immune escape.
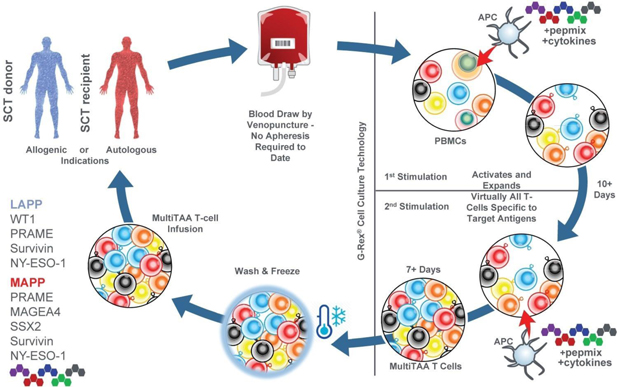
The Company’s approach is to avoid genetic engineering by relying upon the native T cell receptor, which has evolved over millions of years to provide T cells with an exquisite capacity to recognize and kill cancer cells. Use of the native T cell receptor is the bedrock of the Company’s versatile immunotherapy, which is intended to provide a cost-effective and non-toxic strategy to target multiple tumor antigens and lead to durable responses. The process entails expanding tumor-specific T cells from patients, or a patient’s hematopoietic stem cell donor. This is achieved by in vitro manipulation consisting of co-culturing antigen presenting cells with patient (or donor) peripheral blood mononuclear cells (“PBMCs”). As a source of antigen, the Company uses overlapping peptide libraries spanning each of several immunogenic target antigens that are typically associated with certain types of cancer. These peptides are 15 amino acids in length, overlapping by 11 amino acids and span the entire length of each of the target antigens. This typical footprint of peptides allows the Company to induce both CD4 (helper) and CD8 (cytotoxic) T cells. Following manufacture, these cells are frozen and stored for later infusion. Once infused, the natural characteristics of T cells take over and the T cells multiply in quantity, when they encounter the targeted antigens expressed by cancer cells, forming an army of T cells that kill the targeted cancer cells.
Process Development and Manufacturing
The Company is advancing two legacy Marker Cell products through clinical development. Mixed Antigen Peptide Pool (“MAPP”) T cells, which are currently used for patients with lymphoma, multiple myeloma and selected solid tumors, is an autologous product that targets the NY-ESO-1, PRAME, MAGE-A4, Survivin and SSX2 antigens. Leukemia Antigen Peptide Pool (“LAPP”) T cells, which are used for patients with AML, is an allogeneic product targeting the WT1, NY-ESO-1, PRAME, and Survivin antigens using the blood of the stem cell donor as a source of the cells used for therapy. While the blood source and the antigens for stimulation differ between the LAPP and the MAPP products, the manufacturing process for each product is otherwise identical.
| 7 |
In the manufacturing process, blood is drawn from either the individual patient (in the case of the autologous MAPP T cells) or from the allogeneic stem cell transplant donor (in the case of the allogeneic LAPP T cells). Although the T cells that are selected and expanded by the Company process exist in a patient’s circulating blood, these T cells are often present at very low frequencies. More importantly, researchers at BCM believe that these T cells are adversely affected by the suppressive tumor microenvironment. It is a well-accepted concept that cancers not only evade immune detection but often actively suppress the function of the human immune system. The Company’s manufacturing and culturing process is intended to (i) identify the T cells specific for the antigens that the Company intends to target, (ii) restore these T cells to functionality with respect to their anti-tumor capability and (iii) expand the population of those T cells specific for the Company’s targets to achieve the required patient dose.
After blood is drawn, the required component cells, including monocytes and PBMCs, are extracted from the blood, isolated and cryopreserved. Sufficient numbers of cryopreserved monocytes and PBMCs are taken to be used to manufacture a patient-specific product. These cells are placed inside a G-Rex® manufacturing device and combined with an experimentally optimized cocktail of GMP-grade cytokines that is used to restore and enhance the functional capability of the cultured T cells.
In addition, libraries of overlapping peptides (pepmix) spanning the target antigens are combined and added to the cell culture. Each peptide within the pepmix represents a small segment of a target antigen, which a T cell might recognize. Each library represents the entire protein sequence of a target antigen, with each peptide in the pepmix overlapping significantly with the peptides adjacent to it within the antigen’s protein sequence. This overlapping structure ensures that the Company can isolate, activate and expand any T cell that is specific for any segment of every antigen it targets in the unique genetic background of every patient.
The G-Rex® is a cell culture device manufactured by Wilson Wolf used by many cell therapy developers, both in commercial and academic settings. The device allows a user to introduce cells, media and other reagents into a cell culture chamber, which has a gas-permeable membrane at its bottom. The cells settle on this gas-permeable membrane through which oxygen and carbon dioxide are exchanged (i.e. the cells are allowed to breathe), while nutrients required for cell expansion are obtained from the medium above the cells. This system allows for the highly robust growth of cells in culture, by providing them with superior access to oxygen and nutrients. Cells manufactured in the device grow efficiently without need for further manipulation or agitation by a technician, scientist or automated system.
Inside the G-Rex®, PBMCs are co-cultured with dendritic cells that have been exposed to the stimulating pepmixes. This results in the selective expansion of T cells that specifically recognize the target antigens and the loss of other non-specific T cell populations. At the end of the manufacturing process, the resulting product is a mix of helper (CD4) and cytotoxic (CD8) T cells that recognize the antigens that the Company is targeting.
Once cell manufacturing is complete, the product is tested for identity, sterility, phenotype and safety before it is released for infusion into a patient. Sampling of product indicates that, on average, approximately 4,000 different T cell clonotypes are present in a typical 5-antigen-specific patient product.
Upon release of the final patient product, the cells are frozen and transported to the site where the cells will be administered. The standard dose for patients with lymphoma, AML or myeloma ranges from 5 – 20 million cells per meter squared (typically 10 – 40 million cells per adult patient). These cell doses represent a significantly smaller dose of cells, when compared to CAR-T or TCR therapies. As a result, the Company’s therapy requires only a very small infusion volume (less than 10 ml) that can be administered within minutes at an outpatient center. Because the Company’s therapies have generated only one grade 3 adverse event that was considered to be possibly related to the infused T cells out of approximately 60 patients dosed, patients do not need to be hospitalized and monitored overnight. Instead, the Company’s patients are evaluated for any immediate infusion-related reactions and can then usually be discharged within two hours.
| 8 |
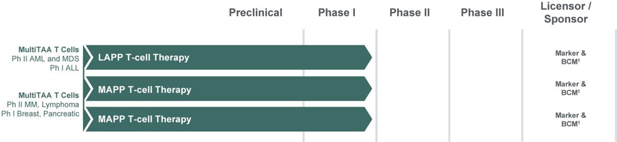
Clinical-stage MultiTAA T Cell Therapy
(1) Baylor College of Medicine
The Company’s MAPP and LAPP product candidates identify and select for substantially all T cells that are specific for any peptide derived from the targeted antigens, thereby recognizing and killing heterogeneous tumors more effectively than single-antigen targeted approaches. These product candidates are currently in Phase I clinical trials for lymphoma, AML/myelodysplastic syndromes (MDS), and MM at BCM and each of these programs is ready for initiation of Phase II. BCM has also initiated Phase I trials in acute lymphocytic leukemia, breast and pancreatic cancers.
In lymphoma, MAPP T cell therapy is currently in a Phase I trial that has treated 13 patients with active disease (“lymphoma active group”), of which 11 patients had follow-up date beyond 3 months post-infusion, and 17 patients in remission (“lymphoma adjuvant group”). No SAEs or CRS have been observed in any of these patients.
| 9 |
Of the 11 patients in the lymphoma active group, 6 patients demonstrated a complete response, 1 patient had durable stable disease and 4 patients had transient disease stabilization (range 5 – 9 months). None of the complete responders has subsequently progressed after receiving MAPP T cells. The duration of response for the complete responders ranged from 5 months to over 2 years (ongoing). Of the 17 patients in the lymphoma adjuvant group, 15 patients were in a continuing complete response, as of the date of data cutoff. The duration of response for these patients ranged from 3 to 37 months.
In post-transplant AML, a setting where currently the only available alternative therapy is a donor lymphocyte infusion (DLI), the Company has seen significant therapeutic benefit for patients, without causing graft-versus-host disease (GVHD) — a frequent side effect of DLIs. LAPP T cell therapy is currently in a Phase I trial that has treated 5 patients with active disease (“AML/MDS active group”) after allogeneic hematopoietic stem cell transplant (HSCT), and 8 patients in remission after HSCT (“AML/MDS adjuvant group”), of which 7 patients were evaluable. One patient had a transient elevation in liver enzymes. Otherwise there were no possibly/probably related SAEs, nor episodes of CRS.
Of the 5 evaluable patients in the AML/MDS active group, 1 patient demonstrated a complete response, 1 patient demonstrated a partial response, and 1 patient demonstrated ongoing stable disease. The duration of response for the complete or partial response patients ranged from 7 to 11 months. Of the 7 evaluable patients in the AML/MDS adjuvant group, 5 patients demonstrated a continued complete response. The duration of response for these patients ranged from 8 to 20 months.
MAPP T cell therapy is also being evaluated in a Phase I/II trial for patients with MM. One arm of this trial assessed patients who received MAPP T cells more than 90 days after an autologous stem cell transplant (“ASCT”), while a second arm assessed patients who received MAPP T cells within 90 days of ASCT. The Company has not seen a meaningful difference in response rates or durability between the two arms and intends to standardize future trials based upon a protocol wherein patients will receive MAPP T cells immediately post ASCT.
Of the patients evaluated in the MM trial, there were 7 patients with residual active disease, 6 of whom were evaluable with greater than 3 months of available follow-up date. Of these evaluable patients, 2 patients demonstrated complete responses and 2 patients demonstrated partial responses. The duration of response ranged from 4 to 22 months. Additionally, there were 7 patients treated in remission after ASCT and all were evaluable. All patients remain in continuing complete response and none have subsequently progressed. The duration of response for these patients ranged from 4 to 22 months.
Intellectual Property
The Company’s commercial success will depend in part on its ability to obtain and maintain patent and other proprietary protection for its technology, inventions, improvements, and know-how related to the business; to defend and enforce proprietary rights, including any patents that the Company may own in the future; to preserve the confidentiality of its trade secrets and other intellectual property; to obtain and maintain licenses to use intellectual property owned by third parties; and to operate without infringing the valid and enforceable patents and other proprietary rights of third parties. The Company’s ability to stop third parties from making, using, selling, offering to sell, or importing its products may depend on the extent to which it has rights under valid and enforceable patents or trade secrets that cover these activities — in other words, the rights obtained under exclusive license arrangements such as those pursuant to the BCM license agreement. With respect to both licensed and Company-owned intellectual property, the Company cannot be sure that patents will be granted with respect to any of its pending patent applications or with respect to any patent applications filed in the future, nor can the Company be sure that any of its existing patents or any patents that may be granted in the future will be commercially useful in protecting its commercial products and methods of manufacturing the same.
The Company also relies on trade secrets and know-how relating to its proprietary technology and product candidates, continuing innovation, and in-licensing opportunities to develop, strengthen and maintain its proprietary position in the field of immuno-oncology. However, trade secrets can be difficult to protect. The Company also plans to rely on regulatory protection afforded through orphan drug designations, data exclusivity, market exclusivity and patent term extensions when available, as well as contractual agreements with our academic and commercial partners.
| 10 |
The Company requires each of its employees, consultants and advisors to execute a confidentiality agreement upon the commencement of any employment, consulting or advisory relationship with the Company. Each agreement provides that all confidential information developed or made known to the individual during the course of the relationship will be kept confidential and not be disclosed to third parties except in specified circumstances. In the case of employees, the agreements provide that all inventions conceived of by an employee shall be the Company’s exclusive property.
There can be no assurance that the Company’s patents, and any patents that may be issued or licensed to it in the future, will afford protection against competitors with similar technology. In addition, no assurances can be given that the patents issued or licensed to the Company will not be infringed upon or designed around by others or that others will not obtain patents that we would need to license or design around. If the courts uphold existing or future patents containing broad claims over technology used by the Company, the holders of such patents could require the Company to obtain licenses to use such technology. Patent coverage may also vary from country to country based on the scope of available patent protection. There are also opportunities to obtain an extension of patent coverage for a product in certain countries, which adds further complexity to the determination of patent life.
To achieve this objective, a strategic focus for the Company has been to identify and license key patents and patent applications that serve to enhance the Company’s intellectual property and technology position. The Company’s intellectual property portfolio currently includes patent applications having: (1) claims directed to methods of generating multi-antigen specific T cell products; and (2) claims directed to therapeutic uses of such multi-antigen specific T cell products. The Company believes its patent portfolio, together with its efforts to develop and patent next generation technologies, provides it with a substantial intellectual property position. However, the area of patent and other intellectual property rights in biotechnology is an evolving one with many risks and uncertainties. Currently, all of the Company’s MultiTAA intellectual property rights are licensed from BCM.
Legacy Marker Cell Intellectual Property
BCM Exclusive License Agreement
On March 16, 2018, the Company entered into an Exclusive License Agreement (the “BCM license agreement”) with BCM, under which the Company received a worldwide, exclusive license to BCM’s rights in and to three patent families to develop and commercialize MultiTAA product candidates in exchange for partial ownership, royalties and milestone payments.
The following is a list of patents and patent applications that the Company has licensed from BCM under the BCM license agreement:
Exclusive license to BCM’s Patent Applications and Patents:
| Title | Country | Application No. | Filing/Issue Date | Patent Number (if issued) |
|||||||||
| PEPMIXES TO GENERATE MULTIVIRAL CTLS WITH BROAD SPECIFICITY | US | 15/905,176 | Filed: 26-Feb-2018 | ||||||||||
| PEPMIXES TO GENERATE MULTIVIRAL CTLS WITH BROAD SPECIFICITY | EP | EP 13746524.1 | Filed: 08-Feb-2013 | ||||||||||
| IMMUNOGENIC ANTIGEN IDENTIFICATION FROM A PATHOGEN AND CORRELATION TO CLINICAL EFFICACY | US | 62/220,884 | Filed: 18-Sep-2015 | N/A | |||||||||
| IMMUNOGENIC ANTIGEN IDENTIFICATION FROM A PATHOGEN AND CORRELATION TO CLINICAL EFFICACY | PCT | PCT/US2016/052487 | Filed: 19-Sep-2016 | N/A | |||||||||
| IMMUNOGENIC ANTIGEN IDENTIFICATION FROM A PATHOGEN AND CORRELATION TO CLINICAL EFFICACY | US | 15/759,501 | Filed: 12-Mar-2018 | ||||||||||
| IMMUNOGENIC ANTIGEN IDENTIFICATION FROM A PATHOGEN AND CORRELATION TO CLINICAL EFFICACY | AU | 2016324479 | Filed: 19-Sep-2016 | ||||||||||
| IMMUNOGENIC ANTIGEN IDENTIFICATION FROM A PATHOGEN AND CORRELATION TO CLINICAL EFFICACY | EP | 16847545.7 | Filed: 19-Sep-2016 | ||||||||||
| IMMUNOGENIC ANTIGEN IDENTIFICATION FROM A PATHOGEN AND CORRELATION TO CLINICAL EFFICACY | IL | 258090 | Filed: 19-Sep-2016 | ||||||||||
| IMMUNOGENIC ANTIGEN IDENTIFICATION FROM A PATHOGEN AND CORRELATION TO CLINICAL EFFICACY | SG | 11201802204S | Filed: 19-Sep-2016 |
| 11 |
Exclusive license to BCM’s Subject Technology:
1. “Generation of CTL Lines with Specificity Against Multiple Tumor Antigens or Multiple Viruses”
2. “Pepmixes to Generate Multiviral CTLs with Broad Specificity”
3. “Immunogenic Antigen Identification from a Pathogen and Correlation to Clinical Efficacy”
In partial consideration for the exclusive rights granted under the BCM license agreement, prior to the Merger, Marker Cell issued shares of Marker Cell common stock to BCM valued at approximately $5.0 million at the time of issuance. Additional consideration includes a royalty paid on net sales by the Company to BCM according to the royalty schedule in the BCM license agreement. The royalty fee schedule is based on aggregate net sales in four different ranges: (1) less than $500M, (2) $500M to $1.0B, (3) $1.0B and over, and (4) $2.0B and over. The corresponding royalty percentages range from 0.65% to 5.0% - increasing in proportion to the aggregate net sales. The royalty fee may be reduced in the event that the Company must pay additional royalties with respect to third-party owned patent rights or technology necessary for the use, manufacture or sale of a licensed product. The Company also agreed to pay BCM one-time milestone payments upon the occurrence of nine particular milestones relating to completion of the first dosing in clinical trials for a first and second distinct product, receipt of approval from the FDA, and hitting certain net sales goals. Under the agreement, the Company may be obligated to make aggregate milestone payments of up to $64.85 million. The Company is also responsible for sublicensing fees. In addition, under the BCM license agreement the Company is responsible for reimbursing BCM for patent-related expenses incurred prior to the execution of the license agreement of approximately $82,000. The Company will be responsible for filing, prosecuting and maintaining all patent applications and patents included in the licensed patent rights and all such related legal costs incurred after the date of the BCM license agreement, except such legal costs shall be reduced on a pro-rata basis on a patent or patent application basis should BCM license such patent or patent application in additional fields of use to any third party.
In addition, upon a liquidity event (as defined in the BCM license agreement) of the Company , BCM will receive a liquidity incentive payment of 0.5% of the liquidity event proceeds (as defined in the BCM license agreement) received by the Company or its stockholders in the liquidity event.
| 12 |
The Company has agreed to indemnify BCM and certain persons affiliated with BCM against claims and liabilities directly or indirectly related to or arising out of the design, process, manufacture or use by any third party of the licensed products, even though such claims and liabilities result in whole or in part from the negligence of the BCM indemnified parties or are based upon doctrines of strict liability or product liability, but not claims or liabilities arising from the gross negligence or intentional misconduct of any such BCM indemnified parties.
Unless terminated sooner, the license will expire on a licensed product-by-product basis and country by country basis, on the later of (i) the date of expiration of the last valid claim of patent rights to expire that covers the sale of such licensed product in such country, or (ii) the first date following the tenth anniversary of the first commercial sale of first licensed product by the Company in such country. After such expiration, but not termination, the licenses granted to the Company shall survive and become a perpetual, paid-in-full license in such country with respect to such licensed product.
The Company has the right in its sole discretion to terminate the BCM license agreement upon 60 days’ written notice to BCM. BCM has the right to terminate the agreement upon material default or failure of the Company of its overall obligation to perform any of the terms, covenants or provisions of the license agreement, including failure to make timely payment, taken as a whole, and which default or failure remains uncured thirty days after written notice from BCM of such material default or failure to correct such default or failure. Notwithstanding the foregoing, if a material default or failure is not susceptible to cure within the 30-day cure period, BCM’s right to terminate shall be suspended if, and for so long as, (i) the Company has provided BCM with a written plan that is reasonably calculated to effect a cure, (ii) such plan is reasonably acceptable to BCM, in its sole but reasonable discretion, and (iii) the Company commits to and does carry out such plan; provided, however, that, unless mutually agreed to by the parties in such plan, such suspension of BCM’s right to terminate shall not extend beyond 60 days after the original cure period. In addition, either party’s right to terminate the license agreement shall be tolled for so long as dispute resolution procedures are being pursued by the allegedly breaching party in good faith, and if it is finally and conclusively determined that the allegedly breaching party is in material breach, then the breaching party shall have the right to cure within 30 days after such determination. BCM also has the right to terminate the agreement if the Company shall (i) become involved in insolvency, dissolution, bankruptcy or receivership proceedings affecting the operation of its business, (ii) make an assignment of all or substantially all of its assets for the benefit of creditors, or (iii) if a receiver or trustee is appointed for the Company and the Company shall, after the expiration of 30 days following any of the enumerated events, have been unable to secure a dismissal, stay or other suspension of such proceedings.
In the event of termination of the BCM license agreement, but not expiration, all rights to the subject technology and patent rights thereunder shall revert to BCM, except to the extent necessary to exercise any surviving right or license thereunder. The Company may sell any licensed products actually in its possession at the effective date of termination, provided that the Company continues to pay to BCM royalties on all such sales in accordance with the license agreement and otherwise complies with the terms of the license agreement and sells all such licensed products within six months after the effective date of the termination.
Other Marker Cell Patent Applications and Patents:
| Title | Country | Application No. | Filing/Issue Date | Patent Number (if issued) |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS | US | 61/236,261 | Filed: 24-Aug-2009 | N/A | |||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | US | 15/246,241 | Filed: 24-Aug-2016 |
| 13 |
| Title | Country | Application No. | Filing/Issue Date | Patent Number (if issued) |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | PCT | PCT/US2010/046505 | Filed: 24-Aug-2010 | N/A | |||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | EP | EP 10814245.6 | Filed: 24-Aug-2010 Issued: 21-Sep-2016 |
EP Patent No. 2470644 |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | CH | 10814245.6 | Filed: 24-Aug-2010 | EP Patent No. 2470644 |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | DE | 10814245.6 | Filed: 24-Aug-2010 | EP Patent No. 2470644 |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | DK | 10814245.6 | Filed: 24-Aug-2010 | EP Patent No. 2470644 |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | FR | 10814245.6 | Filed: 24-Aug-2010 | EP Patent No. 2470644 |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | GB | 10814245.6 | Filed: 24-Aug-2010 | EP Patent No. 2470644 |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | IE | 10814245.6 | Filed: 24-Aug-2010 | EP Patent No. 2470644 |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | NL | 10814245.6 | Filed: 24-Aug-2010 | EP Patent No. 2470644 |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | NO | 10814245.6 | Filed: 24-Aug-2010 | EP Patent No. 2470644 |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | SE | 10814245.6 | Filed: 24-Aug-2010 | EP Patent No. 2470644 |
| 14 |
| Title | Country | Application No. | Filing/Issue Date | Patent Number (if issued) |
|||||||||
| GENERATION OF CTL LINES WITH SPECIFICITY AGAINST MULTIPLE TUMOR ANTIGENS OR MULTIPLE VIRUSES | EP | EP 16180607.0 | Filed: 24-Aug-2010 | ||||||||||
| PEPMIXES TO GENERATE MULTIVIRAL CTLS WITH BROAD SPECIFICITY | US | 61/596,875 | Filed: 09-Feb-2012 | N/A | |||||||||
| PEPMIXES TO GENERATE MULTIVIRAL CTLS WITH BROAD SPECIFICITY | PCT | PCT/US2013/025342 | Filed: 08-Feb-2013 | N/A |
Legacy TapImmune Intellectual Property
Patents
| Applicantion / Publication / Patent No. | Title | Ownership | Jurisdiction Where Granted/Filed |
||||||||||
| Peptide Based Vaccine (Folate Receptor Alpha, Breast and Ovarian Cancer) | |||||||||||||
| Patent No. 8,486,412 | Immunity to Folate Receptors | Exclusive License | USA | ||||||||||
| Patent No. 9,243,033 | Immunity to Folate Receptors | Exclusive License | USA | ||||||||||
| Patent No. 9,915,646 | Immunity to Folate Receptors | Exclusive License | USA | ||||||||||
| Patent No. 2,685,300 | Immunity to Folate Receptors | Exclusive License | Canada | ||||||||||
| Peptide Based Vaccine (HER2/neu+ Breast Cancer) | |||||||||||||
| Patent No. 8,858,952 | Methods and Materials for Generating T Cells | Exclusive License | USA | ||||||||||
| Patent No. 2013221309 | Methods and Materials for Generating CD8+ T Cells Having the Ability to Recognize Cancer Cells Expressing a HER2/neu+ Polypeptide | Exclusive License | Australia | ||||||||||
| 15 |
| Applicantion / Publication / Patent No. | Title | Ownership | Jurisdiction Where Granted/Filed |
||||||||||
| Patent No. ZL2013380019913.1 | Same as above | Exclusive License | China | ||||||||||
| Patent No. 2,814,836 | Same as above | Exclusive License | Europe | ||||||||||
| Patent No. 6,170,076 | Same as above | Exclusive License | Japan | ||||||||||
| Patent No. 9,814,767 | Same as above | Exclusive License | USA | ||||||||||
| Patent No. ZL200890124030.6 | HLA-DR Binding Peptides and Their Uses | Exclusive License | China | ||||||||||
| Patent No. 2,704,397 | Same as above | Exclusive License | Canada | ||||||||||
| Nucleic Acid Based Vaccine (PolyStart™; infectious disease, breast and ovarian Cancer) | |||||||||||||
| Patent No. 9,364,523 | Chimeric Nucleic Acid Molecule with Non-AUG Translation Initiation Sequences | Owned | USA | ||||||||||
| Patent No. 9,655,956 | Chimeric Nucleic Acid Molecules with Non-AUG Translation Initiation Sequences and Uses Thereof | Owned | USA | ||||||||||
| Patent No. 9,988,643 | Chimeric Nucleic Acid Molecules with Non-AUG Translation Initiation Sequences and Uses Thereof | Owned | USA | ||||||||||
| Patent No. 10,030,252 | Chimeric Nucleic Acid Molecules with Non-AUG Translation Initiation Sequences and Uses Thereof | Owned | USA | ||||||||||
| 16 |
| Applicantion / Publication / Patent No. | Title | Ownership | Jurisdiction Where Granted/Filed |
||||||||||
| HLA DR Peptide Vaccines | |||||||||||||
| Patent No. 6,006,265 | HLA-DR Binding Peptides And Their Uses | Exclusive License | Japan | ||||||||||
| Patent No. 2,215,111 | HLA-DR Binding Peptides And Their Uses | Exclusive License | Europe (DE, FR, GB, IE) |
We have exclusively licensed the intellectual property for our TPIV100/110 HER2/neu breast cancer vaccine and TPIV200 folate receptor alpha vaccine product candidates from the Mayo Foundation for Medical Education and Research (the “Mayo Foundation”).
The effect of the issued patents is that they provide us with patent protection for the claims covered by the patents. While the expiration of a product patent normally results in a loss of market exclusivity for the covered product or product candidate, commercial benefits may continue to be derived from: (i) later-granted patents on processes and intermediates related to the most economical method of manufacture of the active ingredient of such product; (ii) patents relating to the use of such product; (iii) patents relating to novel compositions and formulations; and (iv) in the United States and certain other countries, market exclusivity that may be available under relevant law. The effect of patent expiration on our product candidates also depends upon many other factors such as the nature of the market and the position of the product in it, the growth of the market, the complexities and economics of the process for manufacture of the active ingredient of the product and the requirements of new drug provisions of the Federal Food, Drug and Cosmetic Act or similar laws and regulations in other countries.
Our pending patent applications cover a range of technologies, including specific embodiments and applications for treatment of various medical indications, improved application methods and adjunctive utilization with other therapeutic modalities. The coverage claimed in a patent application can be significantly reduced before the patent is issued. Accordingly, we do not know whether any of the applications we acquire or license will result in the issuance of patents, or, if any patents are issued, whether they will provide significant proprietary protection or will be challenged, circumvented or invalidated. Because unissued U.S. patent applications are maintained in secrecy for a period of eighteen months and U.S. patent applications filed prior to November 29, 2000 are not disclosed until such patents are issued, and since publication of discoveries in the scientific or patent literature often lags behind actual discoveries, we cannot be certain of the priority of inventions covered by pending patent applications. Moreover, we may have to participate in opposition proceedings in a foreign patent office, or for United States patent applications filed before March 16, 2013, in interference proceedings declared by the United States Patent and Trademark Office “USPTO”) to determine priority of invention, or in United States inter partes review or post-grant review procedures, any of which could result in substantial cost to us, even if the eventual outcome is favorable to us. There can be no assurance that the patents, if issued, would be held valid by a court of competent jurisdiction. An adverse outcome could subject us to significant liabilities to third parties, require disputed rights to be licensed from third parties or require us to cease using such technology.
We have patents and patent applications in other countries, as well as in the European Patent Office that we believe provide equivalent or comparable protection for our product candidates in jurisdictions internationally that we consider to be key markets. Because of the differences in patent laws and laws concerning proprietary rights, the extent of protection provided by U.S. patents or proprietary rights owned by us may differ from that of their foreign counterparts.
| 17 |
We believe that our patents, the protection of discoveries in connection with our development activities, our proprietary products, technologies, processes and know-how and all of our intellectual property are important to our business. To achieve a competitive position, we rely on trade secrets, non-patented proprietary know-how and continuing technological innovation, where patent protection is not believed to be appropriate or attainable. In addition, as outlined above, we have a number of patent licenses from third parties, some of which are important to our business. There can be no assurance that any of our patents, licenses or other intellectual property rights will afford us any protection from competition.
Trademarks
We currently have pending with the USPTO applications for registration of the marks POLYSTART™ and “Marker Therapeutics.” We currently have the mark “TapImmune” registered with the USPTO. We also have rights to use other names essential to our business. Federally registered trademarks have a perpetual life, as long as they are maintained and renewed on a timely basis and used properly as trademarks, subject to the rights of third parties to seek cancellation of the trademarks if they claim priority or confusion of usage. We regard our trademarks and other proprietary rights as valuable assets and believe they have significant value to us.
| 18 |
RISK FACTORS
See the Glossary at the end of this Form 8-K for definitions of certain technical terms frequently used herein.
An investment in the Company’s common stock involves a high degree of risk. You should carefully consider the risks described below before making an investment decision in the Company’s securities. These risk factors are effective as of the date of this Current Report on Form 8-K and shall be deemed to be modified or superseded to the extent that a statement contained in the Company’s future filings modifies or replaces such statement. All of these risks may impair the Company’s business operations. The forward-looking statements in this Current Report on Form 8-K involve risks and uncertainties and actual results may differ materially from the results we discuss in the forward-looking statements. If any of the following risks actually occur, the Company’s business, financial condition or results of operations could be materially adversely affected. In that case, the trading price of the Company’s common stock could decline, and you may lose all or part of your investment.
Risks Related to the Company’s Business and Intellectual Property
The Company is a development stage company with a history of operating losses.
The Company is a clinical-stage immunotherapy company with a history of losses, and it may always operate at a loss. The Company expects that it will continue to operate at a loss throughout its development stage, and as a result, it may exhaust its financial resources and be unable to complete the development of its products. The Company anticipates that its ongoing operational costs will increase significantly as it continues conducting its clinical development program. The deficit of the Company will continue to grow during its drug development period. The Company has no sources of revenue to provide incoming cash flows to sustain its future operations. As outlined above, the ability of the Company to pursue its planned business activities depends upon its successful efforts to raise additional financing.
The Company has sustained losses from operations in each fiscal year since its inception, and it expects losses to continue for the indefinite future due to the substantial investment in research and development. As of December 31, 2017, the Company had an accumulated deficit of approximately $157 million since inception. The Company expects to spend substantial additional sums on the continued administration and research and development of licensed and proprietary products and technologies with no certainty that its approach and associated technologies will become commercially viable or profitable as a result of these expenditures. If the Company fails to raise a significant amount of capital, it may need to significantly curtail operations or cease operations in the near future. If any of the product candidates of the Company fails in clinical trials or does not gain regulatory approval, the Company may never generate revenue. Even if the Company generates revenue in the future, it may not be able to become profitable or sustain profitability in subsequent periods.
The Company’s future success is highly dependent upon its key personnel, and its ability to attract, retain, and motivate additional qualified personnel.
The Company’s ability to compete in the highly competitive biotechnology and pharmaceutical industries depends upon its ability to attract and retain highly qualified managerial, scientific, and medical personnel. The Company is highly dependent on its management, scientific, and medical personnel, including Peter Hoang, its President and Chief Executive Officer, Ann Leen, Ph.D., its Chief Scientific Officer, Juan Vera, M.D., its Chief Development Officer, and Dr. Richard Kenney, its Acting Chief Medical Officer, as well as the services of several key consultants. The loss of the services of any of the Company’s executive officers, other key employees, and other scientific and medical advisors, and the Company’s inability to find suitable replacements could result in delays in product development and harm to the Company’s business. In particular, Dr. Leen is the key person who has produced the Company’s MultiTAA T cell therapy-based product. A priority of the Company is to quickly train additional qualified scientific and medical personnel in the Company to ensure the ability to maintain business continuity following the Merger. Any delays in training such personnel could delay the development, manufacture, and clinical trials of the Company’s product candidates.
| 19 |
The Company’s ability to attract and retain highly skilled personnel is critical to its operations and expansion. The Company faces competition for these types of personnel from other biotechnology companies and more established organizations, many of which have significantly larger operations and greater financial, technical, human and other resources than the Company. The Company may not be successful in attracting and retaining qualified personnel on a timely basis, on competitive terms, or at all. If the Company is not successful in attracting and retaining these personnel, or integrating them into its operations, its business, prospects, financial condition and results of operations will be materially adversely affected. In such circumstances the Company may be unable to conduct certain research and development programs, unable to adequately manage its clinical trials and other products, and unable to adequately address its management needs.
The Company’s strategic relationship with Baylor College of Medicine, or BCM, is dependent, in part, upon its relationship with key medical and scientific personnel and advisors.
The legacy Marker Cell therapy has been developed through its collaboration with the Center for Cell and Gene Therapy at BCM, founded by Malcom K. Brenner, M.D., Ph.D., a recognized pioneer in immuno-oncology. In addition to Dr. Brenner, Marker Cell’s founders include Ann Leen, Ph.D., Juan Vera, M.D., Helen Heslop, M.D., DSc (Hon) and Cliona Rooney, Ph.D., who have significant experience in this field and are all affiliated with the Center for Cell and Gene Therapy at BCM. Dr. Leen and Dr. Vera are the Company’s Chief Scientific Officer and Chief Development Officer, respectively. In addition, Dr. Brenner, Dr. Heslop and Dr. Rooney have joined the Company’s newly formed Scientific Advisory Board.
The Company’s strategic relationship with BCM is dependent, in part, on its relationship with these key employees and advisors, and in particular Dr. Leen and Dr. Vera, who are also employed with the Center for Cell and Gene Therapy at BCM. If the Company loses Dr. Leen or Dr. Vera, or if either leaves their position at BCM, the Company’s relationship with BCM may deteriorate, and its business could be harmed.
The Company, and certain of its key medical and scientific personnel, will need additional agreements in place with BCM to expand its development, manufacture, and clinical trial efforts.
Although the Company has an exclusive license agreement with BCM under which the Company received a worldwide, exclusive license to BCM’s rights in and to three patent families to develop and commercialize the MultiTAA product candidates, the Company will need to enter into additional agreements with BCM with respect to (i) a strategic alliance to advance pre-clinical research, early stage clinical trials, and Phase II clinical trials with respect to the Company’s product candidates, as well as continued access to its clinical data, (ii) sponsored research for investigators within the Center for Cell and Gene Therapy at BCM, and (iii) product manufacturing and support, including personnel and space at the institution for the foreseeable future. Any delays in entering into new strategic agreements with BCM related to the Company’s product candidates could delay the development, manufacture, and clinical trials of its product candidates.
The multiple roles of certain of the Company’s officers and directors could limit their time and availability to the Company, and create, or appear to create, conflicts of interest.
Dr. Leen and Dr. Vera are employees of BCM and are contractually obligated to spend a significant portion of their time for BCM. In addition, Dr. Leen and Dr. Vera are co-founders and members of ViraCyte, and perform services from time to time for ViraCyte LLC (“ViraCyte”). ViraCyte is owned by the same principal stockholder group as Marker Cell prior to the Merger, and has technology which is being developed under a license agreement with BCM by the same research group at BCM. More specifically, ViraCyte is a clinical stage biopharmaceutical company, which is investigating and developing virus-specific T cell therapy technology for the prevention and/or treatment of viral infections. Accordingly, Dr. Leen and Dr. Vera may have other commitments that would, at times, limit their availability to the Company, and other research being conducted by Dr. Leen and Dr. Vera may, at times, receive higher priority than research on the Company’s programs, which may, in turn, delay the development or commercialization of the Company’s product candidates.
| 20 |
In addition, John Wilson is a member, director and officer of ViraCyte and is a director of the Company. Dr. Leen and Dr. Vera are also co-founders and members of ViraCyte, and perform services for ViraCyte from time to time, and Dr. Vera is a director of the Company. All of these individuals have certain fiduciary or other obligations to the Company and certain fiduciary or other obligations to ViraCyte and, in the case of Dr. Leen and Dr. Vera, to BCM. Such multiple obligations may in the future result in a conflict of interest with respect to presenting other potential business opportunities to the Company or to ViraCyte. A conflict of interest also may arise concerning the timing of the parties’ planned and ongoing clinical trials, investigational new drug application filings and the parties’ opportunities for marketing their respective product candidates. In addition, they may be faced with decisions that could have different implications for the Company than for ViraCyte. Consequently, there is no assurance that these members of the Company’s board and management will always act in the Company’s best interests in all situations should a conflict arise.
The Company has not yet sold any products or received regulatory approval to sell its products.
The Company has no approved products or products pending approval. As a result, the Company has not derived any revenue from the sales of products and has not yet demonstrated ability to obtain regulatory approval, formulate and manufacture commercial-scale products, or conduct sales and marketing activities necessary for successful product commercialization. Without revenue, the Company can only finance its operations through debt and equity financings.
Product development involves a lengthy and expensive process with an uncertain outcome, and results of earlier pre-clinical and clinical trials may not be predictive of future clinical trial results.
Clinical testing is expensive and generally takes many years to complete, and the outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of pre-clinical testing and early clinical trials of the Company’s product candidates may not be predictive of the results of larger, later-stage controlled clinical trials. Product candidates that have shown promising results in early-stage clinical trials may still suffer significant setbacks in subsequent clinical trials. The Company’s clinical trials to date have been conducted on a small number of patients in a single clinical site for a limited number of indications. The Company will have to conduct larger, well-controlled trials in its proposed indications at multiple sites to verify the results obtained to date and to support any regulatory submissions for further clinical development of the Company’s product candidates. The Company’s assumptions related to the Company’s products, such as with respect to lack of toxicity and manufacturing cost estimates, are based on early limited clinical trials and current manufacturing process at BCM and may prove to be incorrect. In addition, the initial estimates of the clinical cost of development may prove to be inadequate, particularly if clinical trial timing or outcome is different than predicted or regulatory agencies require further testing before approval. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or adverse safety profiles despite promising results in earlier, smaller clinical trials. Moreover, clinical data are often susceptible to varying interpretations and analyses. The Company does not know whether any Phase II, Phase III, or other clinical trials it may conduct will demonstrate consistent or adequate efficacy and safety with respect to the proposed indication for use sufficient to receive regulatory approval or market its product candidates.
The biotechnology and immunotherapy industries are characterized by rapid technological developments and a high degree of competition. The Company may be unable to compete with more substantial enterprises.
The biotechnology and biopharmaceutical industries are characterized by rapid technological developments and a high degree of competition. As a result, the Company’s actual or proposed immunotherapies could become obsolete before it recoups any portion of its related research and development and commercialization expenses. Competition in the biopharmaceutical industry is based significantly on scientific and technological factors. These factors include the availability of patent and other protection for technology and products, the ability to commercialize technological developments and the ability to obtain governmental approval for testing, manufacturing and marketing. The Company competes with specialized biopharmaceutical firms in the United States, Europe and elsewhere, as well as a growing number of large pharmaceutical companies that are applying biotechnology to their operations. Many biopharmaceutical companies have focused their development efforts in the human therapeutics area, including cancer. Many major pharmaceutical companies have developed or acquired internal biotechnology capabilities or made commercial arrangements with other biopharmaceutical companies. These companies, as well as academic institutions, governmental agencies and private research organizations, also compete with the Company in recruiting and retaining highly qualified scientific personnel and consultants. The Company’s ability to compete successfully with other companies in the pharmaceutical field will also depend to a considerable degree on the continuing availability of capital to it.
| 21 |
The Company is aware of certain investigational new drugs under development or approved products by competitors that are used for the prevention, diagnosis, or treatment of certain diseases we have targeted for drug development. Various companies are developing biopharmaceutical products that have the potential to directly compete with the Company’s immunotherapies even though their approach may be different. The biotechnology and biopharmaceutical industries are highly competitive, and this competition comes from both biotechnology firms and from major pharmaceutical companies. Many of these companies have substantially greater financial, marketing, and human resources than the Company. The Company also experiences competition in the development of its immunotherapies from universities, other research institutions and others in acquiring technology from such universities and institutions.
In addition, certain of the Company’s immunotherapies may be subject to competition from investigational new drugs and/or products developed using other technologies, some of which have completed numerous clinical trials.
The Company is subject to numerous risks inherent in conducting clinical trials.
The Company outsources some of the management of its clinical trials to third parties. Agreements with clinical investigators and medical institutions for clinical testing and with other third parties for data management services, place substantial responsibilities on these parties that, if unmet, could result in delays in, or termination of, the Company’s clinical trials. If any of the Company’s clinical trial sites fail to comply with FDA-approved good clinical practices, the Company may be unable to use the data gathered at those sites. If these clinical investigators, medical institutions or other third parties do not carry out their contractual duties or obligations or fail to meet expected deadlines, or if the quality or accuracy of the clinical data they obtain is compromised due to their failure to adhere to the Company’s clinical protocols or for other reasons, the Company’s clinical trials may be extended, delayed or terminated, and it may be unable to obtain regulatory approval for, or successfully commercialize, agents. The Company cannot be certain that it will successfully recruit enough patients to complete its clinical trials nor that it will reach its primary endpoints. Delays in recruitment, lack of clinical benefit or unacceptable side effects would delay the Company’s Phase II clinical trials.
The Company, or its regulators, may suspend or terminate its clinical trials for a variety of reasons. The Company may voluntarily suspend or terminate its clinical trials at any time if it believes they present an unacceptable risk to the patients enrolled in its clinical trials or do not demonstrate clinical benefit. In addition, regulatory agencies may order the temporary or permanent discontinuation of the Company’s clinical trials at any time if they believe that the clinical trials are not being conducted in accordance with applicable regulatory requirements or that they present an unacceptable safety risk to the patients enrolled in the Company’s clinical trials.
The Company’s clinical trial operations are subject to regulatory inspections at any time. If regulatory inspectors conclude that the Company or its clinical trial sites are not in compliance with applicable regulatory requirements for conducting clinical trials, the Company may receive reports of observations or warning letters detailing deficiencies, and it will be required to implement corrective actions. If regulatory agencies deem the Company’s responses to be inadequate, or are dissatisfied with the corrective actions the Company or its clinical trial sites have implemented, its clinical trials may be temporarily or permanently discontinued, and it may be fined, the Company or its investigators may be precluded from conducting any ongoing or any future clinical trials, the government may refuse to approve the Company’s marketing applications or allow it to manufacture or market its products, and it may be criminally prosecuted.
The lengthy approval process, as well as the unpredictability of future clinical trial results, may result in the Company failing to obtain regulatory approval for its product candidates, which would materially harm its business, results of operations and prospects.
| 22 |
The successful development of immunotherapies is highly uncertain.
Successful development of biopharmaceuticals is highly uncertain and depends on numerous factors, many of which are beyond the Company’s control. Immunotherapies that appear promising in the early phases of development may fail to reach the market for several reasons including:
| • | clinical study results that may show the immunotherapy to be less effective than expected (e.g., the study failed to meet its primary endpoint) or to have unacceptable side effects; |
| • | failure to receive the necessary regulatory approvals or a delay in receiving such approvals. Among other things, such delays may be caused by slow enrollment in clinical studies, length of time to achieve study endpoints, additional time requirements for data analysis, or Biologics License Application (“BLA”) preparation, discussions with the FDA, an FDA request for additional preclinical or clinical data, or unexpected safety or manufacturing issues; |
| • | manufacturing costs, formulation issues, pricing or reimbursement issues, or other factors that make the immunotherapy uneconomical; and |
| • | the proprietary rights of others and their competing products and technologies that may prevent the immunotherapy from being commercialized. |
Success in preclinical and early clinical studies does not ensure that large-scale clinical studies will be successful. Clinical results are frequently susceptible to varying interpretations that may delay, limit or prevent regulatory approvals. The length of time necessary to complete clinical studies and to submit an application for marketing approval for a final decision by a regulatory authority varies significantly from one immunotherapy to the next and may be difficult to predict.
Even if the Company is successful in getting market approval, commercial success of any of its product candidates will also depend in large part on the availability of coverage and adequate reimbursement from third-party payors, including government payors such as the Medicare and Medicaid programs and managed care organizations, which may be affected by existing and future health care reform measures designed to reduce the cost of health care. Third-party payors could require the Company to conduct additional studies, including post-marketing studies related to the cost effectiveness of a product, to qualify for reimbursement, which could be costly and divert its resources. If government and other health care payors were not to provide adequate coverage and reimbursement levels for one any of the Company’s products once approved, market acceptance and commercial success would be reduced.
In addition, if one of the Company’s products is approved for marketing, it will be subject to significant regulatory obligations regarding the submission of safety and other post-marketing information and reports and registration, and will need to continue to comply (or ensure that the Company’s third-party providers comply) with current Good Manufacturing Practices (“cGMPs”) and current Good Clinical Practices (“cGCPs”) for any clinical trials that the Company conducts post-approval. In addition, there is always the risk that the Company or a regulatory authority might identify previously unknown problems with a product post-approval, such as adverse events of unanticipated severity or frequency. Compliance with these requirements is costly, and any failure to comply or other issues with the Company’s product candidates’ post-market approval could have a material adverse effect on its business, financial condition and results of operations.
It may take longer and cost more to complete the Company’s clinical trials than the Company projects, or the Company may not be able to complete them at all.
For budgeting and planning purposes, the Company has projected the dates for the commencement, continuation, and completion of the Company’s various clinical trials. However, a number of factors, including scheduling conflicts with participating clinicians and clinical institutions, and difficulties in identifying and enrolling patients who meet trial eligibility criteria, may cause significant delays. The Company may not commence or complete clinical trials involving any of the Company’s products as projected or may not conduct them successfully.
| 23 |
During the second half of 2012, BCM began enrollment of the investigator-sponsored, Phase 1 clinical trial to establish the feasibility of one of the Company’s lead products, MAPP, and to assess its overall safety, inclusion of multiple antigens, and dosage tolerance in patients with lymphoma. During the second quarter of 2016, BCM began enrollment of the investigator-sponsored Phase 1 clinical trial to establish the feasibility of one of the Company’s lead products, LAPP, and to assess its overall safety, inclusion of multiple antigens, and dosage tolerance in patients with acute myeloid leukemia (AML)/myelodysplastic syndromes (MDS). However, the Company may experience difficulties in patient enrollment in the Company’s future clinical trials for a variety of reasons. The timely completion of clinical trials in accordance with their protocols depends, among other things, on the Company’s ability to enroll a sufficient number of patients who remain in the study until its conclusion. In addition, the Company’s clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as the Company’s product candidates, and this competition will reduce the number and types of patients available to the Company, because some patients who might have opted to enroll in the Company’s trials may instead opt to enroll in a trial being conducted by one of the Company’s competitors. Accordingly, the Company cannot guarantee that its clinical trials will progress as planned or as scheduled. Delays in patient enrollment may result in increased costs or may affect the timing or outcome of the Company’s ongoing clinical trial and planned clinical trials, which could prevent completion of these trials and adversely affect the Company’s ability to advance the development of the Company’s product candidates.
The Company relies on medical institutions, academic institutions, and clinical research organizations to conduct, supervise, or monitor some or all aspects of clinical trials involving the Company’s products. The Company may have less control over the timing and other aspects of these clinical trials than if the Company conducted them entirely on its own. If the Company fails to commence or complete, or experience delays in, any of its planned clinical trials, the Company may experience delays in its clinical development and/or commercialization plans.
In particular, while BCM will continue to support the Company trials with production of MAPP and LAPP T cells under contract, the Company anticipates that it will have to rely on third parties (CMOs) or internal facilities yet to be developed for the commercial manufacture of its multi-antigen specific T cell therapy products for clinical trials and eventual licensure. If they fail to commence or complete, or experience delays in, manufacturing the Company’s multi-antigen specific T cell therapy products, the Company’s planned clinical trials with respect to such products will be delayed, and the Company may experience delays in its clinical development and/or commercialization plans.
Clinical trials are expensive, time-consuming, and difficult to design and implement, and the Company’s clinical trial costs may be higher than for more conventional therapeutic technologies or drug products.
Clinical trials are expensive and difficult to design and implement, in part because they are subject to rigorous regulatory requirements. Because the Company’s product candidates are based on new technologies and, with respect to the Company’s MultiTAA T cell product candidates, manufactured on a patient-by-patient basis, the Company expects that they will require extensive research and development and have substantial manufacturing costs. In addition, costs to treat patients with relapsed/refractory cancer and to treat potential side effects that may result from the Company’s product candidates can be significant. Some clinical trial sites may not bill, or obtain coverage from, Medicare, Medicaid, or other third-party payors for some or all of these costs for patients enrolled in the Company’s clinical trials, and the Company may be required by those trial sites to pay such costs. Accordingly, the Company’s clinical trial costs may be significantly higher per patient than those of more conventional therapeutic technologies or drug products. In addition, the Company’s proposed personalized product candidates involve several complex manufacturing and processing steps, the costs of which will be borne by the Company. Depending on the number of patients the Company ultimately enrolls in its trials, and the number of trials it may need to conduct, the Company’s overall clinical trial costs may be higher than for more conventional treatments.
The Company’s clinical trials may fail to demonstrate adequately the safety and efficacy of its product candidates, which would prevent or delay regulatory approval and commercialization.
The clinical trials of the Company’s product candidates are, and the manufacturing and marketing of its products will be, subject to extensive and rigorous review and regulation by numerous government authorities in the United States and in other countries where the Company intends to test and market its product candidates. Before obtaining regulatory approvals for the commercial sale of any of the Company’s product candidates, the Company must demonstrate through lengthy, complex, and expensive preclinical testing and clinical trials that its product candidates are both safe and effective for use in each target indication. In particular, because the Company’s product candidates are subject to regulation as biological drug products, the Company will need to demonstrate that they are safe, pure and potent for use in their target indications. Each product candidate must demonstrate an adequate risk versus benefit profile in its intended patient population and for its intended use. The risk/benefit profile required for product licensure will vary depending on these factors and may include not only the ability to show tumor shrinkage, but also adequate duration of response, a delay in the progression of the disease, and/or an improvement in survival.
| 24 |
For example, response rates from the use of the Company’s product candidates may not be sufficient to obtain regulatory approval unless the Company can also show an adequate duration of response. Clinical testing is expensive and can take many years to complete, and its outcome is inherently uncertain. Failure can occur at any time during the clinical trial process. The results of preclinical studies and early clinical trials of the Company’s product candidates may not be predictive of the results of later-stage clinical trials. The results of studies in one set of patients or line of treatment may not be predictive of those obtained in another. In addition, the Company expects that there may be greater variability in results for products processed and administered on a patient-by-patient basis, as anticipated for the Company’s MultiTAA T cell product candidates, than for “off-the-shelf” products, like many other drugs. There is typically an extremely high rate of attrition from the failure of product candidates proceeding through clinical trials. Product candidates in later stages of clinical trials may fail to show the desired safety and efficacy profile despite having progressed through preclinical studies and initial clinical trials. A number of companies in the biopharmaceutical industry have suffered significant setbacks in advanced clinical trials due to lack of efficacy or unacceptable safety issues, notwithstanding promising results in earlier trials. Most product candidates that begin clinical trials are never approved by regulatory authorities for commercialization.
In addition, even if such trials are successfully completed, the Company cannot guarantee that the FDA or foreign regulatory authorities will interpret the results as the Company does, and more trials could be required before the Company submits its product candidates for approval. To the extent that the results of the trials are not satisfactory to the FDA or foreign regulatory authorities for support of a marketing application, the Company may be required to expend significant resources, which may not be available to the Company, to conduct additional trials in support of potential approval of its product candidates.
If the Company encounters difficulties enrolling patients in the Company’s clinical trials, its clinical development activities could be delayed or otherwise adversely affected.
The timely completion of clinical trials in accordance with their protocols depends, among other things, on the Company’s ability to enroll a sufficient number of patients who remain in the trial until its conclusion. The Company may experience difficulties in patient enrollment in the Company’s clinical trials for a variety of reasons, including:
| • | the size and nature of the patient population; |
| • | the patient eligibility criteria defined in the protocol; |
| • | the size of the study population required for analysis of the trial’s primary endpoints; |
| • | the proximity of patients to trial sites; |
| • | the design of the trial; |
| • | the Company’s ability to recruit clinical trial investigators with the appropriate competencies and experience; |
| • | competing clinical trials for similar therapies or other new therapeutics not involving cell-based immunotherapy; |
| • | clinicians’ and patients’ perceptions of the potential advantages and side effects of the product candidate being studied in relation to other available therapies, including any new drugs or treatments that may be approved for the indications the Company is investigating; |
| • | the Company’s ability to obtain and maintain patient consents; and |
| • | the risk that patients enrolled in clinical trials will not complete a clinical trial. |
| 25 |
In addition, the Company’s clinical trials will compete with other clinical trials for product candidates that are in the same therapeutic areas as the Company’s product candidates. This competition will reduce the number and types of patients available to the Company, because some patients who might have opted to enroll in the Company’s trials may instead opt to enroll in a trial being conducted by one of the Company’s competitors. Because the number of qualified clinical investigators is limited, the Company expects to conduct some of its clinical trials at the same clinical trial sites that some of the Company’s competitors use, which will reduce the number of patients who are available for the Company’s clinical trials at such clinical trial sites. Moreover, because the Company’s product candidates represent a departure from more commonly used methods of cancer treatment, potential patients and their doctors may be inclined to use conventional therapies, such as chemotherapy and approved immunotherapies, rather than enroll patients in any future clinical trial. In addition, potential enrollees in the Company’s MultiTAA T cell product clinical trials may opt to participate in alternate clinical trials because of the length of time between the time that the patient’s or the donor’s blood is drawn and the time when the product is infused back into the patient.
Even if the Company can enroll a sufficient number of patients in the Company’s clinical trials, delays in patient enrollment may result in increased costs or may affect the timing or outcome of the planned clinical trials, which could prevent completion of these trials and adversely affect the Company’s ability to advance the development of its product candidates.
The Company’s product candidates may cause undesirable side effects or have other properties that could halt their clinical development, prevent their regulatory approval, limit their commercial potential, or result in significant negative consequences.
Undesirable side effects caused by the Company’s product candidates could cause the Company or regulatory authorities to interrupt, delay, or halt clinical trials and could result in a more restrictive label or the delay or denial of regulatory approval by the FDA or other comparable foreign regulatory authorities. Results of the Company’s trials could reveal a high and unacceptable severity and prevalence of side effects or unexpected characteristics.
If unacceptable toxicities arise in the development of the Company’s product candidates, the Company or the FDA or comparable foreign regulatory authorities could order the Company to cease clinical trials or deny approval of its product candidates for any or all targeted indications. Treatment-related side effects could also affect patient recruitment or the ability of enrolled subjects to complete the trial or result in potential product liability claims. In addition, these side effects may not be appropriately recognized or managed by the treating medical staff, as toxicities resulting from personalized cell therapy, as with the Company’s MultiTAA T cell therapy products, are not normally encountered in the general patient population and by medical personnel. Any of these occurrences may harm the Company’s business, financial condition and prospects significantly.
The Company’s MultiTAA T cell therapy research and development efforts are to a large extent dependent upon BCM’s investigators.
It will take time to fully develop the Company’s research and development infrastructure. The Company currently depends upon and will continue to depend upon independent investigators and collaborators, such as BCM, and which in the future may include other universities, medical institutions, and strategic partners, to conduct the Company’s preclinical studies and clinical trials. The Company does not yet have its own research and development laboratory or a strategic research and development agreement or manufacturing agreement in place with BCM. If the Company needs to enter into alternative arrangements, its product development activities would be delayed. Agreements with such third parties might terminate for a variety of reasons, including a failure to perform by the third parties.
The Company expects to use the results of BCM’s research to support the filing with the FDA of Investigational New Drug applications, or INDs, to conduct more advanced clinical trials of the Company’s products. However, the Company has limited control over the nature or timing of BCM’s clinical trials and limited visibility into their day-to-day activities. The research the Company is funding constitutes only a small portion of BCM’s overall research. Other research being conducted by Dr. Ann Leen and Dr. Juan Vera may at times receive higher priority than research on the Company’s programs. These factors could adversely affect the timing of the Company’s IND filings and its ability to conduct future planned clinical trials.
| 26 |
The Company will be unable to commercialize its products if its trials are not successful.
The Company’s research and development programs are at an early stage. The Company must demonstrate its products’ safety and efficacy in humans through extensive clinical testing. The Company may experience numerous unforeseen events during, or as a result of, the testing process that could delay or prevent commercialization of the Company’s products, including but not limited to the following:
| • | safety and efficacy results in various human clinical trials reported in scientific and medical literature may not be indicative of results the Company obtains in its clinical trials; |
| • | after reviewing trial results, the Company or its collaborators may abandon products that the Company might previously have believed to be promising; |
| • | the Company, its collaborators or regulators, may suspend or terminate clinical trials if the participating subjects or patients are being exposed to unacceptable health risks; and |
| • | the effects the Company’s potential products have may not be the desired effects or may include undesirable side effects or other characteristics that preclude regulatory approval or limit their commercial use if approved. |
Clinical testing is very expensive, can take many years, and the outcome is uncertain. For example, it can take as much as 12 months or more before the Company learns the results from any clinical trial using the Company’s MultiTAA T cell therapy. The data collected from the Company’s clinical trials may not be sufficient to support approval by the FDA of the Company’s MultiTAA T cell therapy-based product candidates for the treatment of hematological malignancies, or the Company’s Folate Receptor Alpha (TPIV200) product for breast and ovarian cancers, HER2/neu+ peptide antigen prpduct (TPIV100/110) or DNA expression PolyStart™ product. The clinical trials for the Company’s products under development may not be completed on schedule and the FDA may not ultimately approve any of the Company’s product candidates for commercial sale. If the Company fails to adequately demonstrate the safety and efficacy of any product candidate under development, the Company may not receive regulatory approval for those products, which would prevent the Company from generating revenues or achieving profitability.
The Company may not be able to expand its manufacturing processes to other third-party manufacturing facilities or successfully create its own manufacturing infrastructure for supply of its requirements of product candidates for use in clinical trials and for commercial sale.
The Company does not own any facility that may be used as its clinical-scale manufacturing and processing facility. The Company currently relies on third-party Contract Manufacturing Organizations, or CMOs, for manufacture of its TapImmune legacy products. The Company anticipates it will initially rely solely on the cGMP manufacturing facility within BCM for the manufacturing of its MultiTAA T cell therapy-based product candidates. If the cGMP manufacturing facility of BCM, which does manufacturing for itself and other parties, experiences capacity constraints, disruptions, or delays in manufacturing the Company’s MultiTAA T cell therapy-based product candidates products, the Company’s planned clinical trials and necessary manufacturing capabilities will be disrupted or delayed, which will adversely affect the Company’s ability to conduct and further develop its business as currently planned. Further, the cGMP manufacturing facility is most likely too small to conduct the pivotal clinical studies being planned by the Company, so the Company will need to develop its own cGMP manufacturing capacity that will be adequate for such clinical trials with respect to its MultiTAA T cell therapy-based product candidates.
In 2019, the Company currently intends to begin developing additional cGMP manufacturing capacity of its own that would be capable of supporting its manufacturing needs with respect to its clinical trials, particularly with respect to pivotal studies. The Company’s manufacturing strategy going forward will involve the use of one or more CMOs or the Company will establish its own capabilities and infrastructure, including a manufacturing facility. Establishment of the Company’s own manufacturing facility is subject to many risks. For example, the establishment of a cell-therapy manufacturing facility is a complex endeavor requiring knowledgeable individuals. Creating an internal manufacturing infrastructure will rely upon building out a complex facility and finding personnel with an appropriate background and training to staff and operate the facility. Should it be unable to find these individuals, the Company may need to rely on external contractors or train additional personnel to fill needed roles. There are a small number of individuals with experience in cell therapy, and the competition for these individuals is high.
| 27 |
The Company expects that development of its own manufacturing facility could provide it with enhanced control of material supply for both clinical trials and the commercial market, enable the more rapid implementation of process changes, and allow for better long-term margins. However, the Company does not have any experience in developing a manufacturing facility and may never be successful in developing the Company’s own manufacturing facility or capability. The Company may establish multiple manufacturing facilities as it expands its commercial footprint to multiple geographies, which may lead to regulatory delays or prove costly. Even if the Company is successful, its manufacturing capabilities could be affected by cost-overruns, unexpected delays, equipment failures, labor shortages, natural disasters, power failures, transportation difficulties and numerous other factors that could prevent the Company from realizing the intended benefits of its manufacturing strategy and have a material adverse effect on the Company’s clinical development and/or commercialization plans.
In addition, the manufacturing process for any products that the Company may develop is subject to the FDA and foreign regulatory authority approval process, and the Company will need to contract with manufacturers who can meet all applicable FDA and foreign regulatory authority requirements on an ongoing basis. If the Company or its CMOs are unable to reliably produce products to specifications acceptable to the FDA, or other regulatory authorities, the Company may not obtain or maintain the approvals it needs to commercialize such products. Even if the Company obtains regulatory approval for any of its product candidates, there is no assurance that either the Company or its CMOs will be able to manufacture the approved product to specifications acceptable to the FDA or other regulatory authorities, to produce it in sufficient quantities to meet the requirements for the potential launch of the product, or to meet potential future demand. Any of these challenges could delay completion of clinical trials, require bridging clinical trials or the repetition of one or more clinical trials, increase clinical trial costs, delay approval of the Company’s product candidate, impair commercialization efforts, increase its cost of goods, and have an adverse effect on its clinical development and/or commercialization plans.
Regardless of whether the Company engages additional CMOs to manufacture its products or establishes its own manufacturing facility, in order to transfer the Company’s MultiTAA T cell manufacturing from or expand its manufacturing capabilities beyond BCM pursuant to its development plans, whether through additional third parties or by developing its own manufacturing capabilities, the Company will need access to the Standard Operating Procedures and the specific Batch Production Records that are used to manufacture the product candidates. If BCM fails to transfer The Company’s manufacturing processes, or impedes the Company’s ability to transfer the manufacturing processes of its products to the Company or third-party manufacturers, the Company’s planned clinical trials and additional necessary manufacturing capabilities will be delayed, which will adversely affect the Company’s ability to conduct and further develop its business as currently planned.
The Company will be dependent on third-party vendors to design, build, maintain and support its manufacturing and cell processing facilities.
As a result of the Company’s strategy to outsource its manufacturing, it will rely very heavily on BCM and other third-party manufacturers to perform the manufacturing of the Company’s products for its clinical trials. The Company also licenses a significant portion of its technology from others and, at this time, does not own any intellectual properties or technologies. The Company intends to rely on its contract manufacturers to produce large quantities of materials needed for clinical trials and potential product commercialization. Third-party manufacturers may not be able to meet the Company’s needs concerning timing, quantity, or quality. If the Company is unable to contract for a sufficient supply of needed materials on acceptable terms, or if it should encounter delays or difficulties in its relationships with manufacturers, its clinical trials may be delayed, thereby delaying the submission of products for regulatory approval or the market introduction and subsequent sales of its products. Any such delay may lower the Company’s revenues and potential profitability. If any third party breaches or terminates its agreement with the Company or fails to conduct its activities in a timely manner, the commercialization of the Company’s products under development could be slowed down or blocked completely. It is possible that third parties relied upon by the Company will change their strategic focus, pursue alternative technologies, or develop alternative products, either on their own or in collaboration with others, as a means for developing treatments for the diseases targeted by the Company’s collaborative programs, or for other reasons. The effectiveness of these third parties in marketing their own products may also affect the revenues and earnings of the Company.
| 28 |
The Company intends to continue to enter into additional third-party agreements in the future. However, the Company may not be able to negotiate any additional agreements successfully. Even if established, these relationships may not be scientifically or commercially successful.
The Company’s manufacturing process is reliant upon the specialized equipment, and other specialty materials, which may not be available to the Company on acceptable terms or at all. For some of this equipment and materials, the Company relies or may rely on sole source vendors or a limited number of vendors, which could impair its ability to manufacture and supply its products.
The Company will depend on a limited number of vendors for supply of certain materials and equipment used in the manufacture of its MultiTAA T cell therapy-based product candidates. For example, the Company will purchase equipment and reagents critical for the manufacture of its product candidates from Wilson Wolf Manufacturing Corporation (a company controlled by John Wilson, who is a director of the Company), JPT Peptide Technologies and other suppliers. Some of the Company’s suppliers may not have the capacity to support commercial products manufactured under cGMP by biopharmaceutical firms or may otherwise be ill-equipped to support the Company’s needs. The Company also may not have supply contracts with many of these suppliers and may not be able to obtain supply contracts with them on acceptable terms or at all. Accordingly, the Company may not be able to obtain key materials and equipment to support clinical or commercial manufacturing.
For some of this equipment and materials, the Company will rely, and may in the future rely, on sole-source vendors or a limited number of vendors. An inability to continue to source product from any of these suppliers, which could be due to regulatory actions or requirements affecting the supplier, adverse financial, or other strategic developments experienced by a supplier, labor disputes or shortages, unexpected demands, or quality issues, could adversely affect the Company’s ability to satisfy demand for its product candidates, which could adversely and materially affect the Company’s operating results or its ability to conduct clinical trials, either of which could significantly harm its business.
As the Company continues to develop and scale its manufacturing process, it may need to obtain rights to and supplies of specific materials and equipment to be used as part of that process. For example, The Company’s MultiTAA T cell manufacturing process is based, in part, upon the G-Rex® cell culture device manufactured by Wilson Wolf Manufacturing Corporation, which is used by many cell therapy developers, both in commercial and academic settings. The Company will not own any exclusive rights to the G-Rex® that could be used to prevent third parties from developing similar and competing processes. The Company may not be able to obtain rights to such materials and equipment on commercially reasonable terms, or at all, and if the Company is unable to alter its process in a commercially viable manner to avoid the use of such materials or find a suitable substitute, it would have a material adverse effect on its business.
The manufacture of the Company’s product candidates is complex, and the Company may encounter difficulties in production, particularly with respect to process development or scaling up of the Company’s manufacturing capabilities. If the Company, or any of the Company’s third-party manufacturers encounter such difficulties, the Company’s ability to supply its product candidates for clinical trials, or its products for patients, if approved, could be delayed or stopped, or the Company may be unable to maintain a commercially viable cost structure.
The Company’s product candidates are biologics, and the process of manufacturing its products is complex, highly regulated and subject to multiple risks. For example, the manufacture of the Company’s MultiTAA T cell therapy-based product candidates involves complex processes, including drawing blood from patients/donors, manufacturing the clinical product, and ultimately infusing the product into a patient. As a result of the complexities, the cost to manufacture biologics is generally higher than traditional small molecule chemical compounds, and the manufacturing process is less reliable and is more difficult to reproduce. The Company’s manufacturing processes will be susceptible to product loss or failure due to any of the following: logistical issues associated with the collection of blood cells, or starting material, from the patient, shipping such material to the manufacturing site, shipping the final product back to the patient, and infusing the patient with the product; manufacturing issues associated with the differences in patients’ starting cells; interruptions in the manufacturing process; contamination; equipment failure; improper installation or operation of equipment, vendor or operator error; inconsistency in cell growth; and variability in product characteristics. Even minor deviations from normal manufacturing processes could result in reduced production yields, product defects, and other supply disruptions. If for any reason the Company loses a patient’s cells, or later-developed product at any point in the process, the manufacturing process for that patient will need to be restarted and the resulting delay may adversely affect that patient’s outcome and/or the results of clinical trials. If microbial, viral, or other contaminations are discovered in the Company’s product candidates or in the manufacturing facilities in which the Company’s product candidates are made, such manufacturing facilities may need to be closed for an extended period of time to investigate and remedy the contamination.
| 29 |
Because the Company’s MultiTAA T cell therapy-based product candidates are manufactured for each particular patient, the Company will be required to maintain a chain of identity with respect to the patient’s/donor’s blood cells as it moves from the patient to the manufacturing facility, through the manufacturing process, and back to the patient. Maintaining such a chain of identity is difficult and complex, and failure to do so could result in adverse patient outcomes, loss of product, or regulatory action including withdrawal of the Company’s products from the market. Further, as product candidates are developed through preclinical to late stage clinical trials towards approval and commercialization, it is common that various aspects of the development program, such as manufacturing methods, are altered along the way in an effort to optimize processes and results. Such changes carry the risk that they will not achieve these intended objectives, and any of these changes could cause the Company’s product candidates to perform differently and affect the results of planned clinical trials or other future clinical trials.
Currently, the Company’s product candidates are manufactured using processes by BCM, its third-party research institution collaborator, that the Company may not intend to use for more advanced clinical trials or commercialization. Although the Company is working to develop commercially viable processes, doing so is a difficult and uncertain task, and there are risks associated with scaling to the level required for advanced clinical trials or commercialization, including, among others, cost overruns, potential problems with process scale up, process reproducibility, stability issues, lot consistency, and timely availability of raw materials. As a result of these challenges, the Company may experience delays in the Company’s clinical development and/or commercialization plans. The Company may ultimately be unable to reduce the cost of goods for the Company’s product candidates to levels that will allow for an attractive return on investment if and when those product candidates are commercialized.
No assurance can be given that the Company will be able to develop a new, FDA-compliant, more efficient, lower cost manufacturing process upon which the Company’s business plan to commercialize MultiTAA-based products is dependent.
In cooperation with the Company’s potential contract manufacturers, the Company intends to develop improved methods for generating and selecting T cells, and to develop methods for large-scale production of its current product candidates that are in accordance with current Good Manufacturing Practices (“cGMP”) procedures. Developing a new, scaled-up, pharmaceutical manufacturing process that can more efficiently and cost effectively, and in a more automated manner produce, measure and control the physical and/or chemical attributes of the Company’s products in a cGMP facility is subject to many uncertainties and difficulties. The Company has never manufactured its adoptive T cell therapy product candidate on any scale, commercial or otherwise. As a result, the Company cannot give any assurance that it will be able to establish a manufacturing process that can produce its products at a cost or in quantities necessary to make them commercially viable. Moreover, the Company’s third-party manufacturers will have to continually adhere to current cGMP regulations enforced by the FDA through its facilities inspection program. If the facilities of these manufacturers cannot pass a pre-approval plant inspection, the FDA premarket approval of the Company’s products will not be granted. In complying with cGMP and foreign regulatory requirements, the Company and any of the Company’s third-party manufacturers will be obligated to expend time, money and effort in production, record-keeping and quality control to assure that the Company’s products meet applicable specifications and other requirements. If the Company or any of its third-party manufacturers fail to comply with these requirements, the Company may be subject to regulatory action. No assurance can be given that the Company will be able to develop such manufacturing process, or that its partners will thereafter be able to establish and operate such a production facility.
| 30 |
The deviations in the Company’s proposed new MultiTAA-based products from existing products may require the Company to perform additional testing, which will increase the cost, and extend the time for obtaining approval.
The Company’s MultiTAA T cell therapy platform is based on the adoptive T cell therapy technology that the Company licensed from BCM and that is presently available as a physician-sponsored investigational therapy at BCM for the treatment of lymphoma, AML/MDS and multiple myeloma in the U.S.. The current method of treatment is labor intensive and expensive. The Company is performing process optimization that it anticipates will enable more efficient manufacturing of its products. The Company may have difficulty demonstrating that the products produced from its new processes are identical to the existing products. The FDA may require additional clinical testing before permitting a larger clinical trial with the new processes, and also the product may not be as efficacious in the new clinical trials. Cellular products are not considered to be well characterized products because there are hundreds of markers present on these cells, and even small changes in manufacturing processes could alter the cell types. It is unclear at this time which of those the markers are critical for success of these cells to combat cancer, so the Company’s ability to predict the outcomes with newer manufacturing processes is limited. The changes that the Company may make to the existing manufacturing process may require additional testing, which may increase costs and timelines associated with these developments. In addition to developing a multi-antigen T cell-based therapy on existing adoptive T cell therapy technology, the Company is currently evaluating the desirability of conducting clinical trials of the Company’s products in combination with other existing drugs. These combination therapies will require additional testing, and clinical trials will require additional FDA regulatory approval and will increase the Company’s future cost of development.
The Company may enter into one or more transactions with entities controlled by one of its directors, which could pose a conflict of interest.
John Wilson, a director of the Company, is also CEO and co-founder of Wilson Wolf Manufacturing Corporation, which is the sole source vendor that provides the Company with the G-Rex® cell culture device for the large-scale production of T cells used in the Company’s manufacturing process. The Company does not currently have a supply contract with Wilson Wolf Manufacturing for the G-Rex®. The Company plans to negotiate a supply contract with Wilson Wolf Manufacturing for the purchase of G-Rex® devices. The Company also plans to engage Wilson Wolf Manufacturing in discussions to customize the G-Rex® further to optimally match the Company’s manufacturing requirements, as well as to develop a scalability plan to drive efficiencies for a commercial product. There may be conflicts of interest between the Company and Wilson Wolf Manufacturing. There can be no assurance that Wilson Wolf Manufacturing will agree to enter into any contract with the Company, or that the terms of any such agreements will be in the best interests of the Company or will have terms no less favorable to the Company than could have been obtained from unaffiliated third parties.
The Company may not be able to develop products successfully or develop them on a timely basis.
The Company’s immunotherapy product candidates are at various stages of research and development. Further development and extensive testing will be required to determine their technical feasibility and commercial viability. The Company will need to complete significant additional clinical trials demonstrating that its product candidates are safe and effective to the satisfaction of the FDA and other non-U.S. regulatory authorities. The drug approval process is time-consuming, which involves substantial expenditures of resources, and depends upon a number of factors, including the severity of the illness in question, the availability of alternative treatments, and the risks and benefits demonstrated in the clinical trials. The Company’s success depends on its ability to achieve scientific and technological advances and to translate such advances into licensable, FDA-approvable, commercially-competitive products on a timely basis. Failure can occur at any stage of the process. If such programs are not successful, the Company may be unable to develop revenue-producing products. As the Company enters a more extensive clinical program for its product candidates, the data generated in these studies may not be as compelling as the earlier results.
| 31 |
Immunotherapies that the Company may develop are not likely to be commercially available for at least five years. Any delay in obtaining FDA and/or other necessary regulatory approvals in the United States and in countries outside the United States for any investigational new drug and failure to receive such approvals would have an adverse effect on the investigational new drug’s potential commercial success and on the Company’s business, prospects, financial condition and results of operations. The time required to obtain approval by the FDA and non-U.S. regulatory authorities is unpredictable but typically takes many years following the commencement of clinical trials and depends upon numerous factors, including the substantial discretion of the regulatory authorities. For example, the FDA or non-U.S. regulatory authorities may disagree with the design or implementation of the Company’s clinical trials or study endpoints; or the Company may be unable to demonstrate that a product candidate’s clinical and other benefits outweigh its safety risks. In addition, the FDA or non-U.S. regulatory authorities may disagree with the Company’s interpretation of data from preclinical studies or clinical trials or the data collected from clinical trials of its product candidates may not be sufficient to support the submission of a new drug application (“NDA”) or other submission or to obtain regulatory approval in the United States or elsewhere. The FDA or non-U.S. regulatory authorities may fail to approve the manufacturing processes or facilities of third-party manufacturers with which the Company contracts for clinical and commercial supplies; and the approval policies or regulations of the FDA or non-U.S. regulatory authorities may significantly change in a manner rendering the Company’s clinical data insufficient for approval. In addition, approval policies, regulations, or the type and amount of clinical data necessary to gain approval may change during the course of a product candidate’s clinical development and may vary among jurisdictions. The proposed development schedules for the Company’s immunotherapy product candidates may be affected by a variety of other factors, including technological difficulties, clinical trial failures, regulatory hurdles, competitive products, intellectual property challenges and/or changes in governmental regulation, many of which will not be within its control.
Any delay in the development, approval, introduction or marketing of the Company’s products could result either in such products being marketed at a time when their cost and performance characteristics would not be competitive in the marketplace or in the shortening of their commercial lives. In light of the long-term nature of the Company’s projects, the unproven technology involved and the other factors described elsewhere in this section, the Company might not be able to successfully complete the development or marketing of any new products, and as a result, its business, prospects, financial condition and results of operations could be materially and adversely affected. The Company may be required to reduce its staff, discontinue certain research or development programs of its future products and cease to operate.
The Company may encounter substantial delays in its clinical trials or may not be able to conduct its trials on the timelines it expects.
Clinical testing is expensive, time-consuming, and subject to uncertainty. The Company cannot guarantee that any clinical studies will be conducted as planned or completed on schedule, if at all. BCM has submitted INDs to the FDA, which allow the use of the Mixed Antigen Peptide Pool, or MAPP, T cells and the Leukemia Antigen Peptide Pool, or LAPP, T cells for human clinical testing. BCM initiated its first clinical trials for the Company’s product candidate, MAPP, in 2012, and clinical trials for LAPP in 2016. Issues may yet arise that could suspend or terminate such clinical trials. The Company intends to file one or more new INDs to advance these products into Phase II clinical trials, and any delay in filing these INDs may have a material adverse impact on the Company’s ability to advance clinical studies in accordance with management’s plans. A failure of one or more clinical studies can occur at any stage of testing, and the Company’s future clinical studies may not be successful. Events that may prevent successful or timely completion of clinical development include:
| • | inability to generate sufficient preclinical data to support the initiation of clinical studies; |
| • | delays in reaching a consensus with regulatory agencies on study design; |
| • | the FDA may not allow the Company to use the clinical trial data from a research institution to support an IND if the Company cannot demonstrate the comparability of its product candidates with the product candidate used by the relevant research institution in its clinical studies; |
| • | delays in reaching agreement on acceptable terms with prospective contract research organizations, or CROs, and clinical study sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and clinical study sites; |
| • | delays in obtaining required Institutional Review Board (“IRB”) approval at each clinical study site; |
| • | the departure of a principal investigator from a clinical site, which could cause delays in conducting the clinical trial at a particular clinical site; |
| 32 |
| • | imposition of a temporary or permanent clinical hold by regulatory agencies; |
| • | delays in recruiting suitable patients to participate in the Company’s clinical studies; |
| • | failure by the Company’s CROs, other third parties, or the Company to adhere to clinical study requirements; |
| • | failure to perform in accordance with the FDA’s current good clinical practices (“cGCPs”) requirements, or applicable regulatory guidelines in other countries; |
| • | patients dropping out of a study; |
| • | occurrence of adverse events associated with the product candidate that are viewed to outweigh its potential benefits; |
| • | changes in regulatory requirements and guidance that require amending or submitting new clinical protocols; |
| • | changes in the standard of care on which a clinical development plan was based, which may require new or additional trials; |
| • | the cost of clinical studies of the Company’s product candidates being greater than The Company anticipates; |
| • | clinical studies of the Company’s product candidates producing negative or inconclusive results, which may result in the Company’s deciding, or regulators requiring the Company, to conduct additional clinical studies or abandon product development programs; |
| • | delays in transfer of manufacturing processes for MultiTAA T cells from BCM to the Company’s contract manufacturers or other larger-scale facilities operated by a CMO, delays or failure by the Company’s CMOs or the Company to make any necessary changes to such manufacturing process, and any inability to obtain all necessary reagents for manufacturing the product; |
| • | any shutdown of the Company’s sole manufacturing site at BCM for MultiTAA T cells, which would render the Company unable to produce such products for clinical trials; |
| • | disruptions in transportation between the clinical site and manufacturing facility; and |
| • | delays in manufacturing, testing, release, validating, or import/export of sufficient stable quantities of the Company’s product candidates for use in clinical studies or the inability to do any of the foregoing, including any quality issues associated with the contract manufacturer. |
The Company also may conduct clinical and preclinical research in collaboration with other biotechnology and biologics entities in which the Company combines its technologies with those of the Company’s collaborators. Such collaborations may be subject to additional delays because of the management of the trials and the necessity of obtaining additional approvals for therapeutics used in the combination trials. These combination therapies will require additional testing and clinical trials will require additional FDA regulatory approval and will increase the Company’s future expenses.
Any inability to successfully complete preclinical and clinical development could result in additional costs to the Company or impair the Company’s ability to generate revenue. In addition, if the Company makes manufacturing or formulation changes to its product candidates, the Company may be required, or may elect, to conduct additional studies to bridge its modified product candidates to earlier versions. Clinical study delays could also shorten any periods during which the Company’s products have patent protection and may allow the Company’s competitors to bring products to market before the Company does, which could impair the Company’s ability to commercialize its product candidates successfully and may harm the Company’s business and the results of its operations.
| 33 |
The Company’s commercial success depends upon attaining significant market acceptance of its product candidates, if approved, among physicians, patients, healthcare payors and the medical community.
Even if the Company obtains regulatory approval for its product candidates, they may not gain market acceptance among physicians, healthcare payors, patients or the medical community. Market acceptance of the Company’s product candidates, if it receives approval, depends on a number of factors, including the:
| • | efficacy and safety of the Company’s product candidates as demonstrated in clinical trials and post-marketing experience; |
| • | clinical indications for which the Company’s product candidates may be approved; |
| • | acceptance by physicians and patients of the Company’s product candidates as safe and effective; |
| • | potential and perceived advantages of the Company’s product candidates over alternative treatments; |
| • | safety of the Company’s product candidates seen in a broader patient group, including its use outside the approved indications should physicians choose to prescribe for such uses; |
| • | prevalence and severity of any side effects; |
| • | product labeling or product insert requirements of the FDA or other regulatory authorities; |
| • | timing of market introduction of the Company’s product candidates as well as competitive products; |
| • | cost in relation to alternative treatments; |
| • | availability of coverage and adequate reimbursement and pricing by third-party payors and government authorities; |
| • | relative convenience and ease of administration; and |
| • | effectiveness of any sales and marketing efforts. |
Moreover, if the Company’s product candidates are approved but fail to achieve market acceptance among physicians, patients, healthcare payors and the medical community, it may not be able to generate significant revenues, which would compromise its ability to become profitable.
The Company may not be able to establish or maintain the third-party relationships that are necessary to develop or potentially commercialize some or all of its product candidates.
The Company expects to depend on collaborators, partners, licensees, clinical research organizations and other third parties to support its discovery efforts, to formulate product candidates, to manufacture its product candidates, and to conduct clinical trials for some or all of its product candidates. The Company cannot guarantee that it will be able to successfully negotiate agreements for or maintain relationships with collaborators, partners, licensees, clinical investigators, vendors and other third parties on favorable terms, if at all. The Company’s ability to successfully negotiate such agreements will depend on, among other things, potential partners’ evaluation of the superiority of its technology over competing technologies and the quality of the preclinical and clinical data that it has generated, and the perceived risks specific to developing its product candidates. If the Company is unable to obtain or maintain these agreements, it may not be able to clinically develop, formulate, manufacture, obtain regulatory approvals for or commercialize its product candidates.
| 34 |
Issued patents covering the Company’s product candidates could be found invalid or unenforceable if challenged in court or with the USPTO.
If the Company, its licensing partners, or any potential future collaborator initiates legal proceedings against a third party to enforce a patent directed to one of the Company’s product candidates, the defendant could counterclaim that the patent is invalid and/or unenforceable in whole or in part. In patent litigation in the United States, defendant counterclaims alleging invalidity and/or unenforceability are commonplace. Grounds for a validity challenge include an alleged failure to meet any of several statutory requirements, including lack of novelty, obviousness or non-enablement. Grounds for an unenforceability assertion could include an allegation that someone connected with prosecution of the patent withheld relevant information from the USPTO, or made a misleading statement during prosecution. Third parties may also raise similar claims before administrative bodies in the United States or abroad, even outside the context of litigation. Such mechanisms include re-examination, post grant review, and equivalent proceedings in foreign jurisdictions (e.g., opposition proceedings). Such proceedings could result in revocation or amendment to the Company’s patents in such a way that they are no longer directed to the Company’s product candidates. The outcome following legal assertions of invalidity and unenforceability is unpredictable, and prior art could render the Company’s patents or those of the Company’s licensors invalid or could prevent a patent from issuing from one or more of its pending patent applications. There is no assurance that all potentially relevant prior art relating to the Company patents and patent applications has been found. There is also no assurance that there is not prior art of which the Company is aware, but which the Company does not believe affects the validity or enforceability of a claim in the Company patents and patent applications, which may, nonetheless, ultimately be found to affect the validity or enforceability of a claim. Furthermore, even if the Company patents are unchallenged, they may not adequately protect the Company’s intellectual property, provide exclusivity for the Company product candidates, prevent others from designing around the Company claims or provide the Company with a competitive advantage. If a defendant were to prevail on a legal assertion of invalidity and/or unenforceability, The Company would lose at least part, and perhaps all, of the patent protection on the Company’s product candidates. In addition, if the breadth or strength of protection provided by the Company’s patents and patent applications is threatened, it could dissuade companies from collaborating with the Company to license, develop or commercialize current or future product candidates. Such a loss of patent protection could have a material adverse impact on the Company’s business development.
If the Company is unable to protect its proprietary rights, the Company may not be able to compete effectively or operate profitably.
The Company’s commercial success is dependent in part on its ability to obtain, maintain, and enforce the patents and other proprietary rights that it has licensed and may develop, and on its ability to avoid infringing the proprietary rights of others. The Company generally seeks to protect its proprietary position by filing patent applications in the United States and abroad related to its product candidates, proprietary technologies and their uses that are important to its business. The Company’s patent applications cannot be enforced against third parties practicing the technology claimed in such applications unless, and until, patents issue from such applications, and then only to the extent the issued claims are directed to the technology. There can be no assurance that the Company’s patent applications or those of its licensor will result in additional patents being issued or that issued patents will afford sufficient protection against competitors with similar technology, nor can there be any assurance that the patents issued will not be infringed, designed around or invalidated by third parties. Even issued patents may later be found invalid or unenforceable or may be modified or revoked in proceedings instituted by third parties before various patent offices or in courts. The degree of future protection for the Company’s proprietary rights is uncertain. Only limited protection may be available and may not adequately protect the Company’s rights or permit the Company to gain or keep any competitive advantage. This failure to properly protect the intellectual property rights relating to the Company’s product candidates could have a material adverse effect on the Company’s financial condition and results of operations.
The Company seeks to protect its proprietary technology and processes, in part, by entering into confidentiality agreements with relevant employees, consultants, scientific advisors, and contractors. The Company also seeks to preserve the integrity and confidentiality of its data and trade secrets by maintaining physical security of the premises and physical and electronic security of the information technology systems. While the Company has confidence in these individuals, organizations, and systems, agreements or security measures may be breached, and the Company may not have adequate remedies for any breach. In addition, trade secrets may otherwise become known or be independently discovered by competitors. To the extent that the consultants, contractors or collaborators use intellectual property owned by others in their work for the Company, disputes may arise as to the rights in related or resulting know-how and inventions.
| 35 |
Although the Company has patents and patent applications in other countries, the Company cannot be certain that the claims in other pending U.S. or European patent applications, international patent applications, and patent applications in certain other foreign territories directed to methods of generating multi-antigen specific T cell products, or the Company’s other product candidates, will be considered patentable by the USPTO, courts in the United States or by the patent offices and courts in foreign countries, nor can the Company be certain that the claims in its issued European patent will not be found invalid or unenforceable if challenged.
Most of the Company’s intellectual property rights are currently licensed from BCM and the Mayo Foundation, so that the preparation and prosecution of these patents and patent applications was not performed by the Company or under the Company’s control. Furthermore, patent law relating to the scope of claims in the biotechnology field in which it operates is still evolving and, consequently, patent positions in the Company’s industry may not be as strong as in other more well-established fields. The patent positions of biotechnology companies can be highly uncertain and involve complex legal and factual questions for which important legal principles remain unresolved. No consistent policy regarding the breadth of claims allowed in biotechnology patents has emerged to date. The patent application process is subject to numerous risks and uncertainties, and there can be no assurance that the Company or any of its potential future collaborators will be successful in protecting the Company product candidates by obtaining and defending patents. These risks and uncertainties include the following:
| • | the USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other provisions during the patent process, the noncompliance with which can result in abandonment or lapse of a patent or patent application, and partial or complete loss of patent rights in the relevant jurisdiction; |
| • | patent applications may not result in any patents being issued; |
| • | patents that may be issued or in-licensed may be challenged, invalidated, modified, revoked, circumvented, found to be unenforceable or otherwise may not provide any competitive advantage; |
| • | the Company’s competitors, many of whom have substantially greater resources than the Company, and many of whom have made significant investments in competing technologies, may seek or may have already obtained patents that will limit, interfere with or eliminate the Company’s ability to make, use and sell the Company’s potential product candidates; |
| • | there may be significant pressure on the U.S. government and international governmental bodies to limit the scope of patent protection both inside and outside the United States for disease treatments that prove successful, as a matter of public policy regarding worldwide health concerns; and |
| • | countries other than the United States may have patent laws less favorable to patentees than those upheld by U.S. courts, allowing foreign competitors a better opportunity to create, develop and market competing product candidates. |
The patent prosecution process is also expensive and time-consuming, and the Company may not be able to file and prosecute all necessary or desirable patent applications at a reasonable cost or in a timely manner or in all jurisdictions where protection may be commercially advantageous. It is also possible that the Company will fail to identify patentable aspects of the Company’s research and development output before it is too late to obtain patent protection. Moreover, in some circumstances, the Company may not have the right to control the preparation, filing and prosecution of patent applications, or to maintain the patents, directed to technology that the Company licenses from third parties. The Company may also require the cooperation of one of the Company’s licensors in order to enforce the licensed patent rights, and such cooperation may not be provided. Therefore, these patents and applications may not be prosecuted and enforced in a manner consistent with the best interests of the Company’s business. The Company cannot be certain that patent prosecution and maintenance activities by the Company’s licensor have been or will be conducted in compliance with applicable laws and regulations, which may affect the validity and enforceability of such patents or any patents that may issue from such applications. If they fail to do so, this could cause the Company to lose rights in any applicable intellectual property that the Company in-licenses, and as a result the Company’s ability to develop and commercialize products or product candidates may be adversely affected and the Company may be unable to prevent competitors from making, using and selling competing products.
| 36 |
In addition, identification of third-party patent rights that may be relevant to the Company’s technology is difficult because patent searching is imperfect due to differences in terminology among patents, incomplete databases and the difficulty in assessing the meaning of patent claims. The issuance of a patent is not conclusive as to its inventorship, scope, validity or enforceability and it is uncertain how much protection, if any, will be given to the patents the Company has licensed from a licensor if either the licensor or the Company attempts to enforce the patents and/or if they are challenged in court or in other proceedings, such as oppositions, which may be brought in foreign jurisdictions to challenge the validity of a patent. A third party may challenge the Company’s patents, if issued, or the patent rights that it licenses from others in the courts or patent offices in the United States and abroad. It is possible that a competitor may successfully challenge the Company’s patents or that a challenge will result in loss of exclusivity or in patent claims being narrowed, invalidated or held unenforceable, which could limit the Company’s ability to stop others from using or commercializing similar or identical products, or limit the duration of the patent protection of the Company’s products and product candidates. Moreover, the cost of litigation to uphold the validity of patents and to prevent infringement can be substantial. If the outcome of litigation is adverse to the Company, third parties may be able to use the Company’s patented invention without payment to it. Moreover, it is possible that competitors may infringe the Company’s patents or successfully avoid them through design innovation. To stop these activities the Company may need to file a lawsuit. These lawsuits are expensive and would consume time and other resources, even if the Company were successful in stopping the violation of the Company’s patent rights. In addition, there is a risk that a court would decide that the Company’s patents are not valid and that the Company does not have the right to stop the other party from using the inventions. There is also the risk that, even if the validity of the Company’s patents were upheld, a court would refuse to stop the other party on the ground that its activities are not covered by, that is, do not infringe, the Company’s patents.
Should third parties file patent applications, or be issued patents claiming technology also used or claimed by the Company’s licensor(s) or by the Company in any future patent application, the Company may be required to participate in interference proceedings in the USPTO to determine priority of invention for those patents or patent applications that are subject to the first-to-invent law in the United States, or may be required to participate in derivation proceedings in the USPTO for those patents or patent applications that are subject to the first-inventor-to-file law in the United States. The Company may be required to participate in such interference or derivation proceedings involving the Company’s issued patents and pending applications. The Company may be required to cease using the technology or to license rights from prevailing third parties as a result of an unfavorable outcome in an interference proceeding or derivation proceeding. A prevailing party in that case may not offer the Company a license on commercially acceptable terms.
The use of the Company’s technologies could potentially conflict with the rights of others.
The Company’s potential competitors or other entities may have or acquire patent or proprietary rights that they could enforce against the Company’s licensors. There is a substantial amount of litigation, both within and outside the United States, involving patent and other intellectual property rights in the biotechnology and pharmaceutical industries, including patent infringement lawsuits, interferences, oppositions, reexaminations, inter partes review proceedings and post-grant review, or PGR, proceedings before the USPTO and/or corresponding foreign patent offices. Numerous third-party U.S. and foreign issued patents and pending patent applications exist in the fields in which the Company is developing product candidates. There may be third-party patents or patent applications with claims to materials, formulations, methods of manufacture or methods for treatment related to the use or manufacture of the Company’s product candidates. If they do so, then they could limit the Company’s ability to make, use, sell, offer for sale or import its product candidates and products that may be approved in the future, or impair its competitive position by requiring the Company to alter its products, pay licensing fees or cease activities.
| 37 |
As the biotechnology industry expands and more patents are issued, the risk increases that the Company’s product candidates may be subject to claims of infringement of the patent rights of third parties. Because patent applications are maintained as confidential for a certain period of time, until the relevant application is published the Company may be unaware of third-party patents that may be infringed by commercialization of any of the Company’s product candidates, and the Company cannot be certain that it was the first to file a patent application related to a product candidate or technology. Moreover, because patent applications can take many years to issue, there may be currently-pending patent applications that later issue as patents that the Company’s product candidates may infringe. If the Company’s products conflict with patent rights of others, third parties could bring legal actions against the Company or its collaborators, licensees, suppliers or customers, claiming damages and seeking to enjoin manufacturing and marketing of the affected products. If these legal actions are successful, in addition to any potential liability for damages, the Company could be required to obtain a license in order to continue to manufacture or market the affected products. The Company may not prevail in any legal action and a required license under the patent may not be available on acceptable terms or at all.
Changes in U.S. patent law could diminish the value of patents in general, thereby impairing the Company’s ability to protect its products.
As is the case with other biopharmaceutical companies, the Company’s success is dependent on intellectual property, particularly patents. Obtaining and enforcing patents in the biopharmaceutical industry involve both technological and legal complexity, and is therefore costly, time-consuming and inherently uncertain. Changes in either the patent laws or in the interpretations of patent laws in the United States and other countries may diminish the value of the Company’s intellectual property. The Company cannot predict the breadth of claims that may be allowed or enforced in its patents or in third-party patents. For example, on September 16, 2011, the Leahy-Smith America Invents Act, or Leahy-Smith Act, was signed into law. The Leahy-Smith Act includes a number of significant changes to U.S. patent law. These include provisions that affect the way patent applications will be prosecuted and may also affect patent litigation. In particular, under the Leahy-Smith Act, the United States transitioned in March 2013 to a “first inventor to file” system in which the first inventor to file a patent application will be entitled to the patent. Third parties are allowed to submit prior art before the issuance of a patent by the USPTO and may become involved in post-grant proceedings including post grant review, derivation, reexamination, inter-partes review or interference proceedings challenging the Company’s patent rights or the patent rights of others. An adverse determination in any such submission, proceeding or litigation could reduce the scope or enforceability of, or invalidate, the Company’s patent rights, which could adversely affect the Company’s competitive position. In addition, recent U.S. Supreme Court rulings on several patent cases have narrowed the scope of patent protection available in certain circumstances and weakened the rights of patent owners in certain situations. In addition to increasing uncertainty with regard to the Company’s ability to obtain patents in the future, this combination of events has created uncertainty with respect to the value of patents, once obtained. Depending on decisions by the U.S. Congress, the federal courts, and the USPTO, the laws and regulations governing patents could change in unpredictable ways that would weaken the Company’s ability to obtain new patents or to enforce the Company’s existing patents and patents that the Company might obtain in the future. While the Company does not believe that any of the patents owned or licensed by the Company will be found invalid based on these decisions, the Company cannot predict how future decisions by the courts, the U.S. Congress or the USPTO may impact the value of the Company’s patents.
The Company has limited foreign intellectual property rights and may not be able to protect its intellectual property rights throughout the world.
The Company has limited intellectual property rights outside the United States. Filing, prosecuting and defending patents on product candidates in all countries throughout the world would be prohibitively expensive, and the Company’s intellectual property rights in some countries outside the United States can be less extensive than those in the United States. In addition, the laws of some foreign countries do not protect intellectual property rights to the same extent as federal and state laws in the United States. Consequently, the Company may not be able to prevent third parties from practicing its inventions in all countries outside the United States, or from selling or importing products made using its inventions in and into the United States or other jurisdictions. Competitors may use the Company’s technologies in jurisdictions where the Company has not obtained patent protection to develop their own products and further, may export otherwise infringing products to territories where the Company has patent protection, but enforcement is not as strong as that in the United States. These products may compete with the Company’s products and patents or other intellectual property rights may not be effective or sufficient to prevent them from competing.
| 38 |
Many companies have encountered significant problems in protecting and defending intellectual property rights in foreign jurisdictions. The legal systems of certain countries, particularly certain developing countries, do not favor the enforcement of patents, trade secrets and other intellectual property protection, particularly those relating to biopharmaceutical products, which could make it difficult for the Company to stop the infringement of its patents or marketing of competing products in violation of the Company’s proprietary rights generally. Proceedings to enforce the Company’s patent rights in foreign jurisdictions could result in substantial costs and divert the Company’s efforts and attention from other aspects of the Company’s business, could put the Company’s patents at risk of being invalidated or interpreted narrowly and the Company’s patent applications at risk of not issuing and could provoke third parties to assert claims against the Company. The Company may not prevail in any lawsuits that it initiates and the damages or other remedies awarded, if any, may not be commercially meaningful. Accordingly, the Company’s efforts to enforce the Company’s intellectual property rights around the world may be inadequate to obtain a significant commercial advantage from the intellectual property that the Company develops or licenses.
The Company may be subject to claims that its employees, consultants or independent contractors have wrongfully used or disclosed confidential information of third parties.
As is common in the biotechnology and pharmaceutical industries, in addition to the Company’s employees, the Company engages the services of consultants to assist it in the development of its product candidates. The Company has received confidential and proprietary information from third parties. The Company employs individuals or engages consultants who were previously employed at other biotechnology or pharmaceutical companies. The Company may be subject to claims that it or its employees, consultants or independent contractors have inadvertently or otherwise used or disclosed confidential information of these third parties or the Company’s employees’ former employers. Litigation may be necessary to defend against these claims. Even if the Company is successful in defending against these claims, litigation could result in substantial cost and be a distraction to the Company’s management and employees.
If the Company fails to comply with any obligations under its existing license agreements or any future license agreements, or disputes arise with respect to those agreements, it could have a negative impact on its business and its intellectual property rights.
The Company is a party to a license agreements with BCM and the Mayo Foundation that impose, and the Company may enter into additional licensing arrangements with third parties that may impose, diligence, development and commercialization timelines, milestone payment, royalty, insurance and other obligations on it. The Company’s rights to use the licensed intellectual property are subject to the continuation of and the Company’s compliance with the terms of these agreements. Disputes may arise regarding the Company’s rights to intellectual property licensed to it from a third party, including but not limited to:
| • | the scope of rights granted under the license agreement and other interpretation-related issues; |
| • | the extent to which the Company technology and processes infringe on intellectual property of the licensor that is not subject to the licensing agreement; |
| • | the sublicensing of patent and other rights; |
| • | the Company’s diligence obligations under the license agreement and what activities satisfy those diligence obligations; |
| • | the ownership of inventions and know-how resulting from the creation or use of intellectual property by the Company, alone or with its licensors and collaborators; |
| • | the scope and duration of the Company’s payment obligations; |
| • | the Company’s rights upon termination of such agreement; and |
| • | the scope and duration of exclusivity obligations of each party to the agreement. |
| 39 |
If disputes over intellectual property and other rights that the Company has licensed or acquired from third parties prevent or impair its ability to maintain its current licensing arrangements on acceptable terms, the Company may be unable to successfully develop and commercialize the affected product candidates. If the Company fails to comply with its obligations under current or future licensing agreements, these agreements may be terminated or the scope of the Company’s rights under them may be reduced and the Company might be unable to develop, manufacture or market any product that is licensed under these agreements.
The Company may be subject to claims challenging the inventorship or ownership of the Company patents and other intellectual property.
The Company may be subject to claims that former employees, collaborators or other third parties have an ownership interest in the Company patents or other intellectual property. Litigation may be necessary to defend against these and other claims challenging inventorship or ownership. If the Company fails in defending any such claims, in addition to paying monetary damages, the Company may lose valuable intellectual property rights. Such an outcome could have a material adverse effect on the Company’s business. Even if the Company is successful in defending against such claims, litigation could result in substantial costs and distraction to management and other employees.
Patent terms may be inadequate to protect the Company’s competitive position on the Company’s product candidates for an adequate amount of time.
Patents have a limited lifespan. In the United States, if all maintenance fees are timely paid, the natural expiration of a patent is generally 20 years from its earliest U.S. non-provisional filing date. Various extensions may be available, but the life of a patent, and the protection it affords, is limited. Even if patents covering the Company’s product candidates are obtained, once the patent life has expired, the Company may be subject to competition from competitive products, including biosimilars. Given the amount of time required for the development, testing and regulatory review of new product candidates, patents protecting such candidates might expire before or shortly after such candidates are commercialized. As a result, the Company’s owned and licensed patent portfolio may not provide sufficient rights to exclude others from commercializing products similar or identical to the Company’s products.
Certain of the Company’s technologies are in-licensed from third parties, and the protection of those technologies is not entirely within its control.
The Company has world-wide exclusive licenses from the Mayo Foundation on (i) a novel set of Class II HER2/neu peptide antigens, (ii) a novel Class I HER2/neu antigen, and (iii) a novel set of Class II Folate Receptor Alpha peptide antigens. The Company has a world-wide exclusive license from BCM of the rights in and to three patent families to develop and commercialize MultiTAA product candidates. As a result of these in-licenses, the Company could lose the right to develop each of the technologies if:
| • | the owners of the patent rights underlying the technologies that the Company licenses do not properly maintain or enforce the patents and intellectual property underlying those properties, |
| • | the Mayo Foundation or BCM seeks to terminate the Company’s license in contravention of the license agreements; |
| • | the Company fails to make all payments due and owing under any of the licenses; or |
| • | the Company fails to obtain on commercially reasonable terms, if at all, in-licenses from the Mayo Clinic or BCM or other for other rights that are necessary to develop the technology that the Company has already in-licensed. |
| 40 |
If any of the above occurs, the Company could lose the right to use the in-licensed intellectual property, which would adversely affect its ability to commercialize its technologies, products or services. The loss of any current or future licenses from Mayo Clinic or BCM, or the exclusivity rights provided such license agreements could materially harm the Company’s financial condition and operating results.
The Company relies upon patents and licensed technologies to protect its technology. The Company may be unable to protect its intellectual property rights, and it may be liable for infringing the intellectual property rights of others.
The Company’s ability to compete effectively depends on its ability to maintain the proprietary nature of its technologies, including PolyStart™, and the proprietary technology of others with whom it has entered into collaboration and licensing agreements. The Company owns or holds licenses to a number of issued patents and U.S. pending patent applications, as well as foreign patents and foreign counterparts. The Company’s success depends in part on its ability to obtain patent protection both in the United States and abroad for its product candidates, as well as the methods for treating patients in the product indications using these product candidates. Such patent protection is costly to obtain and maintain, and sufficient funds might not be available. The Company’s ability to protect its product candidates from unauthorized or infringing use by third parties depends in substantial part on its ability to obtain and maintain valid and enforceable patents. Due to evolving legal standards relating to the patentability, validity and enforceability of patents covering pharmaceutical inventions and the scope of claims made under these patents, the Company’s ability to obtain, maintain and enforce patents is uncertain and involves complex legal and factual questions. Even if the Company’s product candidates, as well as methods for treating patients for prescribed indications using these product candidates are covered by valid and enforceable patents and have claims with sufficient scope, disclosure and support in the specification, the patents will provide protection only for a limited amount of time. Accordingly, rights under any issued patents may not provide the Company with sufficient protection for its product candidates or provide sufficient protection to afford it a commercial advantage against competitive products or processes.
In addition, the Company cannot guarantee that any patents will be issued from any pending or future patent applications owned by or licensed to it. Even if patents have been issued or will be issued, the Company cannot guarantee that the claims of these patents are or will be valid or enforceable or will provide it with any significant protection against competitive products or otherwise be commercially valuable to it. The laws of some foreign jurisdictions do not protect intellectual property rights to the same extent as in the United States and many companies have encountered significant difficulties in protecting and defending such rights in foreign jurisdictions. Furthermore, different countries have different procedures for obtaining patents, and patents issued in different countries offer different degrees of protection against use of the patented invention by others. If the Company encounters such difficulties in protecting or are otherwise precluded from effectively protecting its intellectual property rights in foreign jurisdictions, the Company’s business prospects could be substantially harmed.
The patent positions of biotechnology and pharmaceutical companies, including the Company’s patent positions, involve complex legal and factual questions, and, therefore, validity and enforceability cannot be predicted with certainty. Patents may be challenged, deemed unenforceable, invalidated, or circumvented. The Company’s patents can be challenged by its competitors who can argue that its patents are invalid, unenforceable, lack sufficient written description or enablement, or that the claims of the issued patents should be limited or narrowly construed. Patents also will not protect the Company’s product candidates if competitors devise ways of making or using these product candidates without infringing its patents.
The Company will be able to protect its proprietary rights from unauthorized use by third parties only to the extent that its technologies, methods of treatment, product candidates, and any future products are covered by valid and enforceable patents or are effectively maintained as trade secrets and it has the funds to enforce its rights, if necessary.
The expiration of the Company’s owned or licensed patents before completing the research and development of its product candidates and receiving all required approvals in order to sell and distribute the products on a commercial scale can adversely affect its business and results of operations.
| 41 |
The Company may be involved in lawsuits to protect or enforce its patents or the patents of its licensors, which could be expensive, time-consuming and unsuccessful.
Competitors may infringe the Company’s intellectual property rights or those of the Company’s licensors. To counter infringement or unauthorized use, the Company may be required to file infringement claims, which can be expensive and time-consuming. In addition, in a patent infringement proceeding, a court may decide that one or more of the patents the Company owns or in-licenses is not valid or is unenforceable, and/or is not infringed. An adverse result in any litigation or defense proceedings could put one or more of the Company’s patents at risk of being invalidated, held unenforceable, or interpreted narrowly and could put the Company’s patent applications at risk of not issuing. Defense of these claims, regardless of their merit, would involve substantial litigation expense and would be a substantial diversion of employee resources from the Company’s business. The Company may not prevail in any lawsuits that it initiates, and the damages or other remedies awarded, if any, may not be commercially meaningful. In the event of a successful claim of infringement against the Company, the Company may have to pay substantial damages, including treble damages and attorneys’ fees for willful infringement, obtain one or more licenses from third parties, pay royalties or redesign the Company’s infringing products, which may be impossible or require substantial time and monetary expenditure.
Periodic maintenance fees, renewal fees, annuity fees and various other governmental fees on any issued patent and/or pending patent applications will be due to the USPTO and foreign patent agencies in several stages over the lifetime of the Company’s patents and/or applications. The USPTO and various foreign governmental patent agencies require compliance with a number of procedural, documentary, fee payment and other similar provisions during the patent application process. The Company employs reputable law firms and other professionals to help it comply, and in many cases, an inadvertent lapse can be cured by payment of a late fee or by other means in accordance with rules applicable to the particular jurisdiction. However, there are situations in which noncompliance can result in abandonment or lapse of the patent or patent application, resulting in partial or complete loss of patent rights in the relevant jurisdiction. Noncompliance events that could result in abandonment or lapse of a patent or patent application include, but are not limited to, failure to respond to official actions within prescribed time limits, non-payment of fees and failure to properly legalize and submit formal documents. In such an event, the Company’s competitors might be able to enter the market, which would have a material adverse effect on the Company’s business development.
Interference or derivation proceedings provoked by third parties or brought by the Company or declared by the USPTO may be necessary to determine the priority of inventions with respect to the Company’s patents or patent applications or those of the Company’s licensors. Should third parties file patent applications or be issued patents claiming technology also used or claimed by the Company, the Company may be required to participate in interference or derivation proceedings in the USPTO to determine priority of invention. The Company may be required to participate in interference or derivation proceedings involving its issued patents and pending applications. An unfavorable outcome could require the Company to cease using the related technology or to attempt to license rights from the prevailing party. The business of the Company could be harmed if the prevailing party does not offer the Company a license on commercially acceptable terms.
The Company may be unable to adequately prevent disclosure of trade secrets and other proprietary information.
The Company also relies on trade secrets to protect its proprietary technologies, especially where it does not believe patent protection is appropriate or obtainable. However, trade secrets are difficult to protect. The Company relies in part on confidentiality agreements with its employees, consultants, outside scientific collaborators, sponsored researchers, and other advisors to protect its trade secrets and other proprietary information. These agreements may not effectively prevent disclosure of confidential information and may not provide an adequate remedy in the event of unauthorized disclosure of confidential information. In addition, others may independently discover the Company’s trade secrets and proprietary information. Costly and time-consuming litigation could be necessary to enforce and determine the scope of the Company’s proprietary rights, and failure to obtain or maintain trade secret protection could adversely affect its competitive business position.
| 42 |
If the Company is unable to obtain licenses needed for the development of its product candidates, or if it breaches any of the agreements under which it licenses rights to patents or other intellectual property from third parties, the Company could lose license rights that are important to its business.
If the Company is unable to maintain and/or obtain licenses needed for the development of its product candidates in the future, it may have to develop alternatives to avoid infringing on the patents of others, potentially causing increased costs and delays in drug development and introduction or precluding the development, manufacture, or sale of planned products. Some of the Company’s licenses provide for limited periods of exclusivity that require minimum license fees and payments and/or may be extended only with the consent of the licensor. The Company might not meet these minimum license fees in the future or these third parties might not grant extensions on any or all such licenses. This same restriction may be contained in licenses obtained in the future.
Additionally, the patents underlying the licenses might not be valid and enforceable. To the extent any products developed by the Company are based on licensed technology, royalty payments on the licenses will reduce its gross profit from such product sales and may render the sales of such products uneconomical. In addition, the loss of any current or future licenses or the exclusivity rights provided therein could materially harm the Company’s business financial condition and its operations.
The Company may face legal claims; litigation is expensive and it may not be able to afford the costs.
The Company may face legal claims involving stockholders, consumers, competitors, entities from whom it licenses technology, entities with whom it collaborates, persons claiming that it is infringing on their intellectual property and others. The biotechnology and pharmaceutical industries have been characterized by extensive litigation regarding patents and other intellectual property rights, and companies have employed intellectual property litigation to gain a competitive advantage. The Company may initiate or become subject to infringement claims or litigation arising out of patents and pending applications of its competitors, or it may become subject to proceedings initiated by its competitors or other third parties or the USPTO or applicable foreign bodies to reexamine the patentability of its licensed or owned patents. In addition, litigation may be necessary to enforce the Company’s issued patents, to protect its trade secrets and know-how, or to determine the enforceability, scope, and validity of the proprietary rights of others.
The costs of litigation or any proceeding relating to the Company’s intellectual property or contractual rights could be substantial even if resolved in its favor. Some of the Company’s competitors or financial funding sources have far greater resources than it does and may be better able to afford the costs of complex legal procedures. Also, in a law suit for infringement or contractual breaches, even if frivolous, the Company will require considerable time commitments on the part of management, its attorneys and consultants. Defending these types of proceedings or legal actions involve considerable expense and could negatively affect the Company’s financial results.
The Company’s research and development programs are subject to uncertainty.
Factors affecting the Company’s research and development programs include, but are not limited to:
| • | competition from companies that are substantially and financially stronger than the Company; |
| • | the need for acceptance of the Company’s immunotherapies; |
| • | the Company’s ability to anticipate and adapt to a competitive market and rapid technological developments; |
| • | the amount and timing of operating costs and capital expenditures relating to expansion of the Company’s business, operations and infrastructure; |
| • | the need to rely on multiple levels of outside funding due to the length of drug development cycles and governmental approved protocols associated with the pharmaceutical industry; and |
| • | the dependence upon key personnel including key independent consultants and advisors. |
| 43 |
The Company’s research and development expenses may not be consistent from time to time. The Company may be required to accelerate or delay incurring certain expenses depending on the results of its studies and the availability of adequate funding.
If the Company is unable to establish sales and marketing capabilities or enter into agreements with third parties to market and sell its product candidates, it may be unable to generate any revenue.
The Company does not currently have an organization for the sale, marketing and distribution of products and the cost of establishing and maintaining such an organization may exceed the cost-effectiveness of doing so. In order to market any products approved by the FDA or comparable foreign regulatory authorities, the Company must build its sales, marketing, managerial and other non-technical capabilities or make arrangements with third parties to perform these services. If the Company is unable to establish adequate sales, marketing and distribution capabilities, whether independently or with third parties, it may not be able to generate product revenue and may not become profitable. The Company will be competing with many companies that currently have extensive and well-funded sales and marketing operations. Without an internal commercial organization or the support of a third party to perform sales and marketing functions, the Company may be unable to compete successfully against these more established companies.
If the Company is unable to establish or manage strategic collaborations in the future, its revenue and drug development may be limited.
The Company’s strategy includes eventual substantial reliance upon strategic collaborations for marketing and commercialization of its cancer vaccines, and it may rely even more on strategic collaborations for research, development, marketing and commercialization of the Company’s other immunotherapies. If the Company is unsuccessful in securing such strategic collaborations, it may be unable to commercialize its products as it has not yet licensed, marketed or sold any of its immunotherapies or entered into successful collaborations for these services in order to ultimately commercialize its immunotherapies. Establishing strategic collaborations is difficult and time-consuming. The Company’s discussions with potential collaborators may not lead to the establishment of collaborations on favorable terms, if at all. Potential collaborators may reject collaborations based upon their assessment of the Company’s financial, clinical, regulatory or intellectual property position. If the Company successfully establishes new collaborations, these relationships may never result in the successful development or commercialization of its immunotherapies or the generation of sales revenue. To the extent that the Company enters into co-promotion or other collaborative arrangements, its product revenues are likely to be lower than if it directly marketed and sold any products that it may develop.
Management of the Company’s relationships with its collaborators will require:
| • | significant time and effort from its management team; |
| • | coordination of the Company’s research and development programs with the research and development priorities of its collaborators; and |
| • | effective allocation of the Company’s resources to multiple projects. |
If the Company continues to enter into research and development collaborations at the early phases of drug development, its success will in part depend on the performance of its corporate collaborators. The Company will not directly control the amount or timing of resources devoted by its corporate collaborators to activities related to its immunotherapies. The Company’s corporate collaborators may not commit sufficient resources to its research and development programs or the commercialization, marketing or distribution of its immunotherapies. If any corporate collaborator fails to commit sufficient resources, the Company’s preclinical or clinical development programs related to this collaboration could be delayed or terminated. Also, the Company’s collaborators may pursue existing or other development-stage products or alternative technologies in preference to those being developed in collaboration with the Company. Finally, if the Company fails to make required milestones or royalty payments to its collaborators or to observe other obligations in its agreements with them, its collaborators may have the right to terminate those agreements.
| 44 |
The Company may not be able to license newly developed MultiTAA T cell technology from BCM and others.
An important element of the Company’s intellectual property portfolio is to license additional rights and technologies from BCM. The Company’s inability to license the rights and technologies that the Company has identified, or newly developed MultiTAA T cell technology that the Company may in the future identify, could have a material adverse impact on the Company’s ability to complete the development of its products or to develop additional products. No assurance can be given that the Company will be successful in licensing any additional rights or technologies from BCM and others. Failure to obtain additional rights and licenses may detrimentally affect the Company’s planned development of additional product candidates and could increase the cost, and extend the timelines associated with the Company’s development of such other products.
The market opportunities for the Company’s product candidates may be limited to those patients who are ineligible for or have failed prior treatments and may be small.
The FDA often approves new therapies initially only for use in patients with relapsed or refractory metastatic disease. The Company expects to initially seek approval of its product candidates in this setting. Subsequently, for those products that prove to be sufficiently beneficial, if any, The Company would expect to seek approval in earlier lines of treatment and potentially as a first line therapy. There is no guarantee, however, that the Company’s product candidates, even if approved, would be approved for earlier lines of therapy, and, prior to any such approvals, The Company may have to conduct additional clinical trials.
The Company’s projections of both the number of people who have the cancers it is targeting, as well as the subset of people with these cancers in a position to receive second or third line therapy, and who have the potential to benefit from treatment with the Company’s product candidates, are based on the Company’s research and estimates. These estimates have been derived from a variety of sources, including scientific literature, surveys of clinics, patient foundations, or market research by third parties, and may prove to be incorrect. Further, new studies may change the estimated incidence or prevalence of these cancers. The number of treatable patients may turn out to be lower than expected. Additionally, the potentially addressable patient population for the Company’s product candidates may be limited or may not be amenable to treatment with the Company’s product candidates, and may also be limited by the cost of the Company’s treatments and the reimbursement of those treatment costs by third-party payors. For instance, the Company expects its lead product candidate, LAPP, to initially target a small patient population that suffers from AML. Even if the Company obtains significant market share for its product candidates, because the potential target populations are small, the Company may never achieve profitability without obtaining regulatory approval for additional indications.
The Company is required to pay substantial royalties and lump sum milestone payments under the Company’s license agreement with BCM, and The Company must meet certain milestones to maintain the Company’s license rights.
Under the Company’s license agreement with BCM for the Company’s MultiTAA T cell therapy technologies, the Company is currently required to pay both substantial milestone payments and royalties to BCM based on its revenues from sales of its products utilizing the licensed technologies, and these payments could adversely affect the overall profitability for the Company of any products that it may seek to commercialize. In order to maintain its license rights under the BCM license agreement, the Company will need to meet certain specified milestones, subject to certain cure provisions, in the development of its product candidates. There is no assurance that the Company will be successful in meeting all of the milestones in the future on a timely basis or at all.
In addition, upon a liquidity event (as defined in the Company’s BCM license agreement with BCM, but shall not include the merger) of the licensee under the BCM license agreement (which, the licensee shall be the Company), BCM will receive a liquidity incentive payment of 0.5% of the liquidity event proceeds (as defined in the BCM license agreement) received by such licensee or its stockholders in the liquidity event, thereby diluting the amount of proceeds available to the licensee or its stockholders in a liquidity event.
| 45 |
Because the Company’s current products represent, and the Company’s other potential product candidates will represent novel approaches to the treatment of disease, there are many uncertainties regarding the development, the market acceptance, third-party reimbursement coverage and the commercial potential of the Company’s product candidates.
There is no assurance that the approaches offered by the Company’s products will gain broad acceptance among doctors or patients or that governmental agencies or third-party medical insurers will be willing to provide reimbursement coverage for proposed product candidates. Moreover, the Company does not have verifiable internal marketing data regarding the potential size of the commercial market for the Company’s product candidates, nor has the Company obtained independent marketing surveys to verify the potential size of the commercial markets for the Company’s current product candidates or any future product candidates. Since the Company’s current product candidates and any future product candidates will represent new approaches to treating various conditions, it may be difficult, in any event, to accurately estimate the potential revenues from these product candidates. Accordingly, the Company may spend large amounts of money trying to obtain approval for product candidates that have an uncertain commercial market. The market for any products that the Company successfully develops will also depend on the cost of the product. The Company does not yet have sufficient information to reliably estimate what it will cost to commercially manufacture the Company’s current product candidates, and the actual cost to manufacture these products could materially and adversely affect the commercial viability of these products. The Company’s goal is to reduce the cost of manufacturing its therapies. However, unless the Company is able to reduce those costs to an acceptable amount, the Company may never be able to develop a commercially viable product. If the Company does not successfully develop and commercialize products based upon its approach, or find suitable and economical sources for materials used in the production of its products, The Company will not become profitable.
The Company’s MultiTAA T cell therapy may be provided to patients in combination with other agents provided by third parties. The cost of such combination therapy may increase the overall cost of MultiTAA T cell therapy and may result in issues regarding the allocation of reimbursements between the Company’s therapy and the other agents, all of which may adversely affect The Company’s ability to obtain reimbursement coverage for the combination therapy from third-party medical insurers.
If product liability lawsuits are brought against the Company, the Company may incur substantial liabilities and may be required to limit commercialization of the Company’s product candidates.
The Company faces an inherent risk of product liability as a result of the clinical testing of its product candidates and will face an even greater risk if the Company commercializes any products. For example, the Company may be sued if its product candidates cause or are perceived to cause injury or are found to be otherwise unsuitable during clinical testing, manufacturing, marketing or sale. Any such product liability claims may include allegations of defects in manufacturing, defects in design, a failure to warn of dangers inherent to the product, negligence, strict liability or a breach of warranties. Claims could also be asserted under state consumer protection laws. If the Company cannot successfully defend itself against product liability claims, the Company may incur substantial liabilities or be required to limit commercialization of its product candidates. Even successful defense would require significant financial and management resources. Regardless of the merits or eventual outcome, liability claims may result in:
| • | decreased demand for the Company’s product candidates; |
| • | injury to the Company’s reputation; |
| • | withdrawal of clinical trial participants; |
| • | initiation of investigations by regulators; |
| • | costs to defend the related litigation; |
| • | a diversion of management’s time and the Company’s resources; |
| • | substantial monetary awards to trial participants or patients; |
| • | product recalls, withdrawals or labeling, marketing or promotional restrictions; |
| • | loss of revenue; |
| • | exhaustion of any available insurance and the Company’s capital resources; and |
| • | the inability to commercialize any product candidate. |
| 46 |
The Company’s inability to obtain sufficient product liability insurance at an acceptable cost to protect against potential product liability claims could inhibit or prevent the commercialization of products it develops, alone or with collaborators. The Company’s insurance policies may also have various exclusions, and the Company may be subject to a product liability claim for which the Company has no insurance coverage. While the Company will obtain clinical trial insurance for the Company’s Phase II clinical trials of MAPP and LAPP, the Company may have to pay amounts awarded by a court or negotiated in a settlement that exceed the Company’s coverage limitations or that are not covered by its insurance, and the Company may not have, or be able to obtain, sufficient capital to pay such amounts. Even if the Company’s agreements with any future collaborators entitle it to indemnification against losses, such indemnification may not be available or adequate should any claim arise.
The Company faces significant competition from other biotechnology and pharmaceutical companies and from non-profit institutions.
Competition in the field of cancer therapy is intense and is accentuated by the rapid pace of technological development. Research and discoveries by others may result in breakthroughs that may render the Company’s products obsolete even before they generate any revenue. There are products currently under development by others that could compete with the products that the Company is developing. Many of the Company’s potential competitors have substantially greater research and development capabilities and manufacturing, marketing, financial and managerial resources than the Company has. The Company’s competitors may:
| • | develop safer or more effective immunotherapies and other therapeutic products; |
| • | reach the market more rapidly, reducing the potential sales of the Company’s products; or |
| • | establish superior proprietary positions. |
Potential competitors in the market for treating hematological malignancies are companies such as Bristol-Myers Squibb, Roche/Genentech, Merck, Novartis, Gilead, Amgen, Pfizer, and GlaxoSmithKline, which already have products on the market or in development. Other companies, such as Celgene, Cellectis and Adaptimmune, which are focused on genetically engineered T cell technologies to treat cancer, may also be competitors. Furthermore, companies such as Iovance are developing non-genetically modified T cell therapies such as Tumor Infiltrating Lymphocyte (“TIL”) therapies that may compete with the Company’s products. All of these companies, and most of the Company’s other current and potential competitors have substantially greater research and development capabilities and financial, scientific, regulatory, manufacturing, marketing, sales, human resources, and experience than the Company does. Many of the Company’s competitors have several therapeutic products that have already been developed, approved and successfully commercialized, or are in the process of obtaining regulatory approval for their therapeutic products in the United States and internationally.
Universities and public and private research institutions in the U.S. and around the world are also potential competitors. While these universities and public and private research institutions primarily have educational objectives, they may develop proprietary technologies that lead to other FDA approved therapies or that secure patent protection that the Company may need for the development of its technologies and products.
The Company’s lead product candidate, LAPP, is a therapy for the treatment of refractory AML. Currently, there are numerous companies that are developing various alternate treatments for AML. Accordingly, LAPP faces significant competition in the AML treatment space from multiple companies. Even if the Company obtains regulatory approval for LAPP, the availability and price of competitors’ products could limit the demand and the price the Company will be able to charge for its therapy. The Company may not be able to implement its business plan if the acceptance of its products is inhibited by price competition or the reluctance of physicians to switch from other methods of treatment to the Company’s product, or if physicians switch to other new therapies, drugs or biologic products or choose to reserve the Company’s products for use in limited circumstances.
| 47 |
The Company’s business and operations would suffer in the event of cybersecurity/information systems risk.
Despite the implementation of security measures, the Company’s internal computer systems, and those of its manufacturers and other third parties on which it relies, are vulnerable to damage from computer viruses, unauthorized access, natural disasters, fire, terrorism, war and telecommunication and electrical failures. In addition, the Company’s systems safeguard important confidential personal data regarding its subjects. If a disruption event were to occur and cause interruptions in the Company’s operations, it could result in a material disruption of its drug development programs. For example, the loss of clinical trial data from completed, ongoing or planned clinical trials could result in delays in the Company’s regulatory approval efforts and significantly increase its costs to recover or reproduce the data. To the extent that any disruption or security breach results in a loss of or damage to the Company’s data or applications, or inappropriate disclosure of confidential or proprietary information, it could incur liability and the further development of its product candidates could be delayed.
The recently passed U.S. federal income tax reform could adversely affect the Company.
On December 22, 2017, U.S. federal tax legislation, commonly referred to as the Tax Cuts and Jobs Act (TCJA), was signed into law, significantly reforming the U.S. Internal Revenue Code. The TCJA, among other things, includes changes to U.S. federal tax rates, limitation of the deduction for net operating losses to 80% of current year taxable income and elimination of net operating loss carrybacks. The Company has evaluated the effect of the TCJA on its net operating losses for the quarter and the year ending December 31, 2017. The estimated impact of the TCJA is based on the management of the Company’s current knowledge and assumptions and recognized impacts that could be materially different from current estimates based on its actual results and its further analysis of the new law. The impact of the TCJA on holders of common shares is uncertain and could be adverse. Investors should consult with their own tax advisors with respect to such legislation and the potential tax consequences of investing in the Company’s common stock.
Risks Related to Government Regulation of the Company
The Company is subject to extensive regulation, which can be costly, time consuming and can subject the Company to unanticipated delays; even if the Company obtains regulatory approval for some of its products, those products may still face regulatory difficulties.
All of the Company’s potential products, cell processing and manufacturing activities, are subject to comprehensive regulation by the FDA in the United States and by comparable authorities in other countries. The process of obtaining FDA and other required regulatory approvals, including foreign approvals, is expensive and often takes many years and can vary substantially based upon the type, complexity and novelty of the products involved. In addition, regulatory agencies may lack experience with the Company’s technologies and products, which may lengthen the regulatory review process, increase the Company’s development costs and delay or prevent their commercialization.
No adoptive T cell therapy using MultiTAA T cells has been approved for marketing in the U.S. by the FDA. Consequently, there is no precedent for the successful commercialization of products based on the Company’s technologies. In addition, the Company has had only limited experience in filing and pursuing applications necessary to gain regulatory approvals, which may impede the Company’s ability to obtain timely FDA approvals, if at all. The Company has not yet sought FDA approval for any adoptive T cell therapy product. The Company will not be able to commercialize any of its potential products until the Company obtains FDA approval, and so any delay in obtaining, or inability to obtain, FDA approval would harm the Company’s proposed business.
If the Company violates regulatory requirements at any stage, whether before or after marketing approval is obtained, the Company may be fined, forced to remove a product from the market and experience other adverse consequences including delay, which could materially harm the Company’s business development. Additionally, the Company may not be able to obtain the labeling claims necessary or desirable for the promotion of its products. The Company may also be required to undertake post-marketing trials. In addition, if the Company or others identify side effects after any of the Company’s adoptive T cell therapy products are on the market, or if manufacturing problems occur, regulatory approval may be withdrawn and reformulation of the Company’s products may be required.
| 48 |
The FDA regulatory approval process is lengthy and time-consuming, and the Company may experience significant delays in the clinical development and regulatory approval of its product candidates.
The Company has not previously submitted a Biologics License Application (“BLA”) to the FDA, or similar approval filings to comparable foreign authorities. A BLA must include extensive preclinical and clinical data and supporting information to establish the product candidate’s safety and effectiveness for each desired indication. The BLA must also include significant information regarding the chemistry, manufacturing and controls (“CMC”) for the product. The Company expects the novel nature of the Company’s product candidates to create further challenges in obtaining regulatory approval. For example, the FDA has limited experience with commercial development of cell therapies for cancer. Accordingly, the regulatory approval pathway for the Company’s product candidates may be uncertain, complex, expensive and lengthy, and approval may not be obtained. The Company may also experience delays in completing planned clinical trials for a variety of reasons, including delays related to:
| • | the availability of financial resources to commence and complete the planned trials; |
| • | reaching agreement on acceptable terms with prospective CROs and clinical trial sites, the terms of which can be subject to extensive negotiation and may vary significantly among different CROs and trial sites; |
| • | obtaining approval by an independent IRB at each clinical trial site; |
| • | recruiting suitable patients to participate in a trial; |
| • | having patients complete a trial or return for post-treatment follow-up; |
| • | clinical trial sites deviating from trial protocol or dropping out of a trial; |
| • | adding new clinical trial sites; or |
| • | manufacturing sufficient quantities of qualified materials under cGMPs and applying them on a subject by subject basis for use in clinical trials. |
The Company could also encounter delays if physicians face unresolved ethical issues associated with enrolling patients in clinical trials of the Company’s product candidates in lieu of prescribing existing treatments that have established safety and efficacy profiles. Further, a clinical trial may be suspended or terminated by the Company, the IRB for the institutions in which such trials are being conducted, the Data Monitoring Committee for such trial, or by the FDA or other regulatory authorities due to a number of factors. Those factors could include failure to conduct the clinical trial in accordance with regulatory requirements or the Company’s clinical protocols, inspection of the clinical trial operations or trial site by the FDA or other regulatory authorities resulting in the imposition of a clinical hold, unforeseen safety issues or adverse side effects, failure to demonstrate a benefit from using a product candidate, changes in governmental regulations or administrative actions or lack of adequate funding to continue the clinical trial. If the Company experiences termination of, or delays in the completion of, any clinical trial of the Company’s product candidates, the commercial prospects for the Company’s product candidates will be harmed, and its ability to generate product revenue will be delayed. In addition, any delays in completing the Company’s clinical trials will increase the Company’s costs, slow down the Company’s product development and approval process and jeopardize the Company’s ability to commence product sales and generate revenue.
| 49 |
Obtaining and maintaining regulatory approval of the Company’s product candidates in one jurisdiction does not mean that the Company will be successful in obtaining regulatory approval of its product candidates in other jurisdictions.
Obtaining and maintaining regulatory approval of the Company’s product candidates in one jurisdiction does not guarantee that the Company will be able to obtain or maintain regulatory approval in any other jurisdiction, while a failure or delay in obtaining regulatory approval in one jurisdiction may have a negative effect on the regulatory approval process in others. For example, even if the FDA grants marketing approval of a product candidate, comparable regulatory authorities in foreign jurisdictions must also approve the manufacturing, marketing and promotion of the product candidate in those countries. Approval procedures vary among jurisdictions and can involve requirements and administrative review periods different from, and greater than, those in the United States, including additional preclinical studies or clinical trials as clinical studies conducted in one jurisdiction may not be accepted by regulatory authorities in other jurisdictions. In many jurisdictions outside the United States, a product candidate must be approved for reimbursement before it can be approved for sale in that jurisdiction. In some cases, the price that the Company intends to charge for the Company’s products is also subject to approval.
The Company may also submit marketing applications in other countries. Regulatory authorities in jurisdictions outside of the United States have requirements for approval of product candidates with which the Company must comply prior to marketing in those jurisdictions. Obtaining foreign regulatory approvals and compliance with foreign regulatory requirements could result in significant delays, difficulties and costs for the Company and could delay or prevent the introduction of its products in certain countries. If the Company fails to comply with the regulatory requirements in international markets and/or receive applicable marketing approvals, its target market will be reduced and the Company’s ability to realize the full market potential of its product candidates will be harmed.
Even if the Company receives regulatory approval of its product candidates, it will be subject to ongoing quality and regulatory obligations and continued regulatory review, which may result in significant additional expense, and the Company may be subject to penalties if it fails to comply with regulatory requirements or experiences unanticipated problems with its product candidates.
Any regulatory approvals that the Company receives for its product candidates will require surveillance to monitor the safety and efficacy of the product candidate. The FDA may also require a risk evaluation and mitigation strategy in order to approve the Company’s product candidates, which could entail requirements for a medication guide, physician communication plans or additional elements to ensure safe use, such as restricted distribution methods, patient registries and other risk minimization tools. In addition, if the FDA or a comparable foreign regulatory authority approves the Company’s product candidates, the manufacturing processes, labeling, packaging, distribution, adverse event reporting, storage, advertising, promotion, import, export and recordkeeping for the Company’s product candidates will be subject to extensive and ongoing regulatory requirements. These requirements include submissions of safety and other post-marketing information and reports, registration, as well as continued compliance with cGMPs and cGCPs for any clinical trials that the Company conducts post-approval. Later discovery of previously unknown problems with the Company’s product candidates, including adverse events of unanticipated severity or frequency, or with the Company’s third-party manufacturers or manufacturing processes, or failure to comply with regulatory requirements, may result in, among other things:
| • | restrictions on the marketing or manufacturing of the Company’s product candidates, withdrawal of the product from the market, or voluntary or mandatory product recalls; |
| • | fines, warning letters or holds on clinical trials; |
| • | refusal by the FDA to approve pending applications or supplements to approved applications filed by the Company or suspension or revocation of license approvals; |
| • | product seizure or detention, or refusal to permit the import or export of the Company’s product candidates; and |
| • | injunctions or the imposition of civil or criminal penalties. |
| 50 |
The FDA’s and other regulatory authorities’ policies may change and additional government regulations may be enacted that could prevent, limit or delay regulatory approval of the Company’s product candidates. The Company cannot predict the likelihood, nature or extent of government regulation that may arise from future legislation or administrative action, either in the United States or abroad. If the Company is slow or unable to adapt to changes in existing requirements or the adoption of new requirements or policies, or if it is not able to maintain regulatory compliance, the Company may lose any marketing approval that it may have obtained and the Company may not achieve or sustain profitability.
Recently enacted and future legislation in the United States and other countries may affect the prices the Company may obtain for its product candidates and increase the difficulty and cost to commercialize its product candidates.
In the United States and many other countries, rising healthcare costs have been a concern for governments, patients and the health insurance sector, which has resulted in a number of changes to laws and regulations, and may result in further legislative and regulatory action regarding the healthcare and health insurance systems that could affect the Company’s ability to profitably sell any product candidates for which it has obtained marketing approval.
For example, the Patient Protection and Affordable Care Act, as amended by the Health Care and Education Reconciliation Act (“ACA”) was enacted in the United States in March 2010, with the stated goals of containing healthcare costs, improving quality and expanding access to healthcare, and includes measures to change health care delivery, increase the number of individuals with insurance, ensure access to certain basic health care services, and contain the rising cost of care. Since January 2017, President Trump has signed two executive orders and other directives designed to delay, circumvent, or loosen certain requirements mandated by the ACA. Concurrently, Congress has considered legislation that would repeal or repeal and replace all or part of the ACA. While Congress has not passed repeal legislation, two bills affecting the implementation of certain taxes under the ACA have been signed into law. The Tax Cuts and Jobs Act of 2017 includes a provision that repealed, effective January 1, 2019, the tax-based shared responsibility payment imposed by the ACA on certain individuals who fail to maintain qualifying health coverage for all or part of a year that is commonly referred to as the “individual mandate”. Additionally, on January 22, 2018, President Trump signed a continuing resolution on appropriations for fiscal year 2018 that delayed the implementation of certain ACA-mandated fees, including the so-called “Cadillac” tax on certain high cost employer-sponsored insurance plans, the annual fee imposed on certain health insurance providers based on market share, and the medical device excise tax on non-exempt medical devices. Further, the Bipartisan Budget Act of 2018, among other things, amended the ACA, effective January 1, 2019, to increase from 50% to 70% the point-of-sale discount that is owed by pharmaceutical manufacturers who participate in Medicare Part D and to close the coverage gap in most Medicare drug plans, commonly referred to as the “donut hole.” Congress may consider other legislation to repeal or replace elements of the ACA. These executive orders and legislative actions may result in increased health insurance premiums and reduce the number of people with health insurance in the United States and have other effects that could adversely affect U.S. health insurance markets and the ability of patients to have access to therapies that the Company’s product candidates can provide.
In addition, other federal health reform measures have been proposed and adopted in the United States. For example, as a result of the Budget Control Act of 2011, providers are subject to Medicare payment reductions of 2% per fiscal year through 2027 unless additional Congressional action is taken. Further, the American Taxpayer Relief Act of 2012 reduced Medicare payments to several providers and increased the statute of limitations period for the government to recover overpayments to providers from three to five years. The Medicare Access and CHIP Reauthorization Act of 2015 also introduced a quality payment program under which certain individual Medicare providers will be subject to certain incentives or penalties based on new program quality standards. Payment adjustments for the Medicare quality payment program will begin in 2019. At this time, it is unclear how the introduction of the quality payment program will impact overall physician reimbursement under the Medicare program. Any reduction in reimbursement from Medicare or other government programs may result in a similar reduction in payments from private payors. Further, there has been heightened governmental scrutiny in the United States of pharmaceutical pricing practices in light of the rising cost of prescription drugs and biologics.
The combination of healthcare cost containment measures, increased health insurance costs, reduction of the number of people with health insurance coverage, as well as future legislation and regulations focused on reducing healthcare costs by reducing the cost of, or reimbursement and access to, pharmaceutical products, may limit or delay the Company’s ability to commercialize its products, generate revenue or attain profitability.
| 51 |
The Company’s employees, independent contractors, consultants, commercial partners and vendors may engage in misconduct or other improper activities, including noncompliance with regulatory standards and requirements.
The Company is exposed to the risk of employee fraud or other illegal activity by its employees, independent contractors, consultants, commercial partners and vendors. Misconduct by these parties could include intentional, reckless and/or negligent conduct that fails to: comply with the laws of the FDA and other similar foreign regulatory bodies, provide true, complete and accurate information to the FDA and other similar foreign regulatory bodies, comply with manufacturing standards the Company has established, comply with healthcare fraud and abuse laws in the United States and similar foreign fraudulent misconduct laws, or report financial information or data accurately or to disclose unauthorized activities to the Company. If the Company obtains FDA approval of any of its product candidates and begins commercializing those products in the United States, the Company’s potential exposure under such laws will increase significantly, and its costs associated with compliance with such laws are also likely to increase. These laws may impact, among other things, the Company’s current activities with principal investigators and research patients, as well as proposed and future sales, marketing and education programs. In particular, the promotion, sales and marketing of healthcare items and services, as well as certain business arrangements in the healthcare industry, are subject to extensive laws designed to prevent fraud, kickbacks, self-dealing and other abusive practices. These laws and regulations may restrict or prohibit a wide range of pricing, discounting, marketing and promotion, structuring and commission(s), certain customer incentive programs and other business arrangements generally. Activities subject to these laws also involve the improper use of information obtained in the course of patient recruitment for clinical trials.
Efforts to ensure that the Company’s business arrangements comply with applicable healthcare laws may involve substantial costs. It is possible that governmental and enforcement authorities will conclude that the Company’s business practices may not comply with current or future statutes, regulations or case law interpreting applicable fraud and abuse or other healthcare laws and regulations. If any such actions are instituted against the Company, and it is not successful in defending itself or in asserting its rights, those actions could have a significant impact on its business, including the imposition of civil, criminal and administrative penalties, damages, disgorgement, monetary fines, possible exclusion from participation in Medicare, Medicaid and other federal healthcare programs, contractual damages, reputational harm, diminished profits and future earnings, and curtailment of the Company’s operations, any of which could adversely affect the Company’s ability to develop its business. In addition, the approval and commercialization of any of the Company’s product candidates outside the United States will also likely subject the Company to foreign equivalents of the healthcare laws mentioned above, among other foreign laws.
The Company may not obtain or maintain the benefits associated with orphan drug designation, including market exclusivity.
On December 9, 2015, the Company announced that it received Orphan Drug Designation from the FDA’s Office of Orphan Products Development (“OOPD”) for its cancer vaccine TPIV200 in the treatment of ovarian cancer. The TPIV200 ovarian cancer clinical program will now receive benefits including tax credits on clinical research and seven-year market exclusivity upon receiving marketing approval. Even though the Company was granted orphan drug designation, it may not receive the benefits associated with orphan drug designation. This may result from a failure to maintain orphan drug status or result from a competing product reaching the market that has an orphan designation for the same disease indication. Under U.S. regulations for orphan drugs, if such a competing product reaches the market before the Company’s does, the competing product could potentially obtain a scope of market exclusivity that limits or precludes the Company’s product from being sold in the United States for seven years. Even if the Company obtains exclusivity, the FDA could subsequently approve a drug for the same condition if the FDA concludes that the later drug is clinically superior in that it is shown to be safer, more effective or makes a major contribution to patient care. A competitor also may receive approval of different products for the same indication for which the Company’s orphan product has exclusivity or obtain approval for the same product but for a different indication for which the orphan product has exclusivity.
In addition, if and when the Company requests orphan drug designation in Europe, the European exclusivity period is ten years but can be reduced to six years if the drug no longer meets the criteria for orphan drug designation or if the drug is sufficiently profitable so that market exclusivity is no longer justified. Orphan drug exclusivity may be lost if the FDA or European Medicines Evaluation Agency (“EMEA”) determines that the request for designation was materially defective or if the manufacturer is unable to assure sufficient quantity of the drug to meet the needs of patients with the rare disease or condition.
| 52 |
New regulatory pathways for biosimilar competition could reduce the duration of market exclusivity for the Company’s products.
Under the federal Patient Protection and Affordable Care Act (“PPACA”) enacted in 2010, there is an abbreviated path in the United States for regulatory approval of products that are demonstrated to be “biosimilar” or “interchangeable” with an FDA-approved biological product. The PPACA provides a regulatory mechanism that allows for FDA approval of biologic drugs that are similar to (but not generic copies of) innovative drugs on the basis of less extensive data than is required by a full BLA. Under this regulation, an application for approval of a biosimilar may be filed four years after approval of the innovator product. However, qualified innovative biological products will receive 12 years of regulatory exclusivity, meaning that the FDA may not approve a biosimilar version until 12 years after the innovative biological product was first approved by the FDA. However, the term of regulatory exclusivity may not remain at 12 years in the United States and could be shortened. A number of jurisdictions outside of the United States have also established abbreviated pathways for regulatory approval of biological products that are biosimilar to earlier versions of biological products. For example, the European Union has had an established regulatory pathway for biosimilars since 2005.
The increased likelihood of biosimilar competition has increased the risk of loss of innovators’ market exclusivity. Due to this risk, and uncertainties regarding patent protection, if one of the Company’s late-stage product candidates or other clinical candidates are approved for marketing, it is not possible to predict the length of market exclusivity for any particular product with certainty based solely on the expiration of the relevant patent(s) or the current forms of regulatory exclusivity. It is also not possible to predict changes in United States regulatory law that might reduce biological product regulatory exclusivity. The loss of market exclusivity for a product would likely materially and negatively affect revenues from product sales of that product and thus the Company’s financial results and condition.
Changes in laws and regulations affecting the healthcare industry could adversely affect the Company’s business.
As described above, the PPACA and potential regulations thereunder easing the entry of competing follow-on biologics into the marketplace, other new legislation or implementation of existing statutory provisions on importation of lower-cost competing drugs from other jurisdictions, and legislation on comparative effectiveness research are examples of previously enacted and possible future changes in laws that could adversely affect the Company’s business.
The current U.S. administration and Congress could carry out significant changes in legislation, regulation, and government policy (including with respect to the possible repeal of all or portions of the PPACA, possible changes in the existing treaty and trade relationships with other countries, and tax reform), as evidenced by statements and recent actions of the current president. While it is not possible to predict whether and when any such changes will occur, changes in the laws, regulations, and policies governing the development and approval of the Company’s product candidates and the commercialization, importation, and reimbursement of the Company’s product candidates could adversely affect the Company’s business.
| 53 |
Risks Related to the Company’s Securities
The price of the Company’s stock may be volatile.
The trading price of the Company’s common stock may fluctuate substantially. The price of the Company’s common stock that will prevail in the market may be higher or lower than the price at which its shares of common stock, depending on many factors, some of which are beyond the Company’s control and may not be related to its operating performance. These fluctuations could cause you to lose part or all of your investment in the Company’s common stock. Those factors that could cause fluctuations include, but are not limited to, the following:
| • | price and volume of fluctuations in the overall stock market from time to time; |
| • | fluctuations in stock market prices and trading volumes of similar companies; |
| • | actual or anticipated changes in the Company’s net loss or fluctuations in the Company’s operating results or in the expectations of securities analysts; |
| • | results of the Company’s preclinical studies and clinical trials or delays in anticipated timing; |
| • | the issuance of new equity securities pursuant to a future offering, including issuances of preferred stock; |
| • | announcements of new collaboration agreements with strategic partners or developments by the Company’s existing collaboration partners; |
| • | announcements of acquisitions, mergers or business combinations; |
| • | announcements of technological innovations, new commercial products, failures of products, or progress toward commercialization by the Company’s competitors or peers; |
| • | general economic conditions and trends; |
| • | positive and negative events relating to healthcare and the overall pharmaceutical and biotechnology sectors; |
| • | major catastrophic events; |
| • | sales of large blocks of the Company’s stock; |
| • | departures of key personnel; |
| • | changes in the regulatory status of the Company’s immunotherapies, including results of the Company’s clinical trials; |
| • | events affecting BCM, Mayo Clinic, Mayo Foundation for Medical Education and Research or any future collaborators; |
| • | announcements of new products or technologies, commercial relationships or other events by the Company or its competitors; |
| • | regulatory developments in the United States and other countries; |
| • | failure of the Company’s common stock to maintain listing requirements on the Nasdaq Capital Market; |
| • | changes in accounting principles; and |
| • | discussion of the Company or its stock price by the financial and scientific press and in online investor communities. |
| 54 |
In the past, following periods of volatility in the market price of a company’s securities, securities class action litigation has often been brought against that company. Due to the potential volatility of the Company’s stock price, the Company may therefore be the target of securities litigation in the future. Securities litigation could result in substantial costs and divert management’s attention and resources from the Company’s business.
A limited public trading market may cause volatility in the price of the Company’s common stock.
The listing of the Company’s common stock on the Nasdaq Capital Market does not assure that a meaningful, consistent and liquid trading market currently exists or will exist in the future. In recent years, the stock market has experienced extreme price and volume fluctuations that have particularly affected the market prices of many smaller companies like the Company. The Company’s common stock is thus subject to this volatility. Sales of substantial amounts of common stock, or the perception that such sales might occur, could adversely affect prevailing market prices of the Company’s common stock and its stock price may decline substantially in a short time and its stockholders could suffer losses or be unable to liquidate their holdings. The Company’s stock is thinly traded due to the limited number of shares available for trading thus causing large swings in price. There is no established trading market for the Company’s warrants.
The market prices for the Company’s common stock may be adversely impacted by future events.
Market prices for the Company’s common stock will be influenced by a number of factors, including:
| • | the issuance of new equity securities pursuant to a future offering, including issuances of preferred stock; |
| • | changes in interest rates; |
| • | competitive developments, including announcements by competitors of new products or services or significant contracts, acquisitions, strategic partnerships, joint ventures or capital commitments; |
| • | variations in quarterly operating results; |
| • | change in financial estimates by securities analysts; |
| • | the depth and liquidity of the market for the Company’s common stock and warrants; |
| • | investor perceptions of the Company and the pharmaceutical and biotech industries generally; and |
| • | general economic and other national conditions. |
If the Company fails to remain current with its listing requirements, it could be removed from the Nasdaq Capital Market which would limit the ability of broker-dealers to sell its securities and the ability of stockholders to sell its securities in the secondary market.
Companies listed for trading on the Nasdaq Capital Market must be reporting issuers under Section 12 of the Exchange Act. If the Company fails to file such reports in a timely manner, or if the Company fails to meet any other listing requirements, the shares of its common stock would eventually cease to be listed on the Nasdaq Capital Market, and the market liquidity for its securities could be severely adversely affected by limiting the ability of broker-dealers to sell its securities and the ability of stockholders to sell their securities in the secondary market.
| 55 |
Sales of additional equity securities may adversely affect the market price of the Company’s common stock and your rights may be reduced.
The Company expects to continue to incur drug development and sale, general and administrative costs, and to satisfy its funding requirements, it will need to sell additional equity securities, which may be subject to registration rights and warrants with anti-dilutive protective provisions. The sale or the proposed sale of substantial amounts of the Company’s common stock or other equity securities in the public markets may adversely affect the market price of its common stock and its stock price may decline substantially. The Company’s stockholders may experience substantial dilution and a reduction in the price that they are able to obtain upon sale of their shares. Also, new equity securities issued may have greater rights, preferences or privileges than the Company’s existing common stock.
Because the Company has a significant number of additional authorized shares of common stock available for issuance and outstanding warrants to purchase its common stock, its stockholders may experience dilution in the future and it may adversely affect the market price of its securities.
The Company is currently authorized to issue 150 million shares of its common stock. As of October 18, 2018, it had 45,328,510 million shares of its common stock issued and outstanding. Those outstanding shares represent a minority of the Company’s authorized shares, meaning that the ownership position of the current stockholders could be diluted significantly were the Company to issue a large number of additional shares. In addition, as of October 18, 2018, there were outstanding warrants to purchase up to 23,090,038 shares of its common stock at a weighted average exercise price of $4.78 per share, and options exercisable for an aggregate of 439,467 shares of common stock at a weighted average exercise price of $6.77 per share. The Company has committed to register the resale of all the shares issuable upon exercise of these warrants, and they will be freely tradable by the exercising party upon issuance. Upon such registration, the holders may sell these shares in the public markets from time to time, without limitations on the timing, amount, or method of sale. If the Company’s stock price rises, the holders may exercise their warrants and options and sell a large number of shares. This could cause the market price of the Company’s common stock to decline and cause existing stockholders to experience significant further dilution.
The accounting treatment for certain of the Company’s warrants is complex and subject to judgments concerning the valuation of embedded derivative rights within the applicable securities. Fluctuations in the valuation of these rights could cause the Company to take charges to its statement of operations and make its financial results unpredictable.
Certain of the Company’s outstanding warrants contain or contained prior to being amended, or may be deemed to contain from time to time, embedded derivative rights in accordance with U.S. Generally Accepted Accounting Principles (“GAAP”). There is a risk that questions could arise from investors or regulatory authorities concerning the appropriate accounting treatment of these instruments, which could require the Company to restate previous financial statements, which in turn could adversely affect its reputation, as well as its results of operations. These derivative rights, or similar rights in securities the Company may issue in the future, need to be, or may need to be, separately valued as of the end of each accounting period in accordance with GAAP. The Company records these embedded derivatives as liabilities at issuance, valued using the Black Scholes Option Pricing Model and are subject to revaluation at each reporting date. Any change in fair value between reporting periods is reported on the Company’s statement of operations. At December 31, 2017, the fair value of the derivative liability-warrants was $9,000. Changes in the valuations of these rights, the valuation methodology or the assumptions on which the valuations are based could cause the Company to take charges to the Company’s earnings, which would adversely impact its results of operations. Moreover, the methodologies, assumptions and related interpretations of accounting or regulatory authorities associated with these embedded derivatives are complex and in some cases uncertain, which could cause the Company accounting for these derivatives, and as a result, its financial results, to fluctuate.
The Company does not intend to pay cash dividends.
The Company has not declared or paid any cash dividends on its common stock, and it does not anticipate declaring or paying cash dividends for the foreseeable future. Any future determination as to the payment of cash dividends on the Company’s common stock will be at the Company’s board of directors’ discretion and depends on its financial condition, operating results, capital requirements and other factors that its board of directors considers to be relevant.
| 56 |
GLOSSARY
| ACA | Affordable Care Act |
| AML | acute myeloid leukemia |
| ASCT | autologous stem cell transplant |
| BCM | Baylor College of Medicine |
| BLA | Biologics License Application |
| CAGT | Center for Cell and Gene Therapy at BCM |
| CAR | Chimeric antigen receptor |
| cGMP | current Good Manufacturing Practices |
| CMC | chemistry, manufacturing, and controls |
| CMO | Contract Manufacturing Organization |
| CRS | cytokine-release syndrome |
| CTL | cytotoxic T lymphocyte |
| DLI | donor lymphocyte infusion |
| FDA | U.S. Food & Drug Administration |
| GMP | Good Manufacturing Practices |
| GVHD | graft-versus-host disease |
| HSCT | hematopoietic stem cell transplant |
| ICIs | immune checkpoint inhibitors |
| IND | investigational new drug |
| IRB | Institutional Review Board |
| LAPP | Leukemia Antigen Peptide Pool |
| mAbs | monoclonal antibodies |
| MAPP | Mixed Antigen Peptide Pool |
| MDS | myelodysplastic syndromes |
| MM | multiple myeloma |
| MultiTAA | Multi Tumor-Associated Antigen |
| NHL | Non-Hodgkin’s Lymphoma |
| r/r | relapsed/refractory |
| SAE | serious adverse events |
| TCR | T cell receptor |
| TIL | Tumor Infiltrating Lymphocyte |
| USPTO | U.S. Patent & Trademark Office |
| 57 |
SIGNATURES
Pursuant to the requirements of the Securities Exchange Act of 1934, the registrant has duly caused this report to be signed on its behalf by the undersigned hereunto duly authorized.
| MARKER THERAPEUTICS, INC. |
| Date: October 30, 2018 | By: | /s/Michael Loiacono |
| Name: | Michael Loiacono | |
| Title: | Chief Financial Officer |
