Attached files
| file | filename |
|---|---|
| EX-99.1 - EX-99.1 - AMICUS THERAPEUTICS, INC. | a18-36411_1ex99d1.htm |
| 8-K - 8-K - AMICUS THERAPEUTICS, INC. | a18-36411_18k.htm |
Results From ATB200-02: First-in-Human Study of ATB200 Co-Administered With AT2221 for Pompe Disease (18-Month Results) Benedikt Schoser,1 Drago Bratkovic,2 Barry J. Byrne,3 Paula Clemens,4 Tarekegn Geberhiwot,5 Ozlem Goker-Alpan,6 Priya Kishnani,7 Xue Ming,8 Tahseen Mozaffar,9 Peter Schwenkreis,10 Kumaraswamy Sivakumar,11 Ans T. van der Ploeg,12 Jacquelyn Wright,13 Swati Sathe,13 Sheela Sitaraman,13 Hjalmar Lagast,13 Jay A. Barth,13 Mark Roberts14 1Klinikum der Universität München-Neurologische Klinik, Friedrich-Baur-Institut, Munich, Germany; 2PARC Research Clinic, Royal Adelaide Hospital, Adelaide, SA, Australia; 3University of Florida, Gainesville, FL, USA; 4University of Pittsburgh and Department of Veterans Affairs Medical Center, Pittsburgh, PA, USA; 5University Hospital Birmingham NHS Foundation Trust, Queen Elizabeth Medical Center, Birmingham, UK; 6O&O Alpan LLC, Fairfax, VA, USA; 7Duke University Medical Center, Durham, NC, USA; 8Rutgers New Jersey Medical School, Newark, NJ, USA; 9University of California, Irvine, Orange, CA, USA; 10Neurologische Klinik und Poliklinik des Berufsgenossenschaftlichen, Universitätklinikum Bergmannsheil, Bochum, Germany; 11Neuromuscular Research Center, Phoenix, AZ, USA; 12Erasmus MC University Medical Center, Rotterdam, The Netherlands; 13Amicus Therapeutics, Inc., Cranbury, NJ, USA ; 14Salford Royal NHS Foundation Trust, Salford, UK;

Benedikt Schoser Disclosure Information I have the following financial relationships to disclose: Consultant for Amicus Therapeutics, Inc. Consultant and member of speaker bureau for Audentes, Genzyme, Intiva, Lupin, Valerion and Vertex. I will discuss the following off-label use and/or investigational use in my presentation: Data from a phase 1/2 trial of ATB200/AT2221 for the treatment of patients with Pompe disease ATB200/AT2221 is an investigational therapy that has not been approved for commercial use

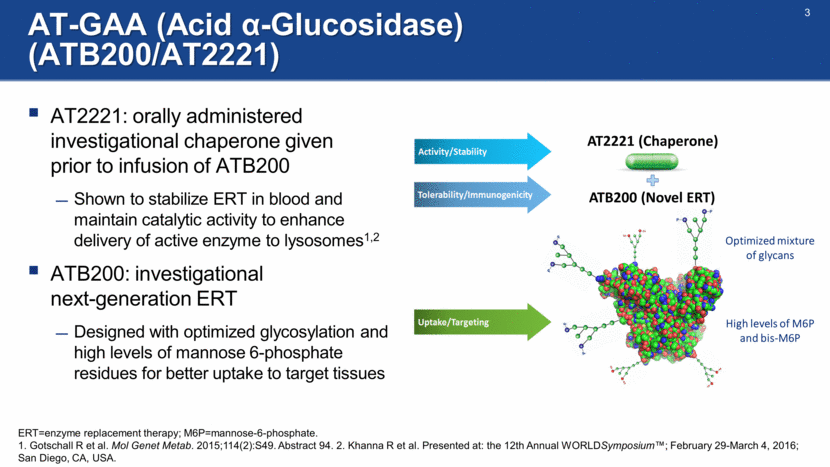
AT-GAA (Acid -Glucosidase) (ATB200/AT2221) AT2221: orally administered investigational chaperone given prior to infusion of ATB200 Shown to stabilize ERT in blood and maintain catalytic activity to enhance delivery of active enzyme to lysosomes1,2 ATB200: investigational next-generation ERT Designed with optimized glycosylation and high levels of mannose 6-phosphate residues for better uptake to target tissues AT2221 (Chaperone) ATB200 (Novel ERT) ERT=enzyme replacement therapy; M6P=mannose-6-phosphate. 1. Gotschall R et al. Mol Genet Metab. 2015;114(2):S49. Abstract 94. 2. Khanna R et al. Presented at: the 12th Annual WORLDSymposium™; February 29-March 4, 2016; San Diego, CA, USA. High levels of M6P and bis-M6P Optimized mixture of glycans
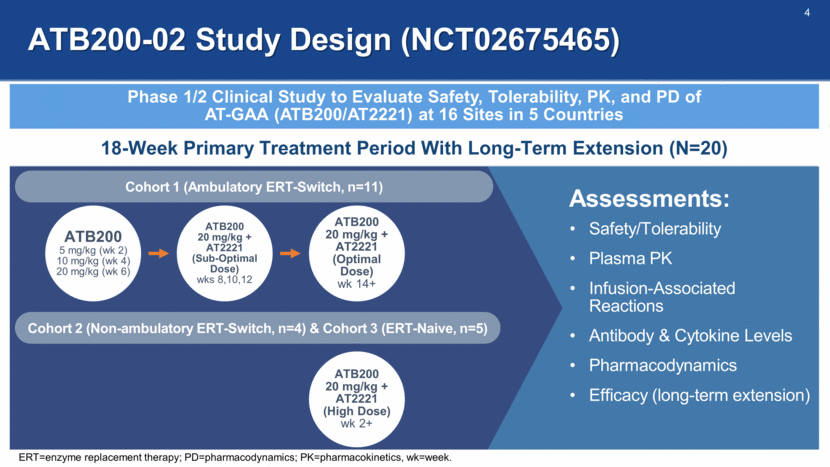
ATB200-02 Study Design (NCT02675465) Phase 1/2 Clinical Study to Evaluate Safety, Tolerability, PK, and PD of AT-GAA (ATB200/AT2221) at 16 Sites in 5 Countries 18-Week Primary Treatment Period With Long-Term Extension (N=20) ATB200 5 mg/kg (wk 2) 10 mg/kg (wk 4) 20 mg/kg (wk 6) ATB200 20 mg/kg + AT2221 (Sub-Optimal Dose) wks 8,10,12 ATB200 20 mg/kg + AT2221 (Optimal Dose) wk 14+ Cohort 1 (Ambulatory ERT-Switch, n=11) Cohort 2 (Non-ambulatory ERT-Switch, n=4) & Cohort 3 (ERT-Naive, n=5) ATB200 20 mg/kg + AT2221 (High Dose) wk 2+ Safety/Tolerability Plasma PK Infusion-Associated Reactions Antibody & Cytokine Levels Pharmacodynamics Efficacy (long-term extension) Assessments: ERT=enzyme replacement therapy; PD=pharmacodynamics; PK=pharmacokinetics, wk=week.
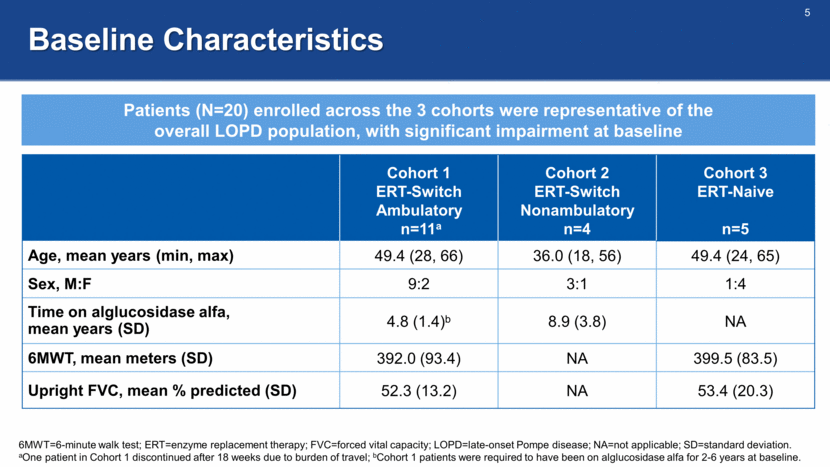
Baseline Characteristics 6MWT=6-minute walk test; ERT=enzyme replacement therapy; FVC=forced vital capacity; LOPD=late-onset Pompe disease; NA=not applicable; SD=standard deviation. aOne patient in Cohort 1 discontinued after 18 weeks due to burden of travel; bCohort 1 patients were required to have been on alglucosidase alfa for 2-6 years at baseline. Cohort 1 ERT-Switch Ambulatory n=11a Cohort 2 ERT-Switch Nonambulatory n=4 Cohort 3 ERT-Naive n=5 Age, mean years (min, max) 49.4 (28, 66) 36.0 (18, 56) 49.4 (24, 65) Sex, M:F 9:2 3:1 1:4 Time on alglucosidase alfa, mean years (SD) 4.8 (1.4)b 8.9 (3.8) NA 6MWT, mean meters (SD) 392.0 (93.4) NA 399.5 (83.5) Upright FVC, mean % predicted (SD) 52.3 (13.2) NA 53.4 (20.3) Patients (N=20) enrolled across the 3 cohorts were representative of the overall LOPD population, with significant impairment at baseline
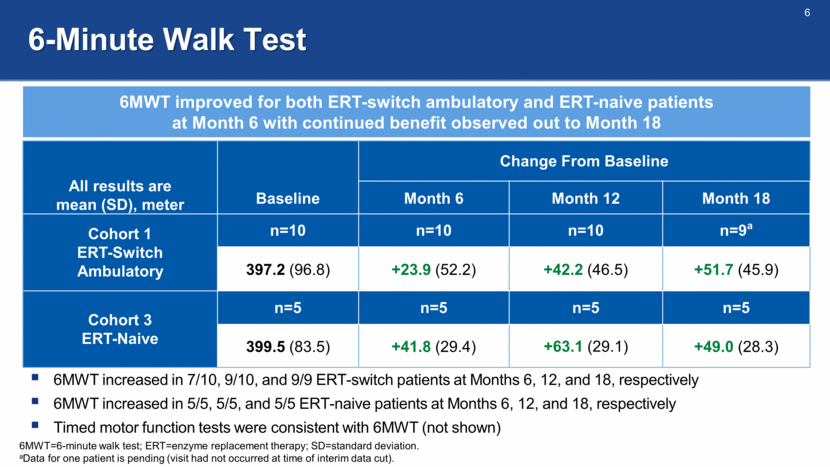
6-Minute Walk Test All results are mean (SD), meter Baseline Change From Baseline Month 6 Month 12 Month 18 Cohort 1 ERT-Switch Ambulatory n=10 n=10 n=10 n=9a 397.2 (96.8) +23.9 (52.2) +42.2 (46.5) +51.7 (45.9) Cohort 3 ERT-Naive n=5 n=5 n=5 n=5 399.5 (83.5) +41.8 (29.4) +63.1 (29.1) +49.0 (28.3) 6MWT improved for both ERT-switch ambulatory and ERT-naive patients at Month 6 with continued benefit observed out to Month 18 6MWT=6-minute walk test; ERT=enzyme replacement therapy; SD=standard deviation. aData for one patient is pending (visit had not occurred at time of interim data cut). 6MWT increased in 7/10, 9/10, and 9/9 ERT-switch patients at Months 6, 12, and 18, respectively 6MWT increased in 5/5, 5/5, and 5/5 ERT-naive patients at Months 6, 12, and 18, respectively Timed motor function tests were consistent with 6MWT (not shown)
Manual Muscle Test Score Increases were observed in manual muscle strengtha in all patients at Months 6, 12, and 18 Quantitative muscle strength testinge results were generally consistent with manual muscle test results Body Area Baseline Change From Baseline Month 6 Month 12 Month 18 mean (SD) n mean (SD) n mean (SD) n mean (SD) n ERT-switch Ambulatory Total Body Max score 80 66.4 (8.1) 10 +2.5 (3.2) 9 +3.3 (3.4) 9 +4.5 (3.2) 9 ERT-switch Non-Ambulatory Upper Body Max score 40 13.3 (12.2) 3b +4.5 (0.7) 2bc +2.7 (2.3) 3b +4.3 (3.5) 3b ERT-Naive Total Body Max score 80 66.9 (3.7) 5 +0.3 (2.8) 5 +1.1 (3.1) 5 +2.0 (2.9) 4d ERT=enzyme replacement therapy; SD=standard deviation. aMeasured via the Medical Research Criteria (MRC) scale; bBaseline data missing for 1 patient; cOne patient did not complete Month 6 assessment; dManual muscle testing not completed for one patient; eMeasured via hand-held dynamometer.
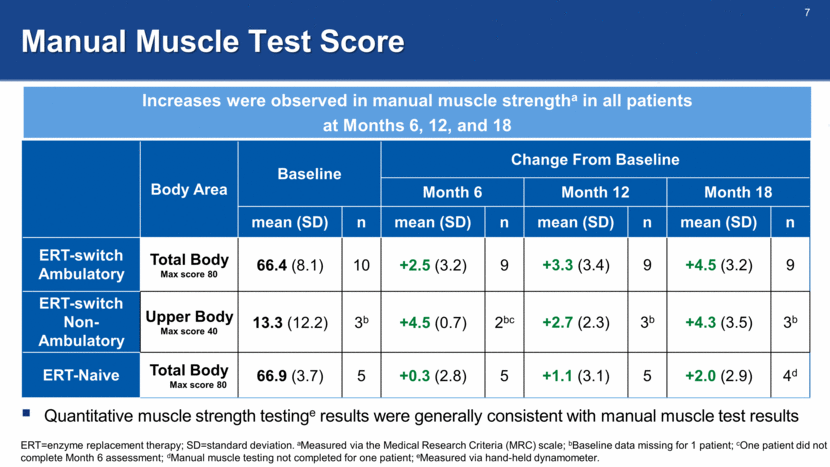
Sitting Forced Vital Capacity (FVC, % Predicted) FVC was stable or increased in 5/9, 6/9, and 5/8 ERT-switch patients at Months 6, 12, and 18, respectively FVC was stable or increased in 5/5, 4/5, and 5/5 ERT-naive patients at Months 6, 12, and 18, respectively FVC was generally stable in ERT-switch ambulatory patients and increased in ERT-naive patients ERT=enzyme replacement therapy; SD=standard deviation. aBaseline FVC not available for 1 patient in Cohort 1; bFVC for one patient in Cohort 1 pending (visit had not occurred at time of interim data cut). . Baseline, mean (SD) Change From Baseline, mean (SD) Month 6 Month 12 Month 18 Cohort 1 ERT-Switch Ambulatory n=9a n=9a n=9a n=8a,b 52.6 (14.7) -1.3 (4.1) -3.3 (6.1) -3.7 (7.0) Cohort 3 ERT-Naive n=5 n=5 n=5 n=5 53.4 (20.3) +4.2 (5.6) +4.4 (8.6) +5.0 (2.9)
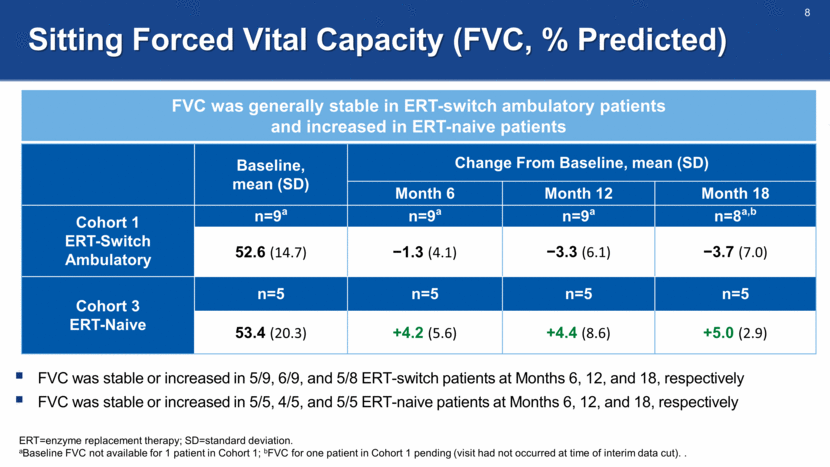
Other Pulmonary Function Tests: MIP and MEP MIP was stable and MEP increased in ERT-switch ambulatory patients; MIP and MEP increased in ERT-naive patients ERT=enzyme replacement therapy; MEP=maximal expiratory pressure; MIP=maximal inspiratory pressure; SD=standard deviation. MIP and MEP measured in centimeters of water. aData for one patient in Cohort 1 pending (visit had not occurred at time of interim data cut). Assessment Baseline, mean (SD) Change From Baseline, mean (SD) Month 6 Month 12 Month 18 Cohort 1 ERT-Switch Ambulatory n=10 n=10 n=10 n=9a MIP 35.7 (11.0) +0.3 (4.6) 0.0 (3.2) -2.8 (4.4) MEP 72.6 (32.6) +16.1 (42.1) +28.6 (44.0) +30.2 (43.0) Cohort 3 ERT-Naive n=5 n=5 n=5 n=5 MIP 32.6 (18.5) +11.0 (5.0) +5.2 (12.2) +6.2 (11.5) MEP 60.6 (8.3) -0.4 (12.4) +8.6 (16.3) +9.8 (19.6)
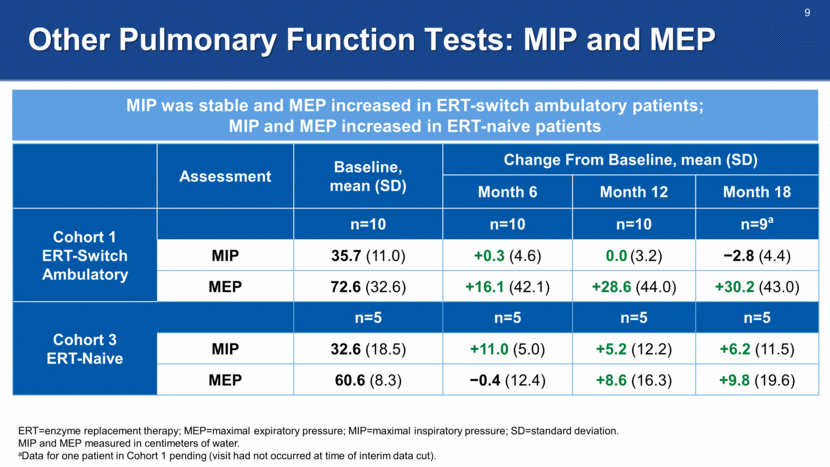
Fatigue Severity Scale (FSS) All cohorts were significantly impacted by fatigue at baseline and demonstrated a mean improvement in fatigue ERT=enzyme replacement therapy; SD=standard deviation. 1. Grace J et al. Parkinsonism Relat Disord. 2007;13(7):442-445. FSS consists of 9 questions, each scored on a scale from 1 to 7. Total scores range from 9 to 63, with higher values representing higher levels of fatigue due to the disease condition. The normative value in the healthy population is ~21.1 Baseline, mean (SD) Change From Baseline, mean (SD) Month 6 Month 12 Month 18 Cohort 1 ERT-Switch Ambulatory n=10 n=10 n=10 n=9 53.5 (7.7) -8.0 (10.7) -8.0 (6.5) -3.8 (12.2) Cohort 2 ERT-Switch Nonambulatory n=4 n=4 n=4 n=3 42.3 (14.6) +2.3 (8.7) -12.5 (10.0) -13.3 (2.1) Cohort 3 ERT Naive n=5 n=5 n=5 n=5 39.2 (12.7) -5.2 (11.7) -7.2 (7.5) -2.0 (7.5)
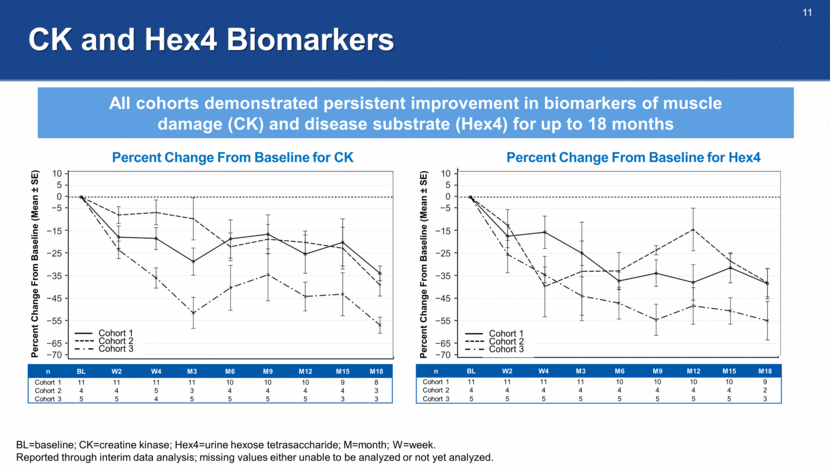
CK and Hex4 Biomarkers All cohorts demonstrated persistent improvement in biomarkers of muscle damage (CK) and disease substrate (Hex4) for up to 18 months Percent Change From Baseline (Mean ± SE) 10 5 0 -5 -15 -25 -35 -45 -55 -65 -70 Percent Change From Baseline for CK Percent Change From Baseline for Hex4 Percent Change From Baseline (Mean ± SE) 10 5 0 -5 -15 -25 -35 -45 -55 -65 -70 n BL W2 W4 M3 M6 M9 M12 M15 M18 Cohort 1 11 11 11 11 10 10 10 9 8 Cohort 2 4 4 5 3 4 4 4 4 3 Cohort 3 5 5 4 5 5 5 5 3 3 n BL W2 W4 M3 M6 M9 M12 M15 M18 Cohort 1 11 11 11 11 10 10 10 10 9 Cohort 2 4 4 4 4 4 4 4 4 2 Cohort 3 5 5 5 5 5 5 5 5 3 BL=baseline; CK=creatine kinase; Hex4=urine hexose tetrasaccharide; M=month; W=week. Reported through interim data analysis; missing values either unable to be analyzed or not yet analyzed. Cohort 1 Cohort 2 Cohort 3 Cohort 1 Cohort 2 Cohort 3
Safety Summary at 18 Months of Treatment AEs were generally mild and transient The most common treatment-emergent AEsa by decreasing frequencies were nasopharyngitis (10/20); fall (9/20); abdominal painb and diarrhea (8/20); upper respiratory tract infection (7/20); arthralgia, nausea, fatigue, pain in extremities, and myalgia (6/20); and headache, tremor, oropharyngeal pain, and muscle spasms (5/20) For SAEs, 5 events occurred in 4 patients (severity: 3 moderate, 2 mild) and were unrelated to treatment. SAEs did not lead to treatment interruption or study discontinuation. 7 incidents of IARs in 5 patients in 890+ infusions, which were controlled by standard medication or premedication 1 IAR event each in 3 ambulatory ERT-switch patients 1 IAR event in a non-ambulatory ERT-switch patient 3 IAR events in a ERT-naive patient Longest duration of treatment is 28+ months Safety data (N=20) for AT-GAA show that AEs have been generally mild and transient with very low rates of IARs (<1%) after 890+ total infusions across all cohorts AE, adverse events; ERT=enzyme replacement therapy; IAR, infusion-associated reaction; SAE=serious adverse event. aNumber of patients experiencing the AE; bIncludes upper and lower abdominal pain.
Conclusions at 18 Months of Treatment 6MWT showed continued benefit in ERT-switch and ERT-naive patients Timed motor function tests were generally consistent with 6MWT results in both ambulatory cohorts Muscle strength increased in all cohorts, including nonambulatory ERT-switch patients Pulmonary function FVC, MIP, and MEP generally increased in ERT-naive patients FVC, MIP, and MEP were generally stable in ERT-switch patients Fatigue Severity Scale Improvement in fatigue score was observed in all cohorts Biomarkers and safety CK and Hex4 levels decreased in all cohorts AT-GAA (ATB200/AT2221) was generally well tolerated 6MWT, an integrated measure of motor, cardiac, and pulmonary function, improved in ERT-switch ambulatory and ERT-naive patients out to Month 18 6MWT=6-minute walk test; CK=creatine kinase; ERT=enzyme replacement therapy; FVC=forced vital capacity; Hex4=urine hexose tetrasaccharide; MEP=maximal expiratory pressure; MIP=maximal inspiratory pressure.
Acknowledgments The authors thank the patients, their families, and Pompe disease patient organizations, as well as the study investigators ATB200-02 Clinical Trial Investigators: Drago Bratkovic, Barry J. Byrne, Paula R. Clemens, Tarekegn Geberhiwot, Ozlem Goker-Alpan, Priya Kishnani, Xue Ming, Mark Roberts, Peter Schwenkreis, Kumaraswamy Sivakumar, Ans T. van der Ploeg, Benedikt Schoser Amicus Therapeutics: Jacquelyn Wright, Sheela Sitaraman, Swati Sathe, Hjalmar Lagast, Jay A. Barth Third-party medical editing assistance was provided by ApotheCom (Yardley, PA)

