Attached files
| file | filename |
|---|---|
| 8-K - FORM 8-K - Aravive, Inc. | d646009d8k.htm |

Aravive Corporate Presentation October 2018 Exhibit 99.1

Important Information Forward-Looking Statements This presentation contains forward-looking statements that may discuss Aravive’s plans, goals, intentions and expectations as to future trends, events, results of operations, financial condition or other matters. Forward-looking statements generally include statements that are predictive in nature and depend upon or refer to future events or conditions, and they often include words such as “may,” “will,” “should,” “would,” “expect,” “anticipate,” “plan,” “likely,” “believe,” “estimate,” “project,” “intend,” and other similar expressions. Statements that are not historical facts are forward-looking statements. Forward-looking statements included in this presentation include statements regarding Aravive’s planned clinical activities, including the design, initiation, patient enrollment and availability of data from clinical studies, future indications and cash position and the anticipated safety, activity and manufacturability of Aravive’s product candidates. Forward-looking statements are based on Aravive’s current beliefs and assumptions, are subject to risks and uncertainties and are not guarantees of future performance. Actual results could differ materially from those contained in any forward-looking statement as a result of various factors, including, without limitation: the unpredictability of clinical development activities; risks related to Aravive’s ability to estimate and control its operating expenses; Aravive’s ability to protect its intellectual property rights; changes in the competitive environment; and legislative, regulatory, political and economic developments. The foregoing review of important factors that could cause actual events to differ from expectations should not be construed as exhaustive and should be read in conjunction with statements that are included herein and elsewhere, including the risk factors included in Aravive’s most recent Annual Report on Form 10-K, Quarterly Report on Form 10-Q and Current Reports on Form 8-K filed with the Securities and Exchange Commission. Except as required by law, Aravive undertakes no obligation to revise or update any forward-looking statement or to make any other forward-looking statements, whether as a result of new information, future events or otherwise.
Aravive at a Glance Promising Validated Target Value Creation GAS6-AXL is a scientifically validated and promising oncology target Elevated GAS6 levels associated with poor prognosis in cancer Target’s significance supported by extensive research Aravive is well positioned to advance the development program to important inflection points AVB-S6-500, a High Affinity, Highly Specific AXL Inhibitor AXL-Fc fusion protein engineered for strong stability and very high affinity for GAS6 AVB-S6-500 has been well tolerated in a Phase 1 trial, where proof of mechanism has been established Company Background Clinical candidate is based on technology originated from the lab of Amato Giaccia , Ph.D and his colleagues at Stanford University. First-patient is expected to be dosed in a Phase 1b/2 trial in 4Q18 Strong cash position expected to reach multiple milestones Plans to advance AVB-S6-500 into multiple additional indications over next two years The company was originally funded in part by a Product Development Award from the Cancer Prevention & Research Institute of Texas (CPRIT).
Highlights of Completed Merger Key Terms Privately-held Aravive Biologics merged with a subsidiary of Versartis, Inc. in a ~50/50, all stock transaction Upon closing of the transaction on October 12, 2018, the merged company now operates as Aravive, Inc. 1:6 reverse split concurrent with merger close resulting in 11.2M shares outstanding Combined company’s common stock now trades on Nasdaq under the symbol “ARAV” Management & Organization Jay Shepard continues in role as CEO of Aravive and director Srinivas Akkaraju, MD, PhD serves as Chairman of the Board Ray Tabibiazar, M.D., Aravive’s former Executive Chairman, continues to serve on the Board of Directors Additional board members: Shahzad Malik, M.D; Amato Giaccia, Ph.D., scientific founder of Aravive Biologics; Eric Zhang, CFA; and, newly elected, Robert Hoffman Combined Cash Position Approximately $60-$62 million unaudited cash and cash equivalents for the combined company as of the close, net of estimated transaction costs Cash expected to get through multiple inflection points and into 2020
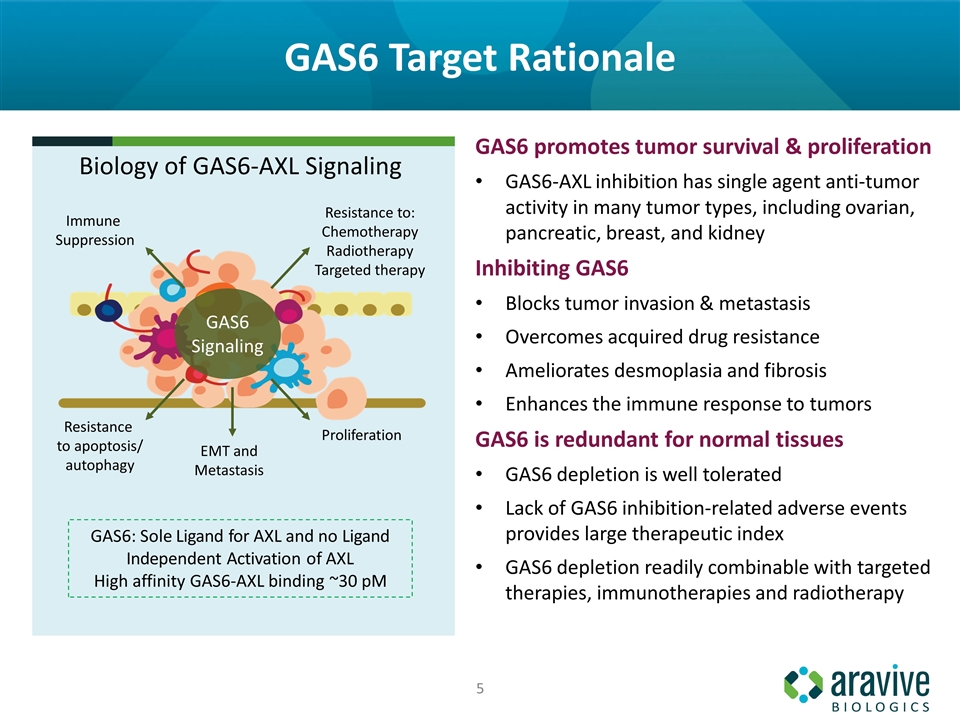
GAS6 Target Rationale GAS6 promotes tumor survival & proliferation GAS6-AXL inhibition has single agent anti-tumor activity in many tumor types, including ovarian, pancreatic, breast, and kidney Inhibiting GAS6 Blocks tumor invasion & metastasis Overcomes acquired drug resistance Ameliorates desmoplasia and fibrosis Enhances the immune response to tumors GAS6 is redundant for normal tissues GAS6 depletion is well tolerated Lack of GAS6 inhibition-related adverse events provides large therapeutic index GAS6 depletion readily combinable with targeted therapies, immunotherapies and radiotherapy Biology of GAS6-AXL Signaling GAS6: Sole Ligand for AXL and no Ligand Independent Activation of AXL High affinity GAS6-AXL binding ~30 pM GAS6 Signaling Immune Suppression Resistance to: Chemotherapy Radiotherapy Targeted therapy Resistance to apoptosis/ autophagy Proliferation EMT and Metastasis
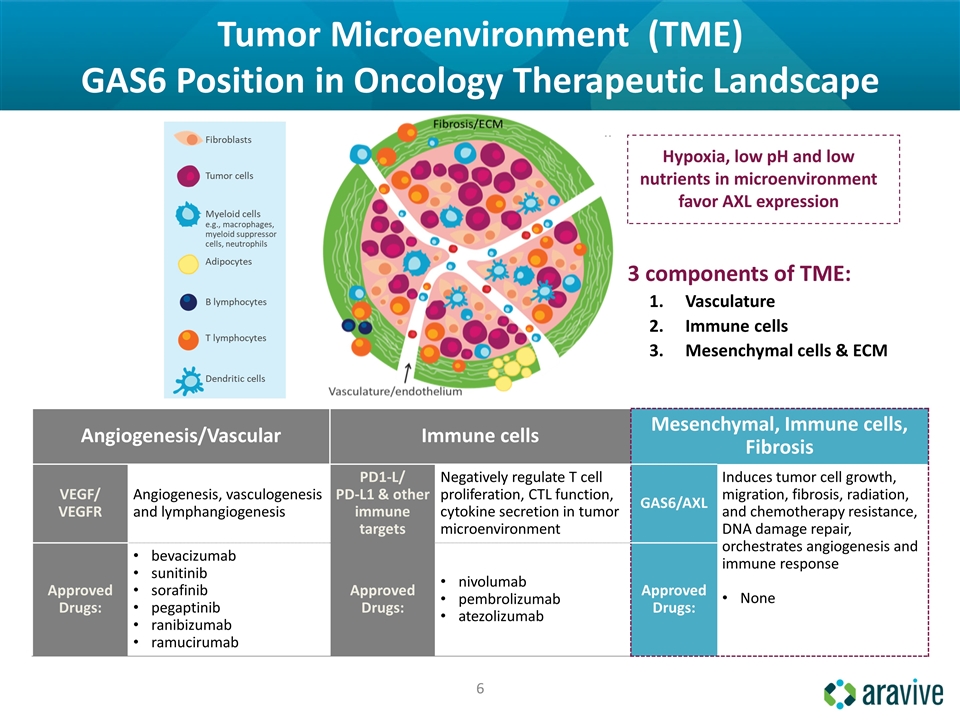
Tumor Microenvironment (TME) GAS6 Position in Oncology Therapeutic Landscape Angiogenesis/Vascular Immune cells Mesenchymal, Immune cells, Fibrosis VEGF/ VEGFR Angiogenesis, vasculogenesis and lymphangiogenesis PD1-L/ PD-L1 & other immune targets Negatively regulate T cell proliferation, CTL function, cytokine secretion in tumor microenvironment GAS6/AXL Induces tumor cell growth, migration, fibrosis, radiation, and chemotherapy resistance, DNA damage repair, orchestrates angiogenesis and immune response None Approved Drugs: bevacizumab sunitinib sorafinib pegaptinib ranibizumab ramucirumab Approved Drugs: nivolumab pembrolizumab atezolizumab Approved Drugs: Hypoxia, low pH and low nutrients in microenvironment favor AXL expression 3 components of TME: Vasculature Immune cells Mesenchymal cells & ECM
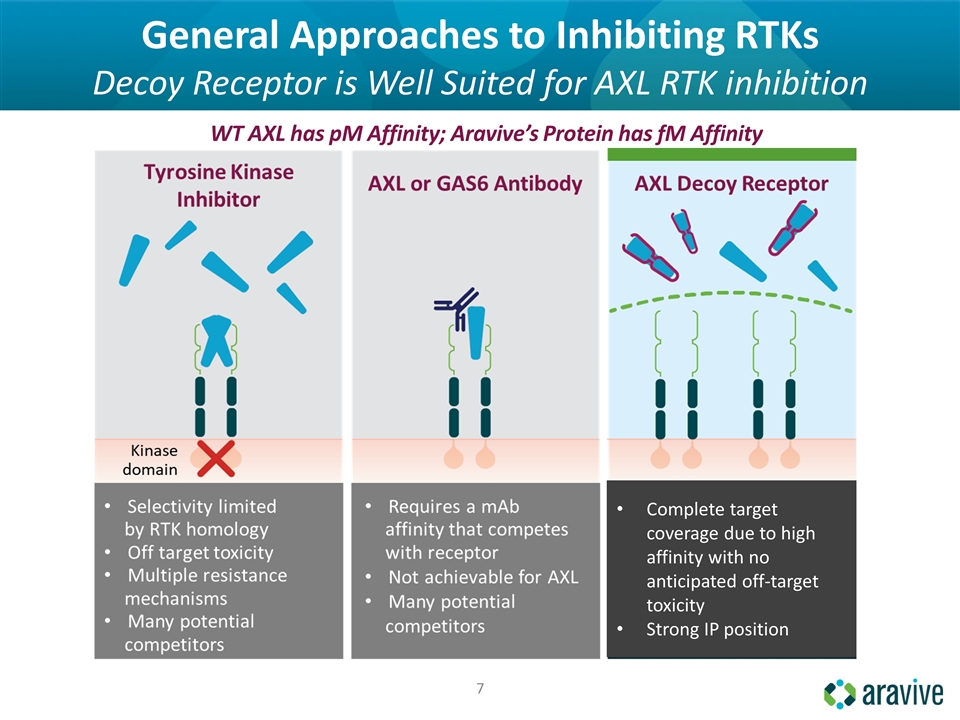
General Approaches to Inhibiting RTKs Decoy Receptor is Well Suited for AXL RTK inhibition WT AXL has pM Affinity; Aravive’s Protein has fM Affinity Complete target coverage due to high affinity with no anticipated off-target toxicity Strong IP position
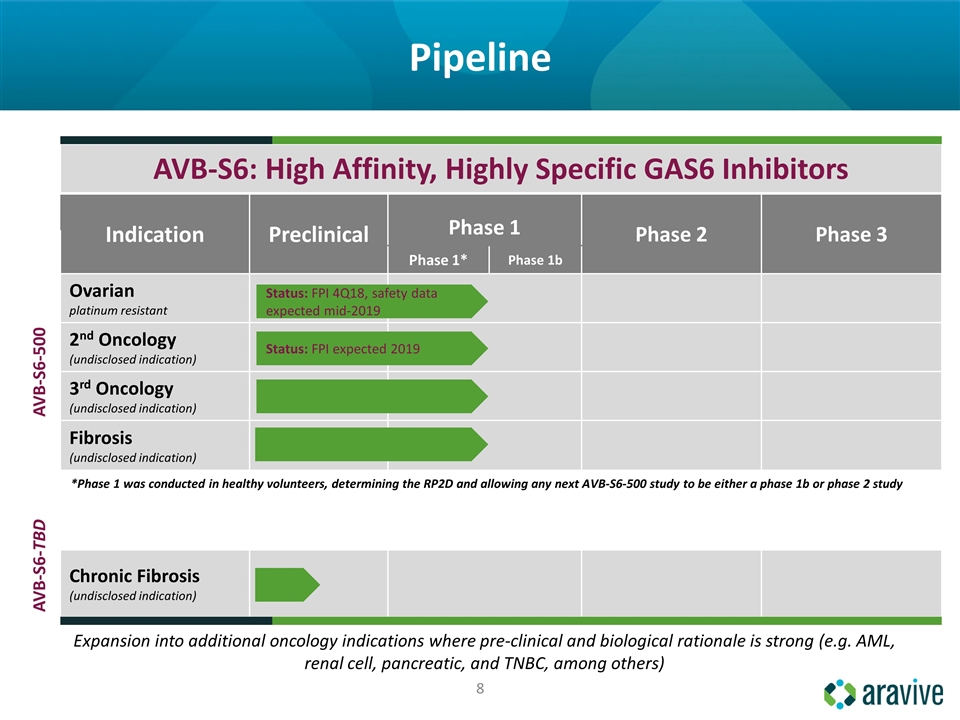
Pipeline Expansion into additional oncology indications where pre-clinical and biological rationale is strong (e.g. AML, renal cell, pancreatic, and TNBC, among others) Indication Preclinical Phase 1 Phase 2 Phase 3 Phase 1 Phase 1b Phase 1* Phase 1b AVB-S6-500 Ovarian platinum resistant 2nd Oncology (undisclosed indication) 3rd Oncology (undisclosed indication) Fibrosis (undisclosed indication) AVB-S6-TBD *Phase 1 was conducted in healthy volunteers, determining the RP2D and allowing any next AVB-S6-500 study to be either a phase 1b or phase 2 study Chronic Fibrosis (undisclosed indication) Status: FPI expected 2019 AVB-S6: High Affinity, Highly Specific GAS6 Inhibitors Status: FPI 4Q18, safety data expected mid-2019
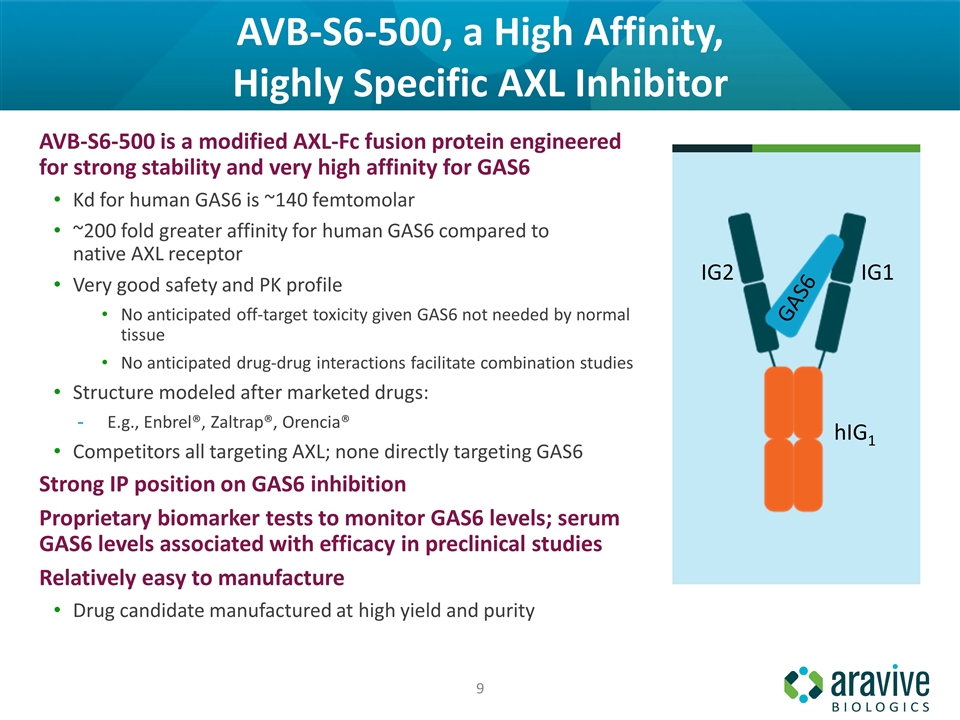
AVB-S6-500, a High Affinity, Highly Specific AXL Inhibitor AVB-S6-500 is a modified AXL-Fc fusion protein engineered for strong stability and very high affinity for GAS6 Kd for human GAS6 is ~140 femtomolar ~200 fold greater affinity for human GAS6 compared to native AXL receptor Very good safety and PK profile No anticipated off-target toxicity given GAS6 not needed by normal tissue No anticipated drug‐drug interactions facilitate combination studies Structure modeled after marketed drugs: E.g., Enbrel®, Zaltrap®, Orencia® Competitors all targeting AXL; none directly targeting GAS6 Strong IP position on GAS6 inhibition Proprietary biomarker tests to monitor GAS6 levels; serum GAS6 levels associated with efficacy in preclinical studies Relatively easy to manufacture Drug candidate manufactured at high yield and purity hIG1 IG1 IG2 GAS6
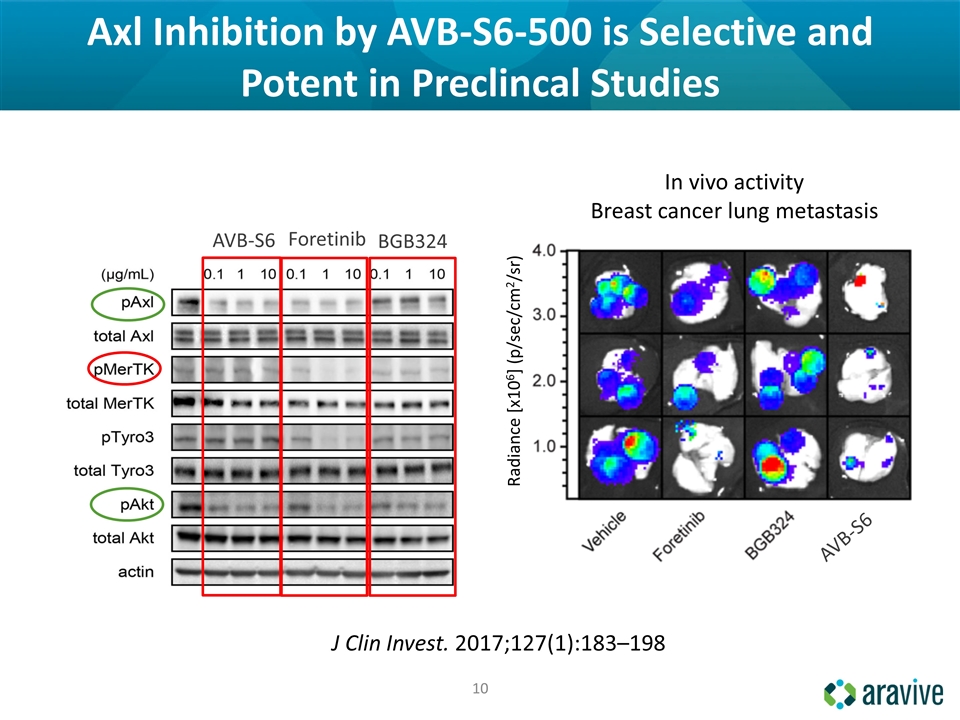
Axl Inhibition by AVB-S6-500 is Selective and Potent in Preclincal Studies In vivo activity Breast cancer lung metastasis AVB-S6 AVB-S6 J Clin Invest. 2017;127(1):183–198 BGB324 Foretinib Radiance [x106] (p/sec/cm2/sr)
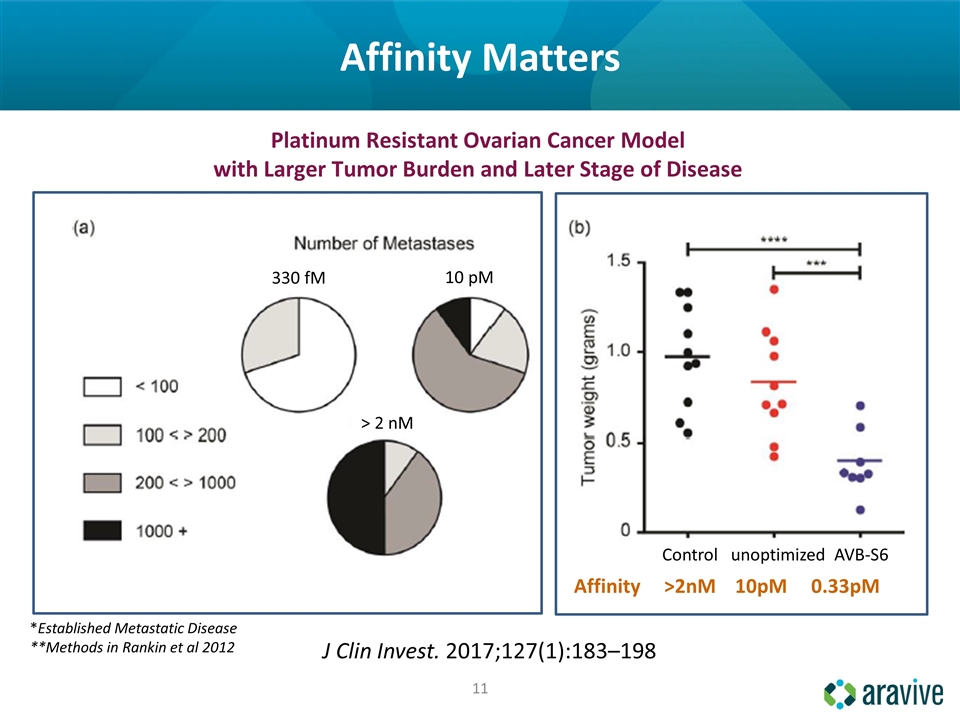
*Established Metastatic Disease **Methods in Rankin et al 2012 Platinum Resistant Ovarian Cancer Model with Larger Tumor Burden and Later Stage of Disease 330 fM > 2 nM 10 pM Affinity >2nM 10pM 0.33pM Control unoptimized AVB-S6 Affinity Matters J Clin Invest. 2017;127(1):183–198
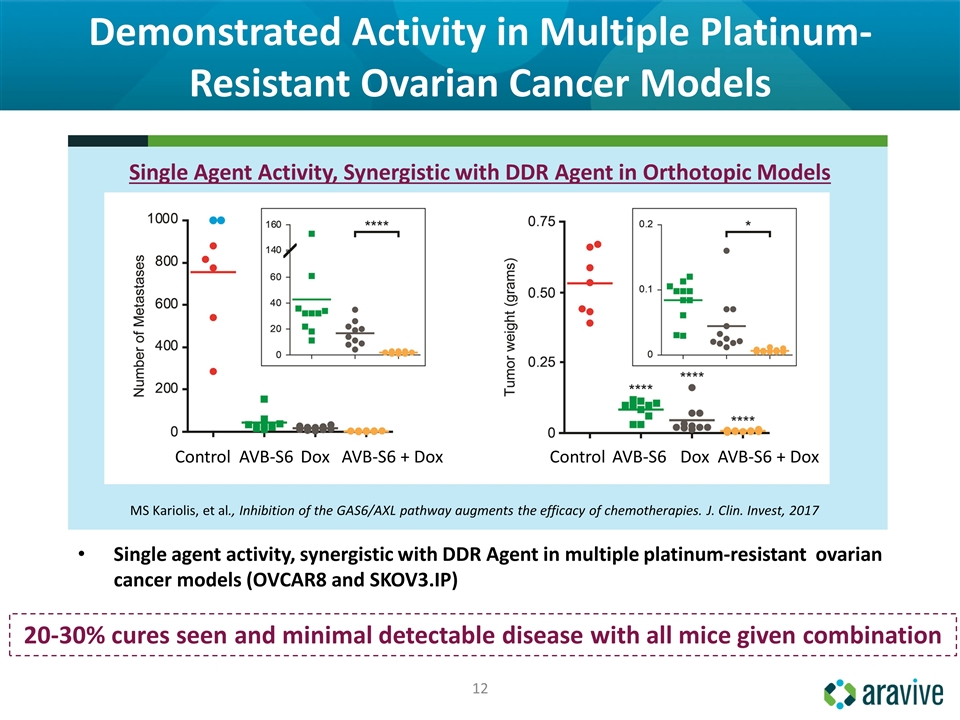
Demonstrated Activity in Multiple Platinum-Resistant Ovarian Cancer Models 20-30% cures seen and minimal detectable disease with all mice given combination Single agent activity, synergistic with DDR Agent in multiple platinum‐resistant ovarian cancer models (OVCAR8 and SKOV3.IP) MS Kariolis, et al., Inhibition of the GAS6/AXL pathway augments the efficacy of chemotherapies. J. Clin. Invest, 2017 Single Agent Activity, Synergistic with DDR Agent in Orthotopic Models Control AVB-S6 Dox AVB-S6 + Dox Control AVB-S6 Dox AVB-S6 + Dox
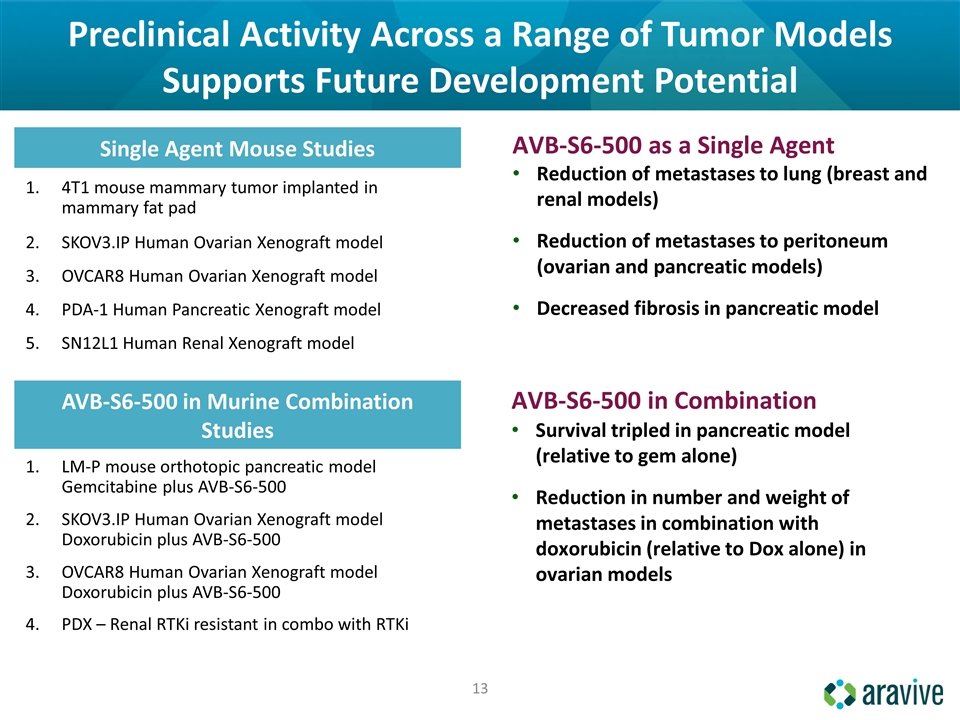
Preclinical Activity Across a Range of Tumor Models Supports Future Development Potential Single Agent Mouse Studies 4T1 mouse mammary tumor implanted in mammary fat pad SKOV3.IP Human Ovarian Xenograft model OVCAR8 Human Ovarian Xenograft model PDA-1 Human Pancreatic Xenograft model SN12L1 Human Renal Xenograft model AVB-S6-500 in Murine Combination Studies LM-P mouse orthotopic pancreatic model Gemcitabine plus AVB-S6-500 SKOV3.IP Human Ovarian Xenograft model Doxorubicin plus AVB-S6-500 OVCAR8 Human Ovarian Xenograft model Doxorubicin plus AVB-S6-500 PDX – Renal RTKi resistant in combo with RTKi Reduction of metastases to lung (breast and renal models) Reduction of metastases to peritoneum (ovarian and pancreatic models) Decreased fibrosis in pancreatic model AVB-S6-500 as a Single Agent Survival tripled in pancreatic model (relative to gem alone) Reduction in number and weight of metastases in combination with doxorubicin (relative to Dox alone) in ovarian models AVB-S6-500 in Combination
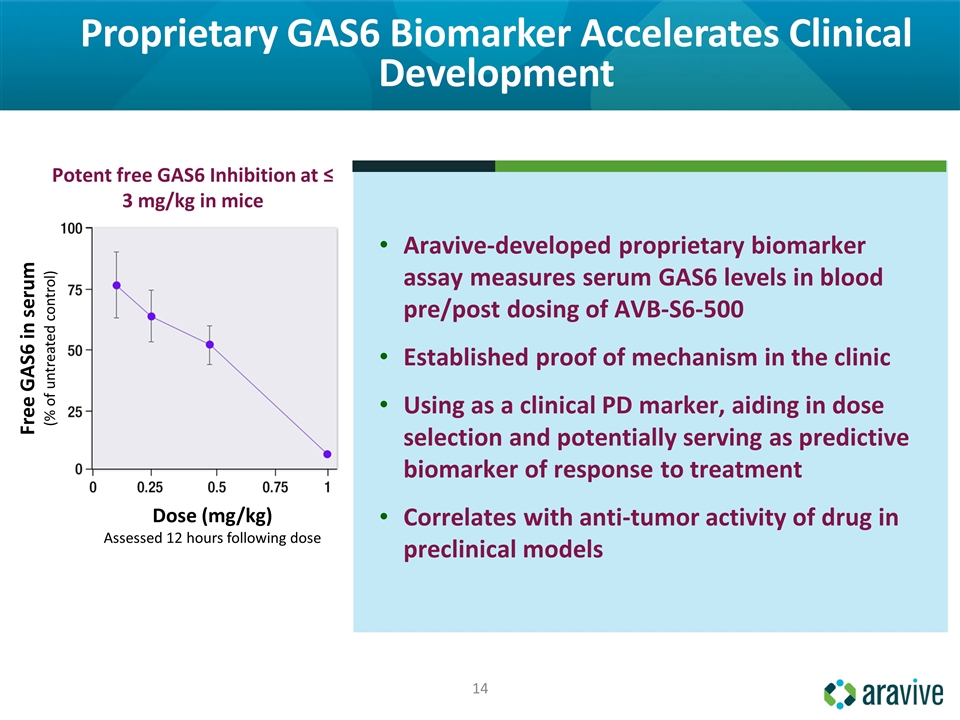
Proprietary GAS6 Biomarker Accelerates Clinical Development Aravive-developed proprietary biomarker assay measures serum GAS6 levels in blood pre/post dosing of AVB-S6-500 Established proof of mechanism in the clinic Using as a clinical PD marker, aiding in dose selection and potentially serving as predictive biomarker of response to treatment Correlates with anti-tumor activity of drug in preclinical models Potent free GAS6 Inhibition at ≤ 3 mg/kg in mice Dose (mg/kg) Assessed 12 hours following dose Free GAS6 in serum (% of untreated control)
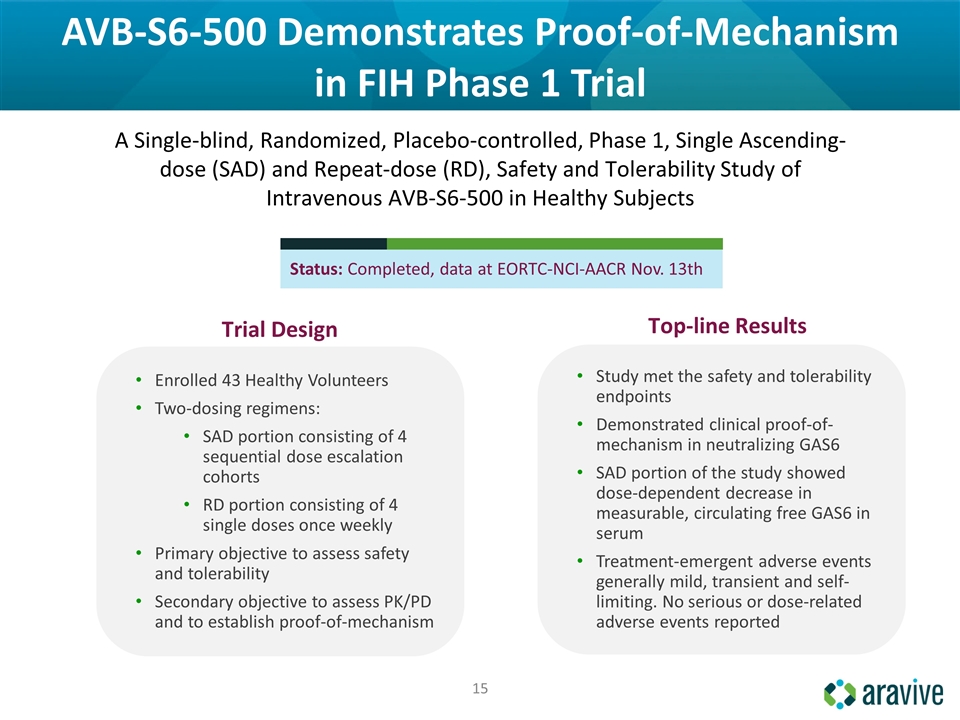
AVB-S6-500 Demonstrates Proof-of-Mechanism in FIH Phase 1 Trial Enrolled 43 Healthy Volunteers Two-dosing regimens: SAD portion consisting of 4 sequential dose escalation cohorts RD portion consisting of 4 single doses once weekly Primary objective to assess safety and tolerability Secondary objective to assess PK/PD and to establish proof-of-mechanism A Single-blind, Randomized, Placebo-controlled, Phase 1, Single Ascending-dose (SAD) and Repeat-dose (RD), Safety and Tolerability Study of Intravenous AVB-S6-500 in Healthy Subjects Study met the safety and tolerability endpoints Demonstrated clinical proof-of-mechanism in neutralizing GAS6 SAD portion of the study showed dose-dependent decrease in measurable, circulating free GAS6 in serum Treatment-emergent adverse events generally mild, transient and self-limiting. No serious or dose-related adverse events reported Top-line Results Trial Design Status: Completed, data at EORTC-NCI-AACR Nov. 13th
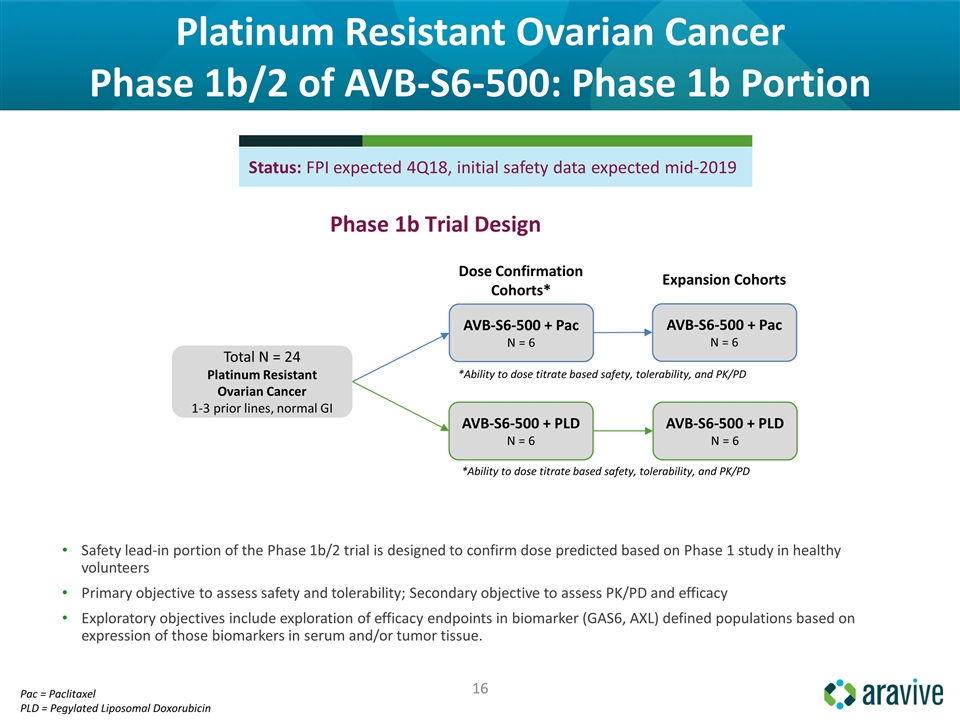
Platinum Resistant Ovarian Cancer Phase 1b/2 of AVB-S6-500: Phase 1b Portion Safety lead-in portion of the Phase 1b/2 trial is designed to confirm dose predicted based on Phase 1 study in healthy volunteers Primary objective to assess safety and tolerability; Secondary objective to assess PK/PD and efficacy Exploratory objectives include exploration of efficacy endpoints in biomarker (GAS6, AXL) defined populations based on expression of those biomarkers in serum and/or tumor tissue. Status: FPI expected 4Q18, initial safety data expected mid-2019 Phase 1b Trial Design AVB-S6-500 + Pac N = 6 AVB-S6-500 + PLD N = 6 AVB-S6-500 + Pac N = 6 AVB-S6-500 + PLD N = 6 Dose Confirmation Cohorts* Expansion Cohorts *Ability to dose titrate based safety, tolerability, and PK/PD *Ability to dose titrate based safety, tolerability, and PK/PD Total N = 24 Platinum Resistant Ovarian Cancer 1-3 prior lines, normal GI Pac = Paclitaxel PLD = Pegylated Liposomal Doxorubicin
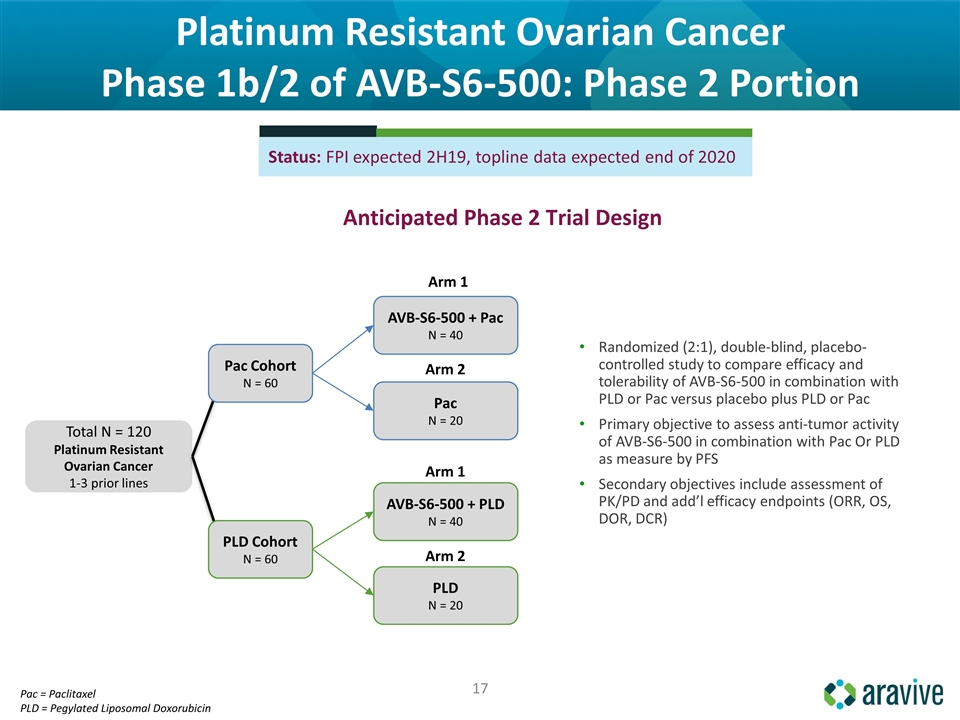
Platinum Resistant Ovarian Cancer Phase 1b/2 of AVB-S6-500: Phase 2 Portion Anticipated Phase 2 Trial Design Status: FPI expected 2H19, topline data expected end of 2020 Total N = 120 Platinum Resistant Ovarian Cancer 1-3 prior lines AVB-S6-500 + Pac N = 40 AVB-S6-500 + PLD N = 40 Pac N = 20 PLD N = 20 Arm 1 Arm 2 Arm 1 Arm 2 Randomized (2:1), double-blind, placebo-controlled study to compare efficacy and tolerability of AVB-S6-500 in combination with PLD or Pac versus placebo plus PLD or Pac Primary objective to assess anti-tumor activity of AVB-S6-500 in combination with Pac Or PLD as measure by PFS Secondary objectives include assessment of PK/PD and add’l efficacy endpoints (ORR, OS, DOR, DCR) Pac = Paclitaxel PLD = Pegylated Liposomal Doxorubicin Pac Cohort N = 60 PLD Cohort N = 60
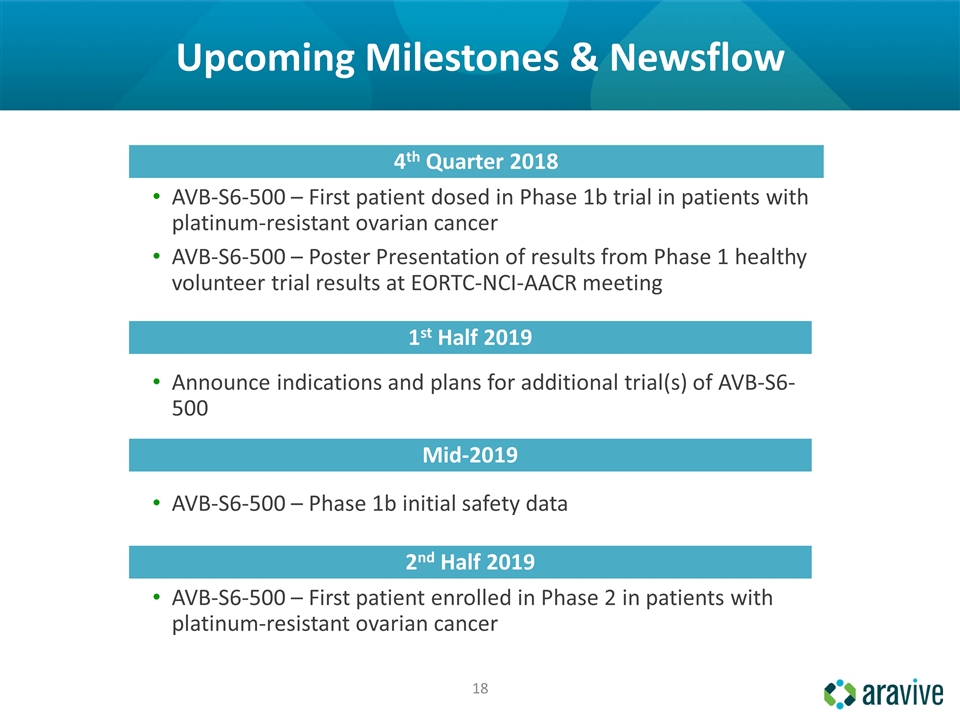
Upcoming Milestones & Newsflow 4th Quarter 2018 AVB-S6-500 – First patient dosed in Phase 1b trial in patients with platinum-resistant ovarian cancer AVB-S6-500 – Poster Presentation of results from Phase 1 healthy volunteer trial results at EORTC-NCI-AACR meeting 1st Half 2019 Announce indications and plans for additional trial(s) of AVB-S6-500 Mid-2019 AVB-S6-500 – Phase 1b initial safety data 2nd Half 2019 AVB-S6-500 – First patient enrolled in Phase 2 in patients with platinum-resistant ovarian cancer
Summary Promising, Validated Target GAS6-AXL is a promising oncology target that has been scientifically validated Established Proof-of-Mechanism in FIH Trial AVB-S6-500, was well tolerated in an Phase 1 trial where proof of mechanism was established -- full GAS6 neutralization at all doses tested Moving into Phase 1b/2 Initiate the Phase 1b portion of a Phase 1b/2 trial in ovarian cancer in 4Q18 Planning to initiate clinical development of AVB-S6-500 in additional tumor types in 2019 Looking Ahead Expansion into oncology indications where pre-clinical and biological rationale is strong (e.g. AML, renal cell, pancreatic, and TNBC) Expansion beyond oncology where pre-clinical and biological rationale is strong (e.g. fibrosis)
