Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - Krystal Biotech, Inc. | d638711dex991.htm |
| 8-K - FORM 8-K - Krystal Biotech, Inc. | d638711d8k.htm |

Gene Therapy for Dermatological Diseases KB103: Gem Study Interim Phase 1/2 Clinical Update October 15, 2018 Exhibit 99.2

Forward-Looking Statements This webcast and the slides accompanying it regarding positive interim data from Krystal’s phase 1/2 trial evaluating KB103 in patients suffering from Dystrophic epidermolysis bullosa, or DEB, contains "forward-looking statements" regarding matters that are not historical facts, including statements relating to the Company's clinical trials, including plans to commence a pivotal Phase 3 study in 2H 2019. There can be no assurance that the data contained in these results will be replicated in additional current and future patients enrolled in this or any future trial or that these results will prove clinically meaningful in the development of KB103 as a potential drug. Because such statements are subject to risks and uncertainties, actual results may differ materially from those expressed or implied by such forward-looking statements. Words such as "anticipates," "plans," "expects," "intends," "will," "potential," "hope" and similar expressions are intended to identify forward-looking statements. These forward-looking statements are based upon current expectations of the Company and involve assumptions that may never materialize or may prove to be incorrect. Actual results and the timing of events could differ materially from those anticipated in such forward-looking statements as a result of various risks and uncertainties. Detailed information regarding factors that may cause actual results to differ materially from the results expressed or implied by statements in the webcast and slides relating to the Company may be found in the Company's periodic filings with the Securities and Exchange Commission, including the factors described in the section entitled "Risk Factors" in its annual report on Form 10-K for the fiscal year ended December 31, 2017, and supplemented from time to time and the Company's Quarter Reports on Form 10-Q and other filings submitted by the Company to the SEC, copies of which may be obtained from the SEC's website at www.sec.gov. The parties do not undertake any obligation to update forward-looking statements contained in this webcast and slides.

KB103 Phase I/II Interim Clinical Update
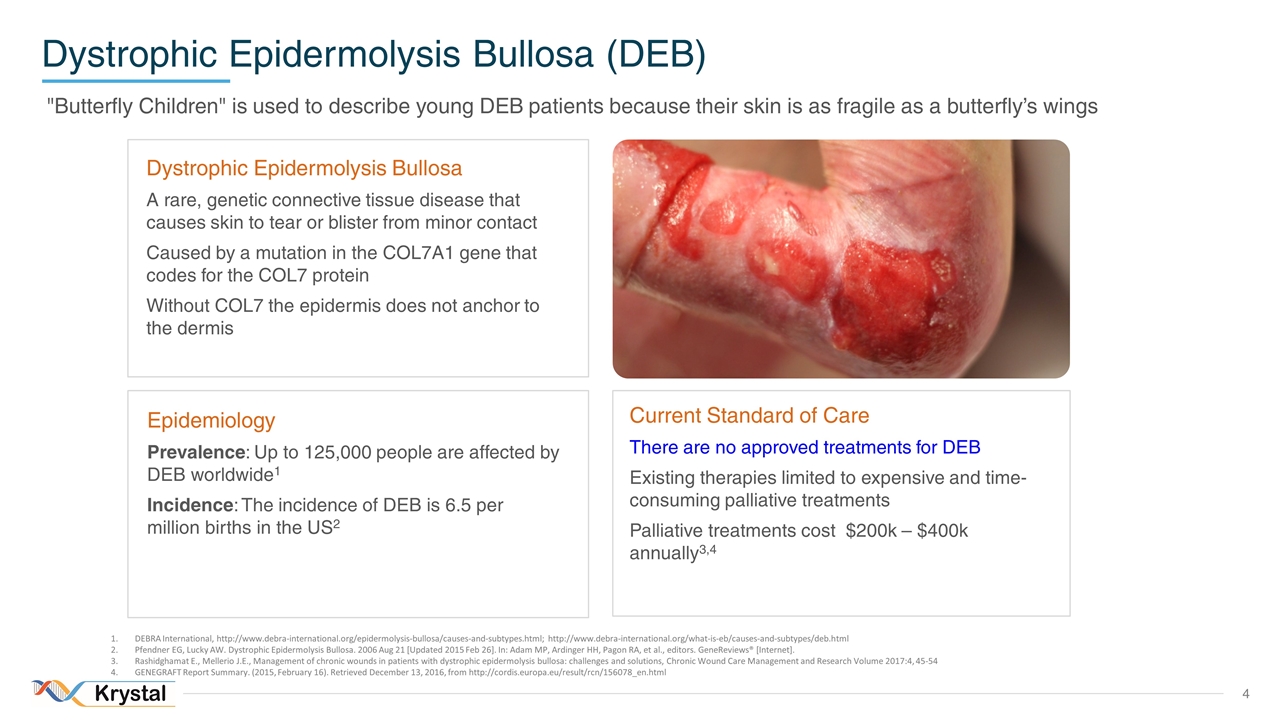
"Butterfly Children" is used to describe young DEB patients because their skin is as fragile as a butterfly’s wings Dystrophic Epidermolysis Bullosa (DEB) Dystrophic Epidermolysis Bullosa A rare, genetic connective tissue disease that causes skin to tear or blister from minor contact Caused by a mutation in the COL7A1 gene that codes for the COL7 protein Without COL7 the epidermis does not anchor to the dermis Epidemiology Prevalence: Up to 125,000 people are affected by DEB worldwide1 Incidence: The incidence of DEB is 6.5 per million births in the US2 Current Standard of Care There are no approved treatments for DEB Existing therapies limited to expensive and time-consuming palliative treatments Palliative treatments cost $200k – $400k annually3,4 DEBRA International, http://www.debra-international.org/epidermolysis-bullosa/causes-and-subtypes.html; http://www.debra-international.org/what-is-eb/causes-and-subtypes/deb.html Pfendner EG, Lucky AW. Dystrophic Epidermolysis Bullosa. 2006 Aug 21 [Updated 2015 Feb 26]. In: Adam MP, Ardinger HH, Pagon RA, et al., editors. GeneReviews® [Internet]. Rashidghamat E., Mellerio J.E., Management of chronic wounds in patients with dystrophic epidermolysis bullosa: challenges and solutions, Chronic Wound Care Management and Research Volume 2017:4, 45-54 GENEGRAFT Report Summary. (2015, February 16). Retrieved December 13, 2016, from http://cordis.europa.eu/result/rcn/156078_en.html
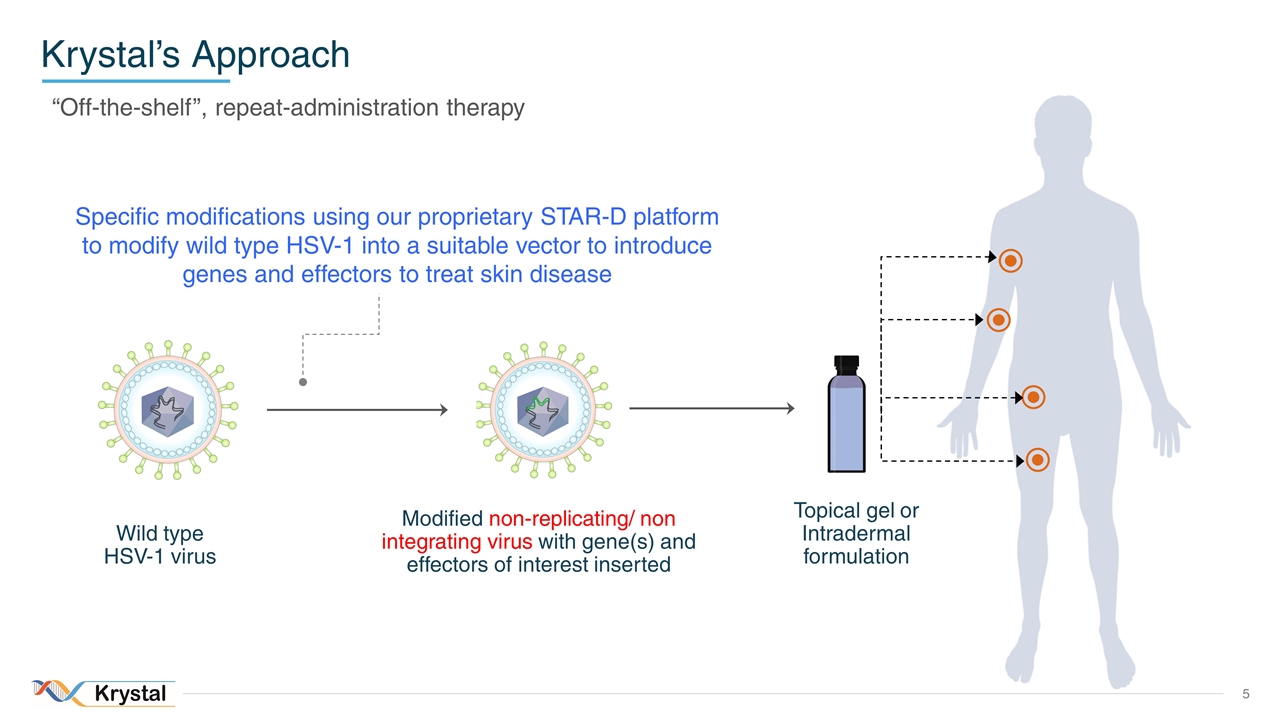
Krystal’s Approach “Off-the-shelf”, repeat-administration therapy Wild type HSV-1 virus Modified HSV-1 with gene(s) of interest inserted Specific modifications using our proprietary STAR-D platform to modify wild type HSV-1 into a suitable vector to introduce genes and effectors to treat skin disease Wild type HSV-1 virus Modified non-replicating/ non integrating virus with gene(s) and effectors of interest inserted Topical gel or Intradermal formulation
Phase 1/2 Trial Design 6 Key objectives: Demonstrate efficacy and safety of KB103 Primary Objectives: Expression of COL7, presence of anchoring fibrils, and safety Secondary Objectives: Change in wound area, duration of wound closure, time to wound closure Principal investigator: Dr. Peter Marinkovich, MD, Dermatologist, Stanford University Trial Design: Randomized, open-label, placebo controlled 2 wounds treated topically: 1 placebo, 1 active 1 intact site treated intradermally Patients were evaluated for COL7 expression by immunofluorescence and for the presence of anchoring fibrils by electron microscopy Initial dosing at Day 0 and a repeat dose a month later. Patient 2 was additionally dosed on Day 14 and Day 42 by PI to understand impact of incremental dose escalation. A Phase I/II Study of KB103, a Non-Integrating, Replication-Incompetent HSV Vector Expressing the Human Collagen VII Protein, for the Treatment of Dystrophic Epidermolysis Bullosa (DEB)
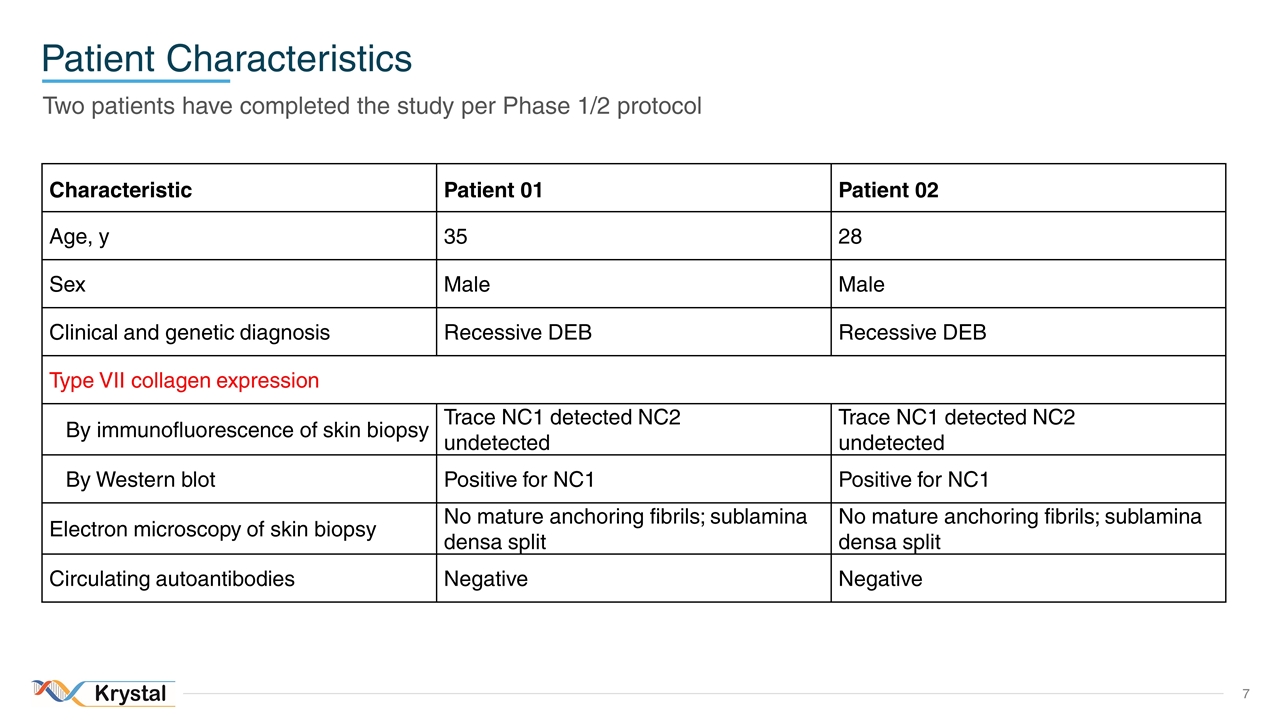
Patient Characteristics Two patients have completed the study per Phase 1/2 protocol Characteristic Patient 01 Patient 02 Age, y 35 28 Sex Male Male Clinical and genetic diagnosis Recessive DEB Recessive DEB Type VII collagen expression By immunofluorescence of skin biopsy Trace NC1 detected NC2 undetected Trace NC1 detected NC2 undetected By Western blot Positive for NC1 Positive for NC1 Electron microscopy of skin biopsy No mature anchoring fibrils; sublamina densa split No mature anchoring fibrils; sublamina densa split Circulating autoantibodies Negative Negative 7
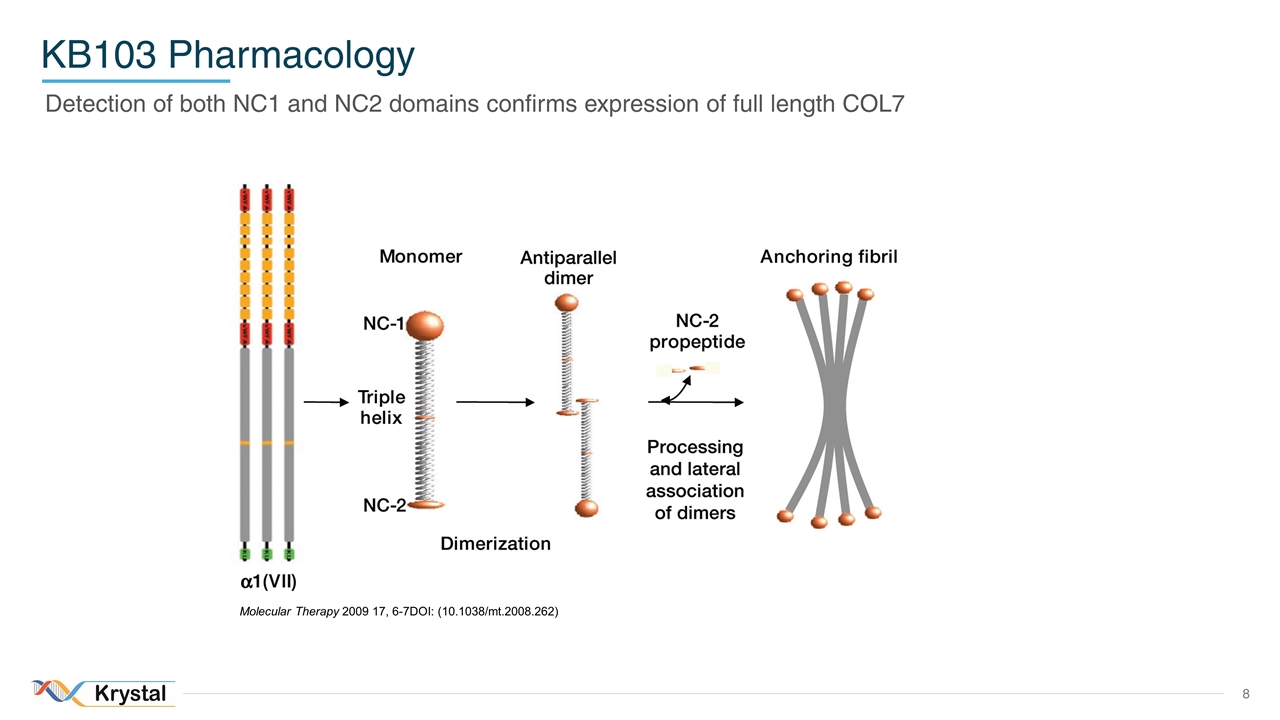
KB103 Pharmacology Detection of both NC1 and NC2 domains confirms expression of full length COL7 Molecular Therapy 2009 17, 6-7DOI: (10.1038/mt.2008.262)

KB103 Safety Update in Wounds with Topical Application No treatment-related adverse events (serious or otherwise) were reported. No immune response or blistering observed around the sites of administration following first and repeat dose. Blood and urine samples collected throughout the study revealed: No systemic viral shedding No adverse events associated with routine labs (chemistry and hematology) No antibodies to COL7 were detected KB103 continues to be well tolerated to date following first and repeat dose Summary

KB103 Efficacy Update in Wounds with Topical Application Results to date on 2 patients met all primary efficacy (presence of functional COL7 expression as early as Day 2 of treatment, observation of NC1 and NC2 reactive anchoring fibrils and continued expression following repeat administration) and safety endpoints (no adverse events, inflammation or irritation) in topically administered KB103 wounds. With respect to secondary endpoints – topically administered KB103 wounds closed in 2 weeks and continue to stay closed to date. Topically administered placebo administered wounds took 10 weeks to close in patient 1 and has not completely closed to date in patient 2. Placebo treated wound in Patient 1 is beginning to get compromised. KB103 when administered intradermally to intact skin shows presence functional COL7 expression and anchoring fibrils in both patients. Empirical observation that one patient discontinued use of bandages at the site of a KB103-treated area, an area which had required bandages for several months prior to administration. Summary
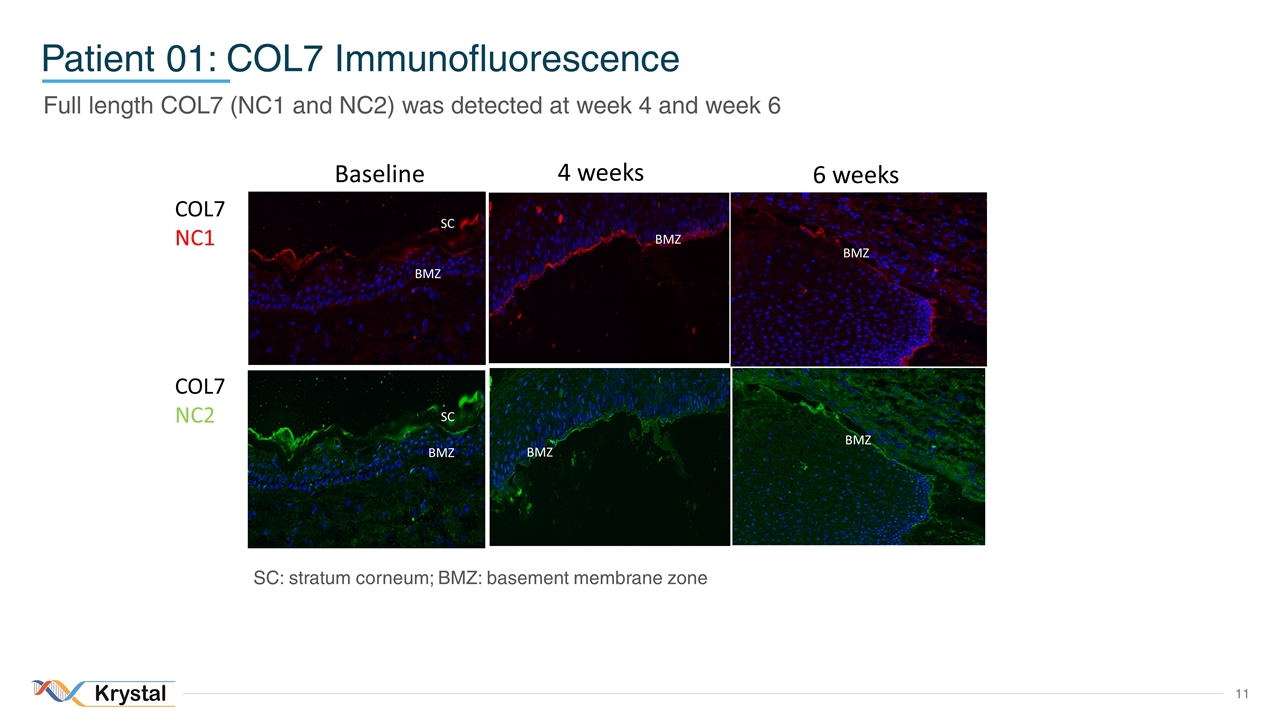
Patient 01: COL7 Immunofluorescence Full length COL7 (NC1 and NC2) was detected at week 4 and week 6 Baseline 4 weeks 6 weeks BMZ BMZ BMZ BMZ BMZ BMZ BMZ SC BMZ SC COL7 NC1 COL7 NC2 SC: stratum corneum; BMZ: basement membrane zone
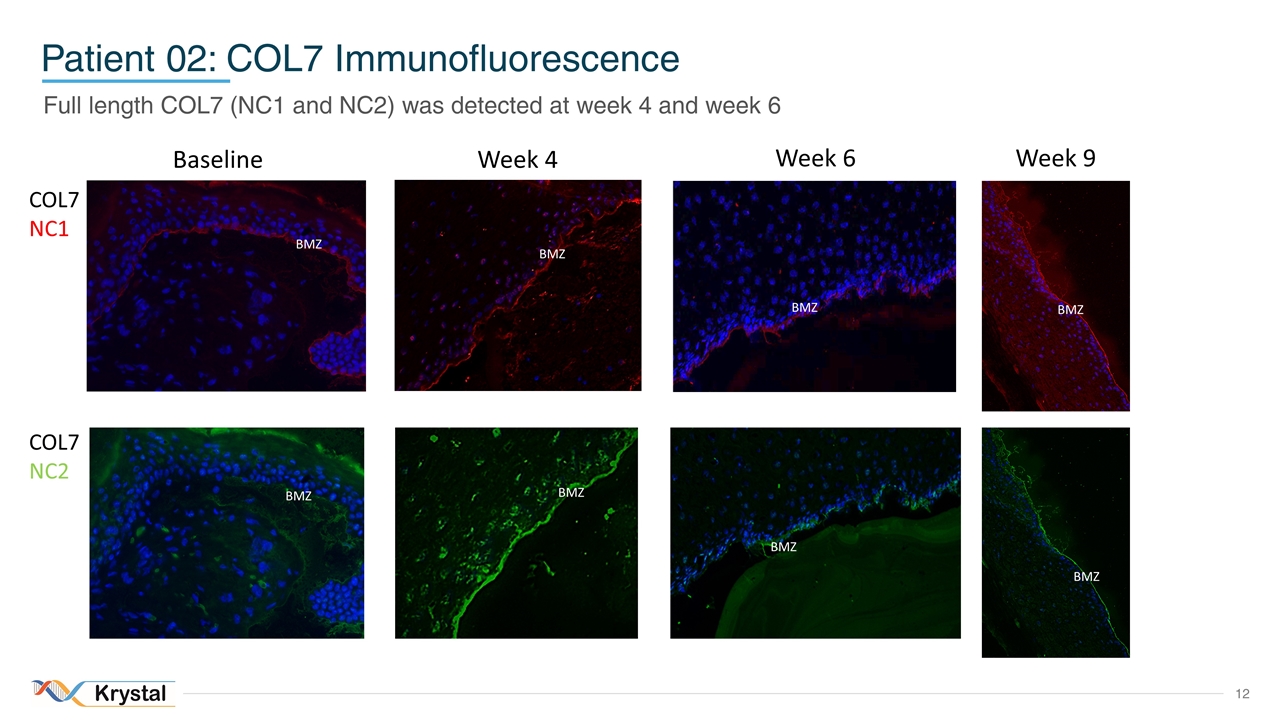
Patient 02: COL7 Immunofluorescence COL7 NC1 COL7 NC2 Baseline Week 4 Week 6 BMZ BMZ BMZ BMZ BMZ BMZ Full length COL7 (NC1 and NC2) was detected at week 4 and week 6 Week 9 BMZ BMZ
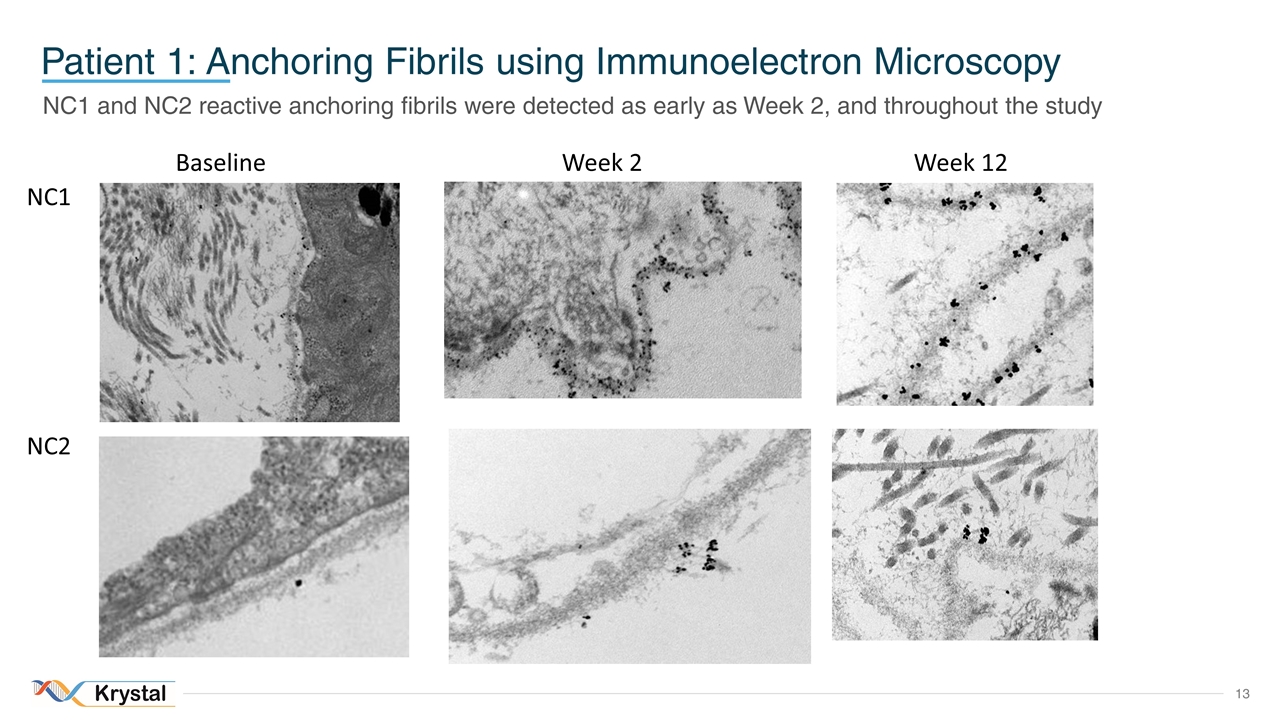
Patient 1: Anchoring Fibrils using Immunoelectron Microscopy NC1 and NC2 reactive anchoring fibrils were detected as early as Week 2, and throughout the study Baseline Week 12 NC1 NC2 Week 2
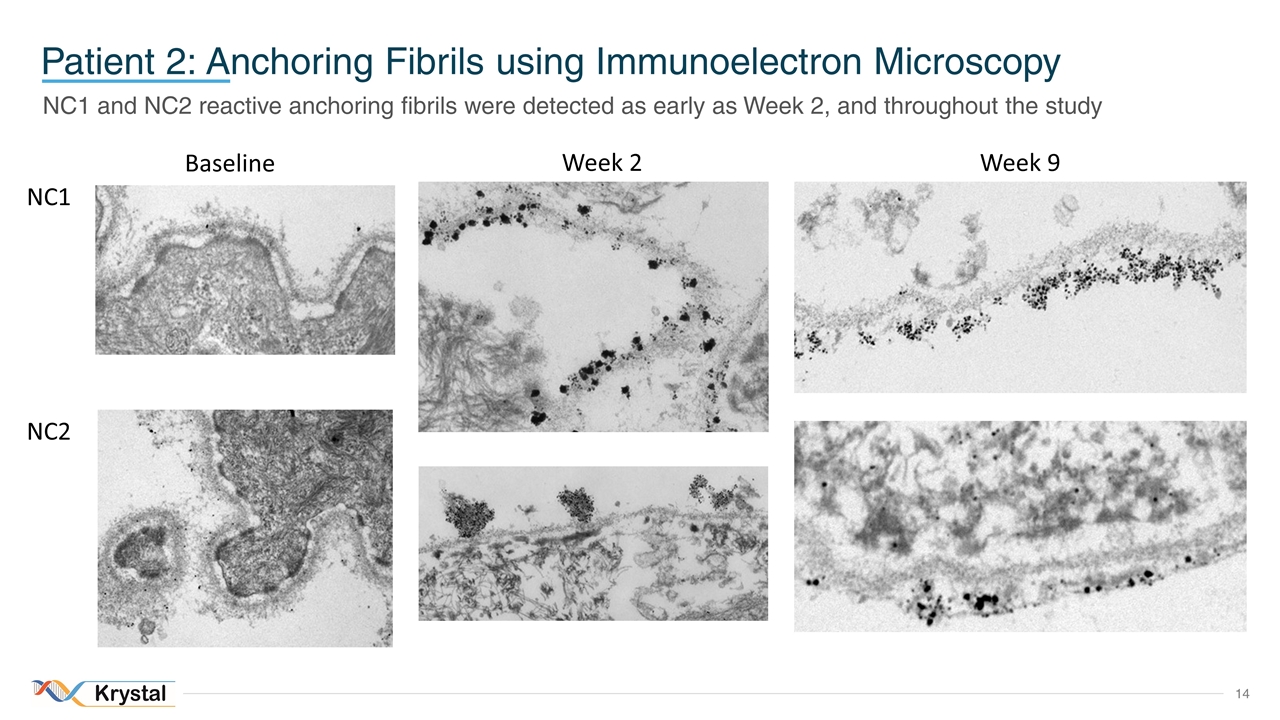
Patient 2: Anchoring Fibrils using Immunoelectron Microscopy NC1 and NC2 reactive anchoring fibrils were detected as early as Week 2, and throughout the study Baseline Week 9 NC1 NC2 Week 2
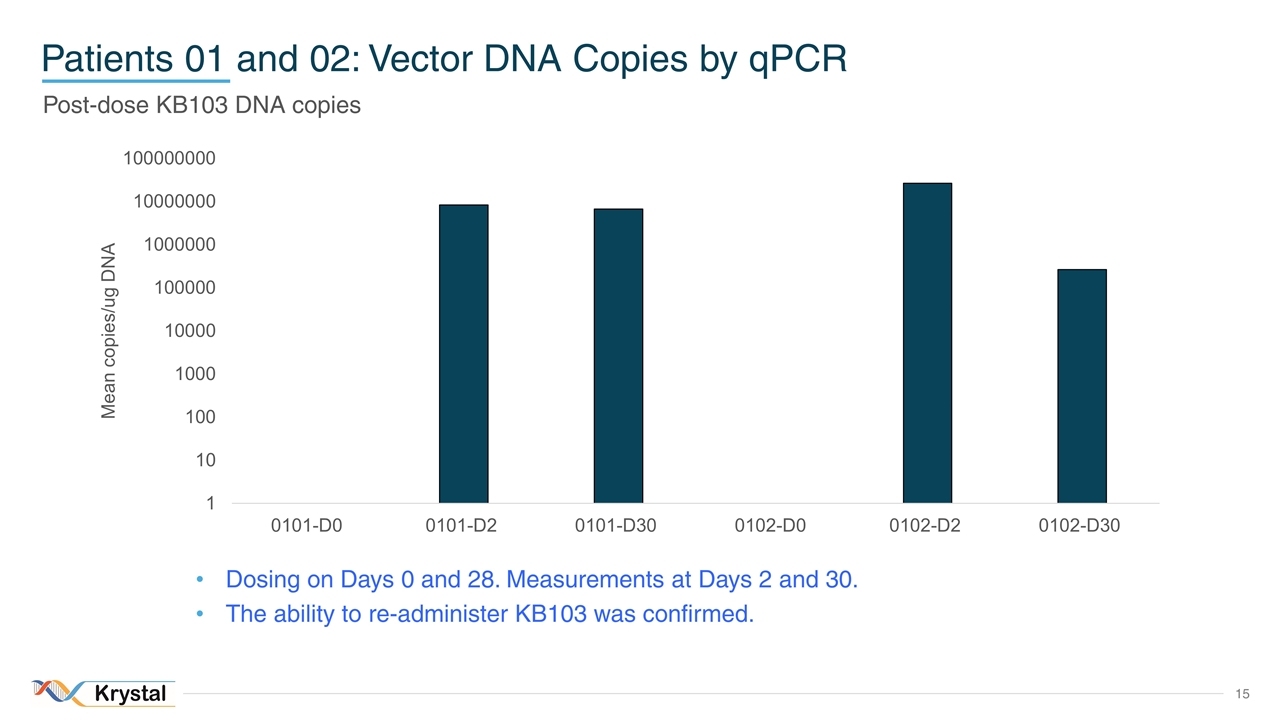
Patients 01 and 02: Vector DNA Copies by qPCR Post-dose KB103 DNA copies Dosing on Days 0 and 28. Measurements at Days 2 and 30. The ability to re-administer KB103 was confirmed.
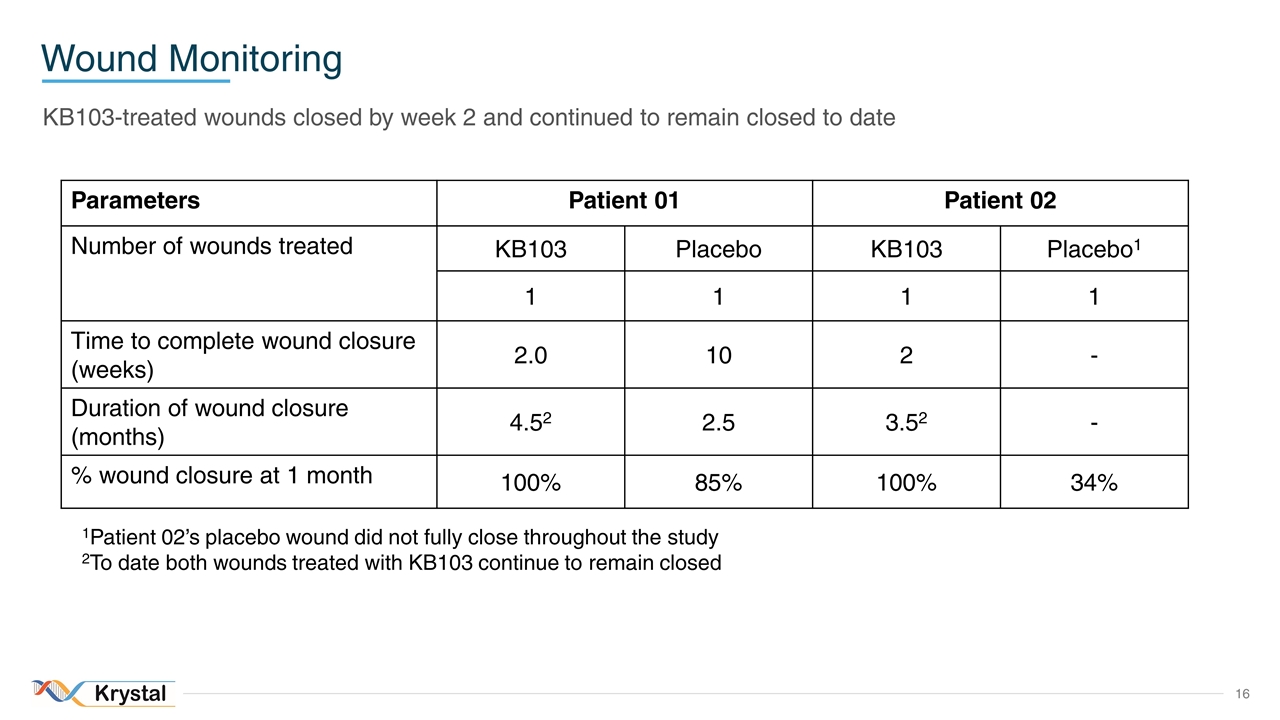
Wound Monitoring KB103-treated wounds closed by week 2 and continued to remain closed to date Parameters Patient 01 Patient 02 Number of wounds treated KB103 Placebo KB103 Placebo1 1 1 1 1 Time to complete wound closure (weeks) 2.0 10 2 - Duration of wound closure (months) 4.52 2.5 3.52 - % wound closure at 1 month 100% 85% 100% 34% 1Patient 02’s placebo wound did not fully close throughout the study 2To date both wounds treated with KB103 continue to remain closed
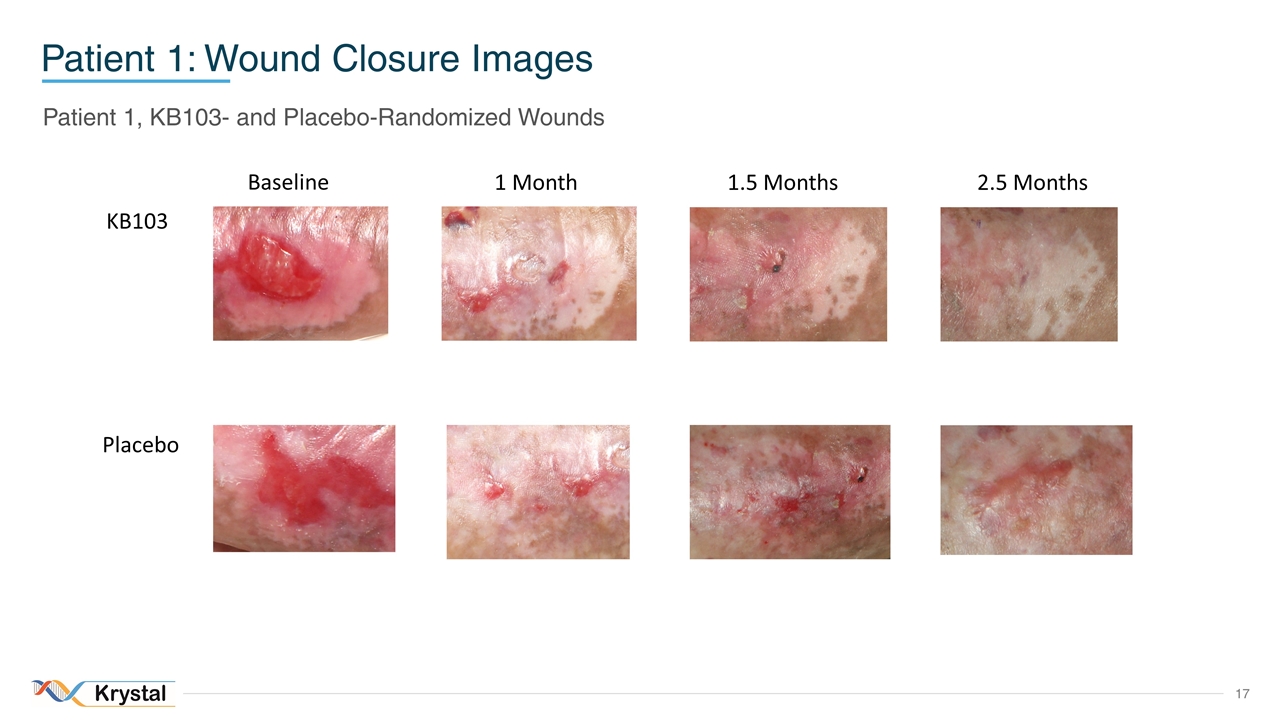
Patient 1: Wound Closure Images Patient 1, KB103- and Placebo-Randomized Wounds Baseline 1 Month 2.5 Months 1.5 Months KB103 Placebo
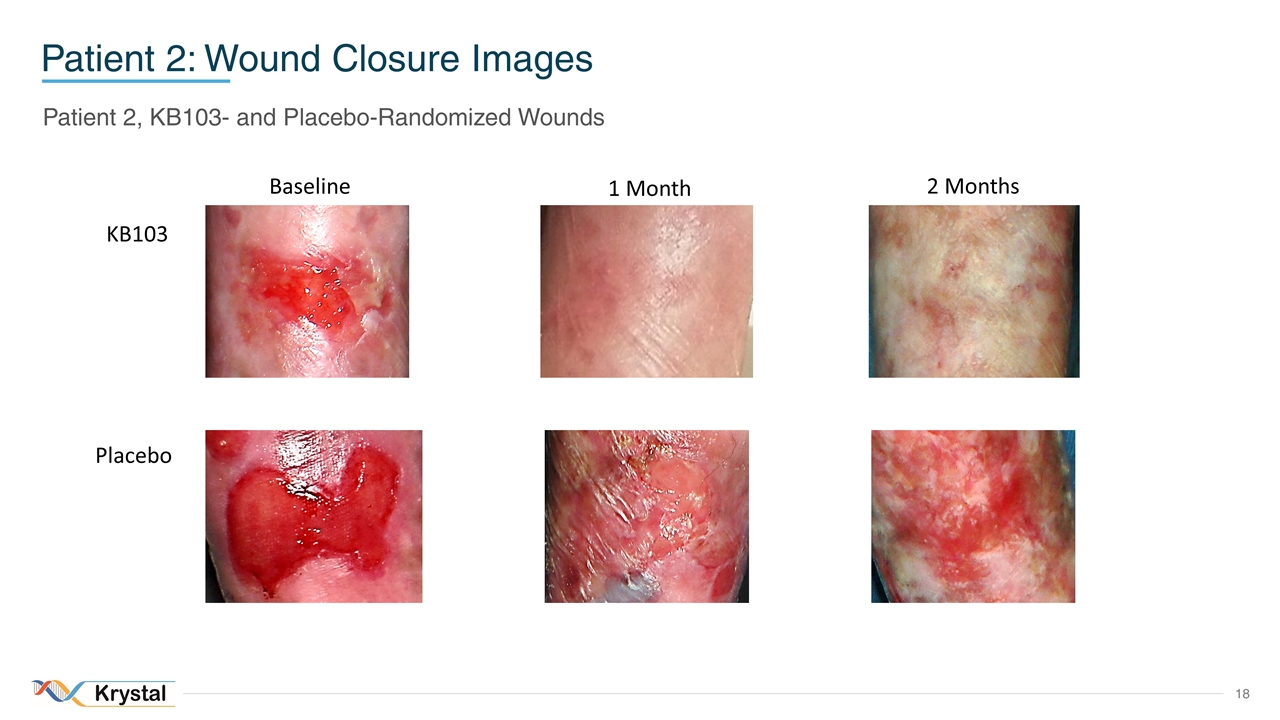
Patient 2: Wound Closure Images Patient 2, KB103- and Placebo-Randomized Wounds Baseline 1 Month 2 Months KB103 Placebo
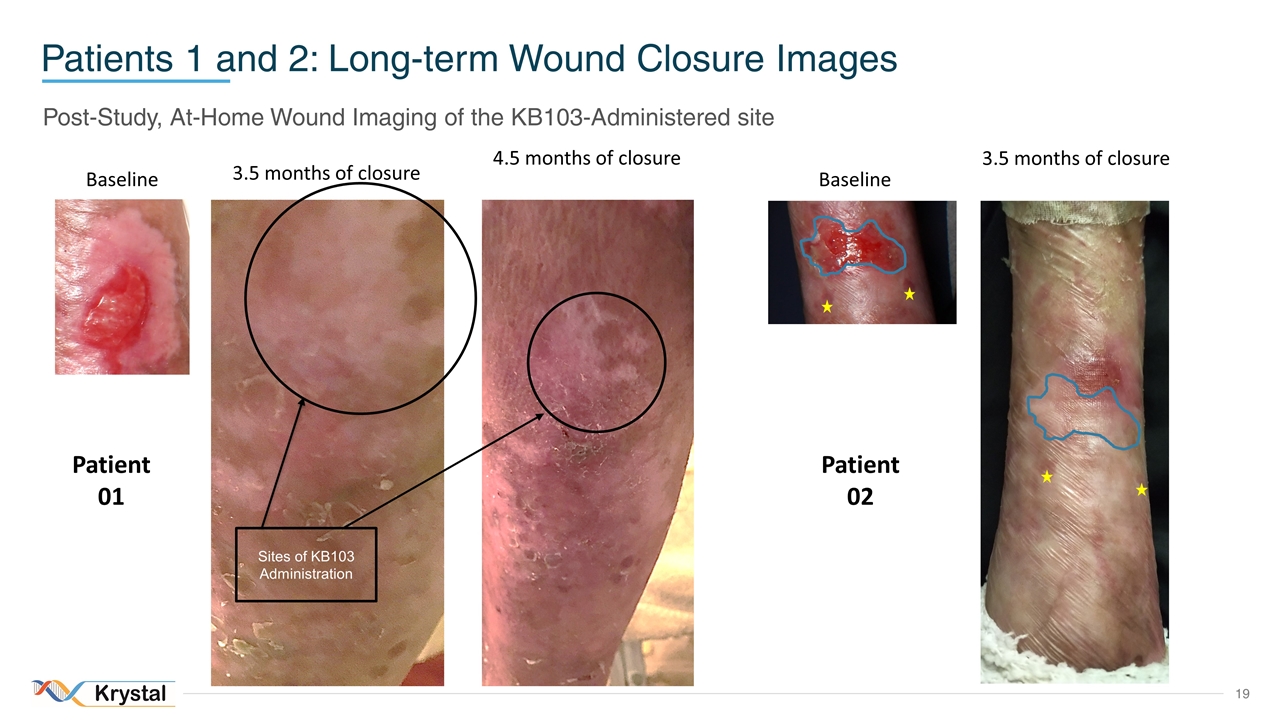
Patients 1 and 2: Long-term Wound Closure Images Post-Study, At-Home Wound Imaging of the KB103-Administered site Sites of KB103 Administration Baseline 4.5 months of closure 3.5 months of closure Baseline 3.5 months of closure Patient 01 Patient 02

KB103 Efficacy Update in Wounds with Topical Application Results to date on 2 patients met all primary efficacy (presence of functional COL7 expression, observation of NC1 and NC2 reactive anchoring fibrils and continued expression following repeat administration) and safety endpoints (no adverse events, inflammation or irritation) in topically administered KB103 wounds. With respect to secondary endpoints – topically administered KB103 wounds closed in 2 weeks and continue to stay closed to date. Topically administered placebo administered wounds took 10 weeks to close in patient 1 and has not completely closed to date in patient 2. Placebo treated wound in Patient 1 is beginning to get compromised. KB103 when administered intradermally to intact skin shows presence functional COL7 expression and anchoring fibrils in both patients. Empirical observation that one patient discontinued use of bandages at the site of a KB103-treated area, an area which had required bandages for several months prior to administration. Summary

Next Steps in KB103 Development Complete Phase 1/2 trial in adults and pediatric patients by 1H 2019 Clinical data from two patients submitted to FDA FDA acknowledges molecular correction as evidenced by expression of COL7 and anchoring fibrils Amended protocol allows for: Removal of the intradermal arm for patients in the ongoing Phase 1/2 trial The enrollment of pediatric patients and a focus on the durability of wound closure Increased dosing and KB103 administration to larger wound areas Initiate pivotal Phase 3 study in 2H 2019 The study is anticipated to be ~ 6 months in duration File the BLAin US for KB103 following successful completion of Phase 3 study Anticipated review time by Agency ~ 6 months based on Fast Track Designation GMP facility in Pittsburgh is expected to be complete by end of 2018 produce clinical materials for pivotal batch and commercialization Efforts underway to initiate clinical trials in Europe and Australia in 2019
