Attached files
| file | filename |
|---|---|
| EX-99.3 - EX-99.3 - AGILE THERAPEUTICS INC | a18-37096_1ex99d3.htm |
| EX-99.1 - EX-99.1 - AGILE THERAPEUTICS INC | a18-37096_1ex99d1.htm |
| 8-K - 8-K - AGILE THERAPEUTICS INC | a18-37096_18k.htm |
1 Thomas D. Kimblea, Alfred Poindexterb, Kurt Barnhartc, Paula M. Castañod, Beatrice A. Chene, Joseph A. Chiodo IIIf, Elizabeth I.O. Garnerf aEastern Virginia Medical School, 601 Colley Ave, Norfolk, VA; bAdvances in Health, Inc., 7515 South Main St Suite 360, Houston, TX; cUniversity of Pennsylvania Medical Center, 3701 Market St Suite 810, Philadelphia, PA; d Columbia University Irving Medical Center, New York, NY; eUniversity of Pittsburgh/Magee-Womens Research Institute, 300 Halket St, Pittsburgh, PA; fAgile Therapeutics, 101 Poor Farm Rd, Princeton, NJ. Body mass index and weight are predictors of pregnancy in a Phase 3 multicenter contraceptive efficacy study of AG200-15, a low-dose combination hormonal contraceptive patch

Disclosures – Thomas D. Kimble, MD Speaker Bureau: Merck, AbbVie, Allergan Research Support: Agile, Allergan, Antiva, Bayer, Chemo, Gynesonics, Inovio, Medicines360, Mithra, Sebela This study was sponsored by Agile Therapeutics, Inc. 2

Background Yamazaki M, et al. Contraception 2015 Meta-analysis concludes that obese women may have a higher pregnancy rate during combination oral contraceptive use compared to non-obese women Objective To perform statistical modeling to identify variables predictive of pregnancy in a prospective Phase III study of an investigational low-dose, weekly transdermal contraceptive containing levonorgestrel and ethinyl estradiol (AG200-15) 3

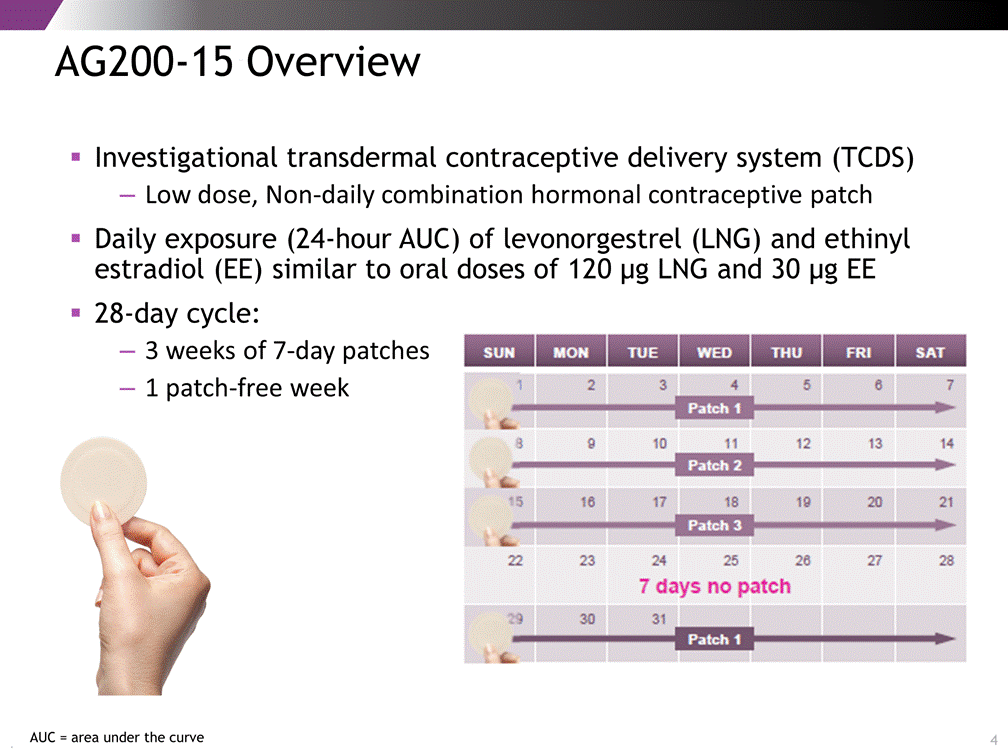
AG200-15 Overview Investigational transdermal contraceptive delivery system (TCDS) Low dose, Non-daily combination hormonal contraceptive patch Daily exposure (24-hour AUC) of levonorgestrel (LNG) and ethinyl estradiol (EE) similar to oral doses of 120 g LNG and 30 g EE 28-day cycle: 3 weeks of 7-day patches 1 patch-free week 4 AUC = area under the curve
SECURE Study Design Elements A Phase 3 Study to Evaluate Contraception Use, Reliability, and Effectiveness (SECURE) ClinicalTrials.gov NCT02158572 Single-arm, open-label, 1-year (13-cycle), healthcare-company funded, Phase 3, IRB-approved study Conducted at 102 US sites 2032 women were enrolled Subjects were 18 years of age or older, sexually active, and with regular menses every 21-38 days No eligibility restrictions for weight or body mass index (BMI) 5

STEP 1 - A series of univariate logistic regression analyses were performed to identify variables predictive of contraceptive failure (i.e., pregnancy) STEP 2 - These variables were used to inform two stepwise regression analyses (SRA1 and SRA2) Note – All analyses were performed for women <35 years of age Methods for Statistical Modeling

Age (years) Race (White, Black or African American, Other) Ethnicity (Hispanic or Latino, Not Hispanic or Latino) Weight (kg) Weight Decile Weight Median Split Weight Quartiles BMI (kg/m2) BMI <25, 25 to <30, >30 BMI <30, >30 BMI Median Split Smoking Status (Current Smoker, Past Smoker, Non-smoker) Alcohol Use in Past 12 Months (Yes, No) STEP 1 - Variables Used In The Univariate Regression Analysis Education Level (No College Degree, College Degree) Naïve to Transdermal Contraception (Yes, No) Prior Use of Hormonal Contraceptives (Current, Recent, Former, Naïve) Number of Prior Pregnancies Prior Pregnancies Category (0, 1, 2, >3) Late or Missed Patch Application (Yes, No) Unscheduled Patch Removal (Yes, No) At Least One Reported Patch Partially Detached (>25%) or Completely Detached (Yes, No) At Least One Reported Complete Detachment of the Patch (Yes, No) Non-compliant, defined as response of No to wearing a patch for 2 or more consecutive days (Yes, No)

Weight (kg) Weight Deciles Weight Quartiles BMI BMI <25, 25 to <30, >30 BMI <30, >30 Number of Prior Pregnancies Prior Pregnancies Category (0, 1, 2, >3) Variables that were Positively Related to Contraceptive Failure (p <0.050) Variables that were Negatively Related to Contraceptive Failure (p < 0.050) Unscheduled Patch Removal (Yes, No) At Least One Reported Complete Detachment of the Patch

Weight and BMI variables were highly correlated in univariate analysis therefore: We performed two stepwise regression analyses SRA1 - BMI >30 kg/m2 and <30 kg/m2 SRA2 - Weight deciles STEP 2 - Stepwise Regression

Age (years) Race (White, Black or African American, Other) Ethnicity (Hispanic or Latino, Not Hispanic or Latino) Smoking Status (Current Smoker, Past Smoker, Non-smoker) Alcohol Use in Past 12 Months (Yes, No) Education Level (No College Degree, College Degree) Prior Use of Hormonal Contraceptives (Current, Recent, Former, Naïve) Number of Prior Pregnancies Late or Missed Patch Application (Yes, No) Unscheduled Patch Removal (Yes, No) At Least One Reported Patch Partially Detached (>25%) or Completely Detached (Yes, No) At Least One Reported Complete Detachment of the Patch (Yes, No) Non-compliant, defined as response of No to wearing a patch for 2 or more consecutive days (Yes, No) Obesity and weight Variables used in Stepwise regression

SRA1 – BMI Number of prior pregnancies: OR = 1.344 (1.140, 1.583; p < 0.001) BMI >30 kg/m2 and <30 kg/m2: OR = 1.957 (1.186, 3.229; p = 0.009) Patch partially detached*: OR = 0.441 (0.260, 0.750; p = 0.002) Age: OR = 0.924 (0.868, 0.983; p = 0.012) Other variables not stat sig (p > 0.050) SRA2 - Weight Number of prior pregnancies: OR = 1.392 (1.175, 1.648; p < 0.001) Weight decile: OR = 1.145 (1.046, 1.253; p = 0.003) Patch partially detached*: OR = 0.405 (0.236, 0.693; p < 0.001) Age: OR = 0.914 (0.858, 0.974; p = 0.005) Other variable not stat sig (p > 0.050) Stepwise Regression Findings * At Least One Reported Patch Partially Detached (>25%) or Completely Detached

These analyses provide strong evidence that weight/BMI are associate with an increased risk of pregnancy in this Phase 3 study There were no restrictions on BMI or weight, resulting in a trial with a representative population of women that reflects current trends of the U.S. population New clinical trials that are being designed should not restrict weight or BMI, in order to have a study population that is representative of the growing obese population in the US and that will allow for an accurate assessment of contraceptive efficacy Conclusion

THANK YOU 13

